Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
Microbiological evaluation of fresh semen from captivity kept collared peccary
(Pecari tajacu) at Brazilian Amazon
Avaliação microbiológica do sêmen fresco de caititus (Percari tajacu) mantidos
em cativeiro na Amazônia Brasileira
DOI:10.34117/bjdv6n9-136
Recebimento dos originais: 08/08/2020 Aceitação para publicação: 08/09/2020
Mário Mansour Pinheiro Bartha
Mestrado
Instituto de Ciências Biológicas - Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Belém-PA, Brazil E-mail: mariobartha@hotmail.com
Hilma Lúcia Tavares Dias
Doutorado
Instituto de Ciências Biológicas - Universidade Federal do Pará Endereço: R. Augusto Corrêa, 01 - Guamá, 66075-110, Belém-PA, Brazil
E-mail: hilmalucia@gmail.com
Natália Inagaki Albuquerque
Doutorado
Empresa Brasileira de Pesquisa Agropecuária, Embrapa Amazônia Oriental Endereço: Tv. Dr. Eneas Pinheiro, s/n - Marco, 66095-903, Belém-PA, Brazil
E-mail: natalia@cpatu.embrapa.br
Priscila Reis Kahwage
Doutorado
Embrapa Amazônia Oriental
Endereço: Tv. Dr. Eneas Pinheiro, s/n - Marco, 66095-903, Belém-PA, Brazil E-mail: priscila.kahwage@hotmail.com
Diva Anelie Araújo Guimarães
Doutorado
Instituto de Ciências Biológicas. Universidade Federal do Pará Endereço: R. Augusto Corrêa, 01 - Guamá, 66075-110, Belém-PA, Brazil
E-mail: diva@ufpa.br
Alexandre Rossetto Garcia
Doutorado
Embrapa Pecuária Sudeste
Rodovia Washigton Luiz, km 234. 13560-970. Caixa Postal 339 - Fazenda Canchim, São Carlos-SP. Brazil.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
Otávio Mitio Ohashi
Doutorado
Instituto de Ciências Biológicas - Universidade Federal do Pará Endereço: R. Augusto Corrêa, 01 - Guamá, 66075-110, Belém-PA, Brazil
E-mail: ohashi@ufpa.br
Ednaldo Silva Filho
Instituto da Saúde e Produção Animal - Universidade Federal Rural da Amazônia Endereço: Av. Presidente Tancredo Neves, 2501 Bairro: Terra Firme, 66077-830, Belém-PA,
Brazil
E-mail: silva.filho@ufra.edu.br
ABSTRACT
This study aimed to identify the existent of microbiota in the fresh semen of collared peccary (Pecari
tajacu), kept in captivity, and their susceptibility patterns to antimicrobial drugs. Ten males of this
species were submitted into experimental induced ejaculation procedures throughout 14 months. The microorganisms found in the semen were evaluated within the following criteria: morphology, biochemistry, colony forming unit (CFU mL‒1), and antibiotic sensitivity. 225 Gram-positive strains were isolated, out of 88 samples. They were identified as following: Streptococcus (30.2%),
Staphylococcus (30.2%), Micrococcus (33.7%), Corynebacterium (2.2%), Enterococcus (1.7%),
and Bacillus (1.7%). The cell counting of contaminated semen material was under 300 CFU mL‒1. The evaluation of sensitivity against eleven antibiotics revealed that most of the bacteria found were sensitive to Gentamicin, Cephalothin, Amikacin, Ampicillin, Ceftriaxone, Cephotaxin and Penicillin. During the evaluation period, the presence of microorganisms in the semen here analyzed did not influence the quality of the semen production of the donor animals. Semen contamination is probably coming from the surrounding environment in which the animals were kept, during collection procedures. This study suggests that Gentamicin and Amikacin are better choices over Tetracycline as diluters of collared peccary’s semen.
Keywords: Tayassuidae, Ejaculated, Microorganisms, antibiotics. RESUMO
O objetivo deste estudo foi identificar a microbiota existente no sêmen fresco de queixada (Pecari
tajacu), mantido em cativeiro, e seus padrões de suscetibilidade a medicamentos antimicrobianos.
Dez machos desta espécie foram submetidos a procedimentos experimentais de ejaculação induzida ao longo de 14 meses. Os microrganismos encontrados no sêmen foram avaliados dentro dos seguintes critérios: morfologia, bioquímica, unidade formadora de colônias (UFC mL‒1) e sensibilidade a antibióticos. Foram isoladas 225 cepas Gram-positivas, de 88 amostras. Eles foram identificados da seguinte forma: Streptococcus (30,2%), Staphylococcus (30,2%), Micrococcus (33,7%), Corynebacterium (2,2%), Enterococcus (1,7%) e Bacillus (1,7%). A contagem de células do sêmen contaminado estava abaixo de 300 UFC mL-1. A avaliação da sensibilidade contra onze antibióticos revelou que a maioria das bactérias encontradas eram sensíveis à gentamicina, cefalotina, amicacina, ampicilina, ceftriaxona, cefotaxina e penicilina. Durante o período de avaliação, a presença de microrganismos no sêmen aqui analisado não influenciou a qualidade da produção de sêmen dos animais doadores. Provavelmente, a contaminação do sêmen é proveniente do ambiente em que os animais foram mantidos, durante os procedimentos de coleta. Este estudo sugere que a gentamicina e a amicacina são melhores escolhas sobre a tetraciclina como diluidoras do sêmen de queixada.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
Palavras-chave: Tayassuidae, ejaculado, microrganismos, antibióticos. 1 INTRODUCTION
Collared peccary (Pecari tajacu) is a species of great environmental diversity and adaptation. In captivity, it represents a highly potential in terms of productivity, when compared to other Amazon mammals (MAYOR et al., 2007; SILVA et al., 2016).
A wide range of bacteria commonly leads to reproductive diseases. Kuster and Althouse (2016) report that the reproductive performance of boar through artificial insemination is threatened by bacteriospermia. They describe a significant list of bacteria registered in semen that come from fecal material, preputial, skin and hair. During the semen collection to be used at in vitro fertilization procedures, it is quite common the occurrence of bacterial contamination coming from either the environment or from the donor animal’s body (RIESENBECK et al., 2015). Such contamination is extremely hard to avoid. Therefore, it is crucial the addition of wide range of antibiotics to the semen’s diluent (YÁNIZ et al., 2010).
A great number of authors have reported the presence of bacteria in the semen of different mammal species, such as: leopards (MORATO et al., 1998), sheep (MENDEZ NÁREZ et al., 1999), swallows (LOMBARDO; THORPE, 2000), pigs (KUSTER; ALTHOUSE, 2016), camel (GHONEIM et al., 2014), bovine and buffalo (SANNAT et al., 2015), goats (SOUZA et al., 2006), fishes (OLIVEIRA et al., 2007) and humans (WENG et al., 2014). However, there is no report found in the literature regarding the presence of such microorganisms in the semen of collared peccary.
It is suggested that the presence of microorganisms in the semen of collared peccary may interfere on its quality when used on in vitro fertilization procedures. As data regarding this topic is absent, studies focused in this topic are in high demand. The goals of this study were to identify the existent micro flora in fresh semen of collared peccary kept in captivity, as well as to evaluate their sensitivity towards the action of antibiotics.
2 MATERIAL AND METHODS
This study has followed all the rules established by the National Council for Animal Experiments of Brazil (CONCEA) and approved by Animal Ethics Committee (CEUA) of the Universidade Federal do Pará, registration nº 184/13 CEPAE-UFPA.
10 individuals, males, with average age of 76.8 (±37.8) months and weighing on average 19.8 (±2.7) kg, kept in the scientific captivity area of Embrapa Amazônia Oriental (Belém-PA, Brazil; 01º24’S; 48º20’W) were used. The animals were kept in 36 m2 collective cubes, grouped by
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
family related individuals of up to 10 animals per cube. The male:female ratios in the groups were 1:2 or 1:3, under natural conditions of temperature, humidity and full darkness-lightness cycle. All the animals received food supplementation composed by pig food based on corn and soy (500 g animal‒1), diverse species of grass, fruit and water ad libitum. At 24 hours before collecting samples, the animals were isolated and submitted into feast from both food and liquids.
The animal’s sedation was done in accordance to the protocol established by Souza et al. (2009) and Kahwage et al. (2010). After sedation, cleaning procedures such as hair removal, prepuce washing, feces removal, and rectus cleaning took place. The external and internal prepuce areas were cleaned up with pre-warmed NaCl 0.9 % solution (35 °C).
The semen collection was done in intervals of 15 days, by rectal electro stimulation, as described by Souza et al. (2009). Aliquots of the collected semen were used for seminal analysis, as well as for microbiological tests.
Samples were first evaluated for the following sperm analysis parameters: general appearance, color, total volume, concentration, pH, sperm’s motility and vitality; morphology, viability and defected sperm cells.
For the microbiological assays, sample aliquots were inoculated in duplicates, in Petri dishes containing enriched bacteriological medium agar with 5% sheep blood defibrinated and MacConkey Agar. Petri dishes were then incubated at 37 °C, during 24 to 48 h. After incubation, the colonies were evaluated through their morphology per stain characteristics and biochemical tests were performed for their identification (QUINN et al., 1994).
The CFU counting was performed in accordance to the protocol preconized by World Organization for Animal Health (OIE, 2008) for cattle, with some few modifications.
The antibiotic susceptibility tests were done by the method of disc diffusion, proposed by Bauer et al. (1966) and reviewed by the Committee for Clinical Laboratory Standards Institute (NCCLS) (CLSI, 2019). The discs were inserted on Müeller-Hinton Agar, throughout 150 mm diameter Petri dishes. The antimicrobial discs used were: Penicillin (10 µg), Gentamicin (10 µg), Ampicillin (10 µg), Amikacin (30 µg), Cephalothin (10 µg), Cephotaxin (30 µg), Ceftazidime (30 µg), Ceftriaxone (30 µg), Chloramphenicol (30 µg), Erythromycin (15 µg), and Tetracycline (30 µg). The Petri dishes were incubated at 37 °C for 24 h. After the incubation, the measurements of growth inhabitation halos were taken.
Odds Ratio tests were used to achieve the frequency based on the number of bacteria in the samples. The established significance level was 0.05.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
3 RESULTS
The donor animals had symmetrical testicular with intermediate consistence. Out of 117 attempts to collect semen from the animals used in this study, only 88 of the ejaculated material contained sperms. The analyzed semen was characterized as the following averages: Volume: 0,81(±0.86) mL; Concentration: 137.44(±153) x 106 sptz mL‒1; Mobility: 52.66(±28.79)%; Vigor: 2.2(±0.8); Viability: 55.84(±28.55)%; Major defects: 22.87(±12.93)%; Minor defects: 9.11(±5.88)% and total defects: 31.52(±13.81)%.
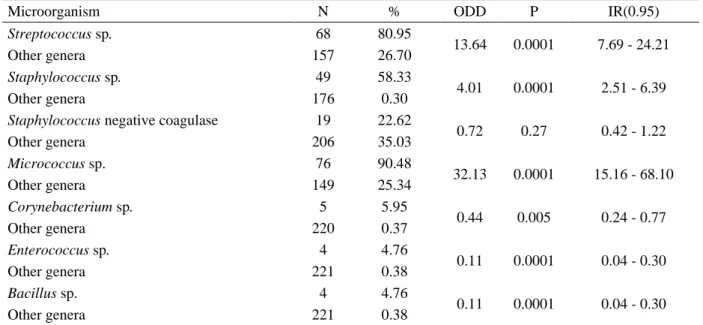
Out of the total of 88 ejaculated obtained, 78 (88.6%) showed bacterial growth, and 225 strains were isolated. From the isolates, 135 (60%) were pure culture and 90 (40%) were mixed culture containing two or more genera. A total of six bacterial genera were identified: Streptococcus (30.2%), Staphylococcus (30.2%), Micrococcus (33.7%), Corynebacterium (2.2%), Enterococcus (1.7%) and Bacillus (1.7%). The estimated frequency and bacterial prevalence are presented on Table 1, in which it can be seen the two most prevalent genera: Streptococcus and Micrococcus. However, Streptococcus sp., Staphylococcus sp. and Micrococcus sp. were the most frequent strains found among the microorganisms isolated in this work.
Table 1. Frequency of isolated microorganisms from a total of 225 colonies isolated from the semen of Collared
peccarys. N= individuals number, ODD= Odds ratio, P= Probability and RI (0.95)= Reference interval at 95%.
Microorganism N % ODD P IR(0.95)
Streptococcus sp. 68 80.95 13.64 0.0001 7.69 - 24.21 Other genera 157 26.70 Staphylococcus sp. 49 58.33 4.01 0.0001 2.51 - 6.39 Other genera 176 0.30
Staphylococcus negative coagulase 19 22.62
0.72 0.27 0.42 - 1.22 Other genera 206 35.03 Micrococcus sp. 76 90.48 32.13 0.0001 15.16 - 68.10 Other genera 149 25.34 Corynebacterium sp. 5 5.95 0.44 0.005 0.24 - 0.77 Other genera 220 0.37 Enterococcus sp. 4 4.76 0.11 0.0001 0.04 - 0.30 Other genera 221 0.38 Bacillus sp. 4 4.76 0.11 0.0001 0.04 - 0.30 Other genera 221 0.38
CFU counting, performed in serial dilutions is presented on Table 2. The following dilutions were: 1:10, 1:100 and 1:1,000 exceeded the limit of 300 CFU mL‒1. The dilutions 1:10,000 and 1:100,000 revealed a decline on the number of bacteria. In regard of CFU mL‒1 counting, it was observed a growth above 300 CFU mL‒1 on the less diluted conditions (Table 2).
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
Table 2. Total counting in CFU mL‒1 from collared peccary semen in its five serial dilution categories. CFU= Colony-forming unit. Microorganism CFU mL ‒1 101 102 103 104 105 Streptococcus sp. >300 >300 >300 139.5 80.2 Staphylococcus sp. >300 >300 >300 175.5 57.2 Micrococcus sp. >300 >300 >300 88.5 58.2 Corynebacterium sp. >300 >300 >300 125.5 66.0 Enterococcus sp. >300 >300 >300 102.5 83.7
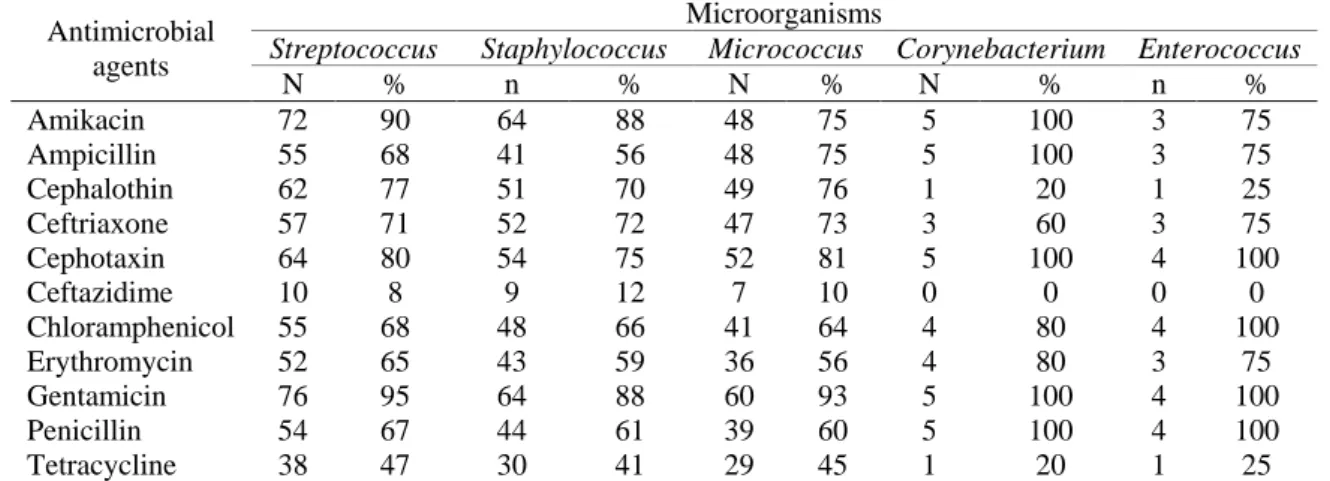
Table 3 presents results of the antibiotic susceptibility assays. Considering a sensitivity of ≥90%, Streptococcus sp. presented sensitivity to Amikacin and Gentamicin; Micrococcus sp. was only sensitive to Gentamicin; Corynebacterium sp. presented sensitivity to: Amikacin, Ampicillin, Cephotaxin, Gentamicin and Penicillin. Additionally, Enterococcus sp. presented sensitivity towards Cephotaxin, Chloramphenicol, Gentamicin and Penicillin.
Table 3. Antimicrobial susceptibility result of microorganisms found in the semen of collared peccary, raised in the metropolitan region of Belém (PA, Brazil).
Antimicrobial agents
Microorganisms
Streptococcus Staphylococcus Micrococcus Corynebacterium Enterococcus
N % n % N % N % n % Amikacin 72 90 64 88 48 75 5 100 3 75 Ampicillin 55 68 41 56 48 75 5 100 3 75 Cephalothin 62 77 51 70 49 76 1 20 1 25 Ceftriaxone 57 71 52 72 47 73 3 60 3 75 Cephotaxin 64 80 54 75 52 81 5 100 4 100 Ceftazidime 10 8 9 12 7 10 0 0 0 0 Chloramphenicol 55 68 48 66 41 64 4 80 4 100 Erythromycin 52 65 43 59 36 56 4 80 3 75 Gentamicin 76 95 64 88 60 93 5 100 4 100 Penicillin 54 67 44 61 39 60 5 100 4 100 Tetracycline 38 47 30 41 29 45 1 20 1 25 4 DISCUSSION
The semen bacterial biodiversity in all animals were similar to found in other studies analyzing semen of several animal groups, such as cattle (HERNÁNDEZ et al., 2002; PRADO; PEREZ, 2005), sheep (SOUZA et al., 2006) pigs (SONE, 1990; CORRÊA et al., 2001), fishes (OLIVEIRA et al., 2007), boar (PRIETO-MARTINEZ et al. 2014), and human (VILVANATHAN et al., 2016). On the other hand, Enterococcus sp., Corynebacterium sp. and Staphylococcus negative coagulase were less frequent. Similar found were obtained in studies of sheep semen (SOUZA et al., 2006), Pig’s semen (ALTHOUSE; LU, 2005), Piracanjuba fish (OLIVEIRA et al., 2007) and cattle (PRADO; PÉREZ, 2005). However, Fernandes et al., (2013) founded frequency of 23.6% for Bacillus sp. in semen of sheep, which is 14 times higher than what was found in collared peccary semen.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
About CFU counting, performed in serial dilutions the result diverges from the ones obtained by Prado and Perez (2005), in cattle’s semen, in which less diluted semen leads to lower CFU mL-1 concentration. However, it corroborates with the results found by Hernández et al. (2002) in bovine’s thawed semen. The 1 in 100,000 dilution did not alter the sperms motility. This observation was also reported by Bennermann et al. (2000), as well as in Prado and Pérez (2005) study. However, Pineda and Santander (2007) stated that at 1 in 10,000 dilution, changes in the quality of the semen of pig could happen.
In regard of antimicrobial agents tested in this work, the sensitivity against Gentamicin, Amikacin and Cephotaxin confirmed the results found by Souza et al. (2006) in semen of sheep, and by Hernández et al. (2002) in cattle’s semen. Nevertheless, in regard of the microorganisms isolated from semen of pig, the study of Gączarzewicz et al. (2016) presented high inhibitory activity against some antimicrobial agents as Gentamicin. In addition, Tetracycline, a very commonly antibiotic used as semen’s dilutor, presented the lowest numbers of sensitivity on antibiotic susceptibility results.
The presence of such microorganisms in the semen has possibly no association or no relation to the efficiency of the male’s reproductive system of the donor animal, once these microorganisms have been found in semen of healthy animals (KUSTER; ALTHOUSE, 2016). Therefore, it was observed that the presence of the six microorganisms did not interfere on the quality of semen analyzed. This fact also corroborates with studies of Sannat et al. (2015), and Kuster and Althouse (2016).
According to Riesenbeck et al. (2015), the semen contamination can be related to the fact the ejaculated material gets direct contact with the animal’s prepuce secretions and hair. In addition, accidental handling by the technician, indirect contact to aerosol, sampling instruments, as well as the process of semen dilution and storage contribute to semen contamination. So, the presence of saprophytic bacteria in collared peccary semen might be occurring due to contamination during sampling and/or from the external environment in which the animals are raised. Yániz et al. (2010) also found microbiological contamination in studies with boar semen that are from other environment.
5 CONCLUSION
The presence of microorganisms in the semen of collared peccary during the entire time of this evaluation does not influence on the seminal quality of the animals. Moreover, it is very likely that the ejaculated material have been contaminated by external microbiota from the surrounding
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
environment. Finally, it is recommended to be reevaluated the use of Tetracycline as a common dilutor in the semen. Additionally, it would be very valuable the see more studies to confirm the use of Gentamicin and/or Amikacin as ideal antibiotics to be used as dilutors in the semen of collared peccaries.
ACKNOWLEDGEMENTS
To all institutions involved in this study: Embrapa Amazônia Oriental, Universidade Federal Rural da Amazônia, and Universidade Federal do Pará.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
REFERENCES
ALTHOUSE, G. C.; LU, K. G. Bacteriospermia in extended porcine semen. Theriogenology, v. 63, p. 573-584. 2005.
BAUER, A. W.; KIRBY, W. M.; SHERRIS, J.C.; et al. Antibiotic susceptibility testing by a standardized single disc method. American Journal of Clinical Pathology, v. 45, p. 493-496. 1966.
BENNERMANN, P. E.; BORTOLOZZO, F.P.; CARDOSO, I.W.R.I. Sperm motility and acrossomal integrity in liquid boar semen dosis inoculated with Escherichia coli and Staphylococcus aureus. Ciência Rural, v. 30, p. 313-318. 2000.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2019. https://clsi.org/media/2663/m100ed29_sample.pdf
CORRÊA, M. N.; MEINCKE, W.; LUCIA JR, T.; et al. Inseminação Artificial em Suínos. Printpar Gráfica e Editora; Pelotas, 2001. 181p.
FERNANDES, G. O.; LEAL, D. R.; MOREIRA, N. H.; et al. Identification of bacteria in the semen in different breeding systems and the effect of Kilol-L®. Ciência Animal Brasileira, v. 14, p. 332-337. 2013.
GĄCZARZEWICZ, D.; UDAŁA, J.; PIASECKA, M.; et al. Bacterial Contamination of Boar Semen and its Relationship to Sperm Quality Preserved in Commercial Extender Containing Gentamicin Sulfate. Polish Journal of Veterinary Sciences, v. 19, p. 451-459. 2016.
GHONEIM, I. M.; WAHEED, M. M.; AL-HOFOFI, A. N.; et al. Evaluation of the microbial quality of fresh ejaculates of camel (Camelus dromedarius) semen. Animal Reproduction Science, v. 149, p. 218-223. 2014.
HERNÁNDEZ, P. J. E.; FÉRNANDEZ, R. F.; GONZÁLEZ, C. C.; et al. Estudio Bacteriológico y Antibiograma de Semen Descongelado de Bovino. Revista de Salud Animal, v. 24, p. 191-197. 2002.
KAHWAGE, P. R.; GARCIA, A. R.; GUIMARÃES, D. A. A.; et al. Testicular biometry, electroejaculation and seminal features of captive collared peccaries (Tayassu tajacu Linnaeus, 1758, Artiodactyla, Tayassuidae) raised in the Eastern Amazon. Acta Amazonica, v. 40, p. 771-778. 2010.
KUSTER, C. E.; ALTHOUSE, G. C. The impact of bacteriospermia on boar sperm storage and reproductive performance. Theriogenology, v. 85, p. 21-26. 2016.
LOMBARDO, M. P.; THORPE, P. A. Microbes in Tree Swallow Semen. Journal of Wildlife Diseases, v. 36, p. 460-468. 2000.
MAYOR, P.; GUIMARÃES, D. A., LE PENDU, Y.; et al. Reproductive performance of captive collared peccaries (Tayassu tajacu) in the eastern Amazon. Animal Reproduction Science, v. 102, p. 88-97. 2007.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
MENDEZ NÁREZ, G.; DÍAZ, A. E.; MORALES ALVAREZ, J. F.; et al. Epidimitis ovina: Estudios bacteriológico y serológico. Veterinaria Mexico, v. 30, p. 329-336. 1999.
MORATO, R. G.; GUIMARÃES, M. A. V. B.; NUNES, A. L. V.; et al. Semen collection and evaluation in the jaguar (Panthera onca). Brazilian Journal of Veterinary Research and Animal Science, v. 35, p. 178-181. 1998.
OIE. World Organization for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 6th ed. Office International des Epizooties, Paris, 2008. 598p.
OLIVEIRA, A. V.; VIVEIROS, A. T. M.; MARIA, A. N.; et al. Sucess of cooling and freezing of pirapitinga (Brycon nattereri) sêmen. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v. 59, p. 1509-1515. 2007.
PINEDA, Y.; SANTANDER, J. Evaluación de la flora bacteriana del semen de verracos en granjas porcinas de Venezuela. Zootecnia Tropical, v. 25, p. 173-177. 2007.
PRADO, E. A. S.; PÉREZ, R. M. Flora bacteriana del semen de toro antes y después de la congelación. Revista electrónica de Veterinaria, v. 6, p. 1-8. 2005.
PRIETO-MARTÍNEZ, N.; BUSSALLEU, E.; GARCIA-BONAVILA, E.; et al. Effects of Enterobacter cloacae on boar sperm quality during liquid storage at 17°C. Animal Reproduction Science, v. 148, p. 72-82. 2014.
QUINN, P. J.; CARTER, M. E.; MARKEY, B.; et al. Clinical Veterinary Microbiology. Wolfe Publishing, London, 1994. 648p.
RIESENBECK, A.; SCHULZE, M.; RÜDIGER, K.; et al. Quality Control of Boar Sperm Processing: Implications from European AI Centres and Two Spermatology Reference Laboratories. Reproduction in Domestic Animals, v. 50, p. 1-4. 2015.
SANNAT, C.; NAIR, A.; SAHU, S. B.; et al. Effect of species, breed, and age on bacterial load in bovine and bubaline semen. Veterinary World, v. 8, p. 461-466. 2015.
SILVA, S. S. B.; GUIMARÃES, D. A.; BIONDO, C.; et al. Dominance relationships between collared peccaries Pecari tajacu (Cetartiodactyla: Tayassuidae) in intensive breeding system. Applied Animal Behaviour Science, v. 184, p. 117-125. 2016.
SONE, M. Investigation on the control of bacteria in boar semen. The Japanese Journal of Animal Reproduction, v. 36, p. 23-29. 1990.
SOUZA, A. F.; GUERRA, M. M. P.; COLETO, Z. F.; et al. Avaliação microbiológica do sêmen fresco e congelado de reprodutores caprinos. Brazilian Journal of Veterinary Research and Animal Science, v. 43, p. 329-336. 2006.
SOUZA, A. L. P.; CASTELO, T. S.; QUEIROZ, J. P. A. F.; et al. Evaluation of anesthetic protocol for the collection of semen from captive collared peccaries (Tayassu tajacu) by electroejaculation. Animal Reproduction Science, v. 116, p. 370–375. 2009.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p. 65920-65930, sep. 2020. ISSN 2525-8761
VILVANATHAN, S.; KANDASAMY, B.; JAYACHANDRAN, A.L.; et al. Bacteriospermia and Its Impact on Basic Semen Parameters among Infertile Men. Interdisciplinary Perspectives on Infectious Diseases, v. 2016, p. e2614692. 2016.
WENG, S. L.; CHIU, C. M.; LIN, F. M.; et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS One, v. 9, p. e110152. 2014.
YÁNIZ, J. L.; MARCO-AGUADO, M. A.; MATEOS, J. A. et al. Bacterial contamination of ram semen, antibiotic sensitivities, and effects on sperm quality during storage at 15°C. Animal Reproduction Science, v. 122, p. 142-149. 2010.