www.ambi-agua.net E-mail: ambi.agua@gmail.com
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Estimation of corrosive and scaling trend in drinking water systems in
the city of Azogues, Ecuador
ARTICLES doi:10.4136/ambi-agua.2237
Received: 05 Feb. 2018; Accepted: 20 Aug. 2018
Fernando García-Ávila1,2*; Lía Ramos-Fernández3; César Zhindón-Arévalo4
1Universidad de Cuenca, Cuenca, Ecuador
Facultad de Ciencias Químicas. E-mail: garcia10f@hotmail.com
2Universidad Nacional Agraria La Molina, La Molina, Lima, Peru
E-mail: garcia10f@hotmail.com
3Universidad Nacional Agraria La Molina, La Molina, Lima, Peru
Departamento Académico de Recursos Hídricos. E-mail: liaseptiembre2012@hotmail.es
4Universidad Católica de Cuenca, Azogues, Ecuador
Unidad Académica de Salud y Bienestar. E-mail: cezhindona@ucacue.edu.ec
*Corresponding author
ABSTRACT
The quality of drinking water flowing in a distribution network can possess corrosive characteristics that may cause the material degradation of pipes and accessories. This problem can result in reduction of the service life of pipes and create a major public health problem. The agreement between the physical-chemical water quality analysis and national standards are not enough to confirm the balance of the water quality in terms of corrosion. In order to predict pipe corrosion in water distribution system networks, the corrosive trend was evaluated using the Langelier (LSI), Ryznar (RSI), and Larson-Skold (LRI) indexes based on measurements of pH, temperature, total dissolved solids, alkalinity, calcium hardness, sulfate and chloride. This study was setup with 180 samples collected in six zones of the distribution network, from July to December of 2017, according to the standard methods for the analysis of drinking water. The results indicate a variation of the LSI from -1.22 to -1.68; RSI from 9.75 to 10.52 and LRI from 0.46 to 0.77. A linear model was fitted for each index to predict the corrosion with the water quality conditions of this study case. Therefore, the drinking water of the city of Azogues, Ecuador has a corrosive tendency from significant to severe. Corrosion indices were calculated to provide useful information on the water's corrosiveness. These results indicate the need to constantly monitor the corrosion rate in the distribution network and conduct a laboratory study to adjust effective parameters such as pH, in order to control corrosion.
Keywords: Azogues city, corrosion rates, potabilization, water quality.
Estimativa da tendência corrosiva e de escala no sistema de água
potável
RESUMO
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
saúde pública. O acordo entre a análise físico-química da qualidade da água e os padrões nacionais não são suficientes para confirmar o equilíbrio da qualidade da água em termos de corrosão. Para prever a corrosão da tubulação nas redes do sistema de distribuição de água, a tendência corrosiva foi avaliada utilizando os índices Langelier (LSI), Ryznar (RSI) e Larson-Skold (LRI) com base em medidas de pH, temperatura, sólidos dissolvidos totais, alcalinidade, dureza de cálcio, sulfato e cloreto. Este estudo foi montado com 180 amostras coletadas em seis zonas da rede de distribuição, de julho a dezembro de 2017, de acordo com os métodos padronizados de análise de água potável. Os resultados obtidos indicam uma variação do LSI de -1,22 para -1,68; RSI de 9,75 a 10,52 e LRI de 0,46 a 0,77. Um modelo linear foi montado para cada índice para prever a corrosão com as condições de qualidade da água do presente estudo. Portanto, a água potável da cidade de Azogues (Equador) tem uma tendência corrosiva de significativa a severa. Os índices de corrosão foram calculados para fornecer informações úteis sobre a corrosividade da água. Esses resultados indicam a necessidade de monitorar constantemente a taxa de corrosão na rede de distribuição e realizar um estudo de laboratório para ajustar parâmetros efetivos como o pH, a fim de controlar a corrosão.
Palavras-chave: cidade de Azogues, potabilização, qualidade da água, taxas de corrosão.
1. INTRODUCTION
Guaranteeing the availability and sustainable management of drinking water in compliance with regulatory requirements will increase consumer confidence (Omaka et al., 2015; Sorlini et al., 2017; Collivignarelli, 2017). Drinking water causes corrosion in the pipes used for transport (Gholizadeh et al., 2017). Iron pipes that undergo a corrosion process could have a great impact on the water quality distributed in the supply network (Yang et al., 2012). The corrosion process in the pipes is related to several factors, such as the pipe material, water quality and hydraulic conditions (García-Avila et al., 2018). The effect of the different water quality parameters including pH, residual chlorine, total organic carbon, conductivity, dissolved oxygen, hardness and alkalinity influences corrosion and the formation of scale inside the metallic pipe (Cui et al., 2016). Vazdirvanidis et al. (2016) noted that the accelerated corrosion of parts of a water pump was probably caused by the presence of sulfates, sulfides and chlorides that caused a severe chemical attack on the various metallic components of the pump. Drinking water is commonly corrosive at a pH of 6.5 to 7.5 and with a low alkalinity. The corrosion potential of iron is affected by sulfate and chloride concentrations, which is why, as a consequence, chemical and physical differences are considered to alter water stability (Jazdzewska et al., 2016). Bigoni et al. (2014) indicated that the turbidity and iron concentration increase the corrosion of the pipes in the drinking water distribution network in a rural hospital in Peru. Corrosion of pipes and fittings that transport potable water is becoming a problem due to the high costs of repair and replacement (Liu et al., 2017). This means that it is important to know the physical and chemical characteristics of drinking water to determine the probable existence of corrosion using the Langelier saturation index (LSI), Ryznar stability index (RSI), the Larson-Skold index (LRI) (Achari et al., 2017).
Based on the LSI, 71% of the waters of the city of Shiraz, Iran is causing incrustation. Based on the RSI, all the zones lacked the characteristic of formation of incrustations’
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018 et al., 2017; Peng et al., 2013; Alsaqqar et al., 2014; Zhang et al., 2014).
Taking into account the corrosion problems caused in drinking water pipes and the lack of studies on the drinking water distribution network of the city of Azogues, Ecuador, this research was carried out to determine the corrosion/incrustations in the pipes of the different zones. The results provided information on the chemical behavior of inorganic contaminants in water and its influence on drinking water quality.
2. MATERIALS AND METHODS
2.1. Description of the study area
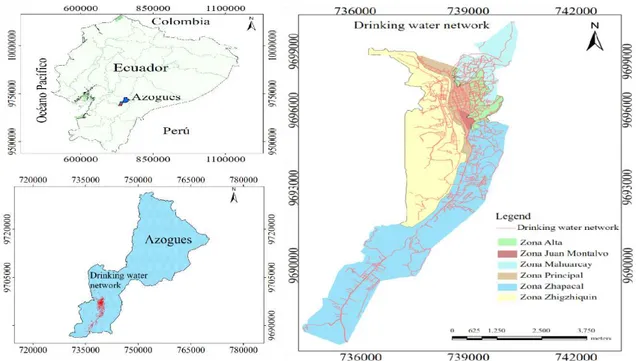
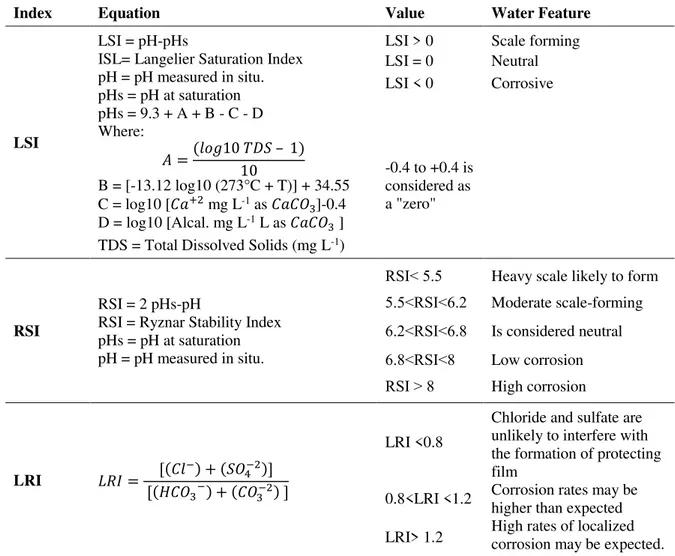
The drinking water distribution network on which this study was conducted is located in the city of Azogues, which is located in the southern part of the Ecuadorian Republic. Its geographical coordinates are: latitude 2°44'22" S, longitude: 78°50'54", and cover approximately 1200 km². The average altitude of the town is 2518 meters above sea level. The city has a population of 70,064 inhabitants. Its average temperature is 17°C; about 42,071 inhabitants receive water from the public network. There are no relevant studies on the corrosion that water can cause in the pipes and fittings of both the public and private networks. Figure 1 shows the location of the network supply, which is made up of pipes with diameters between 315 and 32 mm. The total length of the supply line is 218,105 m. A number of 288 valves have been installed. there are 26 reserves, distributed in six zones. Ten of the reserves have a capacity between 250 and 1000 m3. The altimetric variation of whole network
is 2390-2823 meters above sea level.
Figure 1. Location of the study area.
2.2. Sampling and analysis of water
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
samples for later analysis. The samples were obtained from the faucets in kitchens and bathrooms of homes, commercial places such as restaurants, workshops, washing cars, shops, etc., as well as from distribution tanks. The samples were maintained at 4°C and were transported on the same day for analysis to the laboratory of the city’s municipal drinking water company. The tests on all water samples were analyzed according to the Standard Methods for the examination of water and wastewater (Apha et al., 2012). The calcium hardness and alkalinity were measured by the titration method; the concentration of hydrogen ions (pH), total dissolved solids (TDS) and temperature were measured with the Hach Multiparameter HQ 40d; chloride and sulfate were determined with the HACH DR 2500 Spectrophotometer and compared with national standards. The pH, TDS and temperature were determined in site.
2.3. Determination of corrosion and scaling trend in drinking water
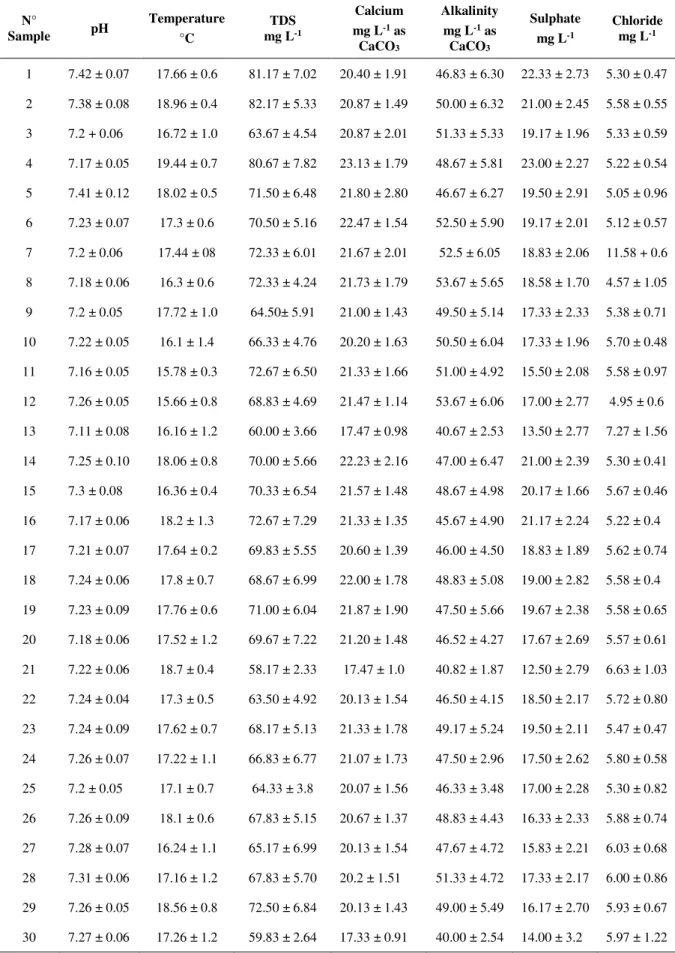
The LSI, RSI and LRI were calculated using the corresponding equations in Table 1. These indices allow the evaluation of the corrosion potential of the pipeline for each point of the network once the physical-chemical analysis has been realized (Bigoni et al., 2014).
Table 1. Description of corrosion indexes used in this study.
Index Equation Value Water Feature
LSI
LSI = pH-pHs
ISL= Langelier Saturation Index pH = pH measured in situ. pHs = pH at saturation pHs = 9.3 + A + B - C - D Where:
𝐴 =(𝑙𝑜𝑔10 𝑇𝐷𝑆 – 1)10
B = [-13.12 log10 (273°C + T)] + 34.55 C = log10 [𝐶𝑎+2 mg L-1 as 𝐶𝑎𝐶𝑂
3]-0.4
D = log10 [Alcal. mg L-1 L as 𝐶𝑎𝐶𝑂
3 ]
TDS = Total Dissolved Solids (mg L-1)
LSI > 0 Scale forming
LSI = 0 Neutral
LSI < 0 Corrosive
-0.4 to +0.4 is considered as a "zero"
RSI
RSI = 2 pHs-pH
RSI = Ryznar Stability Index pHs = pH at saturation pH = pH measured in situ.
RSI˂ 5.5 Heavy scale likely to form
5.5˂RSI˂6.2 Moderate scale-forming
6.2˂RSI˂6.8 Is considered neutral
6.8˂RSI˂8 Low corrosion
RSI ˃ 8 High corrosion
LRI 𝐿𝑅𝐼 = [(𝐶𝑙−) + (𝑆𝑂4−2)]
[(𝐻𝐶𝑂3−) + (𝐶𝑂3−2) ]
LRI <0.8
Chloride and sulfate are unlikely to interfere with the formation of protecting film
0.8<LRI <1.2 Corrosion rates may be higher than expected
LRI> 1.2 High rates of localized corrosion may be expected.
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
3. RESULTS AND DISCUSSION
3.1. Physical and chemical characteristics
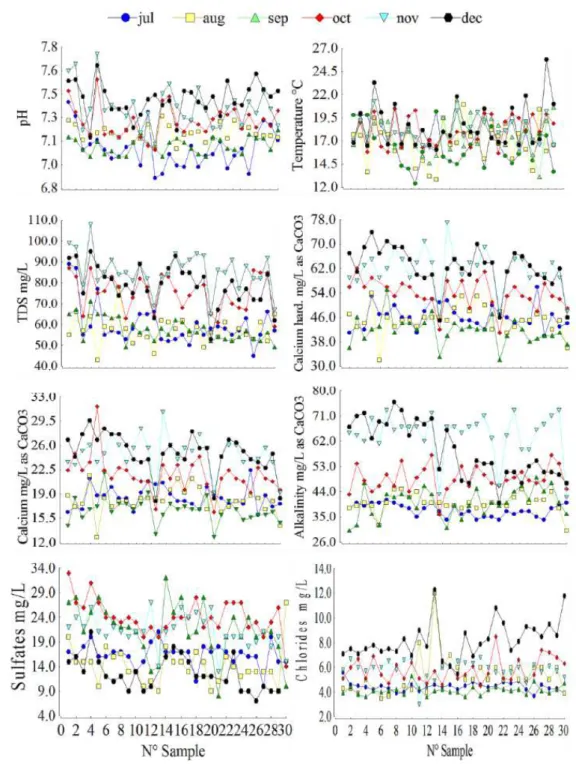
Table 2 shows the average values of temperature, pH, total dissolved solids (TDS), the calcium hardness, alkalinity, chlorides, sulfates and free chlorine of the 180 samples collected at the 30 sites monitored over six months. Figure 2 shows the variation of the physical-chemical parameters of drinking water obtained at each of the sampling points.
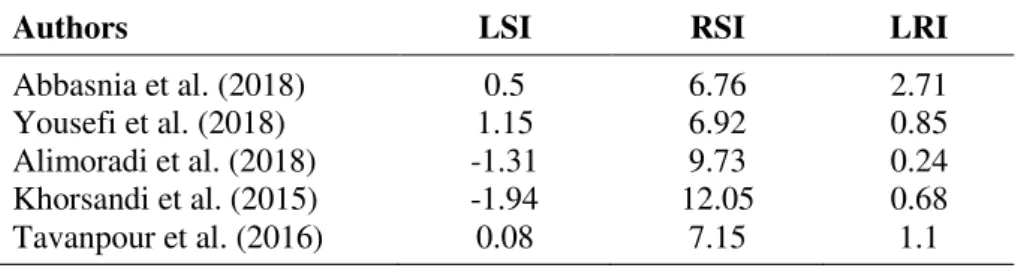
When analyzing the pH values of both Table 2 and Figure 2, conditions observed are almost neutral at each sampling point and during all the months analyzed. In terms of quality of drinking water according to the Ecuadorian standard, the recommended established value is a minimum pH of 6.5 and a maximum of 8.5. While the waters evaluated are within the acceptable range with values between 6.87 and 7.75, certain samples presented a pH less than 7 and were taken in the month of July. This slightly acidic condition can accelerate corrosive processes; this pH record is due to a contribution from natural processes. For its part, the temperature of water presented values near the ambient temperature for all samples; the temperature varied between 12.4 and 24.8°C. The low temperatures that mostly correspond to the months of July, August and September indicate that there is a tendency to corrosion; there are no high temperatures that may cause the precipitation of calcium carbonate, which can cause scale in the pipes and therefore decrease the corrosion. Considering the average values of Figure 2, it can be observed that the temperature values varies according the month, so the lowest temperatures are presented in the months of July and August, while the highest temperatures are presented in the months of October, November and December.
To determine the corrosive or scaling character of the water, it is also important to know the concentration of total dissolved solids (TDS). This parameter in the first instance provides an indicator of the quality of drinking water. Hence, when compared to the maximum permissible value of the Ecuadorian standard equivalent to 1000 mg L-1, it can be observed that
the water distributed in Azogues presents values between 43.1 and 108.0 mg L-1, well below
the established maximums. Even in spite of the low concentrations of TDS, it is important to note that the concentration of total dissolved solids in aqueous solutions is directly proportional to the conductivity, and its increase can favor the corrosive tendency.
When examining the values of drinking water hardness presented in Figure 2, according to the classification, it is observed that this water presents slightly hard values, which is especially related to low concentrations of calcium. This parameter is also an indicator of the quality of drinking water. Hence, when compared with the maximum permissible value of the Ecuadorian standard equivalent to 300 mg L-1, it can be observed that drinking water for the
present study has values between 32.1 and 79.1 mg L-1, which are well below the established
maximums. Waters with low hardness are considered aggressive, causing deterioration and corrosion in supply networks. This fact, together with the low pH presented at certain points, can generate a corrosive effect on the part of these waters.
Figure 2 shows that the alkalinity during the months of July to September has lower values compared to the months of October to December. This is due to the fact that during the months of July and August there are generally fewer rains that cause a decrease in alkalinity; in general there is a corrosive tendency caused by low alkalinity of water, which is at values lower than 76 mg L-1 as calcium carbonate. The Ecuadorian norm for concentrations of sulfate and chloride
ions in drinking water establish maximum permissible values of 200 mg L-1, 250 mg L-1,
respectively. The average values found in the drinking water in the present study were 5.65 mg L-1 and 18.12 mg L-1, which are well below the established norm. Sulfate ions and
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
Table 2. Average values of the analysis of water collected in the sampling sites.
N°
Sample pH
Temperature °C
TDS
mg L-1
Calcium
mg L-1 as
CaCO3
Alkalinity
mg L-1 as
CaCO3
Sulphate
mg L-1
Chloride
mg L-1
1 7.42 ± 0.07 17.66 ± 0.6 81.17 ± 7.02 20.40 ± 1.91 46.83 ± 6.30 22.33 ± 2.73 5.30 ± 0.47
2 7.38 ± 0.08 18.96 ± 0.4 82.17 ± 5.33 20.87 ± 1.49 50.00 ± 6.32 21.00 ± 2.45 5.58 ± 0.55
3 7.2 + 0.06 16.72 ± 1.0 63.67 ± 4.54 20.87 ± 2.01 51.33 ± 5.33 19.17 ± 1.96 5.33 ± 0.59
4 7.17 ± 0.05 19.44 ± 0.7 80.67 ± 7.82 23.13 ± 1.79 48.67 ± 5.81 23.00 ± 2.27 5.22 ± 0.54
5 7.41 ± 0.12 18.02 ± 0.5 71.50 ± 6.48 21.80 ± 2.80 46.67 ± 6.27 19.50 ± 2.91 5.05 ± 0.96
6 7.23 ± 0.07 17.3 ± 0.6 70.50 ± 5.16 22.47 ± 1.54 52.50 ± 5.90 19.17 ± 2.01 5.12 ± 0.57
7 7.2 ± 0.06 17.44 ± 08 72.33 ± 6.01 21.67 ± 2.01 52.5 ± 6.05 18.83 ± 2.06 11.58 + 0.6
8 7.18 ± 0.06 16.3 ± 0.6 72.33 ± 4.24 21.73 ± 1.79 53.67 ± 5.65 18.58 ± 1.70 4.57 ± 1.05
9 7.2 ± 0.05 17.72 ± 1.0 64.50± 5.91 21.00 ± 1.43 49.50 ± 5.14 17.33 ± 2.33 5.38 ± 0.71
10 7.22 ± 0.05 16.1 ± 1.4 66.33 ± 4.76 20.20 ± 1.63 50.50 ± 6.04 17.33 ± 1.96 5.70 ± 0.48
11 7.16 ± 0.05 15.78 ± 0.3 72.67 ± 6.50 21.33 ± 1.66 51.00 ± 4.92 15.50 ± 2.08 5.58 ± 0.97
12 7.26 ± 0.05 15.66 ± 0.8 68.83 ± 4.69 21.47 ± 1.14 53.67 ± 6.06 17.00 ± 2.77 4.95 ± 0.6
13 7.11 ± 0.08 16.16 ± 1.2 60.00 ± 3.66 17.47 ± 0.98 40.67 ± 2.53 13.50 ± 2.77 7.27 ± 1.56
14 7.25 ± 0.10 18.06 ± 0.8 70.00 ± 5.66 22.23 ± 2.16 47.00 ± 6.47 21.00 ± 2.39 5.30 ± 0.41
15 7.3 ± 0.08 16.36 ± 0.4 70.33 ± 6.54 21.57 ± 1.48 48.67 ± 4.98 20.17 ± 1.66 5.67 ± 0.46
16 7.17 ± 0.06 18.2 ± 1.3 72.67 ± 7.29 21.33 ± 1.35 45.67 ± 4.90 21.17 ± 2.24 5.22 ± 0.4
17 7.21 ± 0.07 17.64 ± 0.2 69.83 ± 5.55 20.60 ± 1.39 46.00 ± 4.50 18.83 ± 1.89 5.62 ± 0.74
18 7.24 ± 0.06 17.8 ± 0.7 68.67 ± 6.99 22.00 ± 1.78 48.83 ± 5.08 19.00 ± 2.82 5.58 ± 0.4
19 7.23 ± 0.09 17.76 ± 0.6 71.00 ± 6.04 21.87 ± 1.90 47.50 ± 5.66 19.67 ± 2.38 5.58 ± 0.65
20 7.18 ± 0.06 17.52 ± 1.2 69.67 ± 7.22 21.20 ± 1.48 46.52 ± 4.27 17.67 ± 2.69 5.57 ± 0.61
21 7.22 ± 0.06 18.7 ± 0.4 58.17 ± 2.33 17.47 ± 1.0 40.82 ± 1.87 12.50 ± 2.79 6.63 ± 1.03
22 7.24 ± 0.04 17.3 ± 0.5 63.50 ± 4.92 20.13 ± 1.54 46.50 ± 4.15 18.50 ± 2.17 5.72 ± 0.80
23 7.24 ± 0.09 17.62 ± 0.7 68.17 ± 5.13 21.33 ± 1.78 49.17 ± 5.24 19.50 ± 2.11 5.47 ± 0.47
24 7.26 ± 0.07 17.22 ± 1.1 66.83 ± 6.77 21.07 ± 1.73 47.50 ± 2.96 17.50 ± 2.62 5.80 ± 0.58
25 7.2 ± 0.05 17.1 ± 0.7 64.33 ± 3.8 20.07 ± 1.56 46.33 ± 3.48 17.00 ± 2.28 5.30 ± 0.82
26 7.26 ± 0.09 18.1 ± 0.6 67.83 ± 5.15 20.67 ± 1.37 48.83 ± 4.43 16.33 ± 2.33 5.88 ± 0.74
27 7.28 ± 0.07 16.24 ± 1.1 65.17 ± 6.99 20.13 ± 1.54 47.67 ± 4.72 15.83 ± 2.21 6.03 ± 0.68
28 7.31 ± 0.06 17.16 ± 1.2 67.83 ± 5.70 20.2 ± 1.51 51.33 ± 4.72 17.33 ± 2.17 6.00 ± 0.86
29 7.26 ± 0.05 18.56 ± 0.8 72.50 ± 6.84 20.13 ± 1.43 49.00 ± 5.49 16.17 ± 2.70 5.93 ± 0.67
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
Figure 2. Variation of pH, temperature, TDS, calcium, alkalinity, sulfates and chlorides of drinking water in the distribution network sampling points.
3.2. Corrosion monitoring
Table 3 presents the mean values of the indices obtained by zones of the supply network. The table shows that the values of the indices are similar in each of the zones. All values obtained for the LSI are negative, with an average value of 1.39 (Table 3 and Figure 3), indicating that the water that is distributed in all zones of Azogues is corrosive, considering that at more negative values there is greater corrosivity. The values in Table 3 show that the zones Zhigzhiquin and Zhapacal (2390-2500 altitude meters above sea level) have less negative values with respect to the Mahuarcay zone (altitude 2700-2823 meters above sea level); this could be due to the temperature variation, which in turn affects the pH.
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
of 10.07 shows a slightly to moderately corrosive character of the drinking water distributed in Azogues. Meanwhile, average LRI values of 0.58 were obtained, which does not conform with the strongly corrosive character determined by the Langelier and Ryznar models. This difference is because the Larson-Skold relationship is based on the corrosive influence of the chloride, sulfate and bicarbonate ion, not considering other physicochemical factors such as pH, temperature, total dissolved solids, alkalinity and calcium.
Table 3. Average values of corrosion indexes by zones.
Zone LSI RSI LRI
Alta -1.40 10.03 0.52
Mahuarcay -1.58 10.38 0.53
Media -1.4 10.03 0.67
Zhigzhiquin -1.31 9.94 0.63
Principal -1.43 10.09 0.54
Zhapacal -1.34 9.93 0.60
Average -1.39 10.07 0.58
p <0.0001 <0.0001 <0.0001
Figure 3. Variation of corrosion indexes in the different zones.
The results obtained by several authors are presented in Table 4. When compared with the results obtained in this study, we can observe a similarity to the results obtained by Alimoradi et al. (2018) and Khorsandi et al. (2015). These waters are highly corrosive based on the LSI, RSI and LRI; but they have no tendency to form coatings of calcium carbonate precipitates, due to the low alkalinity and hardness.
Table 4. Average values of LSI, RSI, LRI obtained by several authors.
Authors LSI RSI LRI
Abbasnia et al. (2018) 0.5 6.76 2.71
Yousefi et al. (2018) 1.15 6.92 0.85
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018 Figure 4 shows the variation of corrosion indexes with physical-chemical parameters. pH is one of the main factors that influence the corrosion process (Cui et al., 2016). In Table 2 and Figure 2, it can be observed that the pH varied between 6.87 and 7.75, with an average of 7.24. In Figure 4(a) it can be seen that the LSI increases with increasing pH; meanwhile, the RSI decreased with the increase of pH. Therefore, corrosion increases with the decrease in pH. Another factor that alters the corrosion process is temperature; increase in temperature can cause the precipitation of calcium carbonate, which can cause encrustation in the pipes and therefore reduce corrosion. In Table 2 and Figure 2, it can be observed that the temperature varied between 12.4 and 24.8°C, with an average of 17.6°C. In Figure 4(b), it can be seen that the LSI increased with the temperature increase; meanwhile, the RSI decreased with temperature.
The conventional potabilization process does not affect the dissolved content of treated water. The effect of TDS content on the corrosivity of water is a complex issue; some substances such as carbonate and bicarbonate reduce corrosion, while chloride and sulfate ions notably accelerate corrosion (Alsaqqar et al., 2014). From the data registered in Table 2 and Figure 2,
the TDS concentration fluctuated between 43.1 and 108.0 mg L-1 with an average of
69.29 mg L-1. In Figure 4(c) it can be seen that the LSI increased with the increase of the TDS.
Meanwhile, the RSI was reduced with the increase of TDS; therefore the corrosion increases with the decrease of TDS. Calcium is the second most frequent component in most surface waters and is generally among the three or four most frequent ions in groundwater. The increase Ca2+ concentration decreases water corrosivity (Mirzabeygi et al., 2017), since Ca2+ is important
for the formation of a passivation film on the surface of the pipe, decreasing corrosion. From
the data recorded in Table 2 and Figure 2, the Ca2+ concentration varied between 12.8 and
31.6 mg L-1, with an average of 20.8 mg L-1. In Figure 4(d), it can be seen that the LSI increased
with the calcium increase; the RSI decreased with the calcium increase. The slightly low calcium values in this study influenced the presence of drinking water corrosion.
In water treatment plants, alkalinity is required in the coagulation process for the reaction of alum with water; lime can be added if natural alkalinity is not sufficient for this reaction. Water corrosivity increases as alkalinity decreases (Peng et al., 2013, Choi et al., 2015). According to data obtained from the water analysis, the variation in alkalinity ranged between 30.00 and 76.00 mg L-1, with an average of 48.42 mg L-1 as CaCO
3, as shown in Table 2 and
Figure 2. In Figure 4(e) it can be seen that the LSI increased with the increase in alkalinity; meanwhile, the RSI decreased with the increase in alkalinity. The somewhat low concentrations of alkalinity that have been obtained in this study favor the solubility of CO2, increasing the
drinking water corrosivity; it can also be observed that alkalinity does not have a great relationship with the LRI.
The chloride and sulfate ions drastically reduce corrosion resistance (Vazdirvanidis et al., 2016; Yang et al., 2012). Steel is easily susceptible to pitting corrosion in solutions containing aerated chloride with pH in a wide range (4 - 12.5) at room temperature (Wang et al., 2015). According to data obtained from the water analysis, the chloride ion concentration fluctuated between 1.30 and 12.30 mg L-1, with an average of 5.65 mg L-1; the sulfate varied between 3.0
and 33.0 mg L-1 with an average of 18.12 mg L-1, as shown in Table 2 and Figure 2. In Figure
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
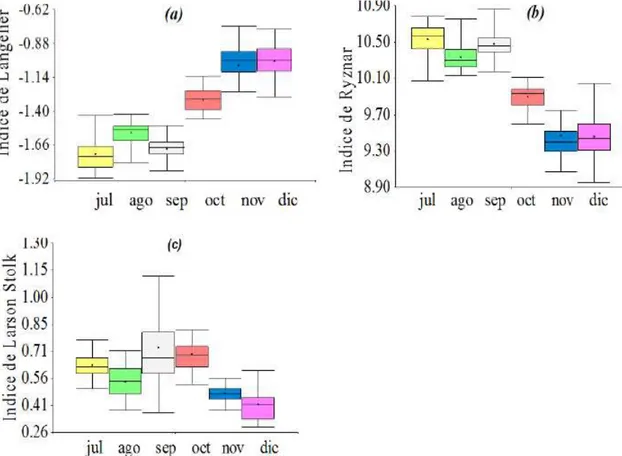
to September, which increased the corrosion. From October to December, a decrease of -1.40 to -0.80 can be observed in the LSI, generating a less corrosive tendency than during the three previous months.
Analyzing the box plot for the RSI, Figure 5(b) shows that during the first three months of sampling (July-September) values between 10.00 and 11.00 are observed, due to a significant decrease in terms of the temperature, pH and alkalinity. During the following three months a decrease in the index of 10.00 to 9.00 can be observed, decreasing this index, but retaining its severe corrosive character.
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018 In Figure 5(c), we can observe a constant trend regarding the LRI; the values calculated and analyzed during July - December, are between the range of 0.29 to 1.26, with an average value of 0.56, presenting a slight corrosion, with a slightly corrosive character, which indicates that there is little amount of chloride and sulfate ions that can cause corrosivity.
Figure 5. Variation of corrosion indexes over time. (a) Variation of LSI with months, (b) Variation of RSI with months, (c) Variation of LRI with months.
3.3. Linear model for corrosion indexes
To facilitate the calculation of corrosion in the drinking water distribution network in Azogues, a mathematical model was obtained for each of the corrosion indexes analyzed in this study. The multiple regression technique was applied using Infostat software, based on data of pH, temperature, SDT, alkalinity, calcium, sulfates and chlorides, obtained from drinking water sampling and analysis. The results of the multiple regression for LSI are presented in Table 5.
Table 5. Regression coefficients and statistical data associated with the LSI model.
Coef Est. E.E. LI (95%) LS (95%) T P-Value CpMallows VIF Const -9.84 0.06 -9.96 -9.71 -155.32 <0.0001
PH 1.01 0.01 0.99 1.03 106.29 <0.0001 11236.03 1.96
T 0.02 6.60E-04 0.02 0.02 28.91 <0.0001 836.03 1.36
SDT -4.30E-04 1.60E-04 -7.50E-04 -1.20E-04 -2.69 0,008 12.18 3.91
Calcium 0.02 5.50E-04 0.02 0.02 38.61 <0.0001 1487.2 3.42
Alkalinity 0.01 1.80E-04 0.01 0.01 44.71 <0.0001 1992.51 3.49
It can be observed that the pH, temperature, TDS, calcium and alkalinity showed a value p <0.05, there being a significant linear relationship with the LSI. The model obtained for the LSI is (Equation 1):
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
The equation is useful to calculate the LSI knowing the values of pH, temperature in °C, SDT in mg/L, calcium hardness in and alkalinity in mg/L as CaCO3.
The results of the multiple regression for RSI are presented in Table 6. It can also be noted that the pH, T, SDT, calcium and alkalinity showed a value p <0.05, there being also a significant linear relationship with the RSI. The model obtained for the RSI is (Equation 2):
𝑅𝑆𝐼 = 19.93 − 1.06 𝑝𝐻 − 0.04𝑇 + 0.00096 𝑆𝐷𝑇 − 0.04 𝐶𝑎𝑙𝑐𝑖𝑢𝑚 − 0.02 𝐴𝑙𝑘𝑎𝑙𝑖𝑛𝑖𝑡𝑦 (2)
Table 6. Regression coefficients and statistical data associated with the RSI model.
Coef Est. E.E. LI (95%) LS (95%) T P-Value CpMallows VIF Const 19.7 0.12 13.67 19.95 157.76 <0.0001
PH -1.02 0.02 -1.06 -0.99 -54.61 <0.0001 2969.87 1.96
T -0.04 1.30E-03 -0.04 -0.04 -29.55 <0.0001 873.33 1.36
SDT 8.40E-04 3.20E-04 2.10E-04 1.50E-03 2.64 0.0091 11.94 3.91
Calcium -0.04 1.10E-03 -0.04 -0.04 -39.07 <0.0001 1522.9 3.42
Alkalinity -0.02 3.60E-04 -0.02 -0.02 -45.06 <0.0001 2023.09 3.49
4. CONCLUSIONS
Although the temperature values, total dissolved solids, alkalinity, calcium hardness, sulfate and chloride obtained in this study were admissible based on Ecuadorian regulations, the average values of the corrosion indexes obtained in this study indicated a significant corrosive tendency. The results obtained were: LSI: -1.39; RSI: 10.02 and LRI: 0.58. These indicated a significant corrosive tendency of the drinking water distributed in Azogues. The most critical parameter analyzed for the LSI and RSI is pH, as well as the low alkalinity and low hardness; while, for the Larson-Stolk index, the most critical parameter is sulfate. It can be concluded that an understanding of the chemical composition of drinking water is vital for predicting the materials behavior that are in contact with drinking water. The corrosion calculation caused by the water is based solely on the physical chemical parameters proposed by Langelier, Ryznar and Larson-Skold; this is not so exact, considering other factors or conditions that prevail in a typical drinking water network, such as dissolved oxygen, residual chlorine and room temperature. the supply system. This factor should be taken into account for the control and prevention of corrosion potential; adjustments in stabilization should be made at the treatment plant.
5. ACKNOWLEDGEMENTS
Fernando García developed this project with his own funds during his doctoral studies in Engineering and Environmental Sciences. The authors thank Eng. Xavier Ramírez, the manager of EMAPAL EP, for his support of this study. We also thank Damián Pauta and Diego Quezada, who helped with the monitoring campaign described, and Lcda Lucy Timbe for his help in translating the document.
6. REFERENCES
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018 ACHARI, V. S.; DEEPA, P.; AMBILI, M. S.; GEORGE, T. R. Corrosion Indices, Drinking
and Irrigation Water Quality of Andhakaranazhy and Cherai Coastal Regions of Kerala.
Academy of Chemistry Teachers, v. 3, n. 1, p. 10-14, 2017.
ALIMORADI, J.; NAGHIPOUR, D.; KAMANI, H. Data on corrosive water in the sources and distribution network of drinking water in north of Iran. Data Brief, v.17, p.105-118, 2018. https://doi.org/10.1016/j.dib.2017.12.057
ALSAQQAR, A.; KHUDAIR, B.; ALI, S. Evaluating Water Stability Indices from Water Treatment Plants in Baghdad City. Journal of Water Resource and Protection, v. 6, n. 14, p. 1344-1351, 2014. http://dx.doi.org/10.4236/jwarp.2014.614124
AMERICAN PUBLIC HEALTH ASSOCIATION – APHA; AMERICAN WATER WORKS ASSOCIATION – AWWA; WATER ENVIRONMENT FEDERATION - WEF.
Standard Methods for examination of water and wastewater. Washington, DC, 2012. BIGONI, R.; SORLINI, S.; COLLIVIGNARELLI, M. C.; BERBENNI, P. Drinking water quality assessment and corrosion mitigation in the hospital water supply system of Chacas village (Perú). Revista Ambiente & Água, v. 9, n. 3, p. 379-389, 2014. http://dx.doi.org/10.4136/ambi-agua.1407
CHOI, J.; GYU, B.; HONG, S. Effects of NF treated water on corrosion of pipe distribution system and its implications to blending with conventionally treated water. Desalination, v. 360, p. 138-145, 2015. https://doi.org/10.1016/j.desal.2015.01.026
COLLIVIGNARELLI, C. Water safety: one of the primary objectives of our time. Revista Ambiente & Água, v. 12, n. 1, p. 1-7, 2017. http://dx.doi.org/10.4136/ambi-agua.1994 CUI, Y.; LIU, S.; SMITH, K.; YU, K.; HU, H.; JIANG, W. Characterization of corrosion scale
formed on stainless steel delivery pipe for reclaimed water treatment. Water Research, v. 88, p. 816-825, 2016. http://dx.doi.org/10.1016/j.watres.2015.11.021
GARCÍA-AVILA, F.; BONIFAZ-BARBA, G.; DONOSO-MOSCOSO, S.; FLORES DEL PINO, L.; RAMOS-FERNÁNDEZ, L. Dataset of copper pipes corrosion after exposure
to chlorine. Data Brief, v. 19, p. 170-178, 2018.
https://doi.org/10.1016/j.dib.2018.05.023
GHOLIZADEH, A.; MOKHTARI, M.; NAIMI, N.; SHIRAVANDC, B.; EHRAMPOUSHA, M. H.; MIRI, M. et al. Assessment of corrosion and scaling potential in groundwater resources; a case study of Yazd-Ardakan Plain , Iran. Groundwater for Sustainable Development, v. 5, p. 59-65, 2017. https://doi.org/10.1016/j.gsd.2017.04.002
JAZDZEWSKA, A.; DAROWICKI, K.; ORLIKOWSKI, J.; KRAKOWIAK, S.; ZAKOWSKI, K.; GRUSZKA, M. et al. Critical analysis of laboratory measurements and monitoring system of water-pipe network corrosion-case study. Case Studies in Construction Materials, v.4, p. 102-107, 2016. https://doi.org/10.1016/j.cscm.2016.01.004
KHORSANDI, H.; MOHAMMADI, A.; KARIMZADEH, S.; KHORSANDI, J. Evaluation of corrosion and scaling potential in rural water distribution network of Urmia, Iran.
Desalination and Water Treatment, p. 1-8, 2015.
http://dx.doi.org/10.1080/19443994.2015.1042058
Rev. Ambient. Água vol. 13 n. 5, e2237 - Taubaté 2018
LIU, H.; GU, T.; LV, Y.; ASIF, M.; XIONG, F.; ZHANG, G. et al. Corrosion inhibition and anti-bacterial efficacy of benzalkonium chloride in artificial CO2-saturated oilfield
produced water. Corrosion Science, v. 117, p. 24-34, 2017. http://dx.doi.org/10.1016/j.corsci.2017.01.006
MIRZABEYGI, M.; ABBASNIA, A.; YOUZI, H.; ALIKHANI, M.; MAHVI, A. Evaluation of groundwater quality and assessment of scaling potential and corrosiveness of water supply networks, Iran. Research and Technology—AQUA, p. 1-10, 2017. https://doi.org10.2166/aqua.2017.128
OMAKA, O. N.; OFFOR, I. F.; ONWE, I. M. Hydrogeochemical attributes and ground water quality of Ngbo Community in Ohaukwu Area Council, Ebonyi State, Nigeria. Revista Ambiente & Água, v. 10, n. 1, p. 35-47, 2015. http://dx.doi.org/10.4136/ambi-agua.1453 PENG, C.; FERGUSON, J.; KORSHIN, G. Effects of chloride, sulfate and natural organic
matter (NOM) on the accumulation and release of trace-level inorganic contaminants from corroding iron. Water Research, v. 47, n. 14, p. 5257-5269, 2013. https://doi.org/10.1016/j.watres.2013.06.004
SORLINI, S.; BIASIBETTI, M.; ABBÀ, A.; COLLIVIGNARELLI, M. C.; DAMIANI, S. Water Safety Plan for drinking water risk management: the case study of Mortara (Pavia, Italy). Revista Ambiente & Água, v. 12, n. 4, p. 513-526, 2017. http://dx.doi.org/10.4136/ambi-agua.2102
TAVANPOUR, N.; NOSHADI, M.; TAVANPOUR, N. Scale Formation and Corrosion of Drinking Water Pipes: A Case Study of Drinking Water Distribution System of Shiraz City. Modern Applied Science, v. 10, n. 3, p. 166-177. 2016. http://dx.doi.org/10.5539/mas.v10n3p166
VAZDIRVANIDIS, A.; PANTAZOPOULOS, G.; RIKOS, A. Corrosion investigation of stainless steel water pump components. Engineering Failure Analysis, v. 82, p. 466-473, 2016. https://doi.org/10.1016/j.engfailanal.2016.09.009
WANG, Y.; CHENG, G.; WU, W.; QIAO, Q.; LI, Y.; LI, X. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions. Applied Surface Science, v. 349, p. 746-756, 2015. https://doi.org/10.1016/j.apsusc.2015.05.053
YANG, F.; SHI, B.; GU, J.; WANG, D.; YANG, M. Morphological and physicochemical characteristics of iron corrosion scales formed under different water source histories in a drinking water distribution system. Water Research, v. 46, n. 16, p. 5423-5433, 2012. https://doi.org/10.1016/j.watres.2012.07.031
YOUSEFI, M.; SALEH, H. N.; MAHVI, A. H.; ALIMOHAMMADI, M.; NABIZADEH, R.; MOHAMMADI,A. A. Data on corrosion and scaling potential of drinking water
resources using stability indices in Jolfa, East Azerbaijan, Iran. Data Brief, v. 16, p. 724-731, 2018. https://doi.org/10.1016/j.dib.2017.11.099