Research Article Open Access
Efficacy and safety of
Citrus sudachi
peel
in obese adults:
A randomized, double-blind, pilot study
Masashi Akaike1, Ken-ichi Aihara2, Hiroaki Yanagawa3, Takashi Iwase4, Sumiko Yoshida2, Chiho Sato3, Tomoka Saijo3, Hiroaki Mikasa5, Yoshizaki Kashiwada6, Yoshihisa Takaishi6, Koichiro Tsuchiya7, Toshiaki Tamaki8, Toshio Matsumoto2,
Masataka Sata4
1
Department of Medical Education, 2Department of Medicine and Bioregulatroy Sciences,
4
Department of Cardiovascular Medicine, 6Department of Natural Medicines, 7Department of Medical Pharmacology, 8Department of Pharmacology, The University of Tokushima Graduate School of Medical Sciences, 3Clinical Trial Center for Developmental Therapeutics, Tokushima University Hospital, 5Support Center for Medical Education, School of Medicine, The University of Tokushima, 3-18-15, Kuramoto-cho, Tokushima 770-8503, Japan
Correspondence author: Masashi Akaike, MD, PhD. Department of Medical Education, University of Tokushima Graduate School of Medical Sciences, 3-18-15 Kuramoto-cho, Tokushima 770-8503, Japan
Submission date: May 9, 2014; Acceptance date: June 27, 2014; Publication date: July 1, 2014
ABSTRACT
Objective: This study was undertaken to explore the efficacy and safety of Citrus sudachi
peel for metabolic risk factors in obese male and female adults.
Background: Citrus sudachi Hort. ex Shirai (Rutaceae), called “sudachi”, is a small, round, green citrus fruit that is mainly cultivated in Tokushima Prefecture in Japan. Our group reported that Citrus sudachi peel powder improved glucose tolerance and dyslipidemia in Zucher-fatty rats and reduced hyperglycemia and hypertriglyceridemia in GK diabetic rats.
Materials and Methods: We conducted a randomized, double-blind, placebo-controlled trial in 40 participants with abdominal obesity and metabolic risk factors including hypertension, impaired glucose tolerance and elevated triglyceride levels. Participants were randomized to receive either tablets that contained 1.3 g dried Citrus sudachi peel powder or placebo tablets for 12 weeks. The sudachi peel group included 14 males and 5 females with a mean age of 54.5 years, and the placebo group included 18 males and 2 females with a mean age of 51.9 years.
than 120 mg/dl, body weight, waist circumference and serum triglyceride levels were significantly decreased at several observation points after the start of treatment in the sudachi peel group but not in the placebo group. No serious adverse events were observed in the sudachi peel group.
Conclusions: Citrus sudachi peel has the potential effect to safely improve abdominal obesity and lower serum levels of TG in obese individuals with hypertriglyceridemia. A large-scale randomized, double-blind clinical study targeting subjects with both abdominal obesity and high TG levels is needed to confirm the metabolic effects of Citrus sudachi peel.
Trial registration: UMIN Clinical Trials Registry (UMIN-CTR) UMIN000002682. Accession number of the Ethics Committee for Clinical Trials of Food in Tokushima University Hospital is F5.
Key words: health functional food, anti-obesity, triglyceride
BACKGROUND:
Citrus sudachi Hort. ex Shirai (Rutaceae), called “sudachi”, is a small, round, green citrus fruit that is mainly cultivated in Tokushima Prefecture in Japan [1]. Slices of this fruit are often used for Japanese dishes as food flavoring in place of vinegar and the peel is also edible. Our group reported that Citrus sudachi peel powder improved glucose tolerance and dyslipidemia in Zucher-fatty rats and reduced hyperglycemia and hypertriglyceridemia in GK diabetic rats [2]. However, the effects of Citrus sudachi peel on lifestyle-related risk factors in humans have not been clarified. Metabolic syndrome is a combination of lifestyle-related risk factors that increase the risk of developing cardiovascular disease and diabetes [3]. The aim of this study was to explore the efficacy and safety of Citrus sudachi peel for improving lifestyle-related risk factors including obesity, hypertension, impaired glucose tolerance and dyslipidemia in humans.
METHODS:
were not appropriate in the attending physicians' opinion.
Experimental design: This study was designed as a randomized, double-blind, placebo-controlled trial. Participants were randomized to receive either 5 tablets that in total contained 1.3 g dried Citrus sud achi peel powder (sudachi peel group) or 5 placebo tablets (placebo group) every day for 12 weeks. Daily intake in the sudachi peel group was equivalent to 1/3 of a whole Citrus sudachi. Since analysis carried out by the Japan Food Research Laboratories showed that the content of synephrine in Citrus sudachi peel is 2.5 mg/g, estimated intake of synephrine, which is contained in abundance in citrus peel and has pharmacological effects similar to those of ephedrine, was only 3.25 mg/day in the sudachi peel group. Analysis carried out by the Japan Food Research Laboratories also showed that the content of hesperidin, a flavonoid found abundantly in citrus fruits, was 7.4 mg/g in Citrus sudachi peel, indicating that the estimated intake of hesperidin was only 9.62 mg/day in the sudachi peel group. The study period was 17 weeks including a 1-week observation period before treatment, 12-week treatment period with Citrus sudachi peel powder or placebo tablets and 4-week observation period after treatment. The subjects made visits to the hospital 1 week before the treatment, 4, 8 and 12 weeks after the start of treatment, and 4 weeks after the treatment period. A life diary including physical activity and exercise was recorded every day during the study period and a diet diary including content of meals was recorded for three days before each visit. The number of remaining tablets was checked at each point of visit to assess the compliance of subjects. Physical examination including measurements of height, body weight, BMI, waist circumference, BP and pulse was performed at each visit. Urine and blood samples were drawn from the antecubital vein after an overnight fast at each visit.
creatinine after correction by urinary creatinine concentration.
Ethics: The present clinical study is in compliance with the Helsinki Declaration. Prior written informed consent was obtained from all subjects before enrollment in this study in accordance with protocols approved by the Ethics Committee for Clinical Trials of Food in Tokushima University Hospital (accession number F5). This study was registered in the UMIN Clinical Trials Registry (UMIN-CTR) with the trial number of UMIN000002682.
Statistical Analysis: All parameters before and after treatment were compared between the sudachi peel group and the placebo group using generalized linear mixed model analysis. Differences in baseline patient characteristics between the two groups were analyzed by the unpaired t-test. The Wilcoxon signed rank test was used to assess significant changes in parameters after the start of treatment in each group. All data are expressed as means ± S.D. The analyses were performed on a Microsoft Windows computer running SPSS software.
Differences were considered statistically significant at p < 0.05.
RESULTS:
Basal characteristics: Forty subjects were enrolled in this study, but one subject could not visit our hospital due to personal reasons. Finally, the sudachi peel group included 14 males and 5 females with a mean age of 54.5 years (one patient having dropped out), and the placebo group included 18 males and 2 females with a mean age of 51.9 years. The patients’ characteristics are summarized in Table 1. The first, second and third quartile of TNFα levels is 1.3, 3.4 and 4.7 pg/mL in the placebo group, respectively, and 2.1, 2.3 and 3.2 pg/mL in the Sudachi peel group, respectively. There were no differences in basal parameters including age, sex, BMI, waist circumference, pulse, BP, TG, HDL-C, HbA1c and FPG between the sudachi peel group and the placebo group.
Intake rate of tablets: There was no difference between the intake rates of tablets in the two groups (95.36.7 % in the sudachi peel group and 96.16.0 % in the placebo group).
Live and diet diary: Physical activity and dietary intake of total calories and nutrients including carbohydrates, proteins and lipids were not different before, during and after treatment in either the sudachi peel group or placebo group. There were also no differences in these parameters at every observation point between the two groups.
Changes in parameters before, during and after treatment: Physical status including body weight, BMI, waist circumference, pulse and BP and laboratory markers including urinalysis, peripheral blood, liver function, renal function, electrolytes, LDL-C, TG, HDL-C, RLP-C, FFA, HbA1c, FPG, IRI, UA, hs-CRP, urinary 8-OHdG, TNF- and adiponectin were not different at any observation point between the two groups. There were also no differences in theses parameter before and 4, 8 and 12 weeks after the start of treatment and at 4 weeks after the treatment period in either the sudachi peel group or placebo group.
expression or worsening of symptoms.
Table 1. Baseline parameters in all subjects
Variables Placebo group
n=20
Sudachi peel group
n=19 P-value
Age 51.911.0 54.5 8.9 ns
Male : Female 18:2 14:5 ns
Body weight (kg) 76.2 10.8 75.610.9 ns
BMI 26.2 3.4 26.8 3.0 ns
Waist circumference(cm) 93.7 6.1 97.3 6.3 Ns
Pulse (bpm) 73.5 5.8 71.9 8.2 ns
Systolic BP (mmHg) 128.9 13.0 134.6 10.5 ns
Diastolic BP (mmHg) 82.3 6.2 87.9 8.3 ns
LDL-C (mg/dL) 134.7 25.2 140.8 20.4 ns
TG (mg/dL) 143.8 67.0 146.6 60.3 ns
HDL-C (mg/dL) 62.9 15.1 67.7 15.8 ns
RLP-C (mg/dL) 9.6 5.8 9.8 5.4 ns
FFA (Eq/L) 538.5 133.1 548.6 197.2 ns
HbA1c (%) 6.0 0.3 5.9 0.4 ns
FPG (mg/dL) 112.2 13.3 108.7 14.1 ns
IRI (g/dL) 8.0 3.1 7.7 3.6 ns
UA (mg/dL) 6.2 1.4 5.8 1.0 ns
hsCRP (g/dL) 100 116 93 98 ns
U-8OHdG(g/gCr) 9.9 4.6 11.9 7.5 ns
TNFα (pg/mL) 4.0 4.1 6.2 15.4 ns
Adiponectin(g/mL) 6.1 2.3 6.5 2.4 ns
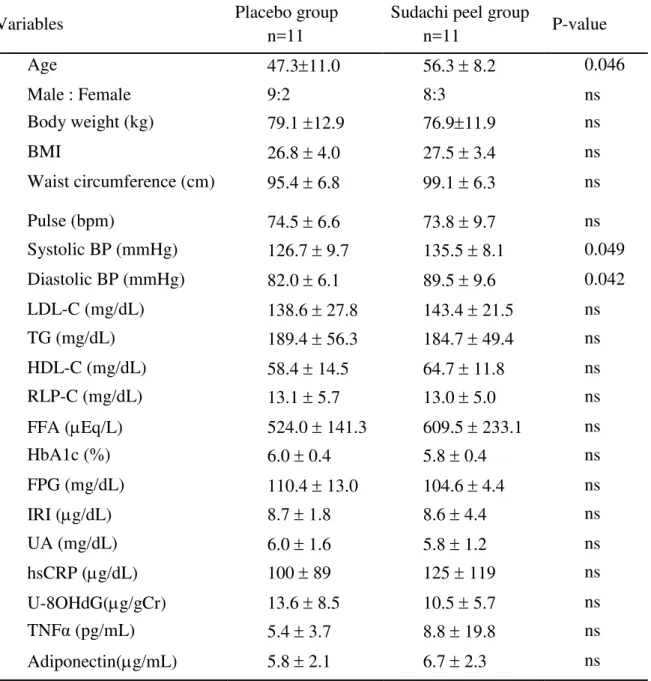
Table 2. Baseline parameters in subjects with serum TG levels of more than 120 mg/dl
Variables Placebo group
n=11
Sudachi peel group
n=11 P-value
Age 47.311.0 56.3 8.2 0.046
Male : Female 9:2 8:3 ns
Body weight (kg) 79.1 12.9 76.911.9 ns
BMI 26.8 4.0 27.5 3.4 ns
Waist circumference (cm) 95.4 6.8 99.1 6.3 ns
Pulse (bpm) 74.5 6.6 73.8 9.7 ns Systolic BP (mmHg) 126.7 9.7 135.5 8.1 0.049
Diastolic BP (mmHg) 82.0 6.1 89.5 9.6 0.042
LDL-C (mg/dL) 138.6 27.8 143.4 21.5 ns
TG (mg/dL) 189.4 56.3 184.7 49.4 ns
HDL-C (mg/dL) 58.4 14.5 64.7 11.8 ns RLP-C (mg/dL) 13.1 5.7 13.0 5.0 ns
FFA (Eq/L) 524.0 141.3 609.5 233.1 ns
HbA1c (%) 6.0 0.4 5.8 0.4 ns
FPG (mg/dL) 110.4 13.0 104.6 4.4 ns
IRI (g/dL) 8.7 1.8 8.6 4.4 ns
UA (mg/dL) 6.0 1.6 5.8 1.2 ns
hsCRP (g/dL) 100 89 125 119 ns
U-8OHdG(g/gCr) 13.6 8.5 10.5 5.7 ns TNFα (pg/mL) 5.4 3.7 8.8 19.8 ns
Adiponectin(g/mL) 5.8 2.1 6.7 2.3 ns
In subjects with serum TG levels of more than 120 mg/dl, body weight significantly decreased from 76.9±11.9 kg before treatment to 76.1±11.1 kg (p<0.05) and 76.0±11.1 kg (p<0.05) at 8 and 12 weeks after the start of treatment, respectively, and the decrease in body weight was maintained 4 weeks after the treatment period (75.6±11.4 kg, p<0.01 vs before the treatment) in the sudachi peel subgroup, but no significant changes in body weight were observed in the placebo subgroup (Figure 1A).
However, there was no difference in these parameters at any observation point between the two groups.
Figure 1. Changes of parameters in the placebo subgroup (□) and the sudachi peel subgroup
(■) with serum triglyceride levels of more than 120 mg/dL. Body weight was significantly decreased at 8 and 12 weeks after the start of treatment and the decrease in body weight was maintained 4 weeks after the treatment period in the sudachi peel subgroup (A). Waist circumference was also significantly decreased at 8 and 12 weeks after the start of treatment in the sudachi peel subgroup (B). Serum triglyceride (TG) levels were significantly decreased at 8 weeks after the start of treatment and at 4 weeks after the treatment period in the sudachi peel subgroup (C). Serum TG levels after the start of treatment significantly decreased compared with the baseline level in the sudachi peel group (p<0.05) (C). All data are expressed as ratios to baseline level before the start of treatment and means ± SEM. * p<0.05 and ** p<0.01 vs before treatment.
DISCUSSION:
This study is the first randomized, double-blind, placebo-controlled trial to clarify the effects and safety of Citrus sudachi peel in humans. We did not find any significant differences in clinical parameters between the placebo group and sudachi peel group in the present study. However, subgroup analysis using serum TG levels showed that intake of Citrus sudachi peel significantly decreased body weight, waist circumference and serum TG level in subjects with serum TG levels of more than 120 mg/dL, but such decreases were not observed in the placebo subgroup. On the other hand, the subgroup analysis did not show significant differences in parameters at any observation point after the start of treatment between the placebo group and the sudachi peel group. These findings suggested that number of enrolled subjects in this study is too small to show a significant difference between the two groups. In addition, Taskinen et al. reported that serum TG level in obese subjects is increased by the combination of increased secretion and severely impaired clearance of TG-rich VLDL1 particles and that increased secretion of TG-rich VLDL1 particles is linked to increased liver and subcutaneous abdominal fat [4], indicating that obesity with high TG levels has a metabolic pathology different from that in other types of obese patients with normal TG levels. Taken together, a large-scale clinical study enrolling obese subjects with high TG levels should be designed to clarify the clinical effects of sudachi peel.
orange juice decreases diastolic blood pressure and postprandially increases endothelium-dependent microvascular reactivity [7]. In Morand’s study, daily intake of hesperidin was 292 mg. However, in our study, estimated intake of hesperidin was quite low (only 9.62 mg/day) in the sudachi peel group. Synephrine contained in the peel of bitter orange citrus also has beneficial effect on obesity as a thermogenic agent like the action of ephedrine. In our study, estimated intake of synephrine was only 3.25 mg/day. Colker et al. conducted the first study on the effects of a bitter orange extract containing 58.5 mg p-synephrine and 528 mg caffeine daily on body fat loss and lipid levels in 20 overweight adult subjects [8]. A review of human studies on Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine showed that products containing a low dose of synephrine, such as in our study, have no anti-obesity effect [9]. Taken together, we speculate that the effect of Citrus sudachi peel on obesity and TG levels may not be due to synephrine.
Nakagawa et al. identified sudachitin (4',5,7-trihydroxy-3',6,8- trimethoxyflavone) and 3¢-demethoxysudachitin from Citrus sudachi peel as the most active compounds with antimicrobial activity against methicillin-resistant Staphylococcus aureus and
Helicobacter pylori [10]. Yuasa et al. also reported that sudachitin inhibited nitric oxide production by suppressing the expression of inducible nitric oxide synthase in lipopolysaccharide-stimulated macrophages, indicating that sudachitin has an anti-inflammatory effect [11]. However, no components having an anti-obesity effect or TG-lowering effect have so far been identified from the peel of Citrus sudachi. Further studies are needed to clarify the mechanisms by which the peel of Citrus sudachi
improves obesity and lowers serum TG levels.
In our study, there were no adverse effects of the intake of 1.3 g dried Citrus sudachi
peel every day for 12 weeks. There are concerns about side effects of citrus peel on the cardiovascular system since it contains a large amount of synephrine with ephedrine-like action. In Guidelines for the Use of Synephrine in Natural Health Products revised by Health Canada in 2010, 30 mg/day is the maximum allowable dose for total synephrine. Peel of Citrus sudachi may be safe for consumption because it contains less synephrine than that in other citrus peels. However, since the subjects of our study had no cardiovascular complications, the safety for patients with cardiovascular diseases was not confirmed. In addition, since the period of Citrus sudachi peel intake was only 12 weeks in our study, further study is needed to show the safety of long-term Citrus sudachi peel intake.
CONCLUSIONS:
Citrus sudachi peel has the potential effect to safely improve abdominal obesity and lower serum levels of TG in obese individuals with hypertriglyceridemia. A large-scale randomized, double-blind clinical study targeting subjects with both abdominal obesity and high TG levels is needed to confirm the metabolic effects of Citrus sudachi peel.
Competing interests: The authors have no financial interests or conflicts of interest.
Author’s contributions: All authors contributed to this study.
C-reactive protein; IRI, immunoreactive insulin; LDL, low-density lipoprotein; OHdG, hydroxydeoxyguanosine; RLP, remnant-like particles; TG, triglycerides; TNF, tumor necrosis factor; UA, uric acid
Acknowledgments and Funding: We thank Kazue Ishikawa for her technical assistance in measurements of TNF-, adiponectin and 8-OHdG. This study was funded by KTT Corporation (Osaka, Japan). Tablets of Citrus Sudachi peel or placebo were synthesized and provided by KTT Corporation.
REFERENCES:
1. Iuchi A, Hayashi K, Tamura K, Kono T, Miyashita M, Chakraborty SK: Technique of quality control for Sudachi (Citrus sudachi Hort. Ex Shirai) juice by high pressure treatment. In: High Pressure Bioscience and Biotechnology (Hayashi R and Balny C, ed.) Elsevier B.V., Amsterdam, 1996, pp. 387-390.
2. Ikeda Y, Tsuchiya K, Tajima S, et al. The effect of SUDACHI peel, a specialty of Tokushima, as anti-metabolic syndrome. J Pharmacol Sci 2010;112(Suppl 1):21. 3. Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a
global public health problem and a new definition. J Atheroscler Thromb
2005;12:295-300.
4. Taskinen MR, Adiels M, Westerbacka J, et al. Dual metabolic defects are required to produce hypertriglyceridemia in obese subjects. Arterioscler Thromb Vasc Biol
2011;31:2144-2150.
5. Wang X, Hasegawa J, Kitamura Y, et al. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J Pharmacol Sci 2011;117:129-138.
6. Akiyama S, Katsumata S, Suzuki K, Nakaya Y, Ishimi Y, Uehara M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem
2009;73:2779-2782.
7. Morand C, Dubray C, Milenkovic D, et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers.
Am J Clin Nutr 2011;93:73–80.
8. Colker CM, Kalman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s wort on body fat loss, lipid levels, and mood states in
overweight healthy adults. Curr Therap Res 1999;60:145-153.
9. Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine.
Int J Med Sci 2012;9:527-538.
10.Nakagawa H, Takaishi Y, Tanaka N, Tsuchiya K, Shibata H, Higuti T. Chemical constituents from the peels of Citrus sudachi. J Nat Prod 2006;69:1177-1179.