ContentslistsavailableatScienceDirect
International
Journal
of
Pharmaceutics
j ou rn a l h om epa ge : w w w . e l s e v i e r . c o m / l o c a t e / i j p h a r m
Pharmaceutical
Nanotechnology
Design
of
cationic
lipid
nanoparticles
for
ocular
delivery:
Development,
characterization
and
cytotoxicity
Joana
F.
Fangueiro
a,
Tatiana
Andreani
a,b,c,
Maria
A.
Egea
d,e,
Maria
L.
Garcia
d,e,
Selma
B.
Souto
f,
Amélia
M.
Silva
b,c,
Eliana
B.
Souto
a,g,∗aFacultyofHealthSciences,FernandoPessoaUniversity(UFP-FCS),RuaCarlosdaMaia,296,4200-150Porto,Portugal bCentreforResearchandTechnologyofAgro-EnvironmentalandBiologicalSciences(CITAB),VilaReal,Portugal cDepartmentofBiologyandEnvironment,UniversityofTrás-os-MonteseAltoDouro(UTAD),VilaReal,Portugal
dDepartmentofPhysicalChemistry,FacultyofPharmacy,UniversityofBarcelona,Av.JoanXXIIIs/n,08028Barcelona,Spain eInstituteofNanoscienceandNanotechnology,UniversityofBarcelona,Av.JoanXXIIIs/n,08028Barcelona,Spain fDivisionofEndocrinology,DiabetesandMetabolism,HospitaldeBraga,Braga,Portugal
gInstituteofBiotechnologyandBioengineering,CentreofGeneticsandBiotechnology,Trás-os-MontesandAltoDouroUniversity(IBB/CGB-UTAD),
VilaReal,Portugal
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11October2013 Receivedinrevisedform 10November2013 Accepted15November2013 Available online 23 November 2013
Keywords: Lipidnanoparticles Oculardelivery Multipleemulsion Cytotoxicity Turbiscan
Y-79humanretinoblastomacells
a
b
s
t
r
a
c
t
Inthepresentstudywehavedevelopedlipidnanoparticle(LN)dispersionsbasedonamultipleemulsion techniqueforencapsulationofhydrophilicdrugsor/andproteinsbyafullfactorialdesign.Inorderto increaseocularretentiontimeandmucoadhesionbyelectrostaticattraction,acationiclipid,namely cetyltrimethylammoniumbromide(CTAB),wasaddedinthelipidmatrixoftheoptimalLNdispersion obtainedfromthefactorialdesign.Therearealimitednumberofstudiesreportingtheidealconcentration ofcationicagentsinLNfordrugdelivery.Thispapersuggeststhatthechoiceoftheconcentrationofa cationicagentiscriticalwhenformulatingasafeandstableLN.CTABwasincludedinthelipidmatrix ofLN,testingfourdifferentconcentrations(0.25%,0.5%,0.75%,or1.0%wt)andhowcompositionaffects LNbehaviorregardingphysicalandchemicalparameters,lipidcrystallizationandpolymorphism,and stabilityofdispersionduringstorage.Inordertodevelopasafeandcompatiblesystemforoculardelivery, CTAB-LNdispersionswereexposedtoHumanretinoblastomacelllineY-79.Thetoxicitytestingofthe CTAB-LNdispersionswasafundamentaltooltofindthebestCTABconcentrationfordevelopmentof thesecationicLN,whichwasfoundtobe0.5wt%ofCTAB.
© 2013 Elsevier B.V. All rights reserved.
1. Introduction
Lipidnanoparticles(LN)havegainedinterest inrecent years asdrugcarriersfor oculardelivery,aiminga betterpermeation and/orprolongeddrugreleaseontotheocularmucosaandallowing drugsreachingthepostsegmentoftheeye(PignatelloandPuglisi, 2011).Oculardrugdeliveryisextremelyaffectedbyeyeanatomy and physiology that leads often to mechanisms that decrease bioavailabilityofapplieddrugs.Thesemechanismsincludereflex processes,suchaslacrimationandblinkingwhichreduces drasti-callythedrugresidencetime,anddifficultytodiffusethoughthe conjunctivaandnasolacrimalduct.Inaddition,thelowvolumeof theconjunctivalsacalsoleadstoapoorcornealorsclera pene-trationofdrugs(DieboldandCalonge,2010).Sinceoculardelivery
∗Correspondingauthorat:FacultyofHealthSciencesofFernandoPessoa
Univer-sity,RuaCarlosdaMaia,296,OfficeS.1,P-4200-150Porto,Portugal. Tel.:+351225074630x3056;fax:+351225504637.
E-mailaddresses:eliana@ufp.edu.pt,souto.eliana@gmail.com(E.B.Souto).
becameaproblemwhentheultimatetargetisintraoculardelivery, duetotheineffectivedrugconcentrationsandtimeresidencereach theinnertissues,alternativesystemsfordrugdeliveryarerequired (PignatelloandPuglisi,2011;Sultanaetal.,2011).Newdrug deliv-erysystemsbasedonlipids,namelyliposomes,andothermaterials suchaspolymers(poly-d-l-lacticacid(PLA)nanopsheres)were abletodeliveranantiviraldrug,acyclovir,intheinnertissuesof theeyecomprisingtheinnovationofthesesystems(Frestaetal., 1999;Giannavolaetal.,2003).
Oculardrugdeliverystrategiesmaybeclassifiedinto3groups: noninvasivetechniques,implants,andcolloidalcarriers.Colloidal drugdeliverysystems,suchasLN,canbeeasilyadministeredin aliquidformandhavetheabilitytodiffuserapidlyandare bet-terinternalizedinoculartissues.Inaddition,theinteractionand adhesionofLNocularsurfacewiththeendotheliummakesthese drugdeliverysystemsinterestingasnewtherapeutictoolsinocular delivery(delPozo-Rodriguezetal.,2013).
LN based onw/o/w emulsion are versatile colloidal carriers fortheadministrationofpeptides/proteinsandhydrophilicdrugs (Fangueiroetal.,2012).Dropletsfromtheinneraqueousphase,
461 (2014) 64–73 65
wherethedrugisdissolvedor/andsolubilized,aresupportedby asolidlipidmatrixsurroundedbyanaqueoussurfactantphase. UsuallyLNarecomposedofphysiological solidlipids(mixtures ofmono-,di-ortriglycerides,fattyacidsorwaxes)stabilizedby surfactants. Inthecase of aw/o/w basedLN dispersion,a high hydrophilic–lipophilic balance (HLB) surfactant is added tothe externalaqueousphaseand,alowHLBsurfactantisaddedtothe lipidphase.Thetwosurfactantsareneededtostabilizethetwo existinginterfaces inthis typeofemulsion. Avarietyof surfac-tantscanbeapplied,suchasphospholipids,bilesalts,polysorbates, polyoxyethyleneethers (Gallarate etal., 2009; Fangueiroet al., 2012).MaterialsusedforLNproductionarelargelyusedin phar-maceuticalindustrywithprovedbiocompatibility(Severinoetal., 2012).
CationicLNhavebeenrecentlyinvestigatedfortargetingocular mucosa,namelytheposteriorsegmentoftheeye(e.g.retina).This isasmartstrategy thatcombinesthepositive surfacechargeof theparticlesandthenegativesurfacechargeofocularmucosaby meansofanelectrostaticattraction.Thisapproachcouldincrease thedrugsretentiontimeintheeyeaswellasimprovenanoparticles bioadhesion(Lallemandetal.,2012).
Intheoculardelivery, itis especiallyrelevant thecontrolof theparticlesizesinceitdirectlyinfluencethedrugrelease rate, bioavailability, and patient comfort and compliance (Shekunov etal.,2007;Soutoetal.,2010).Inaddition,itisknownthatthe smallertheparticlesize,thelongertheretentiontimeandeasier application(Araujoetal.,2009).
Physicochemicalcharacterizationandassessmentof nanotoxic-ityaremajorissuesfordevelopingandlarge-scalemanufacturingof nanocarriers.Furthermore,physicochemicalpropertiesofLNsuch asparticlesize,surfaceandcompositioncansignificantlyinfluence drugdeliveryonoculardelivery(Yingetal.,2013).
Inthepresentwork,thedevelopmentandcharacterizationof asystemofLNbasedonmultipleemulsionsusingablendof tri-acylglycerolsassolidlipid,wascarried out,in whichsonication methodwasemployed.Thefirstaimoftheworkwasthe appli-cation of a full factorial design todeterminewhich dependent variablescouldaffecttheLNdispersionproperties.Theanalyzed independentvariables,namelytheconcentrationofsolidlipidand bothhydrophilicandlipophilicsurfactants,werecheckedfortheir capacitytoinfluencethemeanparticlesize(Z-Ave),polydispersity index (PI) and zeta potential(ZP) of theproduced LNs disper-sions.Theoptimalformulationwasusedtoevaluatethetoxicity ofLN usingY-79 humanretinoblastoma cells employing differ-entcationiclipidconcentrationstoselectthebestformulationfor ocularinstillations.
2. Materialsandmethods
2.1. Materials
Softisan® 100(S100,ahydrogenatedcoco-glyceridesC
10–C18
fattyacidtriacylglycerol)used assolid lipid wasa free sample fromSasolGermanyGmbH(Witten,Germany),Lipoid®S75,75%
soybeanphosphatidylcholine,usedassurfactant,waspurchased fromLipoidGmbH(Ludwigshafen,Germany),Lutrol®F68or
Polox-amer188 (P188)was a free sample fromBASF (Ludwigshafen, Germany).Cetyltrimethylammoniumbromide(CTAB)anduranyl acetatewereacquiredfromSigma–Aldrich(Sintra,Portugal). Anhy-drousglycerolwaspurchasedfromAcopharma(Barcelona,Spain). Ultra-purifiedwaterwasobtainedfromaMiliQPlussystem (Mili-pore,Germany).Allreagentswereusedwithoutfurthertreatment. TheY-79humanretinoblastomacelllinewaspurchasedfromCell LinesService(CLS,Eppelheim,Germany).Reagentsforcellculture werefromGibco(Alfagene,Invitrogene,Portugal).
Table1
Initial3-levelfullfactorialdesign,providingthelower(−1),medium(0)andupper (+1)levelvaluesforeachvariable.
Variables Levels Lowlevel (−1) Medium level(0) Highlevel (+1)
S100(wt%) 2.5 5.0 7.5
Lecithin(wt%) 0.25 0.5 0.75
P188(wt%) 0.5 1.0 1.0
S100:Softisan®100;P188:Poloxamer188.
2.2. Experimentalfactorialdesign
Afactorialdesignapproachusinga33fullfactorialdesign
com-posedof3variableswhichweresetat3-levelseachwasapplied tomaximizetheexperimentalefficiencyrequiringaminimumof experiments.Forthispurposethreedifferentvariablesandtheir influenceonthephysicochemicalpropertiesoftheproducedLN wereanalyzed.Thedesignrequiredatotalof11experiments.The independentvariablesweretheconcentrationofsolidlipidS100, concentrationoflecithin(Lipoid® S75)andtheconcentrationof
hydrophilicsurfactantP188.Theestablisheddependentvariables werethemeanparticlesize(Z-Ave),polydispersityindex(PI)and zetapotential(ZP).Foreachfactor,thelower,mediumandhigher valuesofthelower,mediumandupperlevelswererepresentedby a(−1),a(0)anda(+1)sign,respectively(Table1).Thedatawere analyzedusingtheSTATISTICA7.0(Stafsoft,Inc.)software.
2.3. Lipidnanoparticlesproduction
LNdispersionswerepreparedusinganovelmultipleemulsion (w/o/w)technique(García-Fuentesetal.,2003).Briefly,aninner w/oemulsionwasinitiallyprepared.Avolumeof ultra-purified waterwasaddedtothelipidphase(5wt%)composedofglycerol, S100 and Lipoid® S75at sametemperature (5–10◦C above the
meltingpointofthesolidlipidSoftisan®100(T≈50◦C)and
homog-enized60swithasonicationprobe(6mmdiameter)bymeansofan UltrasonicprocessorVCX500(Sonics,Switzerland).Apower out-putwithamplitudeof40%wasapplied.AfewmillilitersofP188 solutionwasaddedandhomogenizedforadditional90s.This pre-emulsionwaspouredinthetotalvolumeofaP188cooledsolution undermagneticstirringfor15mintoallowtheformationoftheLN. TheobtainedLNdispersionswereusedforsubsequentstudies.The generalcompositionofLNdispersionsisdescribedinTable2.
2.4. Physicochemicalcharacterization
PhysicochemicalparameterssuchasZ-Ave,PIandZPwere ana-lyzedbydynamiclightscattering(DLS,ZetasizerNanoZS,Malvern Instruments,Malvern,UK).Allsamplesweredilutedwith ultra-purifiedwater andanalyzedintriplicate.ForanalysisoftheZP, ultra-purifiedwaterwithconductivityadjustedto−50S/cmwas
used.
Table2
CompositionofSLNdispersions(wt/wt%).
Components %(wt/wt)
Softisan®100 5.0
Glycerol 37.5
Lipoid®S75 0.5
Lutrol®F68 1.0
66 461 (2014) 64–73
2.5. EvaluationoftheconcentrationofCTAB
Inordertoincreaseeyeretentiontimeandmucoadhesiononto theocularmucosa,acationiclipidwasaddedtothelipidmatrix.For thispurpose,CTABwasusedascationiclipidmaintainingthe5% oflipidmatrixvaryingtheproportionsofS100andCTAB.Thus,in theproductionstage,fourdifferentconcentrations(0.25,0.5,0.75 and1.0wt%)ofcationiclipid(CTAB)wasaddedinthelipidphase composedofS100andLipoid®S75oftheoptimalformulation,
pre-viouslyobtainedfromthefactorialdesigntoevaluatetheinfluence inthephysicochemicalproperties.
2.6. StabilityanalysisofLNbyTurbiscanLab®
TheTurbiscanLab® isa techniqueusedtoobservereversible
(creamingandsedimentationduetofluctuationonparticlesize andvolume)andirreversible(coalescenceandsegregationdueto particlesizevariation)destabilizationphenomenainthesample withouttheneed of dilution.TurbiscanLab® is usefultodetect
destabilizationphenomena much earlier and also in a simpler waythanothermethods,sinceit isbasedonthemeasurement ofbackscattering(BS)andtransmission(T)signals(Araújoetal., 2009;Celiaetal.,2009;Marianeccietal.,2010;Liuetal.,2011).
ThephysicalstabilityofLNdispersionswasassessedwithan opticalanalyzerTurbiscanLab®(Formulaction,France).The
disper-sionswereplacedinacylindricalglasscell,atroomtemperature (25◦C).Theequipmentiscomposedofanear-infraredlightsource
(=880nm),and2synchronoustransmission(T)and backscatter-ing(BS)detectors.TheTdetectorreceivesthelightcrossingthe sample,whereastheBSdetectorreceivesthelightscattered back-wardsbythesample(Araújoetal.,2009).Thedetectionheadscans theentireheightofthesamplecell(20mmlongitude),acquiringT andBSeach40m,3timesduring10minatdifferenttimesafter
production(7,15and30days).
2.7. Thermalanalysis
ThecrystallinityprofileofLNwasassessedbydifferential scan-ningcalorimetry(DSC).Thistechniqueis usefultoevaluatethe physicalstate,whichdirectlyaffectsthephysicochemical proper-tiesandthermodynamicstabilityofLNdispersions.Avolumeof LNdispersioncorrespondingto1–2mgoflipidwasscannedusing aMettlerDSC823eSystem(MettlerToledo,Spain).Heatingand coolingrunswereperformedfrom25◦Cto90◦Candbackto25◦C
ataheatingrateof5◦C/min,insealed40
Laluminumpans.An
emptypanwasusedasareference.Indium(purity>99.95%;Fluka, Buchs,Switzerland)wasemployedforcalibrationpurposes.DSC thermogramswererecordedforthefourdifferentCTAB concen-trationformulationsandforthebulklipids(CTABandS100).The DSCparametersincludingonset,meltingpointandenthalpywere evaluatedusingSTAReSoftware(MettlerToledo,Switzerland).
2.8. X-Raystudies
X-raydiffractionpatternswereobtainedusingtheX-ray scat-tering(X’PertPRO,PANalytical)usingaX’Celeratorasadetector. Dataofthescatteredradiationweredetectedwithablend local-sensibledetectorusingananodevoltageof40kVandacurrentof 30mA.FortheanalysisofLNdispersionsandbulkmaterials,the samplesweremountedonastandardsampleholderbeingdriedat roomtemperaturewithoutanyprevioussampletreatment.
2.9. Alamarblueassayinhumanretinoblastomacellline
Y-79(Humanretinoblastomacellline)cellswereusedto per-formthecytotoxicityassay,inwhichfourLNdispersionscontaining
differentCTABconcentrationsweretested.Eachformulationwas testedat fourconcentrations (ingmL−1):10, 25,50 and 100.
Y-79 cells were maintained in RPMI-1640, supplemented with 10%(v/v)fetalbovineserum(FBS),2mMl-glutamine,and
antibi-otics(100UmL−1penicillinand100
gmL−1ofstreptomycin)in
anatmosphereof5%CO2 inairat37◦Cellswerecentrifuged,
re-suspendedinFBS-freeculturemedia,countedandseeded,after appropriatedilution,at1×105cellmL−1densityin96-wellplates
(100L/well).Thedifferentformulationsweredilutedwith
FBS-freeculturemediatoachievethefinalconcentrations,andadded tocells24hafterseeding(100L/well).Cellviabilitywasassayed
withAlamarBlue(Alfagene,Invitrogene,Portugal)byadding10% (v/v)toeachwell,andtheabsorbanceat570nm(reducedform) and620nm(oxidativeform)wasread24,48and72hafter expo-suretotest compounds, datawereanalyzed by calculatingthe percentageofAlamarbluereduction(accordingtothe manufac-tures recommendation)and expressedas percentageof control (untreatedcells).
2.10. Transmissionelectronicmicroscopyanalysis
Transmissionelectronicmicroscopy(TEM)isatechniqueuseful toanalyzetheshape andsizeofLN dispersions.ThechosenLN dispersioncorrespondingtoCTAB-LNdispersionwith0.5wt%CTAB wasmountedonagridandnegativestainedwitha2%(v/v)uranyl acetatesolution.Afterdryingatroomtemperature,thesamplewas examinedusingaTEM(TecnaiSpiritTEM,FEI)at80kV.
2.11. Statisticalanalysis
Statistical evaluation of data was performed using one-way analysisofvariance(ANOVA).TheBonferronimultiplecomparison testwasusedtocomparethesignificanceofthedifferencebetween thegroups,ap-value<0.05wasacceptedassignificant.Datawere expressed as the mean value±standard deviation (Mean±SD) (n=3).
3. Resultsanddiscussion
TheproductionandoptimizationofLNbasedonmultiple emul-sionrequirespre-formulationstudiesandliteratureresearch.The stabilityandcompatibilityoftheemulsifiersandthelipidmatrix areessentialtoprovideastableandfunctionalsystem(Fangueiro etal.,2012).Sincethechoiceofthecomponentsarevitalfor dis-persions formation, Lipoid® S75 (soybean phosphatidylcholine)
wastheselectedlipophilicemulsifierusedwitha HLBvalueof approximately7–9,andPoloxamer188wasselectedashydrophilic emulsifierwithaHLBvalueofapproximately22.
Physicochemicalpropertiesandstabilityofthenewdrug deliv-erysystemsaremajorissuestobeconsideredintheformulation stage, especially those intended for ocular administration. The useof dispersionswithappropriate physicochemical properties ensuresadequatebioavailabilityofadministereddrugs and bio-compatibilitywithocular mucosa.The LN dispersions obtained fromthefactorialdesign,revealedadequatephysicochemical sta-bilityduringtheanalyticaltesting.In addition,themacroscopic stability ofthe particles,monitoredbyvisual analysis,DLSand Turbiscan® Labanalysis,didnot sufferanychanges. Separation
461 (2014) 64–73 67
Table3
Responsevalues(Z-Ave,PIandZP)ofthethreefactorsdepictedinTable1forthe11experimentformulations.
Run S100(wt%) Lecithin(wt%) P188(wt%) Z-Ave(nm)±SD PI±SD ZP(mV)
SLN1 2.5 0.25 0.5 256.40±1.20 0.181±0.02 −1.04
SLN2 7.5 0.25 0.5 268.50± 1.47 0.173± 0.04 −1.06
SLN3 2.5 0.75 0.5 165.85± 2.21 0.162± 0.01 −1.08
SLN4 7.5 0.75 0.5 171.24± 1.78 0.155± 0.03 −1.20
SLN5 2.5 0.25 1.5 241.30±2.54 0.182±0.05 −1.22
SLN6 7.5 0.25 1.5 235.40±1.87 0.192±0.02 −1.04
SLN7 2.5 0.75 1.5 189.75±1.99 0.163±0.08 −1.12
SLN8 7.5 0.75 1.5 164.50±1.14 0.158±0.03 −1.14
SLN9 5.0 0.5 1.0 165.90± 1.20 0.164± 0.04 −1.18
SLN10 5.0 0.5 1.0 164.50± 1.09 0.183± 0.05 −1.17
SLN11 5.0 0.5 1.0 164.70±1.01 0.177±0.02 −1.16
Table4
ANOVAstatisticalanalysisoftheZ-Ave.
Evaluatedfactorsandtheirinteractions Sumofsquares Degreesoffreedom Meansquare F-value p-Value
(1)S100concentration 23.32 1 23.32 0.01962 0.895380
(2)Lecithinconcentration 12032.66 1 12032.66 10.12031 0.033495
(3)P188concentration 120.44 1 120.44 0.10129 0.766206
1by2 84.89 1 84.89 0.07140 0.802522
1by3 295.73 1 295.73 0.24873 0.644149
2by3 533.99 1 533.99 0.44912 0.539455
Error 4755.85 4 1188.96
Total 17846.88 10
Thevaluesinboldarethestatiscallysignificantresults(p<0.5).
Table5
ANOVAstatisticalanalysisofthePI.
Evaluatedfactorsandtheirinteractions SumofSquares Degreesoffreedom Meansquare F-value p-Value
(1)S100concentration 0.000072 1 0.000072 1.02591 0.368411
(2)Lecithinconcentration 0.001352 1 0.001352 19.26425 0.011791
(3)P188concentration 0.000032 1 0.000032 0.45596 0.536541
1by2 0.000032 1 0.000032 0.45596 0.536541
1by3 0.000072 1 0.000072 1.02591 0.368411
2by3 0.000032 1 0.000032 0.45596 0.536541
Error 0.000281 4 0.000070
Total 0.001873 10
Thevaluesinboldarethestatiscallysignificantresults(p<0.5).
Theresultsforthe11producedformulationsisshowninTable3
andvariedfrom164.5±1.09nm(LN8orLN10)to268.50±1.47nm (LN2),whereasPIrangedfrom0.155±0.03(LN4)to0.192±0.02 (LN6).Theparticlesizedistributionwasverynarrowinallcases sincethePIwaslessthan0.2,correspondingtomonodispersed systems.Accordingtotheliterature(ZimmerandKreuter,1995,
Shekunovetal.,2007),theZ-aveforocularadministrationshould bebelow1mwithanassociatednarrowsizedistribution.Thus,all
formulationsrevealedaZ-AveandPIwithinacceptedrange(Fresta
etal.,1999;Giannavolaetal.,2003;Vegaetal.,2008;Soutoetal., 2010).Asexpected,theZPdidnotvary,since allusedreagents havenon-ionicnature.Foreachofthe3variables,analysisof vari-ance(ANOVA)wasperformed.FromTable4andFig.1,theonly factorthatwasshowntohaveasignificanteffect(p-value<0.05) onZ-Avewastheconcentrationoflecithin.Allotherevaluated fac-tors,werenotstatisticallysignificant(p-value>0.05),neitherthe interactionsbetweenthem.Thesameresultswereobservedforthe evaluationofthedependentvariablesonPI(Table5andFig.1).The
68 461 (2014) 64–73
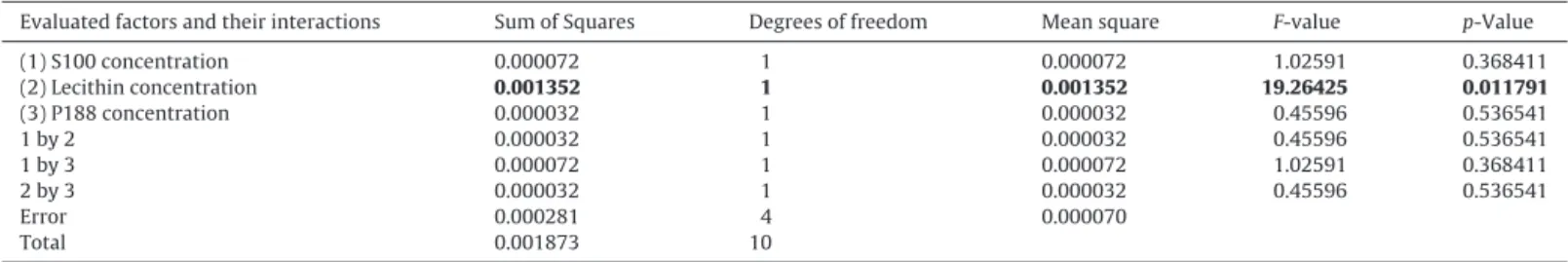
Fig.2.SurfaceresponsechartoftheeffectoftheconcentrationofS100andlecithinontheZ-Ave(A)andPI(C)andtheeffectoftheconcentrationoflecithinandpoloxamer 188ontheZ-Ave(B)andPI(D).
concentration of lecithin was the only independent variable affecting the PI. The lecithin used is composed of 75% of phosphatidylcholine, which is composed by phospholipids that resemblethe cellularmembranes. Theuse of this emulsifier is reportedassafeandbiocompatibleforseveralpurposes,suchas skinproducts(Fiume,2001)andalsoforophthalmic/oculardrug delivery(Bhattaetal.,2012).TheiruseinthedevelopmentofLN dis-persionsisessentialtodecreasetheinterfacialtensionbetweenthe oilphaseandtheinternalandexternalaqueousphase,andalsoto facilitatetheemulsificationofthelipidmatrix.Lecithinisuseddue toitshigherpowerofemulsificationabletoprovideaverygood sta-bilizationoftheoil-in-waterinterfacesandhasalsobeenreported todecreaseparticlesizeinemulsionsthatismainlyexplainedby itsamphiphiliccharacter(Trottaetal.,2002;Schubertetal.,2006; Kawaguchietal.,2008).Thelipophilicportionoflecithindissolves thelipidphase, i.e. lecithin likes tobeat theedge of thelipid phasebeingitslipophilictailsdirectedtothelipidphaseuntilthe hydrophilicportionisdirectedtothewaterphase. Thus,theoil phaseistotallyrecoveredbythelecithinpromotinglongtime stabi-lizationintheinterfaceoftheemulsions(Trottaetal.,2002).From theobtainedresults, acorrelationbetweentheconcentrationof lecithinandmeanparticlesizeoftheparticleswerefound,since highestconcentrationsleadstoadecreaseinZ-Aveand PI.This dependencyofZ-Aveonthetypeofemulsifierisduetheneedfor thecompletecoverageoftheinterface,whichisaffectedbythe selectedconcentration(Fig.2).
Theaimofthisfactorialdesignwastooptimizeaformulation withappropriatephysicochemicalparametersforthe incorpora-tionofhydrophilicdrugsforoculardelivery.Forthispurpose,the limitingfactorsweretheZ-AveandPIandfromtheobtainedresults anoptimalLNdispersionwasfound.ThisLNdispersionwasused forthefollowingstudies.
InordertoimproveLNadhesiontoocularsurfaceandalsoto improvestabilityofthedispersions,acationiclipidwasused.The
approachofusingacationiclipidisinterestingsinceocularmucosa depictsslightlynegativechargeaboveitsisoelectricpointandalso couldimprovesomelimitationsrelatedtoocularadministration, suchaspreventtearwashout(duetoteardynamics),increase ocu-larbioavailabilityandprolongtheresidencetimeofdrugsinthe cul-de-sac(Araujoetal.,2009).
Thestudyreportstheuseofdifferentconcentrationsofacationic lipid(CTAB)inthelipidmatrixofLN.TheresultsoftheCTAB con-centrationontheZ-Ave,PIandZPoftheLNdispersionsoptimized inthefactorialdesign aredepictedin Table6.Asexpected,the parametermostaffectedbythevariationonCTABconcentration istheZP.TheincreaseoftheZPwasdirectlyproportionaltothe increaseonCTABconcentration.Statisticalanalysisofthe physi-cochemicalpropertiesofCTAB-LNdispersionswasnotsignificant (p>0.05),indicatingthattheconcentrationofCTABdidnotaffect drasticallytheZ-Ave,PIandZPoftheformulations.All formula-tionspropertieswereinagreementwiththerequiredparameters foroculardelivery.However,nostatisticaldifferenceswerefound, theconcentrationofCTABimprovedtheZPfollowingaproportional relationship.Thisbehaviorwasexpectedbecauseofthecationic propertiesofCTABandisalsoexpectedthathigherZPvalues con-tributetohigherstabilityoftheparticlesduetoelectronicrepulsion betweenthemmaintaininglongertimeinsuspension.
461 (2014) 64–73 69
Table6
PhysicochemicalparametersfromCTAB-LNdispersionsattheproductiondayandalong-termstabilityafter7,15and30dayafterproductionat25◦
C(Mean±SD)(n=3).
Formulation Parameters Productionday Day7 Day15 Day30
0.25%CTAB-LN
Z-Ave(nm) 230.70±6.71 239.5±0.61 244.9±1.17 255.2±1.32
PI 0.308± 0.09 0.267± 0.01 0.261± 0.03 0.266± 0.01
ZP(mV) +24.80± 2.69 +17.4± 0.65 +16.7± 0.56 +16.4± 0.36
0.5%CTAB-LN
Z-Ave(nm) 194.40±0.43 199.25±0.30 201.45±0.08 213.7±0.74
PI 0.185± 0.02 0.186± 0.01 0.224± 0.02 0.225± 0.01
ZP(mV) +37.20± 1.27 +33.8± 0.45 +32.1± 0.53 +30.5± 0.03
0.75%CTAB-LN
Z-Ave(nm) 172.10±12.64 166.25±1.20 223±0.59 236.8±1.45
PI 0.182± 0.02 0.246± 0.01 0.204± 0.01 0.225± 0.04
ZP(mV) +41.70± 0.71 +40.15± 0.63 +38.6± 0.41 +37.4± 1.21
1.0%CTAB-LN
Z-Ave(nm) 169.10±2.51 144.7±1.61 188.3±1.52 200.7±1.65
PI 0.222± 0.022 0.211± 0.01 0.236± 0.02 0.245± 0.04
ZP(mV) +48.00± 0.31 +44.58± 0.50 +42.0± 0.05 +40.2± 0.63
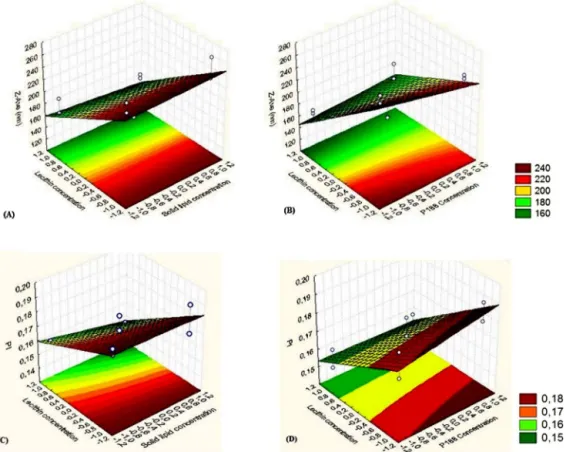
stabilityovertime.Theotherconcentrationsseemtobemore sta-blesincevariationsarelowerthan10%duringthetimeofanalysis. Thisanalysiscouldpredictthegoodstabilityoftheformulations withCTABconcentrationrangingbetween0.5and1.0wt%.These resultsareinagreementwiththeZPvaluesrecordedforthefour formulations(Table6),sincehigherZPvaluescouldalsopredict ahigherstabilityofLNdispersionsasmentionedbefore.Inorder tosupportTurbiscan®Labresultsandalsotomonitoredparticles
overaperiodoftime,zetasizermeasurementsweremadeatthe samedaysafterCTAB-LNdispersionsproductionduringstorageat 25◦C.Alldispersionsshowedamilkycolloidalappearancewhere
noaggregationphenomenaandnophaseseparationweredetected duringtheperiodofanalysisandstorage.Fromtheresultsdepicted
inTable6ispossibletodetectaslightlyincreasebothintheZ-Ave andinthePIafter30daysofstorage.Duringtheperiodof analy-sis,CTAB-LNdispersionsdepictedparticlesizesbelow300nmand PI≤0.3,whichareacceptablevaluesforoculardelivery(Vegaetal., 2008;Gonzalez-Miraetal.,2010;Soutoetal.,2010;Gonzalez-Mira etal.,2011).Thelong-termstabilitystudiesconfirmedthat concen-trationsofCTABupto0.5wt%couldprovideabetterelectrostatic repulsionbetweenparticlesinsuspensionandfromthis sugges-tionprovidebetterstabilityavoidingparticleaggregationand/or flocculation.
Theanalysisofthecristallinity andpolymorphism oftheLN dispersionsobtainedwasanalyzedbyDSCandX-Ray.The thermo-dynamicstabilityofLNdependsmainlyonthelipidmodification
70 461 (2014) 64–73
Fig.4. DSCthermogramsof(a)bulkCTAB,(b)bulkS100andCTAB-LNwithdifferent concentrations(c)0.25wt%,(d)0.50wt%,(e)0.75wt%and(f)1.0wt%.
thatoccursaftercrystallization.ThesolidlipidusedwasS100,a tri-acylglycerolblendofvegetablefattyacidswithC10–C18(Fangueiro
etal.,2013).Polymorphictransitionsaftercrystallizationof triacyl-glycerolbasedLNareslowerforlonger-chaintriacylglycerolsthan forshorter-chaintriacylglycerol(MetinandHartel,2005).Thetype ofemulsifierusedinLNdispersionsalsoaffectstheir thermody-namicbehavior,theirstoragetimeanddegradationvelocity(Han etal.,2008).
Triacylglycerols usually occur in three major polymorphic forms,namelya,ˇ′andˇ(inorderofincreasingthermodynamic
stability)whicharecharacterizedbydifferentsubcellpackingof thelipidchains.Theˇ′modificationisfrequentlyobservedin
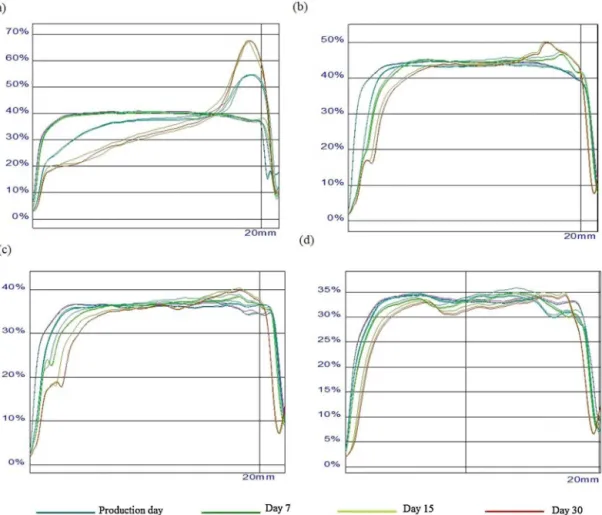
com-plextriacylglycerolssuchasS100(BunjesandUnruh,2007). ThermalanalysisofthebulklipidS100(Fig.4)showsasingle endothermicpeakuponheatingwithaminimumof39.12◦Cand
anenthalpyof−44.76Jg−1(Table7).Ourresultsareinagreement
withthose reportedbyotherauthors(Thomaand Serno,1983; SchubertandMüller-Goymann,2003)foraˇ′modificationof
com-plextriacylglycerolsmixtures.ForCTABbulk,asmallmeltingevent wasobservedbetween40and50◦C,thatcanbeassociatedtothe
meltingofacylchainsandamaintransitionataround106.34◦C
correspondingtocompletemeltingofCTABandattributedtothe meltingofhead-groups(Doktorovovaetal.,2011).
With respect to CTAB-LN dispersions (Fig. 4), a very small endothermiceventwasreportedcomparedtotheother thermo-gramsofthebulklipidandbulkmatrix.Thistransitionisrelatively smallduetothelowestvaluesofenthalpypresentedbytheLN dis-persionscomparedtothebulklipid(Table7).Thepeaktemperature slightlydecreasedforallCTAB-LNdispersionsconfirmingthat poly-morphismofS100.InLNdispersions,aslightdecreaseofenthalpy isusuallyobserved,aswellasadecreaseonthepeaktemperature comparingtothebulkcounterpart,howeversincethislipidhasa
Fig.5. X-raydiffractionpatternsof(a)bulkCTAB,(b)bulkS100andCTAB-LNwith differentconcentrations(c)0.25wt%,(d)0.50wt%,(e)0.75wt%and(f)1.0wt%.
verylowmeltingpoint,itispossibletoformsupercooledmelts. ApossibleexplanationforthereductionofcrystallinityoftheLN dispersionisthecoexistenceoflipidbeingpresentinthea modifi-cationandalsoduetothecolloidalparticlesizeofferinginsufficient numberofdiffractionlevels.Thesedifferencescanbeattributedto thehighsurfacetovolumeratioofLNdispersions(Schubertand Müller-Goymann,2003).
TheX-rayresultsdepictedinFig.5 alsorevealthepresence oftwo signals oneat 0.42nm (2=21.1◦)and otherat 0.38nm
(2=23.2◦) which are both characteristic of the orthorhombic
perpendicularsubcell,i.e.,ˇ′modification(Schubertand Müller-Goymann,2003).TheseresultsareinagreementwithDSCstudies, sinceallformulationsrevealedadecreasedcrystallinity compar-ingwithbulklipidsS100and CTAB.Also, themelting enthalpy ofCTAB-LNdispersionsis increasingwithincreasingCTAB con-tent, indicating higher crystallinity of the matrices containing 0.5–1.0wt%ofCTAB.Thus,theconcentrationofCTABinthelipid matrixisabletoprovidehighercrystallinitytothelipidmatrix; however,otherfeaturessuchasstability andtoxicitywere ana-lyzedtoprovide a full studyin order tochoosethe bestCTAB concentration.
461 (2014) 64–73 71
Table7
Differentialscanningcalorimetry(DSC)analysisoftheSLNformulationswithdifferentCTABconcentrations.
Formulation Onsettemperature(◦C) Meltingpoint(◦C) Integral(mJ) Enthalpy(Jg−1)
Softisan100bulk 36.68 39.12 −3111.15 −44.76
CTABbulk 100.88 106.34 −677.30 −12.72
CTAB-LN0.25wt% 29.58 34.12 −17.50 −0.23
CTAB-LN0.5wt% 31.26 35.03 −116.43 −1.47
CTAB-LN0.75wt% 31.12 34.73 −110.44 −1.41
CTAB-LN1.0wt% 31.65 34.94 −129.98 −1.59
for furtherin vivo studies.Thisassay couldpredictif formula-tionscausecellulardamagewhichconsequentlyresultsinlossof themetaboliccellfunction.Alamarblueisasensitive fluoromet-ric/colorimetricgrowthindicatorusedtodetectmetabolicactivity of cells. Specifically, cells incorporate an oxidation-reduction (REDOX) indicator that, when in a reducing environment of o metabolicallyactivecell,isreduced.Whenreduced,Alamarblue becomes fluorescent and changes color from blueto pink.The reductionofAlamarblueisbelievedtobemediatedby mitochon-drialenzymes(Hamidetal.,2004).However,someauthorsalso suggestthatcytosolicandmicrosomalenzymesalsocontributeto thereductionofAlamarblue(GonzalezandTarloff,2001).
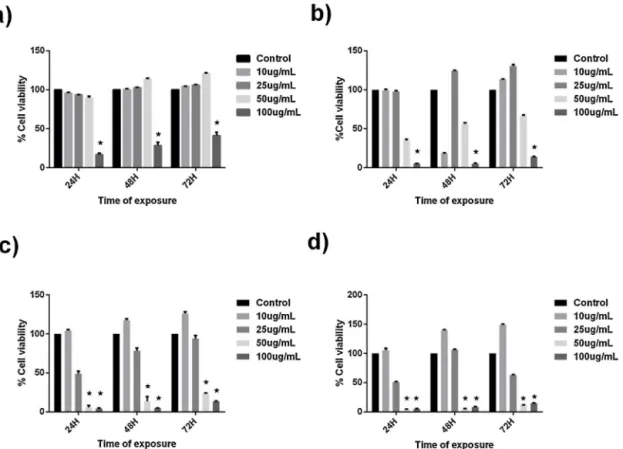
The evaluation of the Alamar blue assaywas based onthe percentviabilityoffourconcentrationsforeachCTAB concentra-tionin thedispersion.Thisassay isimportant topoint outthe importanceof theCTAB quantityin theformulation that could beadministeredforoculardelivery,avoiding celldamage.From
Fig. 6, we can observe that 10gmL−1 of all CTAB-LN
formu-lations is non-toxic to cells as cell viability is not statistically differentfromcontrol (untreatedcells)along the3 time-points of exposure. The concentrationof 50gmL−1 of CTAB-LNonly
reducedsignificantlycellviabilityintheCTAB-LNdispersions con-taining 0.75 and 1.0wt% of CTAB, along the 3 time-points of
exposure.Theconcentrationof100gmL−1ofCTAB-LNreduced
significantlycellviabilityinallCTABpercentages.CTAB-LN formu-lationcontaining0.25%(w/w)ofCTABisnon-toxicfortherangeof 10–50gmL−1(Fig.6a),butreducessignificantlycellviability,in
about50–75%ofcontrol(p<0.05).Theconcentrationrangewhere cellviabilityiskeptsimilartocontrolvaluesisreducedincreasing CTABconcentrationintheformulations.InFig.6b(0.5%ofCTAB, concentrationtwice that usedfor Fig.6a)we can observethat 50gmL−1 offormulationreducescellviabilityfrom40 to60%
ofthecontrolwhile100gmL−1practicallyabolishescell
viabil-ityforthe3timepointsofexposure.RaisingCTABconcentration intheformulationto0.75%(Fig.6c)andto1.0%(Fig.6d)reduces celltolerancetotheformulationasweobservethatwith increas-ing%ofCTABintheformulationreducestheconcentrationthat maintainscellviability.TheseresultssuggestthatahigherCTAB concentrationimplieshighercytotoxicitywhichisexpectedsince theeffectofcationic agentsonthehumanhealthis concentra-tiondependent.Inaddition,higherCTAB-LNconcentrationsalso implymorecytotoxicity.Fromourresults,asafeand biocompat-ibleLNsystemshouldbecomposedof,atmaximum,0.5wt%of CTAB.
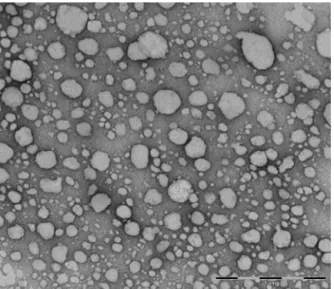
TEManalysishasbeenperformedtoevaluatetheparticleshape andmorphologyofCTAB-LNdispersion.TEMimage(Fig.7)shows
72 461 (2014) 64–73
Fig.7.TEMmicrographof0.5%CTAB-LN.
particlesmainlywithsphericalmorphology.From thisanalysis, theabsenceofaggregationphenomenaofCTAB-LNdispersionwas alsoconfirmed.TheseresultsareinagreementwithTurbiscan®Lab
results.Itispossibletodetectaslightpolydispersityhoweverallthe particlesremainwithinthenanometerrange.Allparticlesshowed ameandiameterthatvariedbetween190and280nm,whichis alsoinagreementwiththezetasizermeasurements.TEManalysis suggestedthatimmediatelyafterproductionCTAB-LNdispersion with0.5wt%CTABcontainedparticlesnohigherthan1m,which
isusefulforoculardeliverypurposes.
4. Conclusions
ThedevelopmentofLNdispersionsforoculardeliveryshould becarriedoutregardingocularmorphologyandbiology.Afull fac-torialdesignwascarriedoutinordertofindoutwhichparameters couldinfluenceLNdispersionsbasedonmultipleemulsion tech-nique.ThesizeandPIofLNdispersionsarehighlydependenton thelecithinconcentrationmainlyduetoitshigheremulsification propertiesandamphiphiliccharacterabletodecreaseparticlesize inemulsions.Inordertoimproveocularmucoadhesion,acationic lipidwasaddedinthelipidmatrix.Theinsufficientinformationand lackofstudiesregardingnanotoxicityofcationicagentsforocular ordrugdeliveryleadsustostudydifferentCTABconcentrations onlipidmatrixanditseffectsonthephysicochemicalparameters andcelltoxicityofCTAB-LNdispersions.Thisstudydemonstrated thatthebetterCTABconcentrationforthedispersionpreviously optimizedbythefactorialdesignwas0.5%,providingbetter stabil-ityandbiocompatibility.Furtherstudiesencapsulatinghydrophilic drugsandevaluatingtheexvivoandinvivoperformanceofthe developedCTAB-LNdispersionarerequiredtoconfirmthese pre-liminaryresults.
Acknowledgements
Ms. Joana Fangueiro and Ms. Tatiana Andreani wish to acknowledge Fundac¸ão para a Ciência e Tecnologia do Min-istério da Ciência e Tecnologia (FCT, Portugal) under the refer-ences SFRH/BD/80335/2011 and SFRH/BD/60640/2009, respec-tively. FCT is also acknowledged under the research project PTDC/SAU-FAR/113100/2009andFCOMP-01-0124-FEDER-022696 (PEst-C/AGR/UI4033/2011).
References
Araujo,J.,Gonzalez,E.,Egea,M.A.,Garcia,M.L.,Souto,E.B.,2009.Nanomedicinesfor ocularNSAIDs:safetyondrugdelivery.Nanomedicine5,394–401.
Araújo,J.,Vega,E.,Lopes,C.,Egea,M.A.,Garcia,M.L.,Souto,E.B.,2009.Effectof polymerviscosityonphysicochemicalpropertiesandoculartoleranceof FB-loadedPLGAnanospheres.ColloidsSurf.B72,48–56.
Bhatta,R.S.,Chandasana,H.,Chhonker,Y.S.,Rathi,C.,Kumar,D.,Mitra,K.,Shukla,P.K., 2012.Mucoadhesivenanoparticlesforprolongedoculardeliveryofnatamycin: invitroandpharmacokineticsstudies.Int.J.Pharm.432,105–112.
Bunjes,H.,Unruh,T.,2007.Characterizationoflipidnanoparticlesbydifferential scanningcalorimetry,X-rayandneutronscattering.Adv.DrugDeliveryRev.59, 379–402.
Celia,C.,Trapasso,E.,Cosco,D.,Paolino,D.,Fresta,M.,2009.Turbiscanlabexpert analysisofthestabilityofethosomesandultradeformableliposomescontaining abilayerfluidizingagent.ColloidsSurf.B72,155–160.
delPozo-Rodriguez,A.,Delgado,D.,Gascon,A.R.,Solinis,M.A.,2013.Lipid nanopar-ticlesasdrug/genedeliverysystemstotheretina.J.Ocul.Pharmacol.Ther.29, 173–188.
Diebold,Y.,Calonge,M.,2010.Applicationsofnanoparticlesinophthalmology.Prog. Retin.EyeRes.29,596–609.
Doktorovova,S.,Shegokar,R.,Rakovsky,E.,Gonzalez-Mira,E.,Lopes,C.M.,Silva,A.M., Martins-Lopes,P.,Muller,R.H.,Souto,E.B.,2011.Cationicsolidlipid nanoparti-cles(cSLN):structure,stabilityandDNAbindingcapacitycorrelationstudies. Int.J.Pharm.420,341–349.
Fangueiro,J.F.,Andreani,T.,Egea,M.A.,Garcia,M.L.,Souto,S.B.,Souto,E.B.,2012. Experimentalfactorialdesignappliedtomucoadhesivelipidnanoparticlesvia multipleemulsionprocess.ColloidsSurf.B100,84–89.
Fangueiro, J.F., Gonzalez-Mira, E., Martins-Lopes, P., Egea, M.A., Garcia, M.L., Souto,S.B.,Souto,E.B.,2013.Anovellipidnanocarrierforinsulindelivery: production,characterization andtoxicitytesting.Pharm.Dev. Technol.18, 545–549.
Fiume,Z.,2001.Finalreportonthesafetyassessmentoflecithinandhydrogenated lecithin.Int.J.Toxicol.20,21–45.
Fresta,M.,Panico,A.M.,Bucolo,C.,Giannavola,C.,Puglisi,G.,1999. Characteriza-tionandin-vivoocularabsorptionofliposome-encapsulatedacyclovir.J.Pharm. Pharmacol.51,565–576.
Gallarate,M.,Trotta,M.,Battaglia,L.,Chirio,D.,2009.Preparationofsolidlipid nanoparticlesfromW/O/Wemulsions:preliminarystudiesoninsulin encap-sulation.J.Microencapsul.26,394–402.
García-Fuentes,M.,Torres,D.,Alonso,M.J.,2003.Designoflipidnanoparticlesfor theoraldeliveryofhydrophilicmacromolecules.ColloidsSurf.B27,159–168. Giannavola,C.,Bucolo,C.,Maltese,A.,Paolino,D.,Vandelli,M.A.,Puglisi,G.,Lee,
V.H.,Fresta,M.,2003.Influenceofpreparationconditionsonacyclovir-loaded poly-d,l-lactic acidnanospheres andeffectofPEGcoatingonoculardrug bioavailability.Pharm.Res.20,584–590.
Gonzalez-Mira,E.,Egea,M.A.,Garcia,M.L.,Souto,E.B.,2010.Designandocular tol-eranceofflurbiprofenloadedultrasound-engineeredNLC.ColloidsSurf.B81, 412–421.
Gonzalez-Mira,E.,Egea,M.A.,Souto,E.B.,Calpena,A.C.,Garcia,M.L.,2011. Opti-mizingflurbiprofen-loadedNLCbycentralcompositefactorialdesignforocular delivery.Nanotechnology22,045101.
Gonzalez,R.J.,Tarloff,J.B.,2001.Evaluationofhepaticsubcellularfractionsfor Ala-marblueandMTTreductaseactivity.Toxicol.InVitro15,257–259.
Hamid,R.,Rotshteyn,Y.,Rabadi,L.,Parikh,R.,Bullock,P.,2004.Comparisonof ala-marblueandMTTassaysforhighthrough-putscreening.Toxicol.InVitro18, 703–710.
Han,F.,Li,S.,Yin,R.,Liu,H.,Xu,L.,2008.Effectofsurfactantsontheformationand characterizationofanewtypeofcolloidaldrugdeliverysystem:nanostructured lipidcarriers.ColloidsSurf.A315,210–216.
Kawaguchi,E.,Shimokawa,K.-i.,Ishii,F.,2008.Physicochemicalpropertiesof struc-turedphosphatidylcholineindrugcarrierlipidemulsionsfordrugdelivery systems.ColloidsSurf.B62,130–135.
Lallemand,F.,Daull,P.,Benita,S.,Buggage,R.,Garrigue,J.S.,2012.Successfully improvingoculardrugdeliveryusingthecationicnanoemulsion,novasorb.J. DrugDeliv.,604204.
Liu,J.,Huang,X.-f.,Lu,L.-j.,Li,M.-x.,Xu,J.-c.,Deng,H.-p.,2011.TurbiscanLab®
Expertanalysisofthebiologicaldemulsificationofawater-in-oilemulsionby twobiodemulsifiers.J.Hazard.Mater.190,214–221.
Marianecci,C.,Paolino,D.,Celia,C.,Fresta,M.,Carafa,M.,Alhaique,F.,2010. Non-ionicsurfactantvesiclesinpulmonaryglucocorticoiddelivery:characterization andinteractionwithhumanlungfibroblasts.J.Control.Release147,127–135. Metin,S.,Hartel,R.W.,2005.Crystallizationoffatsandoils.In:Bailey’sIndustrialOil
andFatProducts.JohnWiley&Sons,Inc.,NewJersey,USA.
Pignatello,R.,Puglisi,G.,2011.Nanotechnologyinophthalmicdrugdelivery:a sur-veyofrecentdevelopmentsandpatentingactivity.RecentPatentsNanomed.1, 42–54.
Schubert,M.A.,Harms,M.,Müller-Goymann,C.C.,2006.Structuralinvestigations onlipidnanoparticlescontaininghighamountsoflecithin.Eur.J.Pharm.Sci.27, 226–236.
Schubert,M.A.,Müller-Goymann,C.C.,2003.Solventinjectionasanewapproach formanufacturinglipidnanoparticles–evaluationofthemethodandprocess parameters.Eur.J.Pharm.Sci.55,125–131.
461 (2014) 64–73 73
lipid nanoparticles (SLN and NLC) for oral drug delivery.J. Drug Deliv., http://dx.doi.org/10.1155/2012/750891.
Shekunov,B.Y.,Chattopadhyay,P.,Tong,H.Y.,Chow,A.H.L.,2007.Particlesize anal-ysisinpharmaceutics:principles,methodsandapplications.PharmRes.24, 203–227.
Souto,E.B.,Doktorovova,S.,Gonzalez-Mira,E.,Egea,M.A.,Garcia,M.L.,2010. Feasi-bilityoflipidnanoparticlesforoculardeliveryofanti-inflammatorydrugs.Curr. EyeRes.35,537–552.
Sultana,Y.,Maurya,D.P.,Iqbal,Z.,Aqil,M.,2011.Nanotechnologyinoculardelivery: currentandfuturedirections.DrugsToday(Barc)47,441–455.
Thoma,K.,Serno,P.,1983.ThermoanalytischerNachweisderPolymorphieder Sup-positoriengrundlageHartfett.Pharm.Ind.45,990–994.
Trotta, M., Pattarino, F., Ignoni, T., 2002. Stability of drug-carrier emulsions containing phosphatidylcholine mixtures. Eur. J. Pharm. Biopharm. 53, 203–208.
Vega,E.,Gamisans,F.,García,M.L.,Chauvet,A.,Lacoulonche,F.,Egea,M.A.,2008. PLGAnanospheresfortheoculardeliveryofflurbiprofen:drugreleaseand interactions.J.Pharm.Sci.97,5306–5317.
Ying,L.,Tahara,K.,Takeuchi,H.,2013.Drugdeliverytotheocularposteriorsegment usinglipidemulsionviaeyedropadministration:effectofemulsion formula-tionsandsurfacemodification.Int.J.Pharm.453,329–335.