Márcia Santos Oliveira
Licenciada
Modulation of α-synuclein aggregation and
toxicity
Dissertação para obtenção do Grau de Mestre em Genética
Molecular e Biomedicina
Orientadores: Tiago Fleming Outeiro, Full Professor, IMM
Hugo Vicente Miranda, PhD, IMM
Setembro, 2013
Márcia Santos Oliveira
Licenciada
Modulation of α-synuclein aggregation and
toxicity
Modulation of α-synuclein aggregation and toxicity
Copyright Márcia Santos Oliveira, FCT/UNL, UNL
A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor.
I
Acknoledgements / Agradecimentos
Este último ano tem sido cheio de pontos altos e baixos. Preciso agradecer às pessoas que estiveram comigo nos bons momentos e que me apoiaram nos momentos menos bons.
Tenho desde já a agradecer ao Prof. Dr. Tiago Outeiro por me ter recebido no seu laboratório e me conceder a incrível oportunidade de fazer parte de um grupo de cientistas extremamente hábil e conhecedor.
Quero agradecer a todo o grupo que para além de serem excelentes profissionais, são também pessoas calorosas e com um imenso sentido de humor. Foram colegas extremamente prestáveis e disponíveis e que fizeram este ano passar certamente de uma forma mais leve. Agradeço em particular ao Dr. Hugo Vicente Miranda, que não só me tem apoiado nesta jornada, incentivando-me e transmitindo-me conhecimentos sem qualquer reserva, como sempre acreditou em mim e nas minhas capacidades.
Não posso deixar de agradecer ao Dr. Francisco Enguita por todo o apoio nas purificações, pelas dicas no manuseamento de proteínas e por todo o conhecimento que me facultou e à unidade de Bioimaging pela disponibilidade e prontidão.
Quero ainda agradecer ao Paulo por todo o apoio, ajuda e por me conseguir animar mesmo nos momentos menos bons.
Finalmente agradeço à minha família, em especial ao meu pai e à minha mãe, que durante toda a minha vida têm acreditado em mim, me têm apoiado e estado presentes. Sem eles não seria o que sou. Obrigada por tudo.
III
Resumo
A α-sinucleína (aSin) é uma proteína amilodoigénica propensa à agregação. Esta proteína é encontrada em inclusões específicas denominadas corpos de Lewys em neurónios sobreviventes de pacientes com doença de Parkinson e outras sinucleinopatias. O processo de agregação é amplamente afectado por diferentes modificações pós-traducionais, como a fosforilação, acetilação e glicação. Recentemente foi demonstrado que as espécies oligoméricas de aSin são mais tóxicas do que os corpos de inclusão. As “heat shock proteins” (HSPs) são chaperonas moleculares com a capacidade de modular o “folding” e o “refolding” de proteínas. A sua sobre-expressão em modelos de doença de Parkinson reduz e previne a agregação de aSin. Como a redução da agregação da aSin pode levar a uma possível acumulação de espécies oligoméricas que podem causar danos celulares, o principal objectivo deste trabalho é melhor entender o papel das HSPs na formação de oligómeros de aSin, clarificando que espécies de aSin são formadas na presença de HSPs. Adicionalmente, como a glicação potencialmente acelera a deposição anormal de proteínas, investigou-se a forma como as HSPs interferem no processo de oligomerização de aSin glicada. Neste estudo a Hsp70 parece induzir a agregação de aSin recombinante, gerando espécies com um elevado peso molecular mas sem toxicidade associada. A Hsp27 reduziu a oligomerização de aSin in
vitro, possivelmente pela indução da formação de pequenos oligómeros não reactivos. A glicação por
metilglioxal aumentou a agregação da proteína e a morte celular. Contudo, a sobre-expressão de Hsp27 reverteu a agregação de aSin glicada e diminuiu a sua toxicidade.
Estes resultados demonstram a importância da modulação das HSPs como alvo de possíveis terapêuticas da doença de Parkinson.
V
Abstract
It is widely known that α-synuclein (aSyn) is an amyloidogenic protein prone to aggregation. This protein is found in specific inclusions named Lewy bodies in the surviving neurons of Parkinsons’s disease patients and other synucleinopathy brains. This aggregation process is greatly affected by different post-translational modifications, such as phosphorylation, acetylation, and glycation. Lately it was shown that aSyn oligomeric species are more toxic than the inclusion bodies. Heat shock proteins (HSPs) are molecular chaperones able to modulate the folding and refolding of proteins. Its overexpression in Parkinson’s disease models reduces and prevents aSyn aggregation. As the reduction of aSyn aggregation can lead to an eventual accumulation of oligomeric species which may cause cell damage, the main goal of this work is to better understand the role of HSPs in aSyn oligomer formation, clarifying which are the aSyn resulting species formed in the presence of HSPs. Moreover, as glycation is suggested to accelerate abnormal protein deposition, we aimed to investigate how HSPs interfere with the oligomerization process of glycated aSyn.
In this study Hsp70 seemed to induce recombinant aSyn oligomerization, generating higher molecular weight species with no associated toxicity. On the other hand, Hsp27 reduced aSyn oligomerization in
vitro possibly by inducing the formation of non-reactive small oligomers. MGO glycation increased
protein aggregation and cell death. Interestingly, Hsp27 overexpression reversed glycated aSyn aggregation and its associated toxicity.
These results demonstrate the importance of HSPs modulation as a possible target of Parkinson’s disease therapeutics.
VII
Table of contents
Acknoledgements / Agradecimentos ... I Resumo ... III Abstract... V Abbreviations ... XI 1. Introduction ... 1 1.1 aSyn ... 1 1.1.1 aSyn sequence... 1 1.1.2 aSyn function... 2 1.1.3 aSyn aggregation ... 3 1.1.4 aSyn toxicity ... 31.1.5 aSyn pathology transmission ... 4
1.1.6 Glycation of aSyn ... 5
1.2 Cell protein quality control systems ... 5
1.2.1 Heat Shock Protein 27 ... 6
1.2.2 Heat shock protein 70 ... 7
1.2.3 Heat shock protein 104 ... 8
1.2.4 Aim of the study... 8
2. Materials and Methods ... 9
2.1 Proteins expression ... 9
2.1.1 Human recombinant aSyn expression and purification... 9
2.1.2 Hsp 27 expression and purification ... 10
2.1.3 Hsp 70 expression and purification ... 10
2.1.4 Hsp 104 expression and purification ... 11
2.2 aSyn oligomerization... 12
2.2.1 SDS-PAGE / Native PAGE Western blot analysis ... 12
2.2.2 Thioflavin T binding assay ... 12
2.2.3 Size exclusion chromatography (SEC) ... 13
2.3 Cell culture ... 13
2.3.1 Transfection of mammalian cells ... 13
VIII
2.3.3 Triton-X 100 solubility assay ... 13
2.3.4 Immunocytochemistry ... 14
3. Results ... 15
3.1 Protein expression an purification ... 15
3.2 Effect of Hsp27 and Hsp70 on aSyn oligomerization in vitro... 16
3.3 Cytotoxicity of aSyn species ... 19
3.4 Effects of glycation on aSyn oligomerization ... 20
3.5 Effects of Hsp27 on aSyn oligomerization in human cells ... 21
4. Discussion ... 25
IX
Table of figures
Figure 1.1 Schematic representation of the primary sequence of aSyn. ... 2
Figure 1.2 Scheme of aSyn oligomerization pathway. ... 3
Figure 1.3 Oligomers are the main toxic aSyn specie in PD. ... 4
Figure 1.4 Protein quality control system. ... 6
Figure 3.1 Protein expression in E. coli BL21+ ... 15
Figure 3.2 Hsp27 decreases aSyn oligomerization in a dose dependent manner... 16
Figure 3.3 Hsp27 decreases aSyn oligomerization over-time in a dose dependent response. ... 17
Figure 3.4. Hsp70 promotes aSyn fibrillization ... 18
Figure 3.5 Hsp27 and Hsp70 do not alter the elution profiles of aSyn in SEC analysis ... 19
Figure 3.6 MGO induces and Hsp27 reverses aSyn fibrillization ... 20
Figure 3.7. MGO induces oligomerization of glycated aSyn ... 21
Figure 3.8 Hsp27 does not alter aSyn solubility ... 22
Figure 3.9 Hsp27 reduces aSyn aggregates in a H4 cell PD model ... 22
Figure 3.10 MGO induces aSyn insolubilization... 23
Figure 3.11 MGO increases aSyn aggregation in a H4 cell PD model ... 23
XI
Abbreviations
A30P Alanine to proline substitution in residue 30 of α-synuclein A53T Alanine to threonine substitution in residue 53 of α-synuclein
Aβ Amyloid β peptide
AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid aSyn Alpha-synuclein
ATP Adenosine triphosphate
Au Arbitrary units
BCA assay Bicinchoninic acid assay
Ctrl Control
C-terminal Carboxyl-terminal DNA Deoxyribonucleic acid
E. coli Escherichia coli
E46K Glutamic acid to lysine substitution in residue 46 of α-synuclein ER Endoplasmic reticulum
FBS Fetal bovine serum
HSF1 Heat shock factor 1 HSP Heat shock protein Hsp104 Heat shock protein 104 Hsp27 Heat shock protein 27 Hsp70 Heat shock protein 70
IPTG Isopropyl β-D-1-thiogalactopyranoside
LB Luria Bertani
LDH Lactate dehydrogenase
MGO Methylglyoxal
NAC Non-amyloid component of Alzheimer’s disease N-terminal Amino-terminal
OD600 Optical density at 600nm
PAGE Polyacrylamide gel electrophoresis PBS Phosphate buffer saline
PD Parkinson’s disease
PLD2 Phospholipase D2
PrP Prion protein
RNA Ribonucleic acid
S129A Serine to alanine substitution in residue 129 of alpha-synuclein SDS Sodium dodecil sulphate
SEC Size exclusion chromatography
XII Tris Trishydroxymethylaminomethane
1
1. Introduction
Parkinson’s disease (PD) is a progressive, neurodegenerative disease affecting ~1% of the population above 60 years old (de Lau and Breteler, 2006). By 2030, the prediction is that it will affect between 8,7 to 9,3 million people throughout the world (Dorsey et al., 2007). The cardinal motor symptoms of PD include resting tremor, bradykinesia, muscular rigidity and postural instability. However, non-motor symptoms like depression, dementia and psychosis are also present in PD (Weintraub et al., 2008). Although several genetic alterations were shown to cause familial cases of disease (Pankratz and Foroud, 2007), ~90% of the cases are sporadic and highly linked to aging (Weintraub et al., 2008). Pathologically, PD is mainly characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta. This results in the depletion of dopamine in the striatum and other brainstem neurons, with the consequent disruption of the cerebral neuronal systems responsible for motor functions (Lotharius and Brundin, 2002). Other hallmark of the disease is the presence of protein inclusions, known as Lewy bodies, in the surviving neurons (Gibb, 1989; Greenamyre and Hastings, 2004). Among other components, Lewy bodies are composed by nearly 80 proteins (Licker et al., 2009) being α-synuclein (aSyn) the main element of these structures (Spillantini et al., 1997).
1.1aSyn
aSyn was originally identified in the neuromuscular junction of the Pacific electric eel Torpedo
Californica (Maroteaux et al., 1988), being highly conserved between vertebrates (George, 2002).
Human aSyn is an abundant presynaptic protein with a perinuclear localization first identified as the precursor of the non-Aβ component (NAC) peptide, present in extracellular amyloid plaques in some forms of Alzheimer’s disease (Iwai et al., 1995). Recently this protein was also identified in bone marrow and peripheral erythrocytes (Nakai et al., 2007).
1.1.1 aSyn sequence
aSyn is a natively unfolded, heat-resistant, acidic protein of 14kDa composed by 140 amino acid residues (Recchia et al., 2004). Its sequence can be divided into three main regions: (i) the first 60 residues, containing five imperfect repeats (coding for amphipathic α-helices) with a conserved motif (KTKEGV); (ii) the residues 61–95, representing the hydrophobic and amyloidogenic NAC region, display three additional KTKEGV repeats; (iii) and the C-terminal region (residues 96-140) which is highly enriched in acidic residues and is therefore though to be responsible for the solubility of the protein. The first two regions include a membrane-binding domain, while the C-terminal is thought to be responsible for protein–protein and protein–small molecule interaction sites (Breydo et al., 2012) (figure1.1). Missense mutations in the aSyn gene (SNCA) (Polymeropoulos, 1997; Krüger et al., 1998; Zarranz et al., 2004; Appel-Cresswell et al., 2013; Kiely et al., 2013) as well as gene multiplications (Singleton et al., 2003) are associated with familial forms of the disease.
2 Figure 1.1 Schematic representation of the primary sequence of aSyn. The sequence can be subdivided into three domains: the N-terminal domain which includes six imperfect KTKEGV repeats (red sequences), the NAC (non-Aβ component of Alzheimer’s disease) domain and the C-terminal domain. Both N-terminal and NAC domain comprise a membrane-binding region.
1.1.2 aSyn function
So far the function of aSyn remains largely unclear. However, several putative functions have been hypothesized. Since this protein is localized in the synaptic terminals and contain membrane-binding protein domains (Jenco et al., 1998), it may be involved in neurotransmitter release. Interestingly, it was observed that membrane-bound aSyn inhibits phospholipase D2 (PLD2), suggesting aSyn may also participate in vesicle regulation (Jenco et al., 1998; Lotharius and Brundin, 2002). PLD2 catalyzes the hydrolysis of phosphatidylcholine to generate phosphatidic acid, which is important in the regulation of vesicle transport and of changes in cell morphology (Lotharius and Brundin, 2002). aSyn may also display a role on dopamine transmission as it has an inhibitory activity on tyrosine hydroxylase, the rate-limiting enzyme involved in dopamine biosynthesis (Perez et al., 2002), and also it may enable the increase of plasma membrane dopamine transporter molecules (Lee et al., 2001). Recently, aSyn was shown to function as a non-classical chaperone, where its activity is essential for the maintenance of continuous presynaptic SNARE complex assembly and disassembly, which is crucial for the repeated release of neurotransmitters by the presynaptic nerve terminals (Burré et al., 2010). aSyn is also believed to play an important role in nerve terminals protection against injury (Chandra et al., 2005), although some studies show that aSyn increases the sensitivity of cells to the toxic effects of proteasome inhibition (Petrucelli et al., 2002).
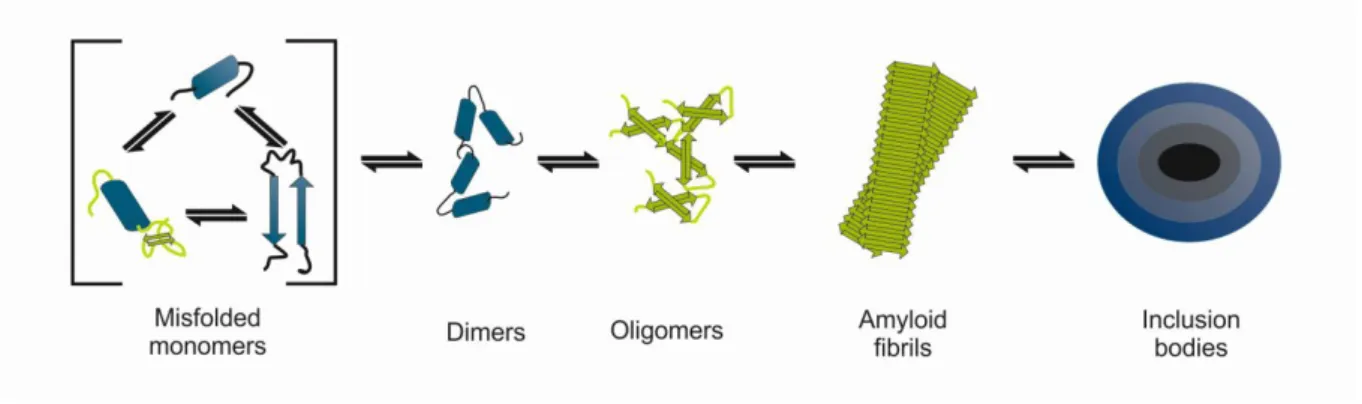
3 1.1.3 aSyn aggregation
Under conditions not currently fully understood, aSyn is able to misfold to assemble into fibrillar-like structures in a nucleation-dependent mechanism (Uversky et al., 2001) (figure1.2). While initial studies suggested fibrils are the main toxic aSyn species inducing cell damage in PD pathology (de la Fuente-Fernandez et al., 1998; Goedert, 2001), it is now widely accepted that the formation of these structures may be related with a protective cellular mechanism analogous to aggresome formation, in which the formation of fibrils serves to sequester potentially cytotoxic soluble aSyn species (McNaught
et al., 2002; Olanow et al., 2004). In addition, a pathological examination of human brains revealed
that dopaminergic neurons containing Lewy bodies appear morphological and biochemically “healthier” than the surrounding neurons (Tompkins and Hill, 1997). Interestingly, Lewy bodies are commonly observed at autopsy of aged individuals with no neurodegenerative disease symptoms (Bloch et al., 2006). Thus it seems likely that protein aggregation intermediates, known as oligomers, rather than the larger intracellular inclusions, might be the pathogenic species in PD. Nevertheless, the neuronal death mechanisms remain unknown, possibly involving multiple molecular pathways (Conway, 2000; Outeiro et al., 2008; Karpinar et al., 2009; Winner et al., 2011).
Figure 1.2 Scheme of aSyn oligomerization pathway. aSyn monomers may misfolded and aggregate into dimers and oligomers, ultimately being sequestered in proteinaceous inclusion bodies.
1.1.4 aSyn toxicity
Different studies showed that aSyn oligomers might promote the cell demise by affecting its proteostasis, namely by inhibiting the activity of the 20S and 26S proteasomes (Petrucelli et al., 2002; Lindersson et al., 2004; Xilouri et al., 2013) and by repressing the protein refolding rate of the Hsp70 chaperone system (Hinault et al., 2010). Impaired proteostasis can result in chronic endoplasmic reticulum (ER) stress that may culminate in neurodegeneration. aSyn oligomers can be formed and/or accumulated within ER, sensitizing neurons to ER stress (Colla et al., 2012a, 2012b). Moreover, this accumulation impairs the ER-Golgi trafficking, disturbing endoplasmic reticulum associated degradation (Cooper et al., 2006). Extracellularly, aSyn oligomers may be associated with different toxicity mechanisms according to their morphologic structure. aSyn oligomers with a globular morphology may act at synapses, altering the function of glutamatergic receptors, activating AMPA
4 receptor-mediated excitatory postsynaptic currents (Hüls et al., 2011; Diógenes et al., 2012). This may lead to dysfunctional synaptic signalling or excitotoxicity and neuronal death, and also to the impairment of hippocampal long-term potentiation (Diógenes et al., 2012; Martin et al., 2012). Either by decreasing the cell membrane lipid bilayer order (Stöckl et al., 2013) or by pore-forming activity (Kayed et al., 2009), aSyn oligomers with an annular structure are associated with the disruption of the cell membrane integrity, which might lead to an increase in intracellular calcium and transition metal dyshomeostasis that result in neuronal death (Pacheco et al., 2012). Both globular and annular aSyn oligomers may promote neuronal death by a seeding phenomenon where misfolded soluble oligomers enter the cell and operate as seeding nuclei that trigger new aggregates formation (Danzer et al., 2007) (figure1.3).
Figure 1.3 Oligomers are the main toxic aSyn specie in PD. aSyn oligomeric species are cytotoxic by mechanisms that include 1. impairment of protein quality control systems, 2. prion-like transmission, 3. seeding effect, 4. endoplasmic reticulum (ER) stress, (5.) membrane pore formation and 6. glutamate receptor disorder, which may all be related with PD pathology (adapted from Kalia et al. 2012).
1.1.5 aSyn pathology transmission
Cell culture studies showed that aSyn oligomers are released either by passive release (like membrane disruption) or by active processes such as exocytosis or calcium-dependent exosomal mechanisms (Danzer et al., 2012) (figure1.3). The uptake mechanism is thought to occur by endocytosis (Volpicelli-Daley et al., 2011). This cell-to-cell transmission of aSyn was recently suggested upon observation of the widespread and acceleration of the synucleinopathy in central
5 nervous system upon injection of aSyn fibrils in mice neocortex and striatum (Luk et al., 2012). This aSyn propagation process was also reported in cases where human PD patients grafts of fetal mesencephalic tissue (Li et al., 2008) were also affected by the synucleinopathy. These results suggest a aSyn self-propagating mechanism such as those of infectious prion proteins (Goedert et al., 2010).
1.1.6 Glycation of aSyn
aSyn is a target of several post-translational modifications including phosphorylation, ubiquitination, truncation, nitration and oxidation, which can modify the protein structure and influence its toxicity (Beyer and Ariza, 2013). Glycation is another aSyn putative post-translational modification in which several non-enzymatic reactions between carbonyl-containing groups and amino groups leads to the formation of advanced glycation end products (Vicente Miranda and Outeiro, 2010). It is suggested to increase aSyn proteolytic degradation resistance (Vicente Miranda and Outeiro, 2010). This type of modifications were first reported in the substancia nigra and locus coeruleus, revealing higher amounts near Lewy bodies in PD patients (Castellani et al., 1996). Additionally, the levels of glycated proteins were shown higher in PD patients than in control cases (Dalfó et al., 2005). In vitro, two glycation agents (methylglyoxal and glyoxal) were shown to induce aSyn oligomerization and to reduce its membrane-binding capacity (Lee et al., 2009a).
1.2 Cell protein quality control systems
Cells possess powerful quality control systems that enable them to cope with protein misfolding. These maintain the proteome homeostasis by ensuring proteins correct folding or, whenever the folding process fails, by targetting these misfolded proteins for degradation, avoiding deleterious effects associated with protein aggregation. Molecular chaperones are part of the protein quality control system and contribute to the correct folding proteins (Buchberger et al., 2010; Theodoraki and Caplan, 2012) (figure1.4). Whenever the refolding of misfolded proteins is not possible, proteins are targeted to elimination, a process that may occur by two major systems: (i) ubiquitin proteasome system, that is the primary route for the degradation of short-lived proteins and the mechanism that provide the control on the steady-state levels of many regulatory proteins; (ii) and the autophagy lysosome pathway, which is responsible for the degradation of long-lived proteins and other cell components (figure1.4). The elimination of misfolded proteins is controlled by both systems (Mittal and Ganesh, 2010; Hartl et al., 2011).
6 Figure 1.4 Protein quality control system. Chaperones are able to correctly assist the folding of the newly-synthetized proteins into their native conformation. When proteins reach a misfolded state, chaperones play a major role by facilitating the generation of intermediate folding states that can further aquire their correct folding. In some cases, chaperones may facilitate the aggregation of proteins that in a more soluble state would be noxious to the cell, or by oposition facilitate the dissociation of proteins to their active conformation state. Whenever the chaperone activity is not possible, proteins are targeted for degradation either by the ubiquitin proteasome system or by autophagy.
1.2.1 Heat Shock Protein 27
The heat shock protein 27 (Hsp27), a small molecular chaperone, is one of the main inducible HSPs, up-regulated in response to a variety of stress conditions including heat shock and oxidative environments (Landry et al., 1989; Dimant et al., 2012). This protein is mainly expressed in motor and sensory neurons in the brainstem, cranial nerve nuclei and cerebellum, having a limited expression in other post-mitotic neuronal types (Stetler et al., 2010). The regulation of Hsp27 activity occurs through its oligomerization and phosphorylation status. This oligomerization process depends on the association of its C-terminal regions in higher molecular weight structures. After its N-terminal serine
7 residues phosphorylation, the high molecular weight targets dissociates into lower oligomeric structures, which are closely related with the promotion of cell survival under stress conditions (Stetler
et al., 2009).
Several studies investigated the role of Hsp27 in neurodegenerative disorders. In a mouse model of PD overexpressing aSyn from a viral vector, Hsp27 was upregulated, evidencing a clear correlation with the disease (St Martin et al., 2007). Hsp27 significantly protected cultured cells against aSyn-induced stress, especially in A30P and A53T aSyn expressing cells (Zourlidou et al., 2004), and to reduce aSyn aggregation and associated toxicity in cultured dopaminergic neurons (Outeiro et al., 2006). Hsp27 is also able to reduce apoptosis in PC12 cells treated with oxidopamine (a cellular model of Parkinsonism), by delaying both cytochrome c release and caspase activation (Gorman et
al., 2005). Moreover, this chaperone also proved to play a role on other misfolding diseases. In
Huntington’s disease Hsp27 suppresses polyglutamine-mediated cell death in both Huntington’s disease rodent and cell line models. This suppression probably occurs by limiting the production of reactive oxygen species and inhibiting caspase activation (Wyttenbach et al., 2002; Perrin et al., 2007). In Alzheimer’s disease, β-amyloid peptide (1-42) fibril formation was inhibited by Hsp27 (Kudva
et al., 1997). This same molecular chaperone has a neuroprotective effect in cortical neurons exposed
to β-amyloid peptide (King et al., 2009). The level of hyperphosphorylated Tau, correlated with Alzheimer’s disease, was found decreased by the presence of Hsp27, suppressing the Tau-mediated cell death (Shimura et al., 2004).
1.2.2 Heat shock protein 70
Heat shock protein 70 (Hsp70) is present in several intracellular compartments, being essential in the cytosol, mitochondria, endoplasmic reticulum, lysosomes and extracellular compartments (Stetler et
al., 2010). Hsp70 is associated with different chaperone processes such as refolding of misfolded or
aggregated proteins, prevention of protein aggregation, folding and assembly of newly synthetized peptides and promotion of the ubiquitination and consequent elimination of misfolded proteins by the proteasome system (Turturici et al., 2011). Its activity is regulated by the interaction with and control of numerous co-chaperone families in a complex manner (Stetler et al., 2010). The Hsp70 has a higher affinity to proteins in unfolded or partially folded states, interacting with an extended protein segment mainly hydrophobic, releasing them in an ATP-dependent manner (Bukau and Horwich, 1998).
Hsp70 reduces the formation of aSyn fibrils by the preferred interaction with prefibrillar species structural features (Dedmon et al., 2005). However, it was shown that Hsp70 modulates aSyn fibril formation by inhibiting its elongation, reducing the quantity of mature fibrils available and therefore the risk of proteasome toxicity (Huang et al., 2006; Luk, Mills, Trojanowski, and Lee, 2008). In addition to inhibiting aSyn aggregation in a mouse model of PD, Hsp70 also decreases aSyn toxicity in a cellular model (Klucken et al., 2004a). Interestingly, in a Drosophila model, a reduction of aSyn toxicity was also observed. However, no effect on aggregation was detected (Auluck et al., 2002). Geldanamycin is a chemical activator of the heat shock response that induces the expression of Hsp70 and other HSPs via inhibition of Hsp90 and subsequent activation of heat shock factor-1 (HSF1) (Zou et al.,
8 1998). This compound prevented aSyn aggregation and toxicity in a cell model (McLean et al., 2004) and also prevented aSyn toxicity in mice (Shen et al., 2005) and in flies (Auluck and Bonini, 2002; Auluck et al., 2005). A recent study with carbenoxolone, a geldanamycin analogue, also reduced aSyn aggregation and associated toxicity in a cell model (Kilpatrick et al., 2013). As previously mentioned aSyn is present extracellularly and is taken up by neighboring cells, having toxic consequences. Interestingly Hsp70 appears to act on extracellular aSyn preventing the formation of oligomeric species, rescuing the oligomer-induced toxicity (Danzer et al., 2011).
1.2.3 Heat shock protein 104
The heat shock protein 104 (Hsp104) is only found in yeast and is essential for yeast survival at high ethanol concentrations. Moreover it also allows the stationary-phase yeast cells and spores to exhibit a naturally high thermo-tolerance (Parsell et al., 1994). Hsp104 belongs to the AAA+ superfamily of ATPases, coupling energy from ATP hydrolysis to remodel an extensive variety of proteins, DNA and RNA (Shorter, 2008). It is mainly localized at the mitochondria, playing a role in the protein quality control by recognizing and solubilizing both structurally poorly defined aggregates and cross-β sheet amyloid fibrils to their native conformation (Stetler et al., 2010; Murray and Kelly, 2012). The Hsp70 system is associated with this process and likely assists the protein refolding after the aggregates dissociation (Glover and Lindquist, 1998).
Interestingly, Hsp104 potently inhibited the fibrillization of wild type aSyn and also to the PD-linked mutant variants A30P, E46K and A53T; and also to serine 129 phospho-resistant mutant S129A and phospho-mimicking S129E. It also reduced the dopaminergic neurodegeneration in rat lentiviral model of PD (Lo Bianco et al., 2008). In a Caenorhabditis elegans Huntington’s disease model, Hsp104 was able to reverse the protein-folding homeostasis imbalance originated by the accumulation of polyglutamine aggregates (Satyal et al., 2000). The reduction of polyglutamine aggregation and cell death by the action of Hsp104 was also observed in a mammalian cell line (Carmichael et al., 2000), in a transgenic mouse (Vacher et al., 2005) and in lentiviral-based rat models of Huntington’s disease (Perrin et al., 2007). Hsp104 likewise inhibited the fibril formation of human PrP106–126, disaggregated mature PrP106–126 (Liu et al., 2011) and suppressed amyloid β protofibrils and fibrils growth and self-assembly (Arimon et al., 2008).
1.2.4 Aim of the study
As some HSPs have the ability to inhibit aSyn aggregation and it was proposed that aSyn toxicity is tightly linked to oligomerization, the role of molecular chaperones as therapeutic targets for amyloidogenic neurodegenerative diseases needs to be further studied. With this study, we aim to better understand the role of HSPs on aSyn oligomerization, clarifying which aSyn species arise in the presence of HSPs. Moreover, being glycation suggested to accelerate abnormal protein deposition, we wanted to investigate how the HSPs interfere with the oligomerization process of glycated aSyn.
9
2. Materials and Methods
2.1 Proteins expression
2.1.1 Human recombinant aSyn expression and purification
Escherichia coli (E. coli) strain BL-21 (GE Healthcare, Buckinghamshire, United Kingdom) was
transformed by heat shock with aSyn pT7-7 construct (a gift from Dr. Hilal Lashuel, Laboratory of Molecular and Chemical Biology of Neurodegeneration, Brain Mind Institute, Switzerland.). A transformed colony was transferred into 10 mL of LB medium supplemented with ampicillin (100 mg/L) and chloramphenicol (34 mg/L) and incubated overnight at 37º C with continuous shaking. 2.5 mL of the bacterial culture was added to a 2 L flask containing 500 mL of LB medium supplemented with antibiotics. The culture was incubated at 37º C under continuous shaking and the protein expression was induced with 0.3 mM isopropyl D-thiogalactopyranoside (IPTG) (Sigma-Aldrich, Missouri, United States of America) at an OD600 of 0.6. After 3 h of expression cells were harvested by a 15 min
centrifugation at 8000 g at 4º C and stored at -20º C until further use.
The cell pellet was resuspended in 20 mL of lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM ethylenediamine tetra-acetic acid, 1 mM phenylmethylsulfonyl fluoride) and lysed by freeze and thaw cycles with liquid nitrogen and five 30 sec sonication cycles (amplitude 10 microns) with 1 min incubation on ice between each sonication step (Soniprep 150 MSE). Taking advantage of aSyn thermostability, the cell extract was heated at 100º C for 20 min to precipitate non-thermostable proteins. After a 22000 g centrifugation for 30 min at 4º C, 30% (w/v) ammonium sulphate was added to the supernatant, allowing aSyn to precipitate by stirring for 30 min at 4º C. A new centrifugation was performed as previously described and the pellet resuspended in 5 mL of 30 mM Tris-HCl pH 7.4. The extract containing aSyn was centrifuged at 45000 g at 4º C for 30 min and the supernatant filtered with 0.22 µm filter and applied in a PD-10 gel filtration column for desalting (GE Healthcare, Buckinghamshire, United Kingdom).
The sample was loaded into an ion-exchange chromatography Q Sepharose TM (GE Healthcare, Buckinghamshire, United Kingdom) fast flow column equilibrated with 30 mM Tris-HCl, pH 8.0. Proteins were eluted with a linear NaCl gradient (0.12– 0.5 M) at a flow rate of 1.5 mL/min and the elution monitored at 280 nm. Protein-containing fractions were collected and probed by immunoblot analysis using Syn-1 anti-aSyn antibody (BD Transduction Laboratories, catalogue number 610786, New Jersey, United State of America). Fractions containing aSyn were collected, concentrated by centrifugation using Amicon filters (Millipore, Massachusetts, United States of America), and applied to a gel filtration Superdex 75 column (GE Healthcare, Buckinghamshire, United Kingdom), equilibrated with 50 mM Tris-HCl buffer, pH 7.4, containing 150 mM NaCl. Proteins were eluted with the same buffer at a flow rate of 1 mL/min. Fractions containing aSyn, probed by immunoblot, were collected, combined and applied in a 30 kDa Amicon filter (Millipore, Massachusetts, United States of America) to further purify monomeric aSyn. Final protein aliquots with ~100% purity were spectrophotometrically quantified in a nano-drop (using a molar extinction coefficient at 280 nm of 5960 M/cm) (Nanodrop
10 1000, Thermo scientific, Massachusetts, United States of America) and stored at -80º C until further use.
2.1.2 Hsp27 expression and purification
E. coli strain BL-21 was transformed by heat shock with Hsp27 PET16b construct (a kind gift from Dr.
Paul Muchowski, Gladstone Institute of Neurological Disease, University of California San Francisco, United States of America). A transformed colony was transferred into 20 mL of LB medium supplemented with ampicillin (100 mg/L) and incubated overnight at 37º C with continuous shaking. 5 mL of the bacterial culture was added to a 2 L flask containing 500 mL of LB medium supplemented with antibiotic. The culture was incubated at 37º C under continuous shaking and the protein expression was induced with 0.3 IPTG (Sigma-Aldrich, Missouri, United States of America) at an OD600 of 0.5. After 3 h of expression cells were harvested by a 15 min centrifugation at 8000 g at 4º C
and stored at -20º C until further use.
The cell pellet was resuspended in 15 mL of lysis buffer and 10 mg/mL of lysozyme (Sigma-Aldrich, Missouri, United States of America) was added. The cell suspension was then incubated on ice with constant stirring for 20 min. 0.33 mL/L benzonase (Sigma-Aldrich, Missouri, United States of America) was added to the cell suspension which was subsequently incubated at room temperature for 20 min with constant stirring. Insoluble cellular debris was removed by centrifugation at 36000g for 30min at 4º C. 53 mL/L of a 200 mM dithiothreitol (Sigma-Aldrich, Missouri, United States of America) solution was added to the soluble supernatant being incubated at room temperature with constant stirring for another 10 min. Insoluble contaminants were removed by centrifugation at 36000 g for 30 min at 4º C and the supernatant was filtered with a 0.22 µm filter.
The sample was loaded into an ion-exchange chromatography Q Sepharose TM fast flow column equilibrated with 20 mM Tris-HCl, pH 8.0. Proteins were eluted with a linear NaCl gradient (0 - 1.0 M) at a flow rate of 1.5 mL/min and the elution monitored at 280 nm. Protein-containing fractions were collected and probed by SDS-PAGE analysis using coomassie staining. Fractions containing the Hsp27 were collected, concentrated by centrifugation using Amicon filters and applied to a gel filtration Superdex 75 column, equilibrated with 20 mM Tris-HCl buffer, pH 7.4, containing 100 mM NaCl. Proteins were eluted with the same buffer at a flow rate of 1 mL/min. Fractions containing Hsp27, as analyzed by SDS-PAGE, were collected, concentrated by centrifugation using Amicon filters and final protein aliquots with 95% purity were stored at -80º C until further use.
2.1.3 Hsp70 expression and purification
E. coli strain BL-21 was transformed by heat shock with Hsp70 pET28A construct with N-terminal His6
tag (a kind gift from Richard Morimoto, Department of Molecular Biosciences, Rice Institute for Biomedical Research, Northwestern University, United States of America). A transformed colony was transferred into 10 mL of LB medium supplemented with ampicillin (100 mg/L) and kanamycin (50 mg/L) and incubated overnight at 37º C with continuous shaking. 5 mL of the bacterial culture was added to a 2 L flask containing 500 mL of LB medium supplemented with antibiotic. The culture was
11 incubated at 37º C under continuous shaking and the protein expression was induced with 0.5 mM IPTG when the culture reached an OD600 of 0.7. After another 3 h growth period, cells were harvested
by a 15 min centrifugation at 8000 g at 4ºC and stored overnight at -20º C.
The cell pellet was resuspended in 20 mL of lysis buffer and lysed by three freeze and thaw cycles with liquid nitrogen and five times 30 sec sonication cycles (amplitude 10 microns) with 1 min incubation on ice between each sonication step. The Hsp70 containing extract was centrifuged at 15000 g at 4 ºC for 40 min and the supernatant was filtered with a 0.22 µm filter.
The sample was loaded into an immobilized Ni2+ affinity chromatography Histrap column (GE Healthcare, Buckinghamshire, United Kingdom) equilibrated with 20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole, pH 7.4. Proteins were eluted with equilibration buffer containing 500 mM imidazole at a flow rate of 1 mL/min and the elution monitored at 280 nm. Protein-containing fractions were collected and probed by SDS-PAGE analysis using coomassie staining. Fractions containing Hsp70 were collected, concentrated by centrifugation using Amicon filter, and applied to a gel filtration Superdex 75 column, equilibrated with 50 mM Tris-HCl buffer, pH 7.4, containing 150mM NaCl. Proteins were eluted with the same buffer at a flow rate of 1 mL/min. Fractions containing the 70 kDa protein were collected, concentrated by centrifugation using Amicon filters and applied in a PD-10 gel filtration column (GE Healthcare, Buckinghamshire, United Kingdom) to exchange for 30 mM Tris-HCl pH 7.4 buffer. Final protein aliquots with 75% purity were stored at -80º C until further use.
2.1.4 Hsp104 expression and purification
E. coli strain BL-21 was transformed by heat shock with Hsp104 pPROEX-Htb construct (a kind gift
from Dr. James Shorter, Department of Biochemistry and Biophysics, Perelman School of Medicine at the University of Pennsylvania, United States of America) with N-terminal His6 tag. A positive colony
was transferred into 100 mL of LB medium supplemented with ampicillin (100 mg/L) and chloramphenicol (34 mg/L) and incubated overnight at 37º C with continuous shaking. 30 mL of the bacterial culture was added to a 2 L flask containing 500 mL of LB medium supplemented with antibiotics and incubated at 37º C under continuous shaking. When the culture reached an OD600 of 1
was placed at 18º C for about an hour. The protein expression was induced with 1 mM IPTG for 18 h at 18º C with continuous shaking. Cells were harvested by a 15 min centrifugation at 8000 g at 4º C and stored overnight at -20º C.
The cell pellet was resuspended in 20 mL of lysis buffer and lysed by three freeze and thaw cycles with liquid nitrogen and five times 30 sec sonication cycles (amplitude 10 microns) with 1 min incubation on ice between each sonication step. The Hsp104 containing extract was centrifuged at 15000 g at 4ºC for 40 min and the supernatant was filtered with a 0.22 µm filter.
The sample was loaded into an immobilized Ni2+ affinity chromatography Histrap column equilibrated with 20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole, pH 7.4. Proteins were eluted with equilibration buffer containing 500 mM imidazole at a flow rate of 1 mL/min and the elution was monitored at 280 nm. Protein-containing fractions were collected and probed by SDS-PAGE analysis using coomassie staining. Fractions containing Hsp104 were collected, concentrated by centrifugation
12 using Amicon filters, and applied to a gel filtration Superdex 75 column, equilibrated with 50 mM Tris-HCl buffer, pH 7.4, containing 150 mM NaCl. Proteins were eluted with the same buffer at a flow rate of 1 mL/min. Fractions containing Hsp104 were collected, concentrated by centrifugation using Amicon filters and eluted through a PD-10 gel filtration column to exchange for 30 mM Tris-HCl pH 7.4 buffer. Final protein aliquots were stored at -80º C until further use.
2.2 aSyn oligomerization
Purified monomeric aSyn was diluted at 140 μM in 30 mM Tris-HCl pH 7.4. Oligomerization was induced by continuous shaking for 4 days at 37° C in a Thermomixer (Eppendorf, Hamburg, Germany) at 800 rpm. aSyn oligomerization was established either with aSyn alone or in the presence of Hsp27 or Hsp70. Controls of the oligomerization of HSPs alone were performed. A glycation environment was also tested in the oligomerization process by adding 0.5 mM of methylglyoxal (MGO). Samples were taken at specific time points, diluted to an aSyn final concentration of 70 μM and stored at -20º C.
2.2.1 SDS-PAGE / Native PAGE Western blot analysis
The composition of different aSyn oligomerization species was evaluated by native or SDS-PAGE. 0.5 μg of each sample was resolved by native or SDS-PAGE using a Tetra Cell (Bio-Rad, California, United States of America) in a 12% polyacrylamide gel (Bio-Rad, California, United States of America) using standard procedures. Proteins were transferred to a nitrocellulose membrane (Bio-Rad, California, United States of America) using the Mini Tans-Blot system (Bio-Rad, California, United States of America). Prestained standard proteins were also loaded on the gel. Membrane was incubated for 1 h with constant shacking at room temperature with blocking solution (5% bovine serum albumin in 50 mM Tris, 150 mM NaCl, pH 7.4). The membrane was incubated overnight at 4° C with the primary antibody anti-aSyn (c-20) (BD Transduction Labs, New Jersey, United State of America) using a dilution of 1:1000 in blocking solution. Membrane was washed in PBS and incubated for 1 h at room temperature with anti-mouse-horseradish peroxide - conjugated secondary antibody (Invitrogen, California, United States of America) using a dilution of 1:10,000 in blocking solution. Detection procedures were performed according to ECL system (Millipore, Massachusetts, United States of America) with appropriate exposure time and films were scanned. Each immunoblot was repeated at least three times from independent experiments.
2.2.2 Thioflavin T binding assay
The formation of β-sheet enriched species was probed by Thioflavin T (ThT) binding assay (Nilsson, 2004). Briefly, ThT (Sigma-Aldrich, Missouri, United States of America) was incubated at a final concentration of 20 µM with 1.4 µM aSyn in 50 mM Tris-HCl, pH 7.4. Emission wavelength scan at 490 nm was performed with an excitation wavelength of 450 nm using a plate reader (Tecan Infinite 200, Männedorf, Switzerland).
13 2.2.3 Size exclusion chromatography (SEC)
aSyn samples (50 μg) were loaded on a SuperdexTM 200 10/300 column (GE Healthcare, Buckinghamshire, United Kingdom) using 30 mM Tris/HCl (pH 7.4, with 0.2 M NaCl) as an eluent at a flow rate of 0.5 mL/min and monitored at 220 nm.
2.3 Cell culture
2.3.1 Transfection of mammalian cells
Human H4 neuroglioma cells (gift from Dr. Bradley T. Hyman, Harvard Medical School) were maintained at 37° C in OPTI-MEM I (Gibco, Invitrogen, California, United States of America) supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, California, United States of America) and seeded in 6 cm dishes (Corning, New York, United States of America) for triton-X 100 solubility assays or 35 mm imaging dishes (µ-Dish, Ibidi, Martinsried, Germany) for microscopy studies 24 h prior transfection. Cells were transfected with pcDNA3.1-SynT (a kind gift from Dr. Bradley T. Hyman), a plasmid encoding for aSyn fused with a truncated form of green fluorescent protein which forms in vivo inclusions (McLean et al., 2001), and pcDNA3.1 (Invitrogen, California, United States of America) or pcDNA3.1-Hsp27 using FuGENE 6 (Roche diagnostics, Basel, Switzerland). 24 h after transfection the media was replaced by fresh media supplemented with either water or 0.5 mM MGO (for glycation experiments). Cells were collected 48 h after transfection.
2.3.2 Cytotoxicity assay
Human H4 neuroglioma cells were maintained in Opti-MEM I supplemented with 10% FBS at 37° C. Cells were plated onto 24-well plates (1.9 cm2) (TPP, Trasadingen, Switzerland), at a density of 25,000 cells/cm2. Samples collected from aSyn oligomerization experiments were incubated at final concentration of 1 µM for 24 h. Media was collected and lactate dehydrogenase activity (LDH) (Clontech, California, United States of America) measured in a plate reader (Tecan Infinite 200, Männedorf, Switzerland), according to the manufacturer’s protocol.
2.3.3 Triton-X 100 solubility assay
48h after transfection, cells were washed twice with PBS, placed at 4º C and lysed in 100 µL of lysis buffer (PBS supplemented with protease inhibitor and phosphatase inhibitor cocktail tablets). Samples were sonicated three times for 30 s (amplitude 10 microns) with 1 min incubation on ice between each sonication step. Total protein concentration was measured using bicinchoninic acid (BCA) assay (Pierce, Thermo scientific, Massachusetts, United States of America) and 1% of Triton-X 100 (Sigma-Aldrich, Missouri, United States of America) was added to 200 µg of protein extracts. Samples were incubated at 4º C for 30 min and the triton-insoluble fractions separated by centrifugation at 15000 g for 1 h at 4ºC. The triton-insoluble fractions were ressuspended with 40 µL of lysis buffer containing
14 2% of SDS and sonicated twice for 30 s (amplitude 10 microns) with 1 min incubation on ice between each sonication step.
2.3.4 Immunocytochemistry
48 h after transfection, cells in 35 mm imaging dishes were washed twice with PBS. 100% ice-cold methanol was added and dishes were incubated at -20º C for 10 min. Cells were washed three times with PBS and incubated with blocking solution (1.5% Normal Goat Serum in PBS) for 1 h at room temperature. Cells were incubated with the primary antibody anti-aSyn (Cell Signaling Technology, catalogue number 2642, Massachusetts, United States of America) using a dilution of 1:75 in blocking solution, overnight at 4º C. Cells were washed with PBS and incubated for 4 h at room temperature with Alexa Fluor® 488 Goat Anti-Rabbit conjugated secondary antibody (Invitrogen, catalogue number A11008, California, United States of America) using a dilution of 1:1000 in blocking solution.
A widefield fluorescent microscope Zeiss Axiovert 200M (Carl Zeiss MicroImaging, Jena, Germany) was used to visualize aSyn inclusions and at least 100 cells per condition were counted.
15
3. Results
3.1 Protein expression an purification
To investigate the effect of selected HSPs on the oligomerization process of aSyn, Hsp27, Hsp70, Hsp104 and aSyn were expressed in E. coli.
For aSyn, we first performed a thermal enrichment step, as previously described (Jakes et al., 1994; Vicente Miranda et al., 2013). By heating the bacterial protein extract, all non-thermo-resistant proteins precipitate, whereas aSyn remained in the soluble fraction (thermo-resistant proteins). After a second ionic exchange purification step, the fractions containing the 14 kDa aSyn (confirmed by Western-blot) were combined and further purified by SEC (figure 3.1A). aSyn positive fractions were again combined, buffer exchanged and the protein concentrated. aSyn was then quantified by spectroscopy and stored at -80º C until further use.
In the case of Hsp27, Hsp70 and Hsp104, the proteins were first purified by immunoaffinity. Next, the proteins were purified by SEC (figure 3.1B, C and D) and the positive fractions (confirmed either by Western-blot or by coomassie staining) combined, buffer exchanged, concentrated and quantified by spectroscopy, prior to storage at -80º C. Even though the expression of Hsp104 was successful after purification by immunoaffinity, the protein was unstable and precipitated irreversibly.
Figure 3.1 Protein expression in E. coli BL21+. Coomassie staining of an SDS-PAGE of eluted protein fractions after SEC: A. Fractions 2 to 4 are mainly composed by a ~14 kDa protein corresponding to aSyn B. Fractions 3 to 5 are mainly composed by a ~70 kDa protein corresponding to Hsp70 C. Fractions 3 and 4 are mainly composed by a ~104 kDa protein corresponding to Hsp104. D. Anti-Hsp27 Western-blotting of 12% SDS-PAGE: Fractions 5 to 9 are mainly composed by a ~27 kDa protein corresponding to Hsp27.
16 3.2 Effect of Hsp27 and Hsp70 on aSyn oligomerization in vitro
To determine the effects of the different chaperones on aSyn oligomerization, we studied aSyn oligomerization either alone or in the presence of each of the recombinant chaperones selected for our study. For this, we used different techniques including gel-based techniques such as native and SDS-PAGE, fluorimetric methods based on ThT reactivity, and SEC.
In native-PAGE, the protein structure and interactions remain unaltered and the proteins are resolved according to both their charge and molecular weight. In the case of aSyn the monomer will be the species with highest migration, and the fibrils will not enter the resolving gel. In SDS-PAGE, only the most stable associations will remain unaltered and the proteins will migrate according to their molecular weight.
In the ThT assay, the biding is proportional to the β-sheet structure content of the protein, a typical characteristic of amyloid fibrils.
Finally, for the SEC, the species populations are resolved according to their molecular weight.
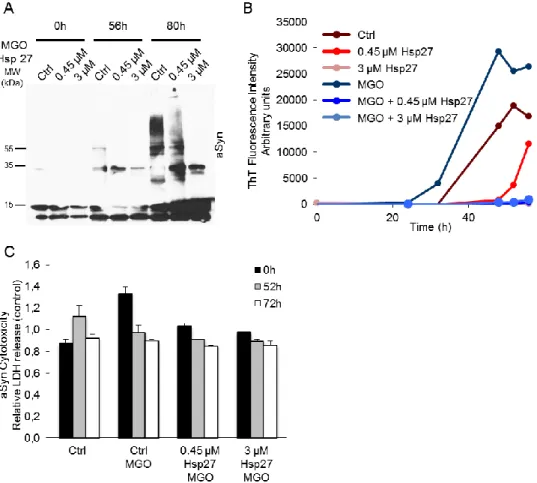
Figure 3.2 Hsp27 decreases aSyn oligomerization in a dose dependent manner. A. Anti-aSyn Western-blotting of 12% native-PAGE. aSyn oligomerization was performed with 140 μM aSyn in 50 mM Tris-HCl pH 7.4 in the absence or the presence of 0.45 and 3 μM of Hsp27, at 37º C, shaking at 800 rpm. B. Analysis of aSyn β-sheet formation by ThT fluorescence at aSyn final concentrations of 1.4 μM.
17 Figure 3.3 Hsp27 decreases aSyn oligomerization over-time in a dose dependent response. A. Anti-aSyn Western-blotting of 12% SDS-PAGE. aSyn oligomerization was performed with 140 μM aSyn in 50 mM Tris-HCl pH 7.4 in the absence or the presence of 0.45 and 3 μM of Hsp27, at 37º C, shaking at 800 rpm. B. Analysis of aSyn β-sheet formation by ThT fluorescence at aSyn final concentrations of 1.4 μM. C. Cytotoxicity of aSyn species population in H4 cells. LDH activity of H4 cells challenged to different oligomerization time-points. LDH release was expressed as relative levels of non-treated samples. Data are expressed as mean ± SD.
To study the kinetics of aSyn aggregation, we incubated the protein under controlled temperature and shaking speed and collected different samples throughout time. We also incubated the different concentrations of HSPs alone. In these conditions, no ThT response was observed. By analyzing the resulting samples, we observed the formation of high molecular weight species along time (figure 3.2A and 3.3A), followed by an increase in the formation of β-sheet structures (figure 3.2B and 3.3B). This formation is most visible after 65 h of incubation where a smear of different-size aSyn species is observed in the native gels followed by a large increase in ThT fluorescence (figure 3.2).
The presence of Hsp27 inverted this tendency, decreasing the formation of high molecular weight species and also their β-sheet structure content (figure 3.2 and 3.3) in a concentration dependent manner. After 65 h the higher molecular weight species decreased in the presence of 0.45 μM of Hsp27, and were almost abolished in the presence of 3 μM of Hsp27 (Fig. 3.2A and 3.3A). In agreement, the ThT fluorescence emission also correlated with this observation (Fig 3.2B and 3.3B).
18 Interestingly, after 80 h of incubation, the presence of 3 μM of Hsp27 almost completely inhibited aSyn high molecular weight species and fibrils (Fig 3.2 and 3.3).
In contrast, the presence of Hsp70 tends to accelerate aSyn oligomerization and the formation of β-sheet structures (figure 3.4). As observed by SDS-PAGE analysis, at both 56 h and 80 h of incubation, at both concentrations of Hsp70, aSyn presents more higher molecular weight species than aSyn incubation alone (figure 3.4A). This data correlates with the increased ThT emission for both concentrations in a concentration dependent response (figure 3.4B).
The oligomerization pattern observed in both Native and SDS-PAGE for aSyn was not observed in SEC analysis (figure 3.5A). Although, an elution peak corresponding to aSyn oligomeric species was observed in the presence of Hsp27 (figure 3.5B, arrow), there were no significant changes in the SEC profile in the aSyn oligomerization in the presence of Hsp70 (figure 3.5C).
Figure 3.4. Hsp70 promotes aSyn fibrillization. A. Anti-aSyn Western-blotting of 12% SDS-PAGE. aSyn oligomerization was performed with 140 μM aSyn in 50 mM Tris-HCl pH 7.4 in the absence or the presence of 0.5 and 1 μM of Hsp70, at 37º C, shaking at 800 rpm. B. Analysis of aSyn β-sheet formation by ThT fluorescence at aSyn final concentrations of 1.4 μM. C. Cytotoxicity of aSyn species population in H4 cells. LDH activity of H4 cells challenged to different oligomerization time-points. LDH release was expressed as relative levels of non-treated samples. Data are expressed as mean ± SD.
19 Figure 3.5 Hsp27 and Hsp70 do not alter the elution profiles of aSyn in SEC analysis. aSyn size exclusion chromatography elution profiles in Superdex-200 column. aSyn oligomerization in the absence A. or presence of 3 μM Hsp27 B. and 1 μM Hsp70 C., 0, 56 and 80 h after oligomerization started. 50 μg of protein were injected. aSyn monomers display a retention time of 1.8 mL, while aSyn oligomers of 1 mL (arrow).
3.3 Cytotoxicity of aSyn species
To evaluate the cytotoxicity of the resulting aSyn species we performed LDH release measurements of cells challenged with aSyn species collected at different time-points, either in the presence or absence of the different chaperones. This method is based on the measurement of this enzyme’s activity that correlates with the loss of membrane integrity and therefore the cytotoxicity.
We observed that the most cytotoxic species were those formed at 52 h of incubation (figure 3.3C). Interestingly, this toxicity was rescued in the samples where aSyn was oligomerized both in the presence of Hsp27 (figure 3.3C) or Hsp70 (figure 3.4C).
20 3.4 Effects of glycation on aSyn oligomerization
To evaluate the effects of glycation on aSyn oligomerization, we incubated aSyn with MGO and evaluated the oligomerization kinetics. Using SDS-PAGE and ThT analysis we observed that MGO increased both aSyn oligomerization and the formation of β-sheet structures (figure 3.6). However, in the presence of Hsp27, aSyn oligomerization was reduced and less β-sheet structures formed (figure 3.6A and 3.6B). Using SEC analysis, we observed MGO induced the formation of small oligomeric species along time (figure 3.7C, arrow). Interestingly, in the presence of Hsp27, an increase in the oligomeric species was also observed (figure 3.7D, arrow).
To evaluate the cytotoxicity of the glycated aSyn species, we performed LDH assays. Interestingly, treating cells with glycated aSyn alone resulted in higher cytotoxicity at time 0. However, in the presence of Hsp27, cytotoxicity was almost reduced to control levels in a concentration dependent manner (figure 3.6C).
Figure 3.6 MGO induces and Hsp27 reverses aSyn fibrillization. A. Anti-aSyn Western-blotting of 12% SDS-PAGE. aSyn oligomerization was performed with 140 μM aSyn and 0,5 μM MGO in 50 mM Tris-HCl pH 7.4, in the absence or presence of 0.45 and 3 μM of Hsp27, at 37º C, shaking at 800 rpm. B. Analysis of aSyn β-sheet formation by ThT fluorescence at aSyn final concentrations of 1.4 μM. C. Cytotoxicity of resulting aSyn species population in H4 cells measured by LDH activity. LDH
21 release was expressed as relative levels to non-treated samples. Data are expressed as mean ± SD.
Figure 3.7. MGO induces oligomerization of glycated aSyn. aSyn size exclusion chromatography elution profiles in Superdex-200 column. aSyn oligomerization in the absence A. or presence of 3 μM Hsp27 B. and in glycation conditions C. and D., 0, 56 and 80 h after oligomerization started. 50 μg of protein were injected. aSyn monomers display a retention time of 1.8 mL, while aSyn oligomers of 1 mL (arrow).
3.5 Effects of Hsp27 on aSyn oligomerization in human cells
To evaluate the oligomerization of aSyn in H4 cells in the presence of Hsp27, we used a well-established paradigm of aSyn aggregation comprising the expression of a C-terminally-modified version of aSyn (synT) together with a control vector or with an Hsp27 expressing vector.
To evaluate the oligomerization of aSyn we used two different techniques: (i) a triton X-100 solubility assay where the proteins are separated according to their solubility at 1% triton X-100; (ii) a microscopy analysis of aggregates formation by immunocytochemistry.
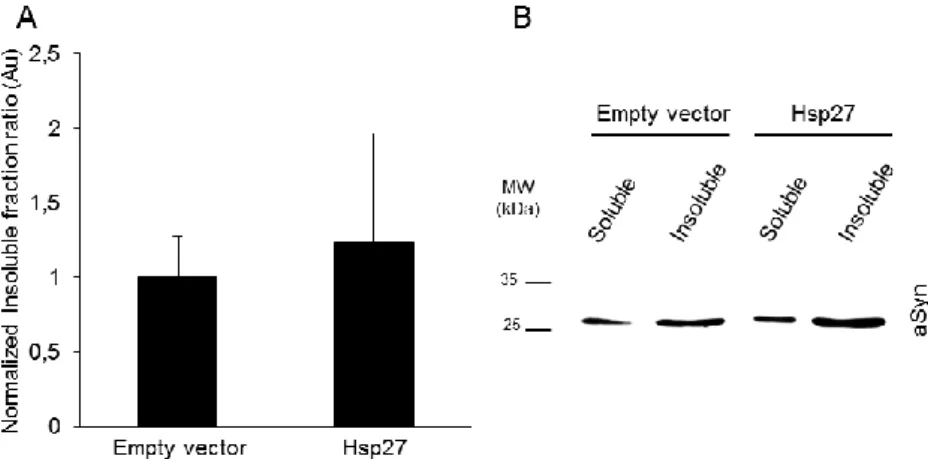
We observed no differences in aSyn triton X-100 solubility in the presence of Hsp27 (figure 3.8). Nevertheless, immunocytochemistry assay, showed a non-significant decrease of cells presenting aSyn aggregates (figure 3.9).
22 Figure 3.8 Hsp27 does not alter aSyn solubility. A. Triton-X normalized insoluble fraction ratio. Data are expressed as mean ± SD, n=3. B. Representative anti-aSyn Western-blot of 12% SDS-PAGE.
Figure 3.9 Hsp27 reduces aSyn aggregates in a H4 cell PD model. Normalized percentage of cells with aggregates. Data are expressed as mean ± SD, n=4.
Interestingly, the challenge of MGO to cells expressing synT alone increased the triton X-100 insoluble aSyn. However, the co-expression of Hsp27 reversed this increase on triton X-100 insoluble aSyn (figure 3.10), followed by a decrease in the number of cells displaying aggregates (figure 3.11).
23 Figure 3.10 MGO induces aSyn insolubilization. A. Triton X-100 normalized insoluble fraction ratio. Data are expressed as mean ± SD, n=3. B. Representative anti-aSyn Western-blot of 12% SDS-PAGE.
Figure 3.11 MGO increases aSyn aggregation in a H4 cell PD model. Normalized percentage of cells displaying aSyn aggregates. Data are expressed as mean ± SD, n=2.
25
4. Discussion
The process of aSyn aggregation has been extensively studied in vitro. In the process that culminates with the formation of aSyn amyloid fibrils, which can be promoted with agitation of monomeric aSyn at 37º C, oligomeric species can be formed (Fink, 2006; Giehm et al., 2011). In our study, we detected aSyn oligomerization using Native/SDS-PAGE (figures 3.2A and 3.3A) and the formation of β-sheet structures using ThT assays (figures 3.2B and 3.3B). Treating H4 cells with the resulting aSyn species populations demonstrated that the oligomeric species (intermediate time-point) were more toxic than the fibrils (later-time point) or monomers (earlier time-point), in agreement with other studies (Outeiro
et al., 2008; Winner et al., 2011). In the presence of Hsp27, the formation of higher molecular aSyn
weight species was decreased (figure 3.2 and 3.3 A and B). This result is again in agreement with other reports where Hsp27 reduced aSyn oligomerization (Bruinsma et al., 2011; Aquilina et al., 2013). The neuroprotective role of Hsp27, previously observed in neuronal cells either exposed to exogenous aSyn species (Zourlidou et al., 2004) or co-expressing both proteins (Outeiro et al., 2006), was also confirmed in our H4 cytotoxicity studies (figure 3.3 C). Hsp27 reduced the toxicity of aSyn species in a concentration dependent manner to levels lower than the control (with monomeric aSyn). In contrast, Hsp70 promoted aSyn aggregation while reducing aSyn-induced toxicity (figure 3.4). Although some studies reported that Hsp70 is able to reduce aSyn cytotoxicity and aggregation when co-expressed with aSyn (Klucken et al., 2004b; McLean et al., 2004; Outeiro et al., 2008; Danzer et al., 2011; Kilpatrick et al., 2013), studies in Drosophila co-expressing Hsp70 (Auluck et al., 2002) or treated with geldanamycin (Auluck and Bonini, 2002; Auluck et al., 2005) showed protection against the aSyn toxicity without affecting aSyn aggregation, since aSyn inclusions were still present in the neurons. Thus, our observation is in line with the potential beneficial role of aSyn inclusion formation, where putative toxic soluble forms of aSyn are arrested, preventing the impairment of different cellular systems (Waxman and Giasson, 2009; Breydo et al., 2012).
Upon glycation of aSyn we observed an increase in the formation of higher molecular weight species and β-sheet-rich structures (figure 3.6A and B). MGO is the most significant and highly reactive glycating agent in cells and is generated as a by-product of glycolysis (Richard, 1993). Incubation of aSyn with MGO was previously shown to induce the formation of aSyn oligomeric or globular structures, rather than fibrils (Lee et al., 2009a; Padmaraju et al., 2011). With Aβ peptide, relevant in the context of Alzheimer’s disease, MGO induced the formation of oligomeric species and larger aggregates, with higher β-sheet content (Chen et al., 2006). These observations are in agreement with our results with aSyn were we found an increase in ThT fluorescence in the presence of MGO, indicating an increase in β-sheet content.
Interestingly, glycated aSyn species displayed higher cytotoxicity when applied exogenously to cells (figure 3.6C). Previously, MGO was described to promoted apoptosis (Ghosh et al., 2011; Antognelli
26 cytotoxic effects (Lee et al., 2009b). Also, rat and hippocampal slices treated with MGO produced increased levels of reactive oxygen species (Heimfarth et al., 2013).
We observed a substantial decrease in the formation of aSyn higher molecular weight species in both the presence of Hsp27 and MGO (figure 3.6A and B). Interestingly, MGO was shown to modify the α-crystallin structure of HSPs (namely, Hsp27) probably by exposing hydrophobic sites that otherwise would not be available for chaperone function. In addition, MGO might induce Hsp27 oligomerization, enhancing its activity (Nagaraj et al., 2003; Oya-Ito et al., 2006). This enhanced Hsp27 activity may explain the effect on reducing aSyn cytotoxicity (figure 3.6C). On one hand, the higher chaperone activity of Hsp27 may explain the reduced formation of aSyn oligomers. On the other hand, glycated Hsp27 was shown to have an extraordinary anti-apoptotic power, being associated with several cancers (Sakamoto et al., 2002; van Heijst et al., 2006; Oya-Ito et al., 2011). To investigate this hypothesis, we aim to investigate whether MGO is able to directly modify Hsp27 and promote its oligomerization, therefore increasing its activity. To that purpose, we will first glycate Hsp27 and further compare aSyn oligomerization kinetics in the presence of unmodified or glycated Hsp27.
Although we observed Hsp27 to reduce aSyn oligomerization in vitro, it did not alter aSyn Triton X-100 solubility in cells (figure 3.8), nor did it significantly decrease the percentage of cells with aSyn inclusions (figure 3.9). Hsp27 has previously shown to reduce the aggregation of aSyn (Outeiro et al., 2006) and of other proteins associated with other neurodegenerative disorders (Kudva et al., 1997; Lee et al., 2006; Wilhelmus et al., 2006; Yerbury et al., 2013) . In some Huntington’s disease models, Hsp27 did not show a neuroprotective effect (Zourlidou et al., 2007) whereas in others it rescued polyglutamine toxicity (Wyttenbach et al., 2002; Perrin et al., 2007).
Some Hsp27 homologues are known to bind misfolded proteins, co-aggregating with them. This process maintains the misfolded proteins in a conformation that allows other HSPs to disaggregate and possibly refold them, being an important stress-protection mechanism (Ehrnsperger et al., 1997; Katoh et al., 2004; Cashikar et al., 2005; Duennwald et al., 2012).
Treating H4 cells with MGO induced the formation of Triton X-100-insoluble aSyn species (figure 3.10). Interestingly, we also observed a trend towards an increase in the formation of aSyn aggregates (figure 3.11). The glycation process is known to increase the levels of intracellular and mitochondrial reactive oxygen species, inducing oxidative stress (Fukunaga et al., 2005; Shangari and O’Brien, 2004; Wang, Liu, and Wu, 2009; Yan et al., 1994). Glycation is also responsible for the impairment of several protein quality control systems, namely the ubiquitin proteasome system and chaperones (Bento et al., 2010), inducing the accumulation of aggregated proteins (Lee et al., 2009a; Oliveira et