Fac
Geneeskunde
KATHOLIEKE UNIVERSITEIT
LEUVEN
AN
IN VITRO
AND
IN VIVO
STUDY ON THE
DURABILITY OF BIOMATERIAL-TOOTH BONDS
o
JAN DE
MUNCK
N.('ham.
CETD
BE
0011
Autor: Munck.
Jan De
Titulo:
An in
vit -0
at
d i 1
vivo study on
II
114 101,1111111111PPIiINI
Ex.I UFSC BSCCSO
tor:
Prof. B. Van
Meerbeek
SC000913694
AQUISICAO Pelb M0A0Ab
DoArr,
orr14
,FEv
2007
REGIS IRO
()DATA DO REGISTRO
-Cover picture:
Background: TEM photomicrograph of an intermediary strong self-etch adhesive (AdheSE, Vivadent) bonded to dentin (chapter 1).
KATHOLIEKE UNIVERSITEIT LEUVEN FACULTEIT GENEESKUNDE
SCHOOL VOOR TANDHEELKUNDE, MONDZIEKTEN EN KAAKCHIRURGIE AFDELING CONSERVERENDE TANDHEELKUNDE
LEUVEN BIOMAT RESEARCH CLUSTER
KATHOLIEKE UNIVERSITEIT LEU VEN
AN
IN VITRO AND
IN VIVO STUDY ON THE
DURABILITY OF
BIOMATERIAL-TOOTH
BONDS
JAN DE
MUNCKMOW4413
'Oa
00Mee
0
0
A
00
g
6 FEV.
2O1
WHOM
Promotor:
B. Van
MeerbeekCo-promotors:
M.
BraemP.
LambrechtsPhD dissertation
Submitted in partial fulfillment of the requirements to obtain the degree of Doctor in Medical Sciences
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
Voorwoord — Acknowledgments
De tandheelkunde erkent steeds meer het belang van conserverende
behandelingen. Ingrijpende restauratieve ingrepen worden vandaag
zoveel mogelijk vernneden of uitgesteld. De K.U.Leuven speelde hierin
een belangrijke rol. Klinisch en laboratorium onderzoek aan deze
universiteit toonde duidelijk het belang van een goede hechting aan
tandweefsel voor de conserverende tandheelkunde.
Prof. Dr. Bart Van Meerbeek wist mij naar aanleiding van mijn
eindwerk Tandheelkunde te boeien met zijn onderzoek naar deze
adhesie aan tandweefsel. Later overhaalde hij mij om samen met hem
het terrein van de adhesieve tandheelkunde verder te verkennen. Hij gaf
mij de mogelijkheid om vier jaar lang door de wondere wereld van het
wetenschappelijk onderzoek te reizen. Zijn uitgebreide expertise, zijn
talrijke buitenlandse contacten, zijn wijze raad en altijd vriendelijke
aanmoedigingen waren onontbeerlijk om dit proefschrift te voleinden. Ik
ben hem daarvoor vandaag heel erg dankbaar.
Prof. Dr. Marc Braem en Prof. Dr. Paul Lambrechts, de
co-promotoren van dit proefschrift ben ik bijzonder erkentelijk voor hun
grote expertise op het vlak van tandheelkundig onderzoek met in het
bijzonder het gedrag van composiet materialen onder verschillende
soorten van belasting. Hun interesse voor mijn onderzoek en hun
aanstekelijke nieuwsgierigheid naar de vorderingen van mijn proefschrift
betekenden zeker een belangrijke steun. Verder is er natuurlijk nog Prof.
Dr. Guido Vanherle die met zijn enthousiasme voor de tandheelkundige
materialen heeft gezorgd voor mijn interesse in het onderzoek.
I would like to thank the members of the jury: Prof Dr. A. De
Laat,
Prof Dr. I.
Naert,
Prof Dr. M.
Wevers,
Prof Dr.
J
Vander
Sloten
and especially both examiners from abroad: Prof Dr. A.
Fellzer (ACTA,
Amsterdam) and Prof Dr. T Watson (Dental School of Guy's &
Thomas, London). They contributed significantly to this manuscript with
their discerning but constructive questions and remarks.
Praktisch was dit werk ook heel afhankelijk van de input van
iedereen die in het onderzoekslabo BIOMAT heeft gewerkt gedurende de
laatste 4 jaar. Vooral Dominique Crombez die keer op keer haar schema
aanpaste om de resultaten op tijd klaar te hebben, wil ik speciaal
vermelden. Zij zorgde voor een efficient labo, waar het heel aangenaam
werken was. Verder was er Jan Geukens die mijn ideeén omzette in
concrete plannen en zelfs werkende apparaten. Ook mijn
collega-onderzoekers, die het werken steeds aangenaam en boeiend maakten
verdienen het om met naam genoemd te worden, nml. Drs. Lars
Bergmans, Drs. Eduardo Coutinho (Rio de Janeiro, Brazil), Mrs. Padmini
Kanumilli (Chennai, India), Mrs. Daniela Mattar
(São
Paulo, Brazil), Dr.
Marleen Peumans, Drs. André Poitevin en Drs. Kirsten Van Landuyt.
Special gratitude goes also to the many foreign researchers that
visited our laboratory to share ideas and to push the limits of our current
knowledge.. Dr. Takatsumi Ikeda (Sapporo, Japan), Dr. Masanori
Hashimoto (Sapporo, Japan), Dr. Kazuhiro Hikita (Sapporo, Japan), Dr.
Jacek Iracki (Warsaw, Poland), Drs. Kenichi Koshiro (Sapporo, Japan), Dr.
Marcos Vargas (Iowa, USA), Dr. Kenichi Shirai (Hiroshima, Japan), Dr.
Satoshi Inoue (Sapporo, Japan) and Dr. Yasuhiro Yoshida (Okayama,
Japan),
Ook de onderzoekers van de afdeling
MIM,
Prof. Dr. J-P Celis, ir.
R. De Vos, Prof. Dr. L. Froyen en Prof. Dr. O. Van der Biest, en van de
afdeling "Menselijke Erfelijkheid", Prof. Dr. J-J Cassiman en Dr.
Bernadette Vanderschueren, ben ik bijzonder erkentelijk voor hun
bereidwillige hulp. Zonder hen waren de vele mooie beelden in dit werk
er niet geweest.
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
The research performed in this manuscript would not have been
possible without the generous supply of materials by the respective
manufacturers, 3M ESPE, Ca vex — Herus Kulzer, GC, Ivoclar-Vivadent
Kerr, Kuraray, Shofu and Ultradent, for which I would like to thank them.
Bij dit dankwoord wil ook gaarne nnijn ouders betrekken. Steeds
hebben zij mij gestimuleerd in het verleggen van mijn grenzen en mij
met raad en daad bijgestaan in mijn studies. Hun raad was meer dan
goud waard.
Tot slot een bijzonder woord van dank aan
mim
n verloofde
Kathleen: Jij zag het proefschrift groeien en sannen genoten we van de
vorderingen. Bij jou kon ik ook steeds terecht met vragen, twijfels en
ontgoochelingen. Maar jouw enthousiasme en trots over mijn resultaten
zijn de mooiste bekroning van mijn werk.
Jan De Munck
April 2004
Index
VOORWOORD
INDEX
INTRODUCTION
CHAPTER 1 9
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES 10
CHAPTER 2 33
WATER DEGRADATION OF ADHESIVES BONDED TO DENTIN
2.1 Four-year water degradation of etch & rinse adhesives bonded to 34 dentin
2.2 Four-year water degradation of a resin-modified glass-ionomer 47 adhesive bonded to dentin
CHAPTER 3 69
EFFECT OF CAVITY CONFIGURATION AND AGING
3.1 Effect of cavity configuration and water storage on the bonding 70 effectiveness of six adhesives to dentin
3.2 Micro-tensile bond strength of adhesives bonded to dentin after 94 thermo-cycling
CHAPTER 4 107
FATIGUE RESISTANCE OF ADHESIVES
4.1 Micro-rotary fatigue resistance of tooth-biomaterial interfaces 108 4.2 Influence of a shock-absorbing layer on the fatigue resistance of a 123 dentin-biomaterial interface.
CHAPTER 5 135
RESTORING CERVICAL LESIONS WITH FLEXIBLE ADHESIVES AND COMPOSITES 136
GENERAL DISCUSSION 147
SUMMARY 176
SAMENVATTING 178
CURRICULUM VITAE 181
REFERENCES 186
AN IN vrrao AND IN V/VOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
This thesis is based upon the following papers
CHAPTER 1
• De Munck 3, Van Meerbeek B, Inoue S, Vargas M, Yoshida Y, Armstrong 5, Lambrechts P,
Vanherle G (2003). Micro-tensile bond strength of one- and two-step self-etch adhesives to bur-cut enamel and dentin Am J Dent 16:414-420.
• De Munck 3, Van Meerbeek B, Vargas M, Iracki 3, Van Landuyt K, Poitevin A, Lambrechts P
(2004). One day bonding effectiveness of new self-etch adhesives to bur-cut enamel and dentin.
Oper Dent (in press).
CHAPTER
2
• De Munck .3, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P,
Vanherle G (2003). Four-year water degradation of total-etch adhesives bonded to dentin. J
Dent Res 82:136-140.
• De Munck 3, Van Meerbeek B, Yoshida Y, Inoue S, Suzuki K, Lambrechts P (2004).
Four-year water degradation of a resin-modified glass-ionomer adhesive bonded to dentin. Eur J Oral Sc! 112:73-83.
CHAPTER
3
• Shirai K, De Munck 3, Yoshida Y, Inoue S, Lambrechts P, Shintani H, Van Meerbeek B (2004). Effect of cavity configuration and aging on the bonding effectiveness of six adhesives to dentin. Dent Mater (in press).
• De Munck 3, Van Landuyt K, Peumans M, Lambrechts P, Van Meerbeek B (2004).
Micro-tensile bond strength of adhesives bonded to dentin after thermo-cycling. (submitted)
CHAPTER
4
• De Munck 3, Van Meerbeek B, Wevers M, Lambrechts P, Braem M (2004). Micro-rotary
fatigue of tooth-biomaterial interfaces. Biomaterials (in press).
• De Munck 3, Van Meerbeek B, Van Landuyt K, Lambrechts P (2004). Influence of a shock
absorbing layer on the fatigue resistance of a dentin-biomaterial interface. (submitted).
CHAPTER
5
• Van Meerbeek B, De Munck 3, Van Landuyt K, Kanumilli P, Lambrechts P, Peumans M (2004). Restoring cervical lesions with flexible adhesives and composites. (Submitted).
GENERAL DISCUSSION
• De Munck 3, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B (2004).
Durability of adhesion to tooth tissue: methods and results. (submitted).
111 Seif-ereh
printer
Adhesive resin
2
Nos
INTRODUCTION
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
During the last decade, restorative dentistry has undergone an important paradigm shift. A mainly 'mechanically' oriented concept that dates from the beginning of the 20th century (Black, 1917), has been replaced by the current trend of 'minimally invasive' dentistry (Degrange and Roulet, 1997). The main principle of this new concept is based upon minimizing the loss of sound tooth structure when restoring teeth. Only since the early nineties, has this concept became achievable thanks to the great progress made in adhesive technology. An adhesive tooth restorative technique enables diseased or lost tooth tissue be replaced by adhering the restorative biomaterial directly to the remaining sound tooth tissue, in contrast to traditional silver amalgam restorations that are only retained macro-mechanically. Because the natural tooth color can be imitated using adhesive restorative materials, this technique even fulfils the growing demand of patients to have their dentition restored not only anatomically and functionally, but also in an esthetic way. It is expected that in the future such adhesive materials will eventually replace silver amalgam that continues to be disputed because of environmental concerns and alleged health risks associated with the mercury contained in dental amalgam (Folwaczny and Hickel, 2002; Swedish government official reports, 2003).
As compared to amalgam, the major shortcoming of today's adhesive restoratives is their limited durability in the mouth. Adhesive restorations only remain in optimum condition for 3 to 5 years (Nickel et al., 2001), after which they not necessarily need to be replaced, but their adaptation to the adjacent tooth tissue mostly requires local adjustment and/or repair in order to prevent any further deterioration of the remaining tooth structure. The most cited reasons for failure of adhesive restorations are loss of retention and marginal adaptation. Hence, a valuable approach to prolong the clinical lifetime might be to focus on improving the stability of the bond of these biomaterials to tooth tissue. The immediate bonding effectiveness of most current adhesive systems is quite favorable, regardless the adhesive approach employed. When these adhesives are, however, tested in a clinical trial, bonding effectiveness of some materials appears dramatically low, whereas of others the bond proves to be more stable.
The main objective of this research project was therefore to investigate/identify in detail the principal factors that cause/accelerate degradation of bonding effectiveness for all classes of adhesives.
INTRODUCTION
CLASSIFICATION OF CONTEMPORARY ADHESIVES
The basic mechanism of bonding to enamel and dentin is essentially an exchange process involving replacement of minerals removed from the hard dental tissue by resin monomers that upon setting become micro-mechanically interlocked in the created porosities. This interlock was first described by Nakabayashi et al. in 1982 and is commonly referred to as 'hybridization' or the formation of a 'hybrid layer'. Based upon the underlying adhesion strategy, three mechanisms of adhesion are currently in use with modern adhesive systems (Fig. 1).
PAA
conditioner
I1
4
GI
1. Etch & rinse adhesives 2. Self-etch adhesives 3. Resin-modified
glass-ionomers
Fig. 1. Classification of contemporary adhesives following adhesion strategy and number of clinical application steps. Gi = Glass-ionomer; PM = polyalkenoic acid.
Etch & rinse adhesives
This adhesion strategy involves at least two steps and, in its most conventional form, three steps with successive application of the conditioner or acid etchant, followed by the primer or adhesion promoting agent, and eventually, application of the actual bonding agent or adhesive resin (Fig. 1). Simplified two-step etch & rinse adhesives combine the primer and adhesive resin into one application, but still following a separate etch-and-rinse phase. This etch & rinse technique is still the most effective
AN IN VITRO AND IN VIVOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
approach to achieve efficient and stable bonding to enamel and basically only requires two steps. Selective dissolution of hydroxyapatite crystals through etching (commonly with a 30-40% phosphoric-acid gel) is followed by in situ polymerization of resin that is readily absorbed by capillary attraction within the created etch pits (Fig. 2).
Fig. 2. Feg-SEM photomicrograph of the interface of a three-step etch & rinse adhesive (OptiBond
FL, Kerr) to enamel. The
phosphoric-acid etchant produced a deep etching pattern. The adhesive resin subsequently infiltrated into the created porosities, producing macro-tags (hand-pointer) in between the enamel prisms.
Fig. 3. TEM photomicrograph of the interface of a three-step etch & rinse adhesive (OptiBond dual
cure, Kerr) to dentin.
Non-demineralized, unstained section. A 4 pm thick hybrid layer is formed that consists of loosely organized collagen fibrils interspersed by resin. Resin-tags seal the opened dentin tubules. The transition of the exposed collagen fibril network towards the underlying unaffected dentin is very abrupt.
At dentin, this phosphoric-acid treatment exposes a micro-porous network of collagen that is nearly totally deprived of hydroxyapatite (Fig. 3). As a result, the primary bonding mechanism of etch & rinse adhesives to dentin is diffusion-based and depends on hybridization or infiltration of resin within the exposed collagen mesh. After in situ polymerization, this hybrid layer provides micro-mechanical retention to the restoration. True chemical adhesion is rather unlikely, as the functional groups of monomers have only weak affinity to 'hydroxyapatite-depleted' collagen. Most critical in the etch & rinse approach is the priming step. When an
dhesive
resin ',V4 •INTRODUCTION
acetone-based adhesive is used, the highly technique-sensitive 'wet-bonding' technique is mandatory. Otherwise, gentle post-conditioning air-drying of acid-etch dentin (and enamel) following a 'dry-bonding' technique still guarantees effective bonding when a water/ethanol-based adhesive is used (Van Meerbeek et al, 1996, 1998).
Self
-etch adhesives
An alternative approach is based on the use of non-rinse acidic monomers that simultaneously condition and prime dentin, the so-called self-etch adhesives. Regarding user friendliness and technique-sensitivity, this approach seems clinically most promising. It no longer needs an etch-and-rinse phase, which not only lessens the clinical application time, but also significantly reduces the technique-sensitivity or the risk to make errors during application. Similar to etch & rinse adhesives, simplified adhesives that combine the (self-etch) primer with the adhesive resin were developed, one-step self-etch adhesives or so-called 'all-in-one' adhesives (Fig. 1).
Fig. 4. TEM photomicrograph of the interface of a 'strong' one-step self-etch adhesive (Adper
Prompt, 3M ESPE) to dentin.
Non-demineralized, unstained section. The adhesive resin infiltrated dentin, producing a thick (±4pm) hybrid layer. In this layer, dentin was completely demineralized, so that an etch & rinse-like morphology is created. Because of the electron-dense resin, the hybrid layer was negatively stained, disclosing the contours of individual collagen fibrils.
Basically, two types of self-etch adhesives can be distinguished (Van Meerbeek etal., 2001): 'mild' and 'strong' ones. 'Strong' self-etch adhesives have a very low pH (<1) and have been documented with a bonding mechanism and interfacial ultra-morphology at dentin resembling that produced by etch & rinse adhesives (Fig. 4). 'Mild' self-etch adhesives (pH = ±2) only partially dissolve the dentin surface, so that a substantial amount of hydroxyapatite remains available within the hybrid layer (Fig. 5). Specific carboxyl or phosphate groups of functional monomers can then chemically interact with this residual hydroxyapatite. This two-fold bonding mechanism is expected to be advantageous in terms of restoration
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
durability. It comprises a micro-mechanical bonding component that may in particular provide resistance to abrupt de-bonding stress. The chemical interaction may result in bonds that better resist hydrolytic break-down and thus keep the restoration margins sealed for a longer period. However, little is known about the long-term effects of incorporating dissolved hydroxyapatite crystals and residual smear layer remnants within the bond. How much of the primer/adhesive solvent is kept within the interfacial structure should also be investigated. Such solvent surplus will directly weaken the bond integrity, provide channels for nanoleakage or may affect polymerization of the infiltrated monomers. The resultant interfacial structure also becomes more hydrophilic and, thus, more prone to hydrolytic degradation.
Fig. 5. TEM photomicrograph of the interface of a 'mild' one-step self-etch adhesive (iBOND,
Hereaus-Kulzer) to dentin.
Non-demineralized, unstained section. The adhesive resin infiltrated dentin, producing a thin (±1pm) hybrid layer. Dentin was only partially demineralized, leaving hydroxyapatite crystals around the collagen fibrils (not visible, as the section was not stained).
Glass-ionomers and glass-ionomer adhesives
Glass-ionomers are still considered the only materials that are self-adhering to tooth tissue (Yoshida et al, 2000). A short polyalkenoic acid pre-treatment cleans the tooth surface; it removes the smear layer and exposes collagen fibrils up to about 0.5-1 pm depth (Fig. 6); herein, glass-ionomer components interdiffuse with the establishment of a micro-mechanical bond following the principle of hybridization. Chemical bonding is additionally obtained by ionic interaction of the carboxyl groups of the polyalkenoic acid with the calcium of the hydroxyapatite that remained attached to the collagen fibrils. As mentioned above for mild self-etch adhesives, this chemical adhesion may be beneficial in terms of resistance to hydrolytic degradation.
gllagopthamw
4g@g phmm
tdd IlauGT
INTRODUCTION
Fig. 6. TEM photomicrograph of the interface of a resin-modified
glass-ionomer adhesive (Fuji
BOND LC, GC) to dentin.
Non-demineralized, unstained section. The poly-alkenoic acid conditioner did not decalcify the surface completely, so that hydroxyapatite remained within the hybrid layer as receptor for additional chemical interaction. On top of the hybrid layer, an amorphous, "gel-phase" represents the reaction product formed through interaction of the poly-alkenoic acid with calcium that was extracted from dentin.
DISSSERTATION OBJECTIVES
A valuable approach to determine bond durability is conducting clinical trials. An inherent disadvantage of clinical studies is however that it takes at least 3 years before clinically valuable data are generated. By the time the data are available, most dental adhesives have however been replaced by new formulations, rendering the data out-dated and less meaningful. Therefore, a need exists for relatively fast and reliable in vitro techniques that can predict in vivo bond durability.
The objectives of this thesis project are therefore: (1) to determine major factors that clinically affect the durability of adhesive tooth-biomaterial bonds, (2) to review and develop artificial aging methodologies that can simulate clinical degradation of adhesive interfaces, and (3) to correlate these findings to clinical trial results.
In a first part, the short-term bonding performance of contemporary dental adhesives was assessed mechanically and ultra-morphologically, to serve as a reference frame (chapter 1).
Subsequently, several in vitro techniques that may predict bond durability were explored. (chapter 2-4). At first, the effect of water exposure on the long-term durability of tooth-biomaterial interfaces was evaluated (chapter 2). Clinically, adhesives are applied in cavities, which results in higher polymerization contraction stresses and technique sensitivity, but may also affect the durability of the created bonds. Therefore, the effect of different factors such as water exposure, protection by adjacent tooth-enamel bonds and artificial aging by thermal cycling in class-I
AN IN VITRO AND IN V/VOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
cavities was studied in the next chapter (chapter 3). All former tests, challenge the interface in a static way; tooth/composite bonds are clinically, however, seldom imposed to such acute tensile stresses, but are more prone to cyclic loading. Up to now, no reliable fatigue test set-up for dental adhesives is available. Therefore, a method, based upon a classic rotating beam experiment, was developed to determine the fatigue resistance of tooth-biomaterial interfaces (chapter 4).
To draw a parallel between the in vitro results of chemically (chapter 2-3) and mechanically (chapter 4) degraded interfaces and the clinical situation, a clinical study was carried out, of which the 5-year data are reported (chapter 5). In this study, the long-term in vivo durability of our 'golden standard' adhesive was determined.
Finally, in the concluding chapter, the fundamental processes that cause degradation of adhesion to enamel and dentin are discussed. Different methodologies that focus on chemical degradation patterns such as hydrolysis and elution of interface components, as well as mechanically oriented test set-ups, for example fatigue and fracture toughness measurements, were critically appraised. Combining these, the most probable degradation pathways were elucidated, along with proposals for the most appropriate set-ups to mimic these in vitro. In this review, also the durability of different types of contemporary adhesives was evaluated.
The eventual objective of this research project is to contribute to further advancement of adhesive techniques, especially in the light of extended longevity.
CHAPTER
1
ONE-DAY BONDING EFFECTIVENESS
OF CONTEMPORARY ADHESIVES TO
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
1. One-day bonding effectiveness of contemporary adhesives
to bur-cut enamel and dentin*
INTRODUCTION
A most recent innovation in dental adhesive technology involves the introduction of 'self-etch' adhesives. 'Self-etch' adhesives make use of acidic monomers that simultaneously condition and prime enamel and dentin, and provide vinyl groups for co-polymerization with the resin composite. The bonding mechanism of 'self-etch' adhesives is based upon changing the chemical composition of the substrate surface, commonly referred to as hybridization; the surface layer of enamel/dentin is partially dissolved and the resultant porosity filled by resin (Inoue etal., 2000).
These self-etch adhesives try to solve difficulties commonly associated with the clinical application of etch & rinse adhesives. Their application procedure is considered less time-consuming and more importantly less technique-sensitive, in particular with regard of keeping the dentin surface in an adequate state of hydration. Self-etch adhesives have, for instance, been associated with less nanoleakage (Sano
et al, 1995). This is most likely attributed to resin impregnation that proceeds
simultaneously with dentin etching (Watanabe etal., 1994). The risk of a discrepancy between the depth of dentin demineralization and hybridization is consequently limited, which is expected to be advantageous in the long term. In support of this effect, Miyazaki et al. (1998) reported a larger decrease in bond strength to dentin after thernno-cycling for 'one-bottle' (or two-step etch & rinse) adhesives than for self-etch adhesives. Also, Sano etal. (1999) found no decrease in micro-tensile bond strength (pTBS) after one-year in-vivo functioning of restorations bonded with a self-etch adhesive. However, self-self-etch adhesive bonds to enamel are thought to be more susceptible to degradation, as was demonstrated by thermo-cycling in a shear-bond strength and SEM study (Miyazaki et al, 2000). This was corroborated by SEM evaluation of resin-enamel interfaces after thermal cycling; small cracks and porosities were observed between enamel and adhesive that were not present before.
*Based in part on the papers: (1) 'De Munck J, Van Meerbeek B, Inoue S, Vargas M, Yoshida Y, Armstrong S, Lambrechts P, Vanherle G (2003). Micro-tensile bond strength of one- and two-step self-etch adhesives to bur-cut enamel and dentin Am J Dent 16:414-420' and (2) 'De Munck 3, Van Meerbeek B, Vargas M, Iracki J, Van Landuyt K, Poitevin A, Lambrechts P (2004). One day bonding effectiveness of new self-etch adhesives to bur-cut enamel and dentin
Oper Dent (in press)'.
Enamel
Dentin Adhesive
3 teeth/ adhesive Diamond
bur
24hr - H 20
icroSpewen former
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
The main objective of this study was to determine the bonding effectiveness to and interaction with enamel/dentin of contemporary one- and two-step self-etch adhesives by pTBS, Feg-SEM and TEM, when compared to a control two-step self-etch and three-step self-etch & rinse adhesive.
MATERIALS AND METHODS
Thirty non-carious human third molars were stored in 0.5% chloramine solution at 4°C and used within 1 month after extraction. The teeth were randomly divided into 3 experimental and 2 control groups. First, all teeth were mounted in gypsum blocks to ease manipulation. The pTBS to enamel and dentin was determined following the protocol described in detail before (Fig. 1, De Munck etal., 2002).
Fig. 1. Schematic presenting the experimental design of micro-tensile bond strength testing.
All adhesives were applied according to manufacturer's instructions (Table 1). The pH of the non-diluted primer, as applied to the tooth surface, was determined at
AN IN VITRO AND IN V/VOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
ambient temperature (20-25°C) using a digital pH meter (Inolab pH Level 2, 1NTW, Weilheim, Germany).
Enamel specimen preparation
Lingual and/or buccal enamel was flattened using a high-speed medium-grit (100 pm) diamond bur (842, Komet, Lemgo, Germany) mounted in the MicroSpecimen Former (The University of Iowa, Iowa City, IA, USA). Subsequently, the adhesives were applied (Table 1), after which the surface was built up with a micro-hybrid resin composite Z100 (3M ESPE, St Paul, MN, USA) in 3-4 layers to a height of 5-6 mm. Because bond strength is also largely influenced by the composite used (Van Noort
etal., 1989), we opted to use the same resin composite with all adhesives to exclude
this variable.
Dentin specimen preparation
The occlusal third of the molars was removed using a slow-speed diamond saw (Isomet 1000, Buehler, Lake Bluff, IL, USA). Dentin surfaces were verified for absence of enamel and/or pulp tissue using a stereo-microscope (Wild M5A, Heerbrugg, Switzerland). A standard smear layer was created by removing a thin layer of the surface using a high-speed medium-grit (100 pm) diamond bur (842, Komet) mounted in the MicroSpecimen Former. The adhesives and restorative composite were applied following the methodology described above.
pTBS testing
The experimental set-up is schematically presented in Fig. 1. After bonding procedures, specimens were stored for 24 hours in tap water at 37°C. The teeth were then sectioned perpendicular to the bonding surface using the Isomet saw to obtain rectangular samples of about 1.8x1.8 mm wide and 8-9 mm long. These specimens were mounted in the pin-chuck of the MicroSpecimen Former and trimmed at the tooth-biomaterial interface to a cylindrical hour-glass shape with a diameter of about 1.2 mm using a cylindrical extrafine-grit (15 pm) diamond bur (835 KREF, Komet) in a high-speed handpiece under air/water spray coolant. Specimens were then fixed to Ciucchi's device (Pashley et al., 1999) with cyanoacrylate glue (Model Repair II Blue, Dentsply-Sankin, Ohtawara, Japan) and stressed at a crosshead speed of 1 mm/min until failure in a LRX testing device
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
(Lloyd, Hampshire, UK) using a load cell of 100 N. The pTBS was expressed in MPa, as derived from dividing the imposed force (in N) at the time of fracture by the bond area (in mm 2 ). When specimens failed before actual testing, the mean pTBS was determined from the specimens that survived specimen processing with an explicit note of the number of pre-testing failures. The mode of failure was determined at a magnification of 50x using a stereo-microscope. For the dentin and enamel group, one-way ANOVA and Tukey HSD multiple comparisons test were used to determine statistical differences in pTBS at a significance level of 0.05.
Feg-SEM
evaluationThe bonding mechanism to enamel was morphologically assessed by Field-emission gun Scanning Electron Microscopy (Feg-SEM) following a protocol described by Perdigk et al. (1997). Two additional enamel surfaces were prepared for each group in the same way as for pTBS testing. One of the self-etch adhesives was then applied and covered with resin composite (Z100). After 24 h water storage at 37°C, the samples were stored in 6N HCI for 8 h to completely dissolve enamel. The remaining composite blocks were then rinsed in Na0CL solution for 10 min and air dried for 24 h. After mounting on aluminum stubs, the resin replica of the enamel etch-pattern was evaluated by Feg-SEM (Philips XL30, Eindhoven, The Netherlands).
TEM evaluation
The bonding mechanism to dentin was morphologically assessed by Transmission Electron Microscopy (TEM). Two dentin surfaces were prepared for each group in the same way as for pTBS testing. Following adhesive treatment, the resin-bonded dentin specimens were cross-sectioned perpendicular to the resin-dentin interface to obtain 1-mm wide sticks using a slow speed diamond saw. Half of the specimens were then demineralized and fixed simultaneously in a 10% formaldehyde-formic acid solution (Gooding and Stewart Fluid, Prosan, Gent, Belgium) for at least 36 h. Further TEM sample preparation of both the demineralized and non-demineralized sections was performed in accordance with common procedures used for ultra-structural TEM examination of biological tissues (Van Meerbeek at al., 1996). Then, 70-90 nm thick sections through the resin-dentin interface were cut using a diamond knife (Diatome, Bienne, Switzerland) in an ultramicrotome (Ultracut UCT, Leica, Vienna, Austria).
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS Table 1. Adhesives investigated in this study.
Product name Manufacturer Composition' Application
Adper Prompt 3M ESPE, Liquid 1: Methacrylated phosphoric St Paul, MN, esters, Bis-GMA, camphorquinone,
USA stabilizers
Liquid 2: Water, HEMA, polyalkenoic acid, stabilizers
AdheSE Ivoclar- Primer: Dimethacrylate, phosphonic
Vivadent, acid acrylate, initiators, stabilizers,
Schaan, water
Liechtenstein Bond: HEMA, dimethacrylate, silicon dioxide, initiators, stabilizers
OptiBond Solo Plus Self-Etch
Kerr, Primer: GPDM, camphorquinone,
Orange, CA, ethanol, water.
USA Bond: Bis-GMA, GDM, HEMA,
GPDM, ethanol
Activate blister. Apply adhesive and rub for 15 sec. Gently air blow. Light cure for 10 sec.
Dry surface. Apply Self-etch primer for at least 30 sec, from which at least 15 sec with rubbing motion. Remove excess of primer with air. Apply bond for at least 10 sec. Gently air blow. Light cure for 10 sec.
Apply self-etch primer and rub for 15 sec. Air thin 3 sec. Apply OptiBond Solo Plus and rub for 15 sec. Air thin 3 sec. Apply OptiBond Solo Plus and rub for 15 sec. Light cure for 20 sec.
Clearfil SE Kuraray, Osaka, Japan
Primer: 10-MDP, HEMA, hydrophilic dimethacrylate, photoinitiator, water
Bond: 10-MDP, Bis-GMA, HEMA, hydrophilic dimethacrylate, microfiller
Apply primer for 20 sec; Gently air blow; Apply bonding agent; Light cure for 10 sec.
Apply etchant for 15 sec; Rinse for 15 sec; Gently air-dry for 5 sec; Scrub surface for 30 sec with primer; Apply thin coat of bonding and light Adhesive: TEGDMA, UDMA, GPDM, cure for 30sec.
HEMA, Bis-GMA, filler, photoinitiator OptiBond FL Kerr, Etchant: 37.5% Phosphoric acid,
Orange, CA, silica thickener
USA Primer: HEMA, GPDM, PAMM,
ethanol, water, photoinitiator
'Composition as provided by respective manufacturer: Bis-GMA = Bisphenol-glycidyl methacrylate; GPDM = Glycerol phosphate dimethacrylate; GDM = Glycerol dimethacrylate; HEMA = Hydroxyethylmethacrylate; 10-MDP = 10-Methacryloyloxydecyl dihydrogen phosphate; PAMM = Phthalic acid monoethyl methacrylate; TEGDMA = Triethylene glycol dimethacrylate; UDMA = Urethane dimethacrylate.
For evaluation of collagen, TEM sections were positively stained with 5% uranyl acetate (UA) for 20 min and saturated lead citrate (LC) for 3 min prior to TEM examination (Philips CM10, Eindhoven, The Netherlands). Unstained sections were evaluated as well. On each section, the minimum and maximum hybrid layer thickness was determined (in pm). The same diamond-knife cut interfaces were gold sputtered and evaluated by Feg-SEM as well.
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
RESULTS
pTBS
The mean pTBS, pH and hybrid-layer thickness are summarized per experimental group in Table 2 and graphically presented in box-whisker plots in Fig. 2 for enamel and in Fig. 3 for dentin. When bonded to enamel, the pTBS of the control etch & rinse adhesive (OptiBond FL) was higher than that of any self-etch adhesive (Fig. 2, Table 2), though not significantly different from OptiBond Solo Plus Self-Etch. When bonded to dentin, no statistically significant differences were observed between the control adhesives and OptiBond Solo Plus Self-Etch. The lowest pTBS was obtained when Adper Prompt, the only one-step adhesive tested, was bonded to dentin as well to enamel; also some pre-testing failures were recorded in this group (Table 2).
Table 2. Results.
pH primer
Enamel pTBS
Mean SD' ptf/n 2
Dentin pTBS
Mean SD I Ptf/n 2
Hybrid layer thickness
Adper Prompt 0.41 18.6` 7.9 0/12 17.8 h 5.2 2/12 1.7-4.5 pm'
AdheSE 1.40 23.2h, ` 10.8 1/12 30.9 h 13.1 0/12 2.1-2.4 pm
OptiBond Solo Plus SE 1.48 32.3" 7.5 0/14 52.2a 9.2 0/12 2.1-2.4 pm
Clearfil SE 1.92 29.8 h 7.3 0/10 48.1a 11.5 0/12 0.7-1 prn h
OptiBond FL 1.78 41.1h 10.3 0/11 47.3a 13.1 0/12 4-5 prn s
Means with the same superscript are not statistically significant different (p<0.05, Tukey HSD multiple comparisons); 'SD = Standard deviation; 2 ptf = Pre-testing Failures, n = Total number of samples; 'Hybrid layers thinner than 3.5 pm were only noticed when no thick bonding layer was present; Inoue & others, 2000; 'Van Meerbeek et al., 1996.
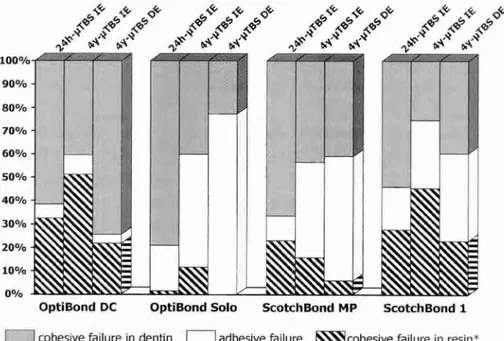
The failure patterns are summarized in Table 3. Most failures were 'mixed' including 'adhesive' failure between tooth and resin and 'cohesive' failure of resin or tooth. Some samples also failed entirely 'adhesively' or 'cohesively' within the resin part of the specimen. Only a few samples failed 'cohesively' within the tooth surface (Table 3), a pattern associated with higher bond strengths. No difference in failure pattern could be detected between the different adhesives tested, except that the 'stronger' adhesives tended to fail more cohesively in dentin than the weaker ones.
F
E-
]-
Adper Prompt
-
[
-
AdheSE
OptiBond Solo Plus SE
_
Clea MI SE Bond
-
OptiBond FL
[ ]
]
' 26 '
r I r T • 1-1- I
40 60 80
o
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL -TOOTH BONDS
pTBS (MPa)
Fig. 2. Box plot of enamel pTBS results. The box represents the spreading of the data between the first and third quartile. The central vertical line represents the median. The whiskers extend to the minimum and maximum value.
Interfacial Morphology
The enamel etching pattern, for the most acidic (Adper Prompt, pH = 0.41) as well as the least acidic self-etch adhesives (OptiBond Solo Plus Self-Etch, pH = 1.48), was non-uniform and seemed dependent on the local smear layer features (Fig. 4). This non-uniform pattern is probably due to the thick and irregular smear layer prepared in this study. All experimental groups were however able to demineralize inter-prismatic enamel and to form acid-resistant resin tags into the created porosities (Fig. 4). The size of the resin tags seemed relatively independent of the pH of the primer, although more detailed TEM observation can probably better discriminate between the different etching patterns (Pashley etal., 2001).
When bonded to dentin, all experimental self-etch adhesives were able to create distinct resin tags (Fig. 5-7). The thickness of the hybrid layer seemed to relate well with the pH of the primer (Table 2). Hydroxyapatite crystals could only be detected in the bottom part of the hybrid layer for AdheSE and OptiBond Solo Plus Self-Etch (Fig. 5, 6). Loose collagen fibrils extending into the bonding resin, known as a 'shag-carpet' appearance (Van Meerbeek et al., 1996), could be detected for AdheSE as well as OptiBond Solo Plus Self-Etch (Fig. 5, 6). Tubule-wall hybridization
II
zio 410 6 10 sio
OptiBond FL
o
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
was very prominent for Adper Prompt, but could also be detected to a smaller extent for AdheSE and OptiBond Solo Plus Self-Etch (Fig. 5-7). When Adper Prompt was bonded to dentin, two different zones could be detected in the bonding resin: first, a uniform layer of 10 pm that had heavily reacted with the staining solution, morphologically resembled the resin that formed the resin tags (Fig. 7a,b); second, an intermediary zone between bonding resin and resin composite. On some spots, only the intermediary zone could be detected (Fig. 7c,d), associated with a thinner hybrid layer (±1.5 pm, Fig 7c). In presence of a separate bonding layer on the other hand, the bonding layer was always at least 3.5 pm thick.
Adper Prompt
AdheSE
OptiBond Solo Plus SE
Clearfil SE Bond
pTBS (MPa)
Fig. 3. Box plot of dentin uTBS results. The box represents the spreading of the data between the first and third quartile. The central vertical line represents the median. The whiskers extend to the minimum and maximum value.
DISCUSSION
In this study, the bonding effectiveness and mechanism to enamel and dentin of three new self-etch adhesives were evaluated by pTBS and electron microscopy. Care was taken that all adhesives were applied to standardized tooth substrates and strictly according to manufacturer's instructions. The buccal/lingual enamel was flattened parallel to the tooth axis to standardize the orientation of enamel prisms.
AN IN VITRO AND IN VNOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
Table 3. Failure patterns of pTBS specimens as analyzed through microscopy
stereo-
Enamel Cohesive in
enamel/dentin
Adhesive
tooth/resin Mixed*
Cohesive in
resin Total
Adper Prompt 9 3 12
AdheSE 3 7 1 11
OptiBond Solo Plus SE 10 4 14
Clearfil SE 1 6 3 10
OptiBond FL 10 1 11
Dentin
Adper Prompt 4 6 10
AdheSE 9 3 12
OptiBond Solo Plus SE 3 4 5 12
Clearfil SE 6 5 1 12
OptiBond FL 5 5 2 12
*Involving cohesive failure in resin and adhesive failure between tooth and resin
For dentin, only the central portion of mid-coronal dentin surfaces was used in order to have all tubuli orientated perpendicular to the surface. In this way, we minimized any regional effects on the pTBS (Shono et al., 1997; Yoshiyama et al., 1998; Carvalho et al., 2000). Although bonding to such laboratory 'model' substrates may clinically be of less relevance, it allowed us to determine the most optimal bonding effectiveness under ideal circumstances, and enabled comparison with previously conducted pTBS studies at Leuven BIOMAT (Inoue et al., 2001a; De Munck et al.,
2002, 2003a; Van Meerbeek etal., 2003a).
All adhesives tested were applied to bur-cut smear layers prepared using the MicroSpecimen Former. This device was equipped with a high-speed dental contra-angle handpiece that held a regular-grit diamond bur, which clinically is commonly used for adhesive cavity preparation. This procedure resulted in a uniform, but rather thick smear layer with a roughness comparable to a smear layer created with 60-grit SIC paper (Wahle and Wendt, 1993). A TEM study revealed that thick smear layers (up to 4.1 pm) did not hinder Clearfil SE to hybridize dentin (Tay etal.,
2000a), although the penetration of the primer might be compromised for weaker one-step self-etch adhesives (Inoue et aí, 2001b). Despite the thick smear layer in
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
this study, all experimental adhesives dissolved the smear layer and created a hybrid layer of at least 2 pm thick (Fig. 5-7) within intact dentin (Tay and Pashley, 2001). Consequently, it can be assumed that the bonding effectiveness of these adhesives is relatively independent of the preparation method used (Tay et al, 2000b; Van Meerbeek etal., 2001).
Besides standardized bur-cut smear-layer formation, the basic capability of the MicroSpecimen Former is to generate specimens that (a) are not only uniform in dimensions, but that (b) are also rounded and (c) narrowed at the resin-tooth interface itself. An inverse relationship between enamel/dentin pTBS and cross-sectional area was found (Sano et al., 1994). This apparatus enabled to prepare specimens with a cylindrical bond area that closely varied around 1 mm2, which corresponds to the bonding area size recommended by Shono et al. (1997) and Phrukkanon etal. (1998a). Using finite element analysis, the stress distribution at the interface was more uniformly distributed within cylindrical specimens as compared to rectangular pTBS specimens, though no significant different pTBS between both shapes were found (Phrukkanon et al., 1998b). Another reason for this indentation is that failure remote from the interface is prevented, as the highest tensile stresses are imposed at the narrowest area. As stated by Phrukkanon et al. (1998b), other reasons to recommend the preparation of cylindrical bond areas are the ease of specimen processing and testing, the reduced risk of pre-testing failures, the appropriate bonding area in compensation for dentin irregularities and potential internal interface defects.
A major drawback of pTBS-testing is that the rather aggressive specimen preparation may induce defects at the interface that may lead to lower bond strength values or even pre-testing failures. So the more gentle (as compared to hand trimming) and standardized specimen preparation of the MicroSpecimen Former may be beneficial, especially taking into account that the highest stresses occur at the outer side of the beam (Phrukkanon etal., 1998b). Hence, any risk on manipulation errors can be considered as minimal. Furthermore, the fact that all specimens were prepared in the same way, and that pre-testing failures only occurred in the weakest groups (Table 2), strongly suggests that the pre-testing failures should be attributed rather to less effective bonding and not to solely manipulation errors.
A
N
IN VI
TR
O
AND
IN
VNOST
UDY
O
N THE DURAB
IL
ITY
O
F
BIO
MATERIAL-T
OOTH
BONDS
V Spot 14agn Dot WO 100 pm 10.0 kV 4.0 250x SE 10.0 -15- Pro t An lenamel
Mc V Spot Magn Dot WO 5 pm
10 0 kV 4.0 5000x SE 10.0 -15- kom t el enamel
cc V Spot Mago Dot WD 50 tun
100 kV 5.0 500x SE 10.0 -15- 0 ti SE - enamel
Fig. 4 Fe-SEM evaluation of the enamel etch-pattern replica after complete decalcification of enamel in HCL. (a) Adper Prompt bonded to enamel. The enamel etching pattern was non-uniform. Some zones show only very limited etching effects (white hand pointer), while on other spots the adhesive was clearly able to form acid-resistant tags (black hand pointer). (b) Higher magnification of acid-resistant tags created by Adper Prompt. Around the dissolved enamel prisms (Ep), resin macro-tags (white hand pointer) can be observed, while nearly no micro-tags were observable. (c) OptiBond Solo Plus Self-Etch bonded to enamel. The enamel etch-pattern was non-uniform. On some spots etching of enamel was very limited (white hand pointer), while on other spots the adhesive was clearly able to penetrate into interprismatic porosities (black hand pointer). The interaction seems to be dependent on the local smear layer properties (horizontal scratches). (d) Higher magnification of (a) at the black pointer. Enamel was preferably dissolved around the prisms, creating resin macro-tags (arrow) that surround the enamel prisms (Ep).
O
NE-DAY
BONDING
EFFECTIVENESS
OF
CONTEMPORARY ADHESI
AN IN VITRO AND IN VI VO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
V
Spat Magn Dot
-
W0 1
.0
Fig. 5 Electron microscopic evaluation of the interface of AdheSE bonded to dentin. (a) Fe-SEM photomicrograph of a diamond-knife cut interface to dentin. On top of dentin (Ud), a hybrid layer (hand pointer) and a bonding layer (Ar, ±11 pm thick, not visible on this picture) can be observed. A resin tag (Rt) was formed in the dentinal tubulus. In the hybrid layer, collagen fibrils can be detected (hand pointer). (b) TEM photomicrograph (non-demineralized, unstained section) of the adhesive-dentin interface. A hybrid layer (H, thickness: ±2.1 pm) is formed with hydroxyapatite crystals clearly scattered within the bottom half (hand pointer). A resin tag (Rt) was formed, but some remnants of the smear plug were still present. (c) TEM photomicrograph (demineralized, stained section) of the interface. Hybrid layer collagen reacted heaviest with the staining solution, revealing a typical 'shag-carpet' appearance (Sc), with collagen fibrils unraveled into their micro-fibrils and directed towards the adhesive resin. Note also some limited tubule wall hybridization (hand pointer). (d) TEM photomicrograph (non-demineralized, unstained section) of the adhesive resin-dentin interface. Hydroxyapatite crystals are clearly scattered within the bottom half of the hybrid layer (hand pointer). Ar — Adhesive resin; Ud = Unaffected dentin; Hy = Hybrid layer (thickness: ±2.2 pm).
O
NE-DAY BONDING EFF
EC
TI
VE
NESS OF CONTEMPO
RARY
A
DHE
SIVE
AN IN VITRO AND IN V/VOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
The only one-step self-etch adhesive tested in this study was Adper Prompt, the successor of Prompt L-Pop, a typical 'strong' self-etch adhesive (Van Meerbeek et al., 2000, 2001). The main difference with the older version is that the amount of non-acidic methacrylates has been increased to obtain a higher viscosity (technical information obtained from 3M ESPE). This may help to provide a sufficient amount of primer to the surface and to obtain a thicker adhesive layer. TEM evaluation in fact revealed the presence of a uniform layer, nearly free from phase separations, on top of the hybrid layer (Fig. 7a), which was not present with its predecessor (Van Meerbeek et al., 2000). On some sites, however, only an intermediary adhesive/composite zone was present (Fig. 7c). Noteworthy is that if the primer was strongly blown out after application (less than with a correct application technique), still some zones exhibited a thick bonding layer (Fig. 7d). Also interesting to note is that if a distinct bonding layer was present, it was at least 10 pm thick. Due to oxygen inhibition, adhesive layers need to have a minimal thickness (Unterbrink and Liebenberg, 1999), consequently it can be hypothesized that if the adhesive layer does not reach this minimal thickness, the adhesive may not cure adequately and may be pushed away by the resin composite. This may explain the marked difference between zones with a thick adhesive layer and zones without adhesive layer at all. A thick adhesive layer, as mentioned before, is beneficial as shock-absorber between tooth and composite (Kemp-Scholte and Davidson, 1990; Van Meerbeek et al., 1993; Uno and Finger, 1995; Choi et al., 2000) and is expected to be especially advantageous on the longer term (Van Meerbeek etal., 1998; Ausiello etal., 2002).
On some spots where the bonding layer was absent, the hybrid layer formed by Adper Prompt was also substantially thinner (Fig. 7c). This local insufficient adhesive penetration may be due to different causes: (a) Locally insufficient adhesive application. (b) Insufficient penetration time; this is less probable, as some spots on the same surface exhibited much thicker hybrid layers. (c) Penetration may have been hampered by an amorphous layer on top of the hybrid layer, formed by the polyalkenoic-acid co-polymer (a component not present in the former version of the adhesive). A similar phenomenon is known for the primers of Scotchbond Multi-purpose and Scotchbond 1 that contain the same polyalkenoic-acid co-polymer (Van Meerbeek et aí, 1996). This hypothetical phenomenon may be corroborated by the presence of a thin electron-dense line at
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
the top of the hybrid layer, which was only present in case of a thin hybrid layer (Fig. 7c), but not when the adhesive was strongly blown out (Fig. 7d). (d) Also a lack of rubbing may have caused the reduced penetration, as this can freshen the adhesive solution at the interface and prevent local concentrations of polyalkenoic acid. Most likely a combination of these factors caused the less optimal infiltration of the adhesive. Note however that the thickness of the hybrid layer is not directly related to its bonding effectiveness (Van Meerbeek etal., 2001).
The long-term durability of Adper Prompt bonded to dentin is questionable (Shirai et aZ, 2003). A hypothesis regarding this effect was advanced by Tay et al.
(2002a); the curing of a combination of acidic monomers, HEMA and water does not result in a uniform, hydrophobic resin layer, but areas of incomplete polymerization and hydrogel formation are present. These areas may then permit water fluxes within the hybrid layer and subsequently accelerate water sorption of the adhesive interface. Combined with degradation and extraction of resin components (Tay etal.
2002b), this process is known to be detrimental for the bond integrity (Hashimoto et al., 2000, 2002; De Munck etal., 2003a).
AdheSE is a two-step self-etch adhesive. Its self-etch capacity is based on phosphonic acid acrylates (opposite to Adper Prompt, which is based on methacrylated phosphoric esters). This particular adhesive is less acidic than Adper Prompt and subsequently results in a thinner hybrid layer (Table 2). At the bottom of the hybrid layer, some residual hydroxyapatite crystals were detected (Fig. 5b), indicating that demineralization was not complete at that location. Consequently, this self-etch approach resulted in a smoother transition to the deeper intact dentin. The system is however more agressive than the control self-etch adhesive Clearfil SE (pH = 1.92). This is reflected in the formation of a thicker hybrid layer and more pronounced resin tags instead of hybridized smear plugs, typically observed with Clearfil SE. Previously, self-etch adhesives were divided in 'mild' self etch adhesives (pH ±2.0, sub-micron hybrid layer formation) and 'strong' self-etch adhesives (pH <1.0, interfacial ultra-morphology similar to etch & rinse adhesives) (Van Meerbeek
et al., 2001, 2003b). As the pH and the interfacial ultra-morphology of AdheSE is intermediary between the structural characteristics produced by the 'mild' and 'strong' self-etch adhesives, it can not be incorporated in one of this two groups, but should be regarded as an 'intermediary strong' self-etch adhesive.
A
N
IN VITRO
AND
IN
VIVO
S
TUDY
O
N THE
DURABI
LITY
OF
BIOMATE
RI
AL
-TOOTH
BONDS
Fig. 6 Electron microscopic evaluation of the interface of OptiBond Solo Plus Self-Etch bonded to dentin. (a) Fe-SEM
photomicrograph of a diamond-knife cut adhesive-dentin interface. On top of unaffected dentin (Ud) a hybrid layer (Hy) is formed (asterisk). In
between the hybrid layer and composite (C), a 27 pm thick adhesive resin (Ar) layer can be observed. Near the interface, the dentinal tubuli seem
to be obturated by resin tags (hand pointer), while further away the tubuli are empty. Consequently, resin tags only extend up to about 10 pm
into the dentin surface. (b) TEM photomicrograph (non-demineralized, unstained section) of the adhesive-dentin interface. (c) TEM
photomicrograph (demineralized, stained section) of the adhesive-dentin interface. A typical 'shag-carpet' appearance (Sc) with collagen fibrils
unraveled into their microfibrils and directed towards the adhesive resin can be observed. Note also the tubule wall hybridization (hand pointer).
Ud = Unaffected dentin; Hy = Hybrid layer (thickness: 2.4 pm); Rt = Resin tag. Ar = Adhesive resin with larger glass particles and nanofillers. (d)
TEM photomicrograph (non-demineralized, unstained section) of the adhesive-dentin interface. Hydroxyapatite crystals are clearly scattered within
the bottom half of the hybrid layer (hand pointer). Ar = Adhesive resin, Ud = Unaffected dentin; Hy = Hybrid layer (thickness: ±2.2 pm); Rt -
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
Fig. 7 Electron microscopic evaluation of the interface of the one-step self-etch adhesive Adper Prompt to dentin. (a) TEM
photomicrograph (non-demineralized, unstained section) of the adhesive-dentin interface. The hybrid layer is about 4 pm thick, the bonding resin
about 9 pm. (b) Higher magnification of (a). The relatively high electron density of resin resulted in a kind of negative staining of the electron
lucent collagen fibrils within the hybrid layer. Notice the abrupt transition of hybrid layer to dentin and the absence of hydroxyapatite crystals,
both characteristics of etch & rinse and 'strong' self-etch adhesives. (c) TEM photomicrograph (demineralized, stained section) of the
adhesive-dentin interface. The stain was strongly picked up by the phosphate-based resin. In contrast to (a), the hybrid layer is much thinner (±1.5 pm)
and no bonding layer is present. On top of the hybrid layer, also a thin electron dense line can be observed (arrow). Note also the extensive
tubule-wall hybridization (hand pointer). (d) TEM photomicrograph (demineralized, stained section) of the adhesive-dentin interface. Although not
recommended by the manufacturer, the adhesive had been strongly blown out after application. Consequently the bonding layer was not present
any more, although in some sections of the same sample still a ±10 pm thick bonding layer was observed. Hybridization however was not
hampered, as the adhesive was able to demineralize dentin for up to about 4.5 pm. Also no electron dense line on top of the hybrid layer (like in
c) can be detected. Due to the high stainability of the resin, the pathway of resin infiltration within the exposed collagen scaffold can be clearly
observed. Within an interfibrillar space, the collagen outer surface appears to be coated by a more dense line (arrows), which results in a kind of
negative staining (resin instead of collagen was stained) of the hybrid layer. Cross-banding, typical of type-I collagen can be observed (asterisk).
Ar = Adhesive resin; C = Composite; Ud = Unaffected dentin; Hy = Hybrid layer; IZ = intermediary zone between adhesive and composite; Rt —
Resin tag.
O
NE
-DAY
B
ON
DING
EFFE
CT
IVE
NESS
O
F CO
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
The major morphologic characteristics of the different types of self-etch-adhesives are summarized in Fig. 8. The application instructions provided with AdheSE included extensive rubbing of the primer. This rubbing motion loosened the collagen fibrils and must so have promoted resin infiltration and adaptation of the resin to the collagen (Fig. 5c). This rubbing motion morphologically resulted in a 'shag-carpet' appearance (Van Meerbeek etal., 2000).
The ultra-morphology of OptiBond Solo Plus Self-Etch (Fig. 6) is very similar to that of AdheSE, because of the similar acidity of both primers (Table 2). Therefore, this adhesive should also be regarded as an 'intermediary strong' self-etch adhesive. Besides the different chemical composition (Table 1), the main difference between both adhesives is the presence of filler particles in the bonding resin. These may help to stabilize the interface and to obtain an adequate thickness of the bonding layer (Fig. 5, 6) in light of reduction of interfacial stresses (Van Meerbeek et al., 1993, 1998; Perdiga6 et al., 1996; Ausiello et al., 2002). In contrast to AdheSE, the bonding resin of OptiBond Solo Plus Self-Etch is not solvent-free. This may induce some technique-sensitivity, as the solvent has to be properly removed by air drying.
AdheSE as well as OptiBond Solo Plus Self-Etch do not remove all hydroxyapatite at the bottom of the hybrid layer (Fig. 5, 6). Some adhesive monomers are known to interact chemically with hydroxyapatite, which may be beneficial to interfacial sealing (Van Meerbeek etal., 2001; Yoshida et al., 2003). For the monomers used in this study, however, no data are yet available. Chemical bonding in this part of the hybrid layer may however be very beneficial, especially considering that this area is more sensitive to nanoleakage (Sano etal., 1995).
When comparing the enamel bonding effectiveness of self-etch adhesives with standard etch & rinse adhesives, the etch & rinse approach remains the 'golden standard'. This is especially true in clinical dentistry because etching is relatively independent from smear layer properties and preparation methods (Van Meerbeek et al., 2003a). The etching pattern created by self-etch adhesives, on the other hand, is less uniform, dependent on the acidity of the primer (Pashley and Tay, 2001) and smear layer properties (Fig. 4). Etching aggressiveness is however not entirely correlated with bonding effectiveness, as some 'mild' and 'intermediary strong' etch adhesives do approach the etch & rinse standard, in contrast to the strong self-etch adhesive tested, which clearly underscored all other adhesives (Table 2). This
ONE-DAY BONDING EFFECTIVENESS OF CONTEMPORARY ADHESIVES
must probably be attributed to properties of the adhesive resin itself (Pashley and Tay, 2001).
When bonded to dentin, it is evident that especially two-step self-etch adhesives are able to compete with etch & rinse adhesives, not only in terms of early bonding effectiveness (Inoue etal., 2000, 2001a, Van Meerbeek et aí, 2003a), but also in terms of durability (Miyazaki et al., 1998; Sano et al., 1999; Nikaido et al.,
2002; Shirai et al., 2003). Taking into account the lower technique-sensivity (Van Meerbeek et al., 2001), the faster application procedure and the lower risk on nanoleakage (Sano et al., 1995), the two-step self-etch approach may become the future standard of adhesion.
Up to now, no clinical data are available for the experimental adhesives tested in this study. The predecessor of Adper Prompt, which is very alike in composition, however performed variably in clinical Class V studies (Brackkett et aí,
2002; Van Dijken, 2002). Whereas the control adhesives (OptiBond FL and Clearfil SE), both performed well in clinical trials (Boghosian 1996; De Munck et aí, 2003b; Peumans et al, 2003; Van Meerbeek et aí, 2003b). For the 'intermediary strong' self-etch adhesives, no data are available yet. Consequently, no predictions can be made regarding the clinical effectiveness of AdheSE and OptiBond Solo Plus Self-Etch.
CONCLUSION
Based upon their interfacial morphology and pH, self-etch adhesives can be subdivided into three classes: 'mild', 'intermediary strong' and 'strong' self-etch adhesives. The early bonding effectiveness of self-etch adhesives varies substantially, depending on the type of adhesive. In particular the 'strong' one-step self-etch adhesive tested seemed to be less effective. The bonding effectiveness of some two-step self-etch adhesives on the other hand was comparable to that of the control etch & rinse adhesive. More elaborate studies on the long-term bonding effectiveness and clinical trials remain necessary.
AN IN VITRO AND IN VIVO STUDY ON THE DURABILITY OF BIONIATERIAL-TOOTH BONDS
Fig. 8 Schematic overview of the interaction of different self-etch adhesives with
dentin (bar at the left represents approximately 5 pm). On the left, unaffected dentin is
represented with a typical smear layer and smear plug occluding a dentinal tubule. On the right, interaction of the three classes of 'self-etch' adhesives with this smear layer covered dentin is represented. The 'mild' self-etch adhesives do not completely remove the smear layer, but do form a submicron hybrid layer. Throughout the whole depth of the hybrid layer, residual hydroxyapatite remains attached to the exposed collagen fibrils and remains available for chemical interaction. The 'intermediary strong' self-etch adhesives dissolve the smear layer and plug, forming short (±10 pm) resin tags. Some residual hydroxyapatite can be found only in the bottom third of the hybrid layer. Also, limited lateral tubule wall hybridization can be observed. The 'strong' self-etch adhesives present with a morphology very alike that produced by etch & rinse adhesives, with a 3-5 pm thick hybrid layer, extensive resin tags, tubule-wall and lateral tubule-wall hybridization.
opaimemun ooelorgq!g
CHAPTER
2
AN IN VITRO AND IN VNOSTUDY ON THE DURABILITY OF BIOMATERIAL-TOOTH BONDS
2.1. Four-year water degradation of etch & rinse adhesives
bonded to dentin
INTRODUCTION
Most current etch & rinse adhesives perform well in bond strength tests, at least when tested shortly after application and under controlled in vitro conditions (Inoue
et aZ, 2001). However, the oral cavity — with temperature changes, chewing loads, and chemical attacks by acids and enzymes — forms a rather severe challenge for tooth-composite bonds to survive for a reasonably long time. Clinically, marginal deterioration of composite restorations remains problematic and forms the major factor that dramatically shortens the lifetime of adhesive restorations (Van Meerbeek
et al., 1998a). A factor known to degrade tooth-composite bonds is exposure to water (Gwinnett and Yu, 1995; Sano et al., 1999; Armstrong et aZ, 2001b). Among different forms of marginal leakage, nano-leakage, or the ingress of oral fluids through nanometer-sized channels along collagen fibrils within the hybrid layer, is considered very detrimental to bond integrity (Sano et al, 1995; Hashimoto et ai.,
2000, 2002). As part of an etch & rinse procedure, the rather aggressive phosphoric-acid-etching nearly completely deprives collagen of hydroxyapatite (Van Meerbeek et
aZ, 1998b). Consequently, adequate infiltration into, wetting of, and molecular interaction with hydroxyapatite depleted collagen by resin monomers is challenging. It may result in incomplete hybridization, leaving collagen unprotected and vulnerable to hydrolytic degeneration (Hashimoto et aZ, 2000). Other degradation promoting factors are, e.g., residual solvent of the adhesive or insufficiently removed surface water. Eventually, resin itself degrades over time and leaches out, causing the restoration-tooth bond to deteriorate (Santerre etal., 2001).
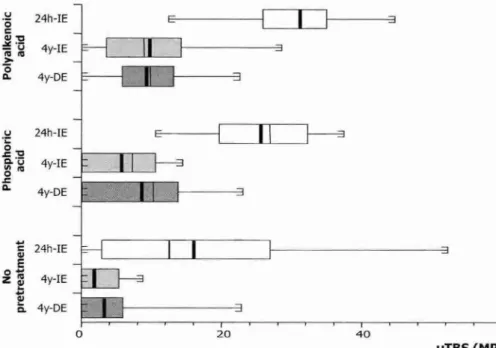
The objective of this laboratory study was to test the hypotheses that: (1) two-step etch & rinse adhesives resist water degradation as well as do three-step etch & rinse adhesives, and that (2) an adjacent composite-enamel bond protects the composite-dentin bond against degradation. Therefore, the micro-tensile bond strength (pTBS) to dentin of 2 three-step etch & rinse adhesives was compared with that of 2 two-step etch & rinse adhesives after 4 yrs of storage in water. Quantitative
*Based in part on the paper: 'De Munck 3, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G (2003). Four-year water degradation of total-etch adhesives bonded to dentin.> Dent Res 82:136-140'.