In vitro
anti-
Candida
activity of selective serotonin reuptake inhibitors
against
fl
uconazole-resistant strains and their activity against bio
fi
lm-forming isolates
Rose Anny Costa Silva
a,b, Cecília Rocha da Silva
a,c, Jo
ao Batista de Andrade Neto
~
a,b,
Anderson Ramos da Silva
a, Rosana Sousa Campos
a,c, Letícia Serpa Sampaio
a,b,
Francisca Bruna Stefany Aires do Nascimento
a,b, Brenda da Silva Gaspar
a,
Said Gonçalves da Cruz Fonseca
a, Maria Aparecida Alexandre Josino
a,b,
Thalles Barbosa Grangeiro
e, Danielle Macedo Gaspar
d, David Freitas de Lucena
d,
Manoel Odorico de Moraes
d, Bruno Co
elho Cavalcanti
^
d, H
elio Vitoriano Nobre Júnior
a,b,*aDepartment of Clinical and Toxicological Analysis, School of Pharmacy, Laboratory of Bioprospection in Antimicrobial Molecules (LABIMAN), Federal
University of Ceara, Fortaleza, CE, Brazil
bDepartment of Pathology and Legal Medicine, School of Medicine, Federal University of Ceara, Fortaleza, CE, Brazil cChristus University Center (UNICHRISTUS), Fortaleza, CE, Brazil
dDepartment of Physiology and Pharmacology, Neuropharmacology Laboratory, Federal University of Ceara, Fortaleza, CE, Brazil e
Department of Biology, Science Center, Molecular Genetics Laboratory, Federal University of Ceara, CE, Brazil
a r t i c l e
i n f o
Article history:
Received 9 February 2017 Received in revised form 1 April 2017
Accepted 1 April 2017 Available online 12 April 2017
Keywords: Candidaspp. Biofilm Fluoxetine Paroxetine Sertraline
a b s t r a c t
Recent research has shown broad antifungal activity of the classic antidepressants selective serotonin reuptake inhibitors (SSRIs). This fact, combined with the increased cross-resistance frequency of the genre Candidaregarding the main treatment today, fluconazole, requires the development of novel therapeutic strategies. In that context, this study aimed to assess the antifungal potential offluoxetine, sertraline, and paroxetine againstfluconazole-resistantCandidaspp. planktonic cells, as well as to assess the mechanism of action and the viability of biofilms treated withfluoxetine. After 24 h, the fluconazole-resistant Candida spp. strains showed minimum inhibitory concentration (MIC) in the ranges of 20
e160mg/mL forfluoxetine, 10e20mg/mL for sertraline, and 10e100.8mg/mL for paroxetine by the broth microdilution method (M27-A3). According to our data byflow cytometry, each of the SSRIs cause fungal death after damaging the plasma and mitochondrial membrane, which activates apoptotic signaling pathways and leads to dose-dependant cell viability loss. Regarding biofilm-forming isolates, the fluoxetine reduce mature biofilm of all the species tested. Therefore, it is concluded that SSRIs are capable of inhibit the growth in vitro ofCandidaspp., both in planktonic form, as biofilm, inducing cellular death by apoptosis.
©2017 Elsevier Ltd. All rights reserved.
1. Introduction
In recent years, hospital-acquired fungal infections have received attention due to their greater prevalence and high mor-tality rates, ranging between 40 and 70% depending on the risk factor involved[1]. Among clinically relevant yeasts, those in the genus Candida are important opportunistic pathogens isolated
from these hospitalized patients, mainly immunocompromised[2]. Some hospital-acquired fungal infections, such as in the blood stream or in the urinary tract, are directly linked to implanted medical devices, which may serve as support for biofilm growth, one of the main virulence factors of the genusCandida[3]. Mature biofilms are much more resistant against both conventional anti-microbials and host defense mechanisms compared to free (planktonic) cells in the medium[3,4].
The high incidence of fungal infections caused byCandidaspp. and the increase in strains resistant against the main treatment
*Corresponding author.
E-mail address:label_ufc@yahoo.com.br(H.V. Nobre Júnior).
Contents lists available atScienceDirect
Microbial Pathogenesis
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / m i c p a t h
(fluconazole), associated with these species' ability to form
bio-films, have led to a growing number of treatment failures, which requires new, effective anti-Candidaagents[4].
The literature carries reports of selective serotonin reuptake inhibitors (SSRIs) impacting antimicrobial activity, such as against gram-positive and some gram-negative bacteria, against fungi, and in the reversal ofPlasmodium falciparumresistance[5e7].
Lass-Florl et al.€ [8]reported in vitro antifungal activity of SSRIs against Aspergillusspp. andCandida parapsilosis. Among thefive SSRIs tested,fluoxetine and sertraline showed high activity against these fungi.
The anti-Candidaactivity of three SSRIs was analyzed in the present study: fluoxetine, paroxetine, and sertraline. Given the antifungal potential of SSRIs, this study aimed to verify the anti-fungal effect of the candidates against fluconazole-resistant
Candida spp. strains while elucidating the possible mechanisms
involved in the cytotoxic action by employing procedures such as
flow cytometry and comet assay. The study also assessed the ac-tivity of SSRIs against biofilms of differentCandidaspecies.
2. Materials and methods
2.1. Yeast strains
Fluconazole-resistant clinical strains ofC.albicans,C.tropicalis,
C.parapsilosisandC. glabrata(four strains from each species) were
used. These strains are from the yeast collection of the Laboratory of Bioprospection in Antimicrobial Molecules (LABIMAN/FF/UFC)
[9,10].
2.2. Molecular identification
Genomic DNA was purified from the yeast strains using a cetyltrimethylammoniumbromide (CTAB)-based protocol as pre-viously described[11]. The nuclear DNA region comprising the in-ternal transcribed spacers (ITS1 and ITS2) and the 5.8S rRNA gene was amplified by polymerase chain reaction (PCR) using the primers ITS4 (50-TCCTCCGCTTATTGATATGC-30) and ITS5 (50
-GGAAGTAAAAGTCGTAACAAGG-30) as suggested by White et al.
[12]. Amplification reactions were performed in afinal volume of 25
m
L, which contained genomic DNA (300e400 ng), 1GoTaq reaction buffer (Promega, Madison, WI, USA), 1.5 mM MgCl2, 200m
M each dNTP (GE Healthcare Life Sciences, Piscataway, NJ, USA), 0.5m
M each primer and 1.25 U of GoTaq DNA Polymerase (Promega). Reactions were carried out in a Mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany) programmed for an initial denaturation step (2 min at 95C) followed by 35 cycles of1 min at 95C, 1 min at 60C, and 3 min at 72C. The last cycle was
followed by afinal incubation of 10 min at 72C. Control samples
containing all reaction components, except DNA, were used to test that no DNA contamination occurred. The amplifications specificity was determined by 1.0% agarose gel electrophoresis [13]. The remaining amplified products were purified using the illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare Life Sciences) and the concentrations of the purified amplicons were determined by measuring the absorbance at 260 nm of ten-fold dilutions. DNA sequencing was performed at the Macrogen Inc. (Seoul, South Ko-rea) using the Sanger's dideoxy chain termination method. Both strands of each amplicon were sequenced using the ITS4 and ITS5 primers. Sequences were then assembled using the Phred/Phrap/ Consed package[14e16]. The start and end boundaries of the ITS1 and ITS2 were identified by comparisons to annotated sequences from the ITSoneDB[17]and ITS2 database[18], respectively. The ITS/5.8S sequences were deposited in the GenBank database (accession numbers: AB861478, AB861479, KJ740174, KJ740175,
KJ740184, AB861491, AB861493, AB861490, AB861486, AB861487, AB861488, AB861485, AB861484) and compared to those available on public DNA sequence databases using the BLAST program[19].
2.3. L929 cell proliferation inhibition using the MTT test
L929 cells were cultivated under standard conditions in MEM with Earle's salts. All culture media were supplemented with 10% fetal bovine serum, 2 mM glutamine, 100
m
g/mL penicillin/strep-tomycin at 37C with 5% CO2. L929 cells were plated in 96-wellplates (0.3106cells/well) and test compounds (0.156e100
m
g/ mL) dissolved in ethanol were added to each well, followed by incubation for 24 h, under standard cultivation conditions. Cells were treated with MTT assay reagents (0.5 mg/mL). Three hours later, MTT formazan product was dissolved in 150m
L DMSO and absorbance was measured using a multiplate reader (Packard Spectra Count, Canada). Test substances effect was quantified as reduced dye control absorbance percentage at 595 nm. Experi-ments were carried out in duplicate and repeated at least three times[9].2.4. In vitro antifungal activity
The broth microdilution (BMD) susceptibility test was per-formed according to the document M27-A3 using RPMI broth (pH 7.0) buffered with 0.165 M MOPS (Sigma Chemical, St. Louis, MO). Fluconazole (FLC; Merck Sharp&Dohme, S~ao Paulo, Brazil) was dissolved in distilled water and tested at concentrations ranging from 0.125 to 64
m
g/mL. The selective serotonin reuptake inhibitors (SSRIs):fluoxetine, sertraline and paroxetine (FLX, SER, PAR; Galena Química e Farmac^eutica, Brasil) were dissolved in 95% ethanol and their effects on yeast cells were evaluated at concentrations ranging from 4.68 to 600m
g/mL. The yeasts and compounds were incubated in 96-well culture plates at 35C for 24 h and the results wereexamined visually, as recommended by CLSI[20]. The minimum inhibitory concentration (MIC) of each compound was determined as the concentration that inhibited 50% of fungal growth. The strains were classified as susceptible (S) or resistant (R) to FLC ac-cording to the following cutoff points, as recommended by the CLSI document M27-S4: MIC2
m
g/mL (S), MIC8m
g/mL (R). The strainsC. parapsilosisATCC 22019 and C. kruseiATCC 6258 were used as controls[21].2.5. Cell treatments
To assess cell density, membrane integrity, mitochondrial transmembrane potential, and DNA damage, one representative FLC-resistant strain ofC. albicans(strain 2) was exposed for 24 h to various concentrations (MIC, 2MIC, and 4MIC) offluoxetine, sertraline or paroxetine. Cells treated with FLC (64
m
g/mL) or amphotericin B (4m
g/mL) were included for comparison. All the tests were performed in triplicate in three independent experi-ments[9,10].2.6. Preparation of yeast suspensions
Cell suspensions were prepared from cultures in the exponential growth phase. The cells were collected, washed, re-suspended and adjusted to 106cells/mL in HEPES buffer (pH 7.2) supplemented with 2% glucose[9,10].
2.7. Determination of cell density and membrane integrity
from yeast cells incubated for 24 h with the tested drugs were analyzed byflow cytometry. A total of 10,000 events was evaluated per experiment (n¼2) and cellular debris was omitted from the analysis. Cellular fluorescence was then determined by flow cytometry using a Guava EasyCyte™ Mini System cytometer (Guava Technologies Inc. Hayward, CA, USA) and analyzed using CytoSoft 4.1 software[9,10].
2.8. Measurement of mitochondrial transmembrane potential
(
Dj
m)Yeast cells were washed with PBS, incubated with 5 mg/L rhodamine 123 at 37C for 30 min in the dark, washed twice with
PBS and theirfluorescence was measured byflow cytometry (Guava EasyCyte™Mini System). A total of 10,000 events was evaluated per experiment (n¼2) and cellular debris was omitted from the analysis[9,10].
2.9. Yeast comet assay
The alkaline comet assay was performed essentially as described by Silva et al.[22]. Images obtained byfluorescence microscopy of 100 randomly selected cells (50 cells from each of 2 replicate slides) were analyzed for each experimental group. The cells were scored visually and assigned to one offive classes according to tail size (from undamaged [class 0] to maximally damaged [class 4]), and a damage index value was calculated for each sample of cells. The damage index values, therefore, ranged from 0 (completely un-damaged: 100 cells x 0) to 400 (maximum damage: 100 cells x 4). The frequency of tailed cells, which were taken as an indicator of DNA damage, was calculated based on the numbers of cells with tails (DNA strand breaks) and without them[9,10].
2.10. Annexin V staining
Yeast cells were harvested by centrifugation and digested with 2 mg/mL zymolyase 20T (Seikagaku Corp. Japan) in potassium phosphate buffer (PPB, 1 M sorbitol, pH 6.0) for 2 h at 30C. The
protoplasts were then stained with FITC-labelled Annexin V and PI using a FITC-Annexin V apoptosis detection kit (Guava Nexin Kit, Guava Technologies). Subsequently, the cells were washed with PPB, incubated for 20 min in Annexin binding buffer containing 5
m
L/mL FITC-Annexin V and 5m
L of PI and analyzed by flow cytometry (Guava EasyCyte™Mini System). For each experiment (n¼2), 10,000 events were evaluated and cell debris was omitted from the analysis[9,10].2.11. Biofilm formation
For the effect offluoxetine on biofilm formation, one strain of
eachCandidaspecies (Table 1) was used andfluoxetine was tested
at concentrations ranging from 20 to 4000
m
g/mL. The yeasts were incubated in 96-well plates (1.0106cells/well). After 24 h, the wells were washed three times with PBS and 200m
L offluoxetine solution was added to each well containing viable 24 h biofilm. The plates were incubated at 35C for 24 h. The measurement of themetabolic activity of biofilm cells was evaluated using the MTT colorimetric assay and the readings were performed in a microplate reader at 540 nm[23].
2.12. Statistical analysis
In vitrosusceptibility experiments were repeated at least three
times on different days and geometric means were used to compare the MIC values. The data obtained from theflow cytometry and
alkaline comet assays were compared using a one-way analysis of variance (ANOVA) followed by the Newman-Keuls test (p<0.05). Mean absorbance values from the biofilm formation assay were compared using one-way ANOVA followed by the Tukey test (p<0.05).
3. Results
3.1. Molecular identification
To confirm the identity of theCandidaspecies used in the pre-sent work, the complete ITS/5.8S region (ITS1, 5.8S, and ITS2) of the nuclear ribosomal DNA from each strain was amplified, sequenced and compared to the sequences deposited in the GenBank database. The BLAST searches revealed that the sequences from the isolates were identical to the ITS/5.8S sequences from different isolates and strains ofC. albicans,C. tropicalis,C. parapsilosisandC. glabrataas shown inTable 1.
3.2. Citotoxic activity in murinefibroblasts
After 72 h exposure to compounds SER, FLX and PAR no showed cytotoxicity against murinefibroblasts (L929 strain), because none of the agents interfered with the proliferation of cells as well as untreated control cells (p<0.05). The median inhibitory concen-tration (IC50), lower concenconcen-tration of an agent which inhibits 50% of cell growth study was higher than the range tested, 100
m
g/mL (Table 2).3.3. SSRIs inhibit the growth of FLC-resistant strains of Candida spp.
Thefluconazole susceptibility profiles of theCandidaspp. strains were assessed using the microdilution technique and all the strains evaluated (Table 1) were resistant to FLC (MIC8
m
g/mL). On the other hand, the SSRIs were able to inhibit the growth of all FLC-resistant strains used in the present work (Table 1). The MIC values ranged from 20 to 160m
g/mL (FLX), from 10 to 20m
g/mL (SER) and from 10 to 100.8m
g/mL (PAR). Based on thesefindings, experiments were devised aiming to elucidate the mechanisms involved in the antifungal action of the SSRIs againstC.albicans2 (Table 1).3.4. Loss of cell viability in C. albicans after treatment with SSRIs
As shown in Fig. 1, there was no significant reduction in the number of viable cells when thefluconazole-resistant strains were treated with the azole, in comparison to untreated cells (p<0.05). In contrast to this, when the fluconazole-resistant cells were treated with the SSRIs (sertraline,fluoxetine, paroxetine) for 24 h they exhibited significant decreases in cell viability (p<0.05).
3.5. Changes in the yeast mitochondrial transmembrane potential
(
Dj
m)Significant (p < 0.05) changes in the mitochondrial trans-membrane potential were observed when the yeast cells were exposed to increasing concentrations of the SSRIs in comparison to untreated cells (Fig. 2). Amphotericin B was used as a positive control.
3.6. Externalization of phosphatidylserine in yeast cells
close to negative control cultures. Yeast cultures treated with the three SSRIs (MIC, 2MIC, 4MIC) after 24 h incubation showed significant increase (p< 0.05) in the apoptotic cells percentages compared to the control group. The treatment of fl uconazole-resistant cells with the sertraline, fluoxetine and paroxetine clearly induced cell death in a similar way to what was found in cells treated with amphotericin B, which was used as a positive control.
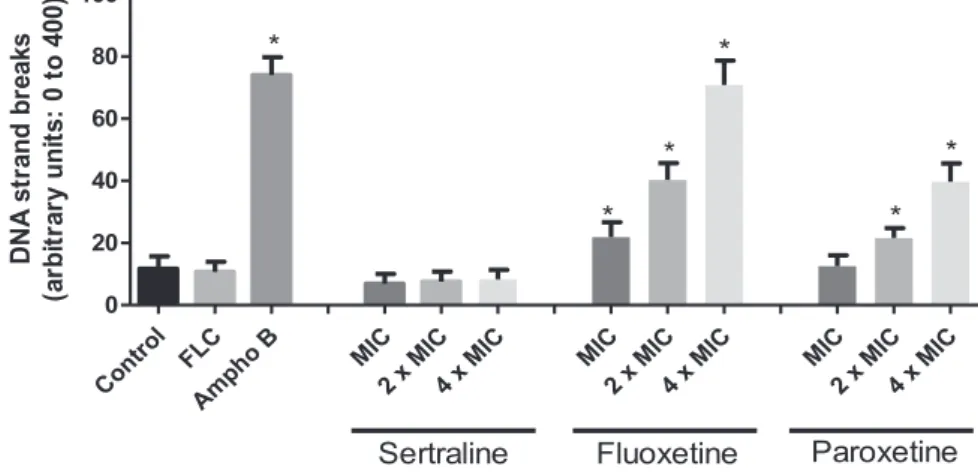
3.7. DNA damage
Fig. 4shows the DNA damage induced by effect of the three SSRIs on strains of C. albicansresistant tofluconazole. The indi-vidual analysis of single cell regarding the distribution of grades of DNA damage showed thatfluconazole and one of the SSRIs, the sertraline, induce low levels of DNA damage. In contrast,C. albicans
exposed with fluoxetine and paroxetine after 24 h incubation resulted in significant increment (p<0.05) on DNA strand break levels. Cells treated with SSRIs during 24 h exhibited damage index. Amphotericin B, used as positive control, induced high levels of DNA strand breaks.
3.8. Effect offluoxetine on mature Candida spp. biofilm
As seen inFig. 5, when the biofilm-forming isolates are exposed tofluoxetine, the minimum inhibitory concentrations (MIC) were similar among the species (MIC50). MIC was approximately 40
m
g/ mL forC. parapsilosis, 80m
g/mL forC. albicans, and 160m
g/mL forC. tropicalis and C. glabrata. The antifungal effects of fluoxetine
caused statistically significant reductions in the biofilm's cell ac-tivity (p<0.05) and the MIC values were similar to those obtained with the same cells in planktonic growth in our study.
4. Discussion
The genus Candida is responsible for about 75% of hospital-acquired fungal infections worldwide [2]. Incorrect diagnosis, short-duration therapy, patients with high microbial load, highly virulent strains, and long exposure to the medication are factors that may cause fungal resistance, a problem correlated with treat-ment failures[24].
In the quest for new treatment strategies, non-antifungal drugs
obtained promising activity even against fluconazole-resistant strains[10]. The mechanism of action of SSRIs is the inhibition of the sodium-dependant serotonin transporter and some of their main representatives, i.e.,fluoxetine and sertraline, have already shown promising antifungal activity alone and combined with
fluconazole[6,7,25], also in in vivo studies[26].
The results of the present study showed that the three SSRIs used (sertraline,fluoxetine, and paroxetine) have antifungal effect within the range tested. Fluoxetine was the drug with the greatest MIC range (20e160
m
g/mL) compared to sertraline and paroxetine. This variation in sensitivity was not directly related to theCandidaspecies and likely occurs due to genotypic variations in each strain
[27]. Silvestri et al.[25]found similar results and reported MIC for
fluoxetine againstC. albicansstrains in the range of 32e122
m
g/mL. Oliveira et al. [6], in turn, found higher MIC values even forfluconazole-sensitiveCandidaspp. strains.
Unlike the other candidates, paroxetine had not yet shown significant antifungal activity results. The antifungal activity of this SSRI (10e100.8
m
g/mL) was below that already reported in the literature forCandida parapsilosis(MIC¼500e2.000m
g/mL)[8].The in vitro antifungal effect of sertralineethe candidate with the best resultehad already been shown againstAspergillusspp.
Cryptococcus neoformans, Plasmodium falciparum, andCandidaspp.
[5,8,25]. The values in the present study corroborate other studies
[7]and show an interesting antifungal profile.
After the fluconazole-resistant Candida albicans strain was Table 1
The effects offluoxetine, paroxetine and sertraline against FLC-resistant strains ofCandidaspp.
Strain GenBank accession number of the ITS/5.8S sequence MIC (mg/mL)
Fluoxetine Paroxetine Sertraline
Candida albicans1 AB861478 40 63.5 20
Candida albicans2a,b AB861479 127 80 20
Candida albicans3 KJ740174 63.5 80 20
Candida albicans4 KJ740175 100.8 80 20
Candida tropicalis1a KJ740184 160 80 20
Candida tropicalis2 AB861491 50.4 100.8 20
Candida tropicalis3 AB861493 40 80 20
Candida tropicalis4 AB861490 80 80 20
Candida parapsilosis1 AB861486 80 40 20
Candida parapsilosis2a AB861487 160 63.5 20
Candida parapsilosis3 AB861488 80 80 10
Candida parapsilosis4 AB861485 20 10 10
Candida glabrata1a AB861484 80 63.5 15.9
Minimum inhibitory concentrations (MIC) offluoxetine, paroxetine and sertraline against clinical strains ofCandidaspp.. The MIC was defined as the lowest concentration that produced 50% reduction in fungal cells growth. The microdilution in broth was performed according to CLSI protocol M27-A3. SSRIs concentrations ranged from 4.68e600mg/
mL. The MICs represent the geometric means of at least three MICs determined on different days. aThese strains were used in the biofilm formation assay.
bThis strain was used to investigate the antifungal action mechanism of the SSRIs.
Table 2
Cytotoxic activity of Sertraline, Fluoxetine and Paroxetine on fibroblasts of murine. Data are presented as IC50 values and 95% confidence interval (CI 95%) from three independent experiments performed in triplicate.
Compounds CI 95%
(mg/mL) Fibroblastos IC50
NCa NEb
Sertraline >100
Fluoxetine >100
Paroxetine >100
aNegative control was treated with the vehicle (DMSO,
0.1%).
Fig. 1. Reduction in the number of viable cells in an FLC-resistantC. albicansstrain treated with SER, FLX, and PAR.Cell viability assessment byflow cytometry of the cells treated with FLC (64mg/mL), Ampho B (4mg/mL), control, only the RPMI medium, and with the compounds sertraline MIC (20mg/mL), 2MIC (40mg/mL), 4MIC (80mg/mL); fluoxetine MIC (127mg/mL), 2MIC (254mg/mL), 4MIC (508mg/mL); and paroxetine MIC (80mg/mL), 2MIC (160mg/mL), 4MIC (320mg/mL); in a single representative FLC-resistant strain,C. albicans 2(Table 1), after 24 h of exposure. p<0.05 compared with control according to ANOVA followed by Newman-Keuls test.
Fig. 2. Assessment of the mitochondrial transmembrane potential in an FLC-resistantC. albicansstrain treated with SER, FLX, and PAR.The cells were stained with Rh123 (50 nM). Cell viability assessment byflow cytometry of the cells treated with FLC (64mg/mL), Ampho B (4mg/mL), control, only the RPMI medium, and with the compounds sertraline MIC (20mg/mL), 2MIC (40mg/mL), 4MIC (80mg/mL);fluoxetine MIC (127mg/mL), 2MIC (254mg/mL), 4MIC (508mg/mL); and paroxetine MIC (80mg/mL), 2MIC (160mg/mL), 4MIC (320mg/mL), over 24 h. p<0.05 compared with control according to ANOVA followed by Newman-Keuls test.
exposed to each compound, the number of viable cells decreased at the concentrations treated (MIC, 2MIC, and 4MIC) compared to the control in the presence of the cytofluorimetric marker propi-dium iodide (PI), which indicates cell-membrane damage that possibly compromised their functions.
The intrinsic apoptosis activation pathway involves mitochon-drial participation in response to some cell damage/stress. When cell death signals reach the mitochondrion, the inner mitochondrial membrane potential (
Dj
m) that controls the opening of transition pores and the release of pro-apoptotic factors into cytosol collapses[26]. The change in
Dj
m prevents rhodamine 123 (Rh123), a nucleophilicfluorescent dye, from accumulating inside mitochon-dria. Our results using Rh123 confirm a mitochondrial disorder, indicating that treatingfluconazole-resistantC. albicanswith SSRIs affects mitochondrial respiratory function and culminates in cell death.In vivostudies show that the chronic treatment withfl uox-etine using doses well above the therapeutic one changes mito-chondrial energy metabolism and integrity in the liver of animals. These changes are due to the increase in oxidative stress inside the cell, identified by the increase in the liver carbonyl protein and MDA markers. The increase in this lipid peroxidation marker may also have led to the membrane damage reported[27,28]. Rainey et al.[29]identified that sertraline, besides its conventional protein target, also targets phospholipids in cytoplasmic surfaces such as Golgi apparatus, vacuoles, and endosomes. Some cells have compensatory mechanisms for this additional stress, but, when this system is not expressed, the membrane suffers damages, which is what likely occurs with the cells of C. albicans and other microorganisms.During the apoptosis process, phosphatidylserine (PS), an inner membrane phospholipid, is externalized and serves as a marker for the cell to be phagocyted by macrophages. PS externalization detection employed annexin V, a protein able to bind to this phospholipid[30]. After exposure (24 h) to the compounds, fungal cells with externalized PS were observed, which highlights the role of apoptosis on cell death during the antifungal activity of SSRIs. This programmed cell death has been reported for Burkitt lym-phoma cells and in human neuroblastoma cells through the acti-vation of caspases[31,32]. Apoptosis was also induced byfluoxetine in a human carcinoma lineage by altering the mitochondrial membrane potential, thus leading to the activation of caspases through reactive oxygen species[33].
Regarding DNA damage, DNA fragmentation is a characteristic
that represents an irreversible step in the cell death process[30]. The alkaline comet assay enables detecting breakages in single and double strands of the DNA molecule induced by substances with genotoxic potential. Lemos et al.[34]concluded thatfluoxetine is genotoxic for the human cell lineage tested only at high in vitro concentrations (5000
m
g/mL), but that does not occurs in vivo, likely due to metabolization. The other drugs tested have no re-ported positive genotoxic activity, however, in long-term assays for carcinogenesis in rats, paroxetine and sertraline yielded positive results[35]. Our results showed that fungal cells are more sensitive to the three candidates than human cells regarding DNA integrity, but, nonetheless, we found no direct correlation with the antifungal mechanism of action since the values were proportional to the concentrations employed. Sertraline, which obtained the best result regarding MIC, caused no DNA damage at the concentrations tested (20, 40, and 80m
g/mL). The candidate that caused the greatest percentage of DNA damage was the one used at the highest concentration (127, 254, and 508m
g/mL), i.e.,fluoxetine.According to our data, each of the SSRIs tested cause fungal death after damaging the membrane, which activates apoptotic signaling pathways and leads to dose-dependant cell viability loss. Aware of the promising effect of sertraline, fluoxetine, and paroxetine and taking into account that an important virulence factor of yeasts of the genusCandidais biofilm formation[36], we analyzed the antifungal activity against biofilm formed over 24 h by differentCandidaspecies. Our results showed that thefluoxetine concentration required to inhibit biofilm viability are similar to or even lower than for planktonic cells. Homogenicity was found in the results among the species. Wang et al.[37]showed that the substance tested, baicalin, is able to induce apoptosis inC. albicans
biofilm the same way it induced apoptosis in planktonic cells, showing that some substances can be equally effective against either form. The membrane disorder caused by fluoxetine may significantly change vital structures in the biofilm, as was the case with free cells. Thisfinding becomes relevant since the character-istic often associated with a biofilm is its reduced sensitivity against commonly used conventional antifungal agents. Lass Fl€orl et al.[38]
showed that sertraline impacts other virulence factors of the genus
Candida, such as lower hypha formation and decreased
phospho-lipase and aspartyl proteinase production.
Although it is known that the common plasma concentration is around 200 ng/mL (forfluoxetine), which is much lower than the values obtained in the MICs, the in vivo plasma concentration at
several anatomical sites, such as vaginal fluid and cerebrospinal
fluid, are not clearly defined. Some studies have already shown in vivo antifungal activity despite the unknown concentration at the action site[6,39]. Thus, these results must be taken into account when choosing the clinical treatment. If the patient falls into the risk factors for Candidainfections, i.e., recurrence, resistance, or biofilm formation, and requires treatment with antidepressants, the drug of choice may befluoxetine, sertraline, or paroxetine given their possible adverse effect againstCandidaspp. Moreover, topical use must be evaluated in order to identify whether a new formu-lation is viable for the treatment of vulvovaginal candidiasis, as also discussed by Oliveira et al.[6].
5. Conclusion
SSRIs show in vitro antifungal activity againstCandida albicans,
C. tropicalis, C. parapsilosis, and C. glabrata strains. Against
C. albicansstrain, it promoted changes in plasma and mitochondrial
membrane integrity, which led to cell death through apoptosis. Fluoxetine also proved able to decrease in vitro biofilm viability of allfluconazole-resistantCandidaspecies tested.
References
[1] D.E. Corzo-Leon, M.J. Satlin, R. Soave, T.B. Shore, A.N. Schuetz, S.E. Jacobs, et al., Epidemiology and outcomes of invasive fungal infections in allogeneic hae-matopoietic stem cell transplant recipients in the era of antifungal prophy-laxis: a single-centre study with focus on emerging pathogens, Mycoses 58 (2015) 325e336.
[2] M.A. Pfaller, D.R. Andes, D.J. Diekema, D.L. Horn, A.C. Reboli, Rotstein, et al., Epidemiology and outcomes of invasive candidiasis due to non-albicans spe-cies of Candidain 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008, PLoS One 9 (7) (2014) e101510. [3] C.F. Rodrigues, S. Silva, J. Azeredo, M. Henriques, Detection and quantification
offluconazole withinCandida glabratabiofilms, Mycopathologia 179 (5e6)
(2015) 391e395.
[4] J.C.O. Sardi, L. Scorzoni, T. Bernardi, A.M.F. Almeida, M.J.S.M. Giannini,Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options, J. Med. Microbiol. 62 (2013) 10e24.
[5] A.F. Coutaux, J.J. Mooney, D.F. Wirth, Neuronal monoamine reuptake in-hibitors enhancein vitrosusceptibility to chloroquine in resistantPlasmodium falciparum, Antimicrob. Agents Chemother. 38 (1994) 1419e1421. [6] A.S. Oliveira, C.A. Gaspar, R.P. Oliveira, J.M. Oliveira, A.P. Oliveira, Anti-Candida
activity offluoxetine alone and combined withfluconazole: a synergistic ac-tion againstfluconazole-resistant strains, Antimicrob. Agents Chemother. 58 (2014) 4224e4226.
[7] C. Lass-Florl, M.P. Dierich, D. Fuchs, E. Semenitz, M. LedochowskI, Antifungal€ activity againstCandidaspecies of the selective serotonin-reuptake inhibitor, Sertraline, Clin. Infect. Dis. 33 (2001) 135e136.
[8] C. Lass-Florl, M.P. Dierich, D. Fuchs, E. Semenitz, M. LedochowskI, Antifungal€ properties of selective serotonin reuptake inhibitors againstAspergillus spe-ciesin vitro, J. Antimicrob. Chemother. 48 (2001) 775e779.
[9] J.B. Andrade Neto, C.R. da Silva, M.A.S. Neta, R.S. Campos, J.T. Siebra, R.A.C. Silva, et al., Antifungal activity of naphthoquinoidal compoundsin vitro againstfluconazole-resistant strains of differentCandidaspecies: a special emphasis on mechanisms of action on Candida tropicalis, PLoS One 9 (2014) e93698.
[10] C.R. Da Silva, J.B. De Andrade Neto, J.J.C. Sidrim, M.R.F. Angelo,^ H.I.F. Magalh~aes, B.C. Cavalcanti, et al., Synergistic effects of amiodarone and fluconazole onCandida tropicalisresistant tofluconazole, Antimicrob. Agents Chemother. 57 (2013) 1691e1700.
[11] S.A.J. Warner, Genomic DNA isolation and lambda library construction, in: G.D. Foster, D. Twell (Eds.), Plant Gene Isolation: Principles and Practice, John Wiley&Sons, West Sussex, 1996, pp. 51e73.
[12] T. White, T. Bruns, S. Lee, J. Taylor, Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, in: M.A. Innis, D.H. Gefland, J.J. Sninsky, T.J. White (Eds.), PCR Protocols: a Guide to Methods and Appli-cations, Academic Press, San Diego, CA, 1990, pp. 315e322.
[13] J. Sambrook, E.F. Fritsch, T. Maniatis, The Condensed Protocols from Molecular Cloning: a Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989.
[14] B. Ewing, P. Green, Base-calling of automated sequencer traces using phred. II. Error probabilities, Genome Res. 8 (1998) 186e194.
finishing, Genome Res. 8 (1998) 195e202.
[17] M. Santamaria, B. Fosso, A. Consiglio, G. De Caro, G. Grillo, F. Licciulli, S. Liuni, M. Marzano, D. Alonso-Alemany, G. Valiente, G. Pesole, Reference databases for taxonomic assignment in metagenomics, Brief. Bioinform 13 (2012) 682e695.
[18] A. Keller, T. Schleicher, J. Schultz, T. Müller, T. Dandekar, M. Wolf, 5.8S-28S rRNA interaction and HMM-based ITS2 annotation, Gene 430 (2009) 50e57. [19] S.F. Altschul, W. Gish, W. Miller, E.W. Myers, D.J. Lipman, Basic local alignment
search tool, J. Mol. Biol. 215 (1990) 403e410.
[20] Clinical and Laboratory Standards Institute, References method for broth dilution antifungal susceptibility testing of yeasts approved standard, in: Document M27-A3, third ed., CLSI, Wayne, PA, 2008.
[21] Clinical and Laboratory Standards Institute, Reference method for broth dilution antifungal susceptibility testing of yeasts, in: Fourth Informational Supplement M27-S4, third ed., CLSI, Wayne, PA, 2012.
[22] A.R. Da Silva, J.B. Andrade Neto, C.R. Da Silva, R.S. Campos, R.A.C. Silva, D.D. Freitas, et al., Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: action mechanism evaluated byflow cytometry and bio-film growth inhibition inCandidaspp. Antimicrob. Agents Chemother. 60 (2016) 3551e3557.
[23] C.G. Pierce, P. Uppuluri, A.R. Tristan, F.L.W Jr., G. Ramage, J.L. Lopez-Ribot, A simple and reproducible 96 well plate-based method for the formation of fungal biofilms ans its application to antifungal susceptibility testing, Nat. Protoc. 3 (9) (2008) 1494e1500.
[24] S. Tobudic, C. Kratzer, E. Presterl, Azole-resistantCandidaspp.eemerging pathogens? Mycoses 55 (2012) 24e32.
[25] R. Silvestri, M. Artico, G. Regina, A. Pasquali, G. Martino, F.D. D'Auria, et al., Imidazole analogues offluoxetine, a novel class of anti-Candidaagents, J. Med. Chem. 47 (2004) 3924e3926.
[26] L. Galluzzi, E. Morselli, O. Kepp, I. Vitale, A. Rigoni, E. Vacchelli, et al., Mito-chondrial gateways to c^ancer, Mol. Asp. Med. 31 (2010) 1e20.
[27] J. Zlatkovic, N. Todorovic, N. Tomanovic, M. Boskovic, S. Djordjevic, T. Lazarevic-Pasti, et al., Chronic administration offluoxetine or clozapine induces oxidative stress in rat liver: a histopathological study, Eur. J. Pharm. Sci. 59 (2014) 20e30.
[28] M. Feldman, L.S. Friedman, L.J. Brandt, Sleisenger&Fordtran's Gastrointestinal
and Liver Disease. Pathophysiology, Diagnosis, Management, eighth ed., Saunders Elsevier, Philadelphia, 2006.
[29] M.M. Rainey, D. Korostshevsky, S. Lee, E.O. Perlstein, The antidepressant ser-traline targets intracellular vesiculogenic membranes in yeast, Genetics 185 (2010) 1221e1233.
[30] I. Hwang, J. Lee, H.G. Jin, E.R. Woo, D.G. Lee, Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death inCandida albi-cans, Mycopathologia 173 (2012) 207e218.
[31] A. Serafeim, M.J. Holder, G. Grafton, A. Chamba, M.T. Drayson, Selective se-rotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells, Blood 101 (2003) 3212e3219.
[32] Y. Levkovitz, I. Gil-Ad, E. Zeldich, M. Dayag, A. Weizman, Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evi-dence for p-c-Jun, cytochrome c, and caspase-3 involvement, J. Mol. Neurosci. 27 (2005) 29e42.
[33] C.S. Lee, Y.J. Kim, E.R. Jang, W. Kim, S.C. Myung, Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor- j B, Basic Clin. Pharmacol. Toxicol. 106 (2009) 446e453.
[34] M.G. Lemos, M.S. Mantovani, V.E.P. Vicentini, Evaluation of genotoxic effect of prozac (fluoxetine) without and with addition of vitamins A and C by means of the comet assay in culture of CHO-K1 cells, Semin. Ci^enc Biol. Saúde 26 (2005) 95e100.
[35] G. Brambilla, F. Mattioli, A. Martelli, Genotoxic and carcinogenic effects of antipsychotics and antidepressants, Toxicology 261 (2009) 77e88. [36] C.S. Lim, R. Rosli, H.F. Seow, P.P. Chong,Candidaand invasive candidiasis: back
to basics, Eur. J. Clin. Microbiol. Infect. Dis. 31 (2012) 21e31.
[37] T.M. Wang, G.X. Shi, J. Shao, D. Wu, Y.Y. Yan, M.X. Zhang, et al.,In vitro anti-fungal activity of baicalin againstCandida albicansbiofilms via apoptotic in-duction, Microb. Pathog. 87 (2015) 21e29.
[38] C. Lass-Fl€orl, M. Ledochowski, D. Fuchs, C. Speth, L. Kacani, M.P. Dierich, et al., Interaction of sertraline withCandidaspecies selectively attenuates fungal virulencein vitro, FEMS Immunol. Med. Mic. 35 (2003) 11e15.