Cecília da Silva Cristelo
Exploring the molecular mechanisms
involved in macrophage’s response to the
antimicrobial peptide LLKKK18
Tese de Mestrado
Mestrado em Genética Molecular
Trabalho efetuado sob a orientação de
Professor Doutor Francisco Miguel Portela da Gama
Departamento de Engenharia Biológica, Universidade do Minhoe co-orientação de
Professora Doutora Olga Maria Pereira Coutinho
DECLARAÇÃO
Nome: Cecília da Silva Cristelo
Endereço eletrónico: ceciliacristelo3@gmail.com Título da dissertação:
Exploring the molecular mechanisms involved in macrophage’s response to the antimicrobial peptide LLKKK18
Orientador(es):
Francisco Miguel Portela da Gama Olga Maria Fernandes Pereira Coutinho
Ano de conclusão: 2018
Designação do Mestrado: Mestrado em Genética Molecular
É AUTORIZADA A REPRODUÇÃO INTEGRAL DESTA DISSERTAÇÃO APENAS PARA EFEITOS DE INVESTIGAÇÃO, MEDIANTE DECLARAÇÃO ESCRITA DO INTERESSADO, QUE A TAL SE COMPROMETE;
Universidade do Minho, ___/___/______ Assinatura:
A
GRADECIMENTOSGostaria de expressar os meus sinceros agradecimentos a todas as pessoas e instituições que de alguma forma contribuíram para a realização desta Tese de Mestrado.
Ao Professor Miguel, pela oportunidade que me deu de realizar este projeto e de trabalhar no seu laboratório, e pela experiência que me possibilitou adquirir. Agradeço ainda pela sua disponibilidade e pelo seu investimento e dedicação a este projeto, pelos seus conselhos e pela sua compreensão mesmo quando os resultados não eram os mais favoráveis e pela perseverança e motivação que me transmitiu.
À Professora Olga, pelo acompanhamento, já desde muito antes desta Tese de Mestrado e sem a qual esta parceria não teria ocorrido. Agradeço-lhe por toda a dedicação, todo o incentivo que sempre me transmitiu e por ser uma fonte de motivação, que foi muito importante para conseguir atingir este objetivo. Obrigada ainda por todas as horas dispensadas a ler e reler a tese, por todas as sugestões e por estar sempre disponível para me fornecer tudo aquilo que precisasse, pela sua sabedoria, que sempre me ajudou a tentar organizar-me e a desenvolver o meu pensamento crítico.
Ao Doutor João Pedro Silva e Professor Rui Appelberg pela colaboração neste projeto e por todas as sugestões, e disponibilidade em ajudar-me a desenvolver estratégias para a realização deste trabalho. A todos os membros grupo LTEB, dos quais vou sentir imensas saudades, sinto muito orgulho por ter feito parte de um grupo de trabalho tão excelente. Agradeço a todos pela companhia, pelos conselhos, pela ajuda e pela motivação. Em especial gostaria de agradecer ao Ricardo pela supervisão, pela ajuda, pela paciência, dedicação e por todos os conhecimentos que me transmitiu. Agradeço ainda por sempre ter acompanhado o meu trabalho e por além de “chefinho” ser um grande amigo, imprescindível para a realização desta Tese.
Às minhas amigas por sempre me terem motivado e animado ao longo deste ano e que sempre me incentivaram. Ao João, por ter estado sempre do meu lado, por ter sido o melhor ouvinte e conselheiro e por me motivar e me ajudar a enfrentar todas as dificuldades com uma melhor atitude.
E por último, mas não menos importante, aos meus pais que sempre me apoiaram e me deram o ponto de partida para poder atingir os meus objetivos.
A
BSTRACTTuberculosis (TB) is an infectious disease that represents a serious health problem worldwide. This condition is caused mainly by Mycobacterium tuberculosis, a bacterium that can manage to evade the immune response, surviving inside macrophages. Globally, TB incidence, prevalence and mortality are decreasing, however, drug resistance to the current treatments makes TB eradication progressively challenging. In this perspective, antimicrobial peptides, molecules used by the innate immune system to combat invading pathogens including mycobacteria, are promising candidates for TB treatment. A cathelicidin derivative peptide, LLKKK18, has recently become of great interest due to its strong antimicrobial activity and high efficiency in mycobacterial killing, which make LLKKK18 a promising alternative for TB treatment. Association of antimicrobial peptides to an intracellular drug delivery system can help to overcome the cytotoxic effects that limit the use of this peptide, while improving the delivery to target infected cells. Hyaluronic acid (HA)-based nanogels arise in this field as a suitable delivery system for LLKKK18, due to its biocompatibility and favorable internalization by cells over-expressing the CD44 receptor, such as activated macrophages. This strategy was previously used and allowed a significant reduction in mycobacteria burden in infected macrophages (Silva et al., 2017). In this project, we expect to further explore the macrophage’s response to this antimicrobial peptide, to unravel other action mechanisms that might benefit mycobacteria elimination. We aim to obtain information on the peptide effects on macrophage metabolism, phenotype and immune response to different bacterial components gaining further data on the LLKKK18 by itself or loaded in the nanogel in TB treatment.
We efficiently synthesized an amphiphilic HA nanogel which allowed LLKKK18-encapsulation and internalization by macrophages. However, cytotoxicity assays revealed that the peptide-loaded nanogels had an unexpected effect causing loss of cell viability, progressively with the increased peptide concentration, in all macrophage cell models used. Cytokine release in response to different Toll-like receptor (TLR) ligands was not significantly affected by the LLKKK18-loaded nanogels and was probably affected by some of the formulation’s cytotoxic effects. When using a lower concentration of free LLKKK18 peptide, we observed a pro-inflammatory effect, enhancing predominantly the TLR4-mediated response. This suggests that in addition to LLKKK18 bactericidal activity, its pro-inflammatory effects might benefit macrophage response against mycobacteria. Further study on this subject is needed to understand the peptide’s action mechanisms and its applicability in TB treatment.
R
ESUMOA tuberculose (TB) é uma doença infeciosa que representa um grave problema de saúde a nível mundial. Esta doença é causada maioritariamente por Mycobacterium tuberculosis, uma bactéria que consegue escapar à resposta imunitária e sobreviver no interior de macrófagos. Globalmente, a incidência, prevalência e a mortalidade associadas à TB estão em decréscimo, no entanto, a resistência aos tratamentos atualmente utilizados torna a erradicação da TB progressivamente desafiante. Nesta perspetiva, os péptidos antimicrobianos, que são moléculas utilizadas pelo sistema imunitário inato para combater agentes patogénicos, incluindo as micobactérias, são candidatos promissores para o tratamento da TB. Recentemente, um péptido derivado da catelicidina, LLKKK18, tornou-se de elevado interesse devido à sua forte atividade antimicrobiana e alta eficiência na eliminação de micobactérias, tornando-o uma alternativa promissora no tratamento da TB. Por outro lado, o desenvolvimento de um sistema de entrega intracelular destes péptidos pode permitir superar os efeitos citotóxicos que limitam o seu uso e melhorar a sua a distribuição para as células infetadas. Nesta perspetiva, o desenvolvimento de nanogéis à base de ácido hialurónico (do inglês HA) surgem como um sistema de entrega intracelular adequado para o péptido LLKKK18, devido à sua biocompatibilidade e internalização preferencial por células que sobre-expressam o recetor CD44, como é o caso dos macrófagos ativados. Esta estratégia foi anteriormente utilizada e permitiu uma redução significativa do número de micobactérias em macrófagos infetados (Silva et al., 2017). Neste projeto, ambicionamos explorar a resposta dos macrófagos a este péptido antimicrobiano, para desvendar outros mecanismos de ação que possam beneficiar a eliminação de micobactérias. Pretendemos obter informações sobre os efeitos do péptido no metabolismo dos macrófagos, no fenótipo e na resposta imunitária a diferentes componentes bacterianos, e assim obter informação mais detalhada acerca da aplicabilidade do péptido LLKKK18, por si só ou encapsulado num nanogel, no tratamento da tuberculose.
Foi-nos possível sintetizar eficientemente um nanogel de HA anfifílico que permitiu a encapsulação de LLKKK18 e a internalização por macrófagos. No entanto, os ensaios de citotoxicidade revelaram que os nanogéis carregados com o péptido tiveram um efeito inesperado causando perda de viabilidade celular, progressivamente com o aumento da concentração de péptido, em todos os modelos celulares de macrófagos utilizados. A libertação de citocinas em resposta a diferentes agonistas dos Toll-like Receptor (TLR) não foi significativamente afetada pelos nanogéis carregados com LLKKK18 e foi provavelmente influenciada por efeitos citotóxicos da formulação. Ao utilizar uma menor concentração
LLKKK18, o seu efeito pró-inflamatório pode beneficiar a resposta dos macrófagos contra micobactérias. É necessário aprofundar a investigação neste âmbito de forma a que se compreendam os mecanismos de ação do péptido e sua aplicabilidade no tratamento da tuberculose.
P
ALAVRAS-
CHAVE:
Tuberculose, macrófagos, péptidos antimicrobianos, nanogéis, ácido hialurónico, LLKKK18, recetores Toll-like, citocinas.Contents
Agradecimentos ... iii Abstract... v Resumo... vii List of Abbreviations ... xi 1. Introduction ... 1 1.1 Tuberculosis ... 11.2 Innate Immune response to Mycobacterium tuberculosis ... 2
1.2.1 Macrophage role in TB ... 3
1.2.2 Toll Like Receptors - TLRs... 5
1.2.3 TLR signaling ... 6
1.2.4 M. tuberculosis recognition by TLRs ... 7
1.3 Antimicrobial peptides ... 9
1.3.1 LL-37 structure, expression and activities ... 10
1.3.2 LL-37 role in TB control ... 11
1.3.3 LLKKK18, a LL37 derivative peptide ... 12
1.4 Drug delivery systems ... 14
1.4.1 CD44 receptor: a target for drug delivery ... 15
1.4.2 HA self-assembling nanogels for TB therapy ... 16
2. Rational and Aims ... 19
3. Materials and Methods ... 21
3.1 Materials ... 21
3.2 Methods ... 21
3.2.1 HA-based nanogel synthesis ... 21
3.2.2 LLKKK18 encapsulation in the HA-Hexa nanogel ... 24
3.2.3 Nanogel characterization ... 25
3.2.4 Cell culture conditions ... 25
3.2.7 Evaluation of the effects of LLKKK18-loaded nanogels on macrophage’s immune response 30
3.2.8 Effects from LLKKK18 on macrophage’s response to inflammatory stimuli ... 32
3.2.9 SRB colorimetric assay ... 33
4. Results ... 35
4.1 Hyaluronic acid nanogel synthesis ... 35
4.2 LLKKK18 encapsulation and Nanogel characterization... 36
4.3 Viability assays ... 41
4.4 Nanogel internalization by cells ... 45
4.5 Evaluation of the effects of LLKKK18-loaded nanogels on macrophage’s immune response 46 4.6 Effects from LLKKK18 on macrophage’s response to inflammatory stimuli ... 48
5. Discussion ... 53
6. Conclusions and Future prospects ... 63
Bibliography ... 65
L
IST OFA
BBREVIATIONSAMP – Antimicrobial peptide APC – Antigen presenting cell
AT – 11-amino-1-undecanethiol hydrochloride BCG – Bacillus Calmette-Guérin
BMMΦ – Bone marrow derived macrophages CAMP – Cathelicidin Antimicrobial peptide CpG – cytosine-phospate-guanine
CSF-1 – Colony stimulating factor 1
DAMP – Danger-associated molecular pattern DLS – Dynamic light scattering
DMSO – Dimethyl sulfoxide DS – Degree of substitution
EDC – 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride EE – Encapsulation efficiency
ELISA – Enzyme-linked immunosorbent assay HA – Hyaluronic acid
hCAP18 – Human cationic antimicrobial peptide-18 Hexa – Hexadecylamine
HIV – Human immunodeficiency virus HRP – Horseradish Peroxidase IFN-γ – Interferon-γ
IL – Interleukin
IRAK – IL-1 receptor-associated kinase IRF – Interferon-regulatory factor LAM – Lipoarabinomannan LM – Lipomannan
LPS – Lipopolysaccharides
MAPK – Mitogen-activated protein kinase M-CSF – Macrophage colony-stimulating factor
MHC – Major histocompatibility complex
MTT – 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MyD88 – Myeloid differentiation factor 88
NF-κB – Nuclear factor-κB NHS – N-hydroxysuccinimide
PAMP – Pathogen-associated molecular pattern PBS – Phosphate buffered saline
PdI – Polydispersity Index
PMA – Phorbol 12-myristate 13-acetate PRR – Pattern recognition receptor ROS – Reactive oxygen species SRB – Sulforhodamine B
TACO – Tryptophan aspartate-containing coat protein (TACO) TB – Tuberculosis
TBA – Tetrabutylammonium
TBA-F – Tetrabutylammonium fluoride TCA – Trichloroacetic
TIR – Toll/IL-1 receptor TLR – Toll-like receptor
1. I
NTRODUCTION1.1 Tuberculosis
Tuberculosis (TB) represents a global health problem, being one of the leading causes of death worldwide. It is an infectious disease that can be easily spread when an individual with active TB coughs and expels bacteria into the air, reaching other individuals. It is estimated that 2-3 billion people in the world are infected, but only 5-15 % will develop the disease during their lifetime (WHO, 2016). In most cases, the immune system can control the infection; however, in immunocompromised states resulting from age, malnutrition, and co-infection with human immunodeficiency virus (HIV), TB can be reactivated (Semba et al., 2010).
Globally, TB incidence, prevalence and mortality are decreasing, however not at the rate that was established by the World Health Organization’s End TB Strategy, set for 2020 (WHO, 2016). The unequal access to TB diagnosis and treatments in different countries makes its eradication very difficult. In addition, drug resistance to the current treatments makes TB eradication progressively challenging (Gutsmann, 2016).
Predominantly, TB is caused by Mycobacterium tuberculosis bacteria. In addition to M. tuberculosis, other closely related bacteria can cause TB, in both humans and animals. Sharing more than 99 % homology in some loci, these bacteria are grouped as the Mycobacterium tuberculosis complex (Forrellad et al., 2013). Mycobacterium genus is comprised of Gram-positive, nonspore forming and aerobic bacteria. Bacteria in this group can grow at different rates, which gives rise to different pathogenicity. Slow-growing species, such as M. tuberculosis, are generally pathogenic and cause problematic diseases; on the other hand, fast-growing species are normally non-pathogenic and opportunistic, only causing TB in immunocompromised hosts (Forrellad et al., 2013).
Spreading of TB and associated mortality is controlled by prevention and treatment of the infected individuals. Prevention of the disease is performed through vaccination, to obtain a faster and efficient immune response upon contact with the actual pathogen. Bacillus Calmette-Guérin (BCG) is the only available vaccine against TB. It was produced in 1921 from a virulent strain of M. bovis, that after several in vitro passages was attenuated and began to be administrated massively from 1930 until today (Andersen and Doherty, 2005). This vaccine has a high efficiency in children, but lower in adults already infected or sensitized to mycobacteria (from 0-80 %), an effect that is not well understood. Even though
was yet approved. Thus, in addition to prevention, there is also a strong effort to develop new treatments for TB (Rivas-Santiago et al., 2013).
The most effective drugs against M. tuberculosis are isoniazid, pyrazinamide and rifampicin (Nuermberger et al., 2010). The efficacy of the treatment requires a long duration therapy to kill all mycobacteria. The prolonged treatment, and associated adverse effects, contributes to the poor adherence of the patients to the recommended treatment regimens. Such problem does not only decreases cure rates, but also the inadequate use of antibiotics may be associated with the emergence of multidrug-resistant and extensively-drug resistant strains of M. tuberculosis (Sarkar and Suresh, 2011). The elimination of these bacteria is far more difficult and requires the use of even more toxic drugs, that are not always efficient, as M. tuberculosis can also acquire resistance to these drugs (Nuermberger et al., 2010).
In the last years, antibiotic research has not been as successful as expected, with only one novel drug being approved in the past 40 years (Osborne, 2013). In this panorama, the use of new strategies and molecules with different killing mechanisms is urgently needed to fight this health problem. One interesting strategy is to explore natural microbicidal factors used by the innate immune system to combat mycobacteria, and introduce these factors in therapies. Therefore, a better understanding of the interaction between the immune system and mycobacteria is essential for the control of this disease and to develop new strategies for TB treatment.
1.2 Innate Immune response to
Mycobacterium tuberculosis
The innate immune system is the first line of defense against invading pathogens, allowing a fast recognition of microbial components and eliciting an antimicrobial response (Sonawane et al., 2011). Infection can occur in different organs, but the lung is where these mycobacteria establish commonly, causing pulmonary TB (Kaufmann, 2004).
M. tuberculosis, present in droplets in the air, can sometimes overcome the physical barriers in the airways and reach the lungs where they settle in the alveoli. There, mycobacteria interact with alveolar macrophages that recognize and engulf these bacteria and are key players in TB immune response (Weiss and Schaible, 2015).
1.2.1 Macrophage role in TB
Macrophages are phagocytic cells responsible for the recognition and ingestion of invading pathogens, being the central cells involved in TB response and outcome, since they serve as a major cell niche for the growth and survival of M. tuberculosis (Guirado et al., 2013).
The local environment where macrophages reside, including the tissue and cells in contact, microbial products or the cytokines and growth factors they are exposed to, have a great influence on macrophage phenotype (Murray et al., 2014). Macrophage phenotype is also dynamic, changing in accordance with its microenvironment (Stout and Suttles, 2004). Although different nomenclatures have been previously used in attempt to divide macrophage phenotypes into different groups (Biswas and Mantovani, 2010; Mills, 2012), it is becoming increasingly apparent that macrophage phenotypes can be more accurately represented by a broad spectrum, with pro- and anti-inflammatory activities as opposing limits (Murray et al., 2014). Macrophage activation through different stimuli can result in a vast range of activities, changes in cell metabolism, cytokine production and gene expression, and different microbicidal activity, which result in different macrophage ability to deal with infection (Murray et al., 2014). Thus, macrophage phenotype can have significant impact on TB control.
The alveolar macrophage has a unique phenotype characterized by the high expression of membrane receptors involved in microbial recognition. These cells have effective phagocytic activity, yet a poor bactericidal activity and suppressive of T cell activation, which prevents excessive inflammation from damaging the thin lining of alveoli (Hussell and Bell, 2014). These tightly regulated activities of alveolar macrophages can be exploited by host-adapted intracellular pathogens like M. tuberculosis, to enhance their survival and persistence (Rajaram et al., 2010).
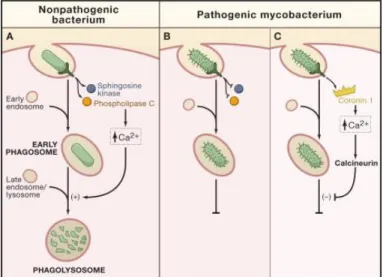
Alveolar macrophages recognize invading pathogens in the lung, in this case mycobacteria, and rapidly engulf them by phagocytosis. Maturation of the bacteria-containing phagosomes involves sequential fusion of vesicles from the endocytic pathway, with increasing microbicidal properties, due to the progressively lower pH and depletion of nutrients essential for mycobacterial survival (Pieters, 2008). Finally, fusion with lysosome should occur as it contains a large range of hydrolases, antimicrobial peptides, and generates a lower pH, creating an adverse environment that ultimately kills pathogens (Figure 1A; Flannagan et al., 2012). However, M. tuberculosis can interfere with this process, inhibiting its elimination. Mycobacterial products, present in the cell wall like lipoarabinomannan (LAM), can interfere with host glycolipids and proteins and impair the phagosome-lysosome fusion (Figure 1B; Welin et al., 2008). Mycobacteria-containing phagosomes have also been characterized by the presence of
coronin 1), which inhibits the phagolysosome formation, allowing bacterial permanence inside the phagosome (Figure 1C; Ferrari et al., 1999; Jayachandran et al., 2007). In consequence, only a mild acidification occurs in the vacuole, and mycobacteria are not effectively eliminated (Sturgill-Koszycki et al., 1994). M. tuberculosis-infected cells also have reduced expression of the major histocompatibility complex (MHC) class II, and consequently reduced antigen presentation. This leads to reduced identification of infected macrophages by T helper cells (Noss et al., 2001; Pai et al., 2004), allowing its survival in the phagosome.
Macrophage recruiting and activation in the following days leads to their accumulation around the infection site, forming a granuloma, a hallmark of TB (Cosma et al., 2003; Mahamed et al., 2017). Granulomas have a defined anatomical structure, intended to imprison mycobacteria, preventing its dissemination, and focusing the immune response to a limited area (Cardoso et al., 2015). Available oxygen in the core decreases with the increased size of the granuloma. The consequent hypoxia and nutrient deprivation may result in necrosis of the central cells and release of lytic enzymes that cause further cell damage (Cardoso et al., 2015). This process results in a caseous center with extracellular mycobacteria that can reach the airways and be transmitted to other individuals while coughing (Owen et al., 2013).
Figure 1. Immune evasion strategies from pathogenic mycobacteria. Recognition and internalization of non-pathogenic bacterium leads to activation of signal transduction enzymes which increase the levels calcium ions inducing the fusion of the early phagosome with late endosomes/lysosomes resulting in phagolysosomes and destroying the bacterium (A). Pathogenic bacteria, like M. tuberculosis, can interfere with this process, since bacterial components can inhibit activation of signal transduction enzymes, preventing the elevation of calcium levels, and thereby arresting maturation at the early phagosome stage (B). Another model, by Jayachandran et al. (2007), suggests that Coronin 1 is recruited to phagosomes containing mycobacteria, which causes a sustained increase in calcium levels. This activates calcineurin which prevents phagosome maturation, allowing mycobacteria to persist (Trimble and Grinstein, 2007).
From M. tuberculosis infection to onset of the disease, mycobacteria recognition by immune cells is the first step in the process of pathogen elimination. Immune cells, including macrophages, contain different receptors that allow them to recognize and act against pathogens. These receptors are termed pattern recognition receptors (PRR), that can recognize pathogen-associated molecular patterns (PAMPs), present in microorganisms, and danger associated molecular patterns (DAMPs) resulting from cell damage. From the different types of PRRs, Toll-like receptors (TLRs) were the first to be identified and are the most extensively characterized, being fundamental for M. tuberculosis recognition and immune response at the onset of infection (Kawai and Akira, 2010).
1.2.2 Toll Like Receptors - TLRs
TLRs enable the recognition of different PAMPs, being activated by a variety of mycobacterial components (Kawai and Akira, 2010). These receptors can facilitate the transcription of genes involved in the immune response but can also participate in M. tuberculosis evasion strategies (Saraav et al., 2014). Thus, it is crucial to understand the molecular mechanisms involved in M. tuberculosis recognition by TLRs and consequent immune system activities for infection’s resolution.
In the TLR family, 10 members have been identified in humans (TLR1-TLR10) and 12 members in mice (TLR1-TLR9, TLR11-TLR13). These receptors are normally expressed in tissues associated with immune response, as spleen, peripheral blood leucocytes including mast cells, macrophages and dendritic cells, and in tissues in contact with external environment such as the lungs and gastrointestinal tract (Vu et al., 2016). TLRs have a strategic localization in the cell membrane of antigen presenting cells (APCs), and in intracellular compartments like endocytic vesicles and lysosome (Kawasaki and Kawai, 2014). Regardless of its lower specificity, these receptors can distinguish a large range of molecules associated with pathogens such as lipids, proteins, lipoproteins and nucleic acids (Kawai and Akira, 2010).
TLRs can be divided into two main sub-families, depending on its intracellular or cell surface localization. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10, are cell surface TLRs, whereas TLR3, TLR7, TLR8, TLR9, TLR11, TLR12, and TLR13 are localized in the membrane of intracellular compartments (Kawai and Akira, 2010). The specific localization of each TLR is associated with the different occurrence of its microbial ligands and simultaneously allows self- or non-self-discrimination (Iwasaki and Medzhitov, 2004). Cell-surface localized TLRs are responsible for recognition of characteristic microbial membrane
hand, intracellular TLRs, can recognize nucleic acids, from internalized bacteria or viruses, nevertheless these molecules are not unique to the microbial world. The intracellular localization of TLRs prevents recognition of self-nucleic acids while favoring recognition of internalized viruses and bacteria after microbial lysis occurs in the late-endosome-lysosome (Iwasaki and Medzhitov, 2004).
1.2.3 TLR signaling
TLRs are membrane spanning glycoproteins composed of: an ectodomain responsible for PAMP recognition; a transmembrane domain that anchors the receptor; and a conserved cytoplasmic domain that contains a Toll/IL-1 receptor (TIR) responsible for signal transduction initiation. Signal transduction allows consequent activation of signaling pathways that result in alteration of the cell metabolism and/or gene expression, needed to eliminate a particular pathogen (Kawasaki and Kawai, 2014).
Upon ligand recognition, a common adaptor protein involved in signal transduction that is recruited to the TIR domain of all TLR, with exception of TLR3, is the myeloid differentiation factor 88 (MyD88) (Figure 2; Kawai and Akira, 2011). MyD88 is a crucial protein that is involved in the initiation of inflammatory cytokines production, mainly Tumor necrosis factor alpha (TNF-α) and interleukin (IL) 6 (Feng et al., 2003), which have a fundamental role in resistance against TB (Fremond et al., 2004).
After signal transduction initiation, a series of IL-1 receptor-associated kinases (IRAKs) associate with MyD88, resulting in a complex, that can consequently activate transcription factors including the nuclear factor-kB (NF-κB), interferon-regulatory factors (IRFs) or mitogen-activated protein kinases (MAPKs) (Figure 2; Picard et al., 2003). These factors regulate the expression of inflammatory cytokines such as IL-1β, IL-6, IL-8, IL-12, TNF-α, chemokines, and co-stimulatory molecules to protect the host against microbial infection (Jones et al., 2001; Kawasaki and Kawai, 2014).
Cytokines produced by activated cells are then detected by T helper lymphocyteswhich secrete interferon-γ (IFN-γ), particularly important in TB (Harding and Boom, 2010). IFN-γ is responsible for upregulation of the MHC II on these cells resulting in enhanced antigen presentation and higher ability to eliminate the pathogen (Sharma et al., 2007).
1.2.4 M. tuberculosis recognition by TLRs
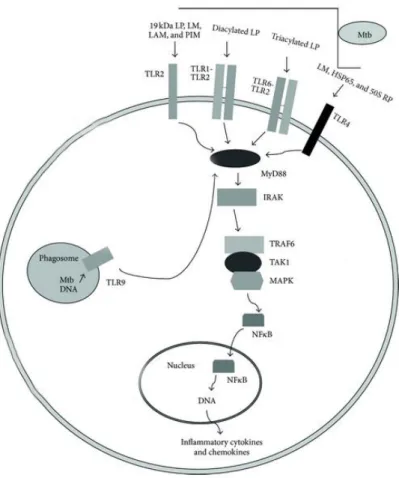
Macrophages can recognize several components of M. tuberculosis through the TLRs, initiating the immune response against infection. The TLRs involved on recognition of this pathogen are TLR2 in association with TLR1 or TLR6, TLR4 and TLR9 (Figure 2; Jo et al., 2007).
TLR2 is the most investigated TLR in mycobacterial infections (Vu et al., 2016). In association with TLR1 or TLR6, TLR2 recognizes a wide range of PAMPs of different pathogens, including lipoproteins/lipopeptides, glycolipids, peptidoglycan and zymosan (Kawai and Akira, 2010). Mycobacteria agonists that can elicit a TLR2-associated response include cell wall glycolipids like lipoarabinomannan (LAM) and lipomannan (LM), 38-kDa and 19-kDa mycobacterial lipoproteins, and diacylated or triacylated lipoproteins (Figure 2; Bulut et al., 2005; Jung et al., 2006; Pitarque et al., 2008). Activation of TLR2 results in production of TNF-α, a vital cytokine for containment and resistance to mycobacterial infections (Roach et al., 2002; Tjarnlund et al., 2006), and in the augmented expression of the vitamin D receptor and vitamin D hydroxylase genes, which increases the expression of antimicrobial peptides, key players in TB control (Liu et al., 2006).
TLR4 is well characterized by the ability to recognize lipopolysaccharides (LPS), a structural component of Gram-negative bacteria (Chow et al., 1999). TLR4 can also recognize mycobacterial endogenous heat shock proteins (HSP) (Figure 2; Bulut et al., 2005; Ohashi et al., 2000). It has been demonstrated that TLR4 is essential in innate immunity against M. tuberculosis, since TLR4 mutant mice infected with these bacteria were more susceptible to TB (Abel et al., 2002).
Regarding TLR9, an intracellular receptor present in endosomal membranes, has demonstrated ability to recognize unmetilated cytosine-phosphate-guanine (CpG) dinucleotide sites mostly present in DNA from bacterial, and viral genomes (Figure 2; Vu et al., 2016). Mammalian DNA shows reduced frequency of these nucleotides, and these are mostly methylated, decreasing the possibility of self-DNA recognition (Vu et al., 2016). TLR9 has a significant role in M. tuberculosis recognition, being demonstrated that TLR9 and TLR2 cooperate for optimal resistance to M. tuberculosis infection (Bafica et al., 2005). Deficiency of both TLRs leads to higher susceptibility to mycobacterial infection than the single deficiency in each TLR2 or TLR9 (Bafica et al., 2005). It was also shown that TLR9 function is not directly connected to production of pro-inflammatory cytokines but is critical for the control of M. avium infection in mice (Carvalho et al., 2011).
Figure 2. M. tuberculosis (Mtb) recognition by TLRs, in macrophages. Several mycobacterial ligands, glycolipids and lipoproteins present in mycobacteria cell wall can be recognized by TLR2 alone or in association with TLR1 or TLR6. TLR4 can also recognize HSP from Mtb and TLR9 can identify its DNA. After TLR activation, signal transduction via MyD88 is a key step in TLR-mediated defense. The downstream signaling leads to activation of the transcription factor NF-κB, which results in increased
expression of inflammatory cytokines and chemokines that activate intracellular mechanisms which help to eliminate Mtb. Adapted from Hossain and Norazmi (2013).
These data suggest that M. tuberculosis recognition by the immune system depends on the cooperation of different TLRs to orchestrate an appropriate immune response (Tjarnlund et al., 2006). The events that follow TLR activation are responsible for the effector mechanisms of macrophages against this pathogen. During this process, several molecules of the innate immune system are involved, including antimicrobial peptides (AMPs). These peptides are normally expressed in response to pathogens, after TLR activation, being key players in the M. tuberculosis immune response and control (Sonawane et al., 2011). For this reason, antimicrobial peptides are being explored as novel therapeutics in TB treatment.
1.3 Antimicrobial peptides
Antimicrobial peptides (AMPs) are important components of the innate immune system. Ubiquitous in nature, these peptides have been found in various organisms, including bacteria, invertebrates, vertebrates and plants (Zhao et al., 2013). AMPs can have direct antimicrobial and modulating functions against different pathogens, from viruses to bacteria and fungi.
Most AMPs maintain a few common and characteristic features: a small size, from 15 to 50 amino acid residues; a positive charge, due to the abundance of lysine and arginine residues, and also hydrophobic residues that can contribute to more than 50 % of the amino acid composition (Powers and Hancock, 2003). The antimicrobial activity of these peptides relies on this specific molecular composition. The presence of hydrophilic and hydrophobic regions, and the overall positive charge, allows this amphipathic molecule to interact with the polyanionic cell membrane of most microorganisms, disrupting the lipid bilayer and triggering bacterial death (Bi et al., 2013).
AMPs are becoming of great interest, due to the broad microbicidal activity against Gram-positive and -negative bacteria, fungi, protozoa, and importantly against mycobacteria (Silva et al., 2016; Sonawane et al., 2011), showing increasing potential as antituberculosis agents. These peptides can interfere with various biological processes in different microbes, and through different mechanisms, with a modest potency – this reduces the selective pressure and decreases the possibility of resistance development, increasing its potential for therapeutic application (Hale and Hancock, 2007). However, it should be noted that some resistance mechanisms to AMPs have been described for a few human pathogens, that may include the modification of the cell membrane, reducing its negative charge (Nizet, 2006). AMPs have also regulatory functions, working as signaling molecules between immune cells with the ability to modulate the immune response (Teng et al., 2015).
Humans synthesize two main families of AMPs - defensins and a single cathelicidin. Differing from the multitude of defensins synthesized, only one cathelicidin AMP (CAMP) gene has been found in humans until now, that ultimately originates the antimicrobial peptide LL-37 (Vandamme et al., 2012). The increasing attention over LL-37 lies on its ability to kill hard-to-eliminate pathogens such as mycobacteria, being also effective against MDR strains (Z. Jiang et al., 2011), increasing its potential introduction in several therapies.
1.3.1 LL-37 structure, expression and activities
LL-37 is produced by various leucocytes and helps to combat different infectious agents. This peptide is the result of the expression of the CAMP gene, that originates a prepeptide termed human cationic antimicrobial peptide-18 (hCAP18). Only upon secretion into phagosomes or extracellularly, through cleavage by proteinase-3, the active and functional LL-37 is obtained (Dürr et al., 2006; Sorensen et al., 2001). The peptide LL-37 is composed of 37 amino acids, containing two leading leucine in the N-terminus, which gives rise to its name. This molecule displays an α-helix secondary structure, similar to other cathelicidin-derived AMPs found in closely related species (Dürr et al., 2006).
Various cell types produce LL-37, mainly neutrophils, monocytes, natural killer cells, mast cells and B cells, but also epithelial cells, keratinocytes and macrophages (Hancock et al., 2016). In addition, LL-37 is present in several body fluids including breast milk, sweat, saliva, wounds and seminal plasma, among others (Dürr et al., 2006). The presence of this peptide seems to be particularly important in the lung, where its concentration in airway fluids can reach several fold higher concentrations than other body fluids (Sorensen et al., 1997).
Regulation of LL-37 concentration is a dynamic process and can vary greatly depending on different factors. Infection significantly increases LL-37 concentration, promoting pathogen elimination through different activities. Expression of LL-37 can also depend on the production of inflammatory cytokines, growth factors, nutrients and bacterial products (Ramos et al., 2011).
LL-37 is a very versatile peptide with a broad range of activities, extending from Gram-negative to Gram-positive bacteria, fungi and viruses. Like most AMPs, LL-37 can interact with the cell membrane of microorganisms causing its disruption, being also able to permeate the bacterial membrane, inhibiting the synthesis of cell wall components, or binding DNA and RNA by electrostatic interactions, affecting vital processes for bacterial survival and growth (Hale and Hancock, 2007).
Apart from this antimicrobial activity, LL-37 has also been associated to significant immunomodulatory activity, playing a central role in innate immunity. Depending on the cell type and on the inflammatory stimulus, LL-37 can have pro- or anti-inflammatory effects (Hancock et al. 2016). LL-37 is a potent chemotactic to human leucocytes, namely monocytes, mast-cells, T-lymphocytes and neutrophils, contributing to host defense against invading pathogens (Ramos et al., 2011). Despite increasing the lifespan of neutrophils by inhibiting spontaneous apoptosis (Nagaoka et al., 2012), LL-37 can stimulate apoptosis in regulatory T-cells (Tregs), inhibiting their suppressor activity (Mader et al., 2011), overall promoting the inflammatory response. Controversially, LL-37 can suppress the TLR4-mediated response in mice macrophages (Brown et al., 2011), and inhibit the TLR2-, TLR4- and
TLR9-mediated response in human monocytes, suppressing the release of pro-inflammatory cytokines (Mookherjee et al., 2006). Although this effect is controversial and somehow contradictory, this is a very essential property, that can inhibit excessive and harmful inflammation (Afacan et al., 2012).
Thus, LL-37 is an antimicrobial peptide, that apart from its modest bactericidal activity, reveals an increasingly significant immunomodulatory effect. Its ability to interfere with gene expression, cell metabolism and inflammatory state, needs to be explored.
1.3.2 LL-37 role in TB control
LL-37 involvement in TB immune response has been extensively reported, from the early stages of infection until the advanced progressive disease (Castañeda-Delgado et al., 2010; Liu et al., 2006; Torres-Juarez et al., 2015). During early infection, LL-37 has an important antimicrobial role, since it contributes to the control of mycobacterial growth. When mycobacteria are not eradicated, and progressive inflammation is established, LL-37 has an immunomodulatory role by protecting the host from excessive inflammation (Castañeda-Delgado et al., 2010).
LL-37 expression is also increased when immune cells are exposed to M. tuberculosis. Interaction of a 19kDa lipopeptide from M. tuberculosis with TLR2 in human leucocytes results in the upregulation of vitamin D receptor and the enzyme 25-Hydroxyvitamin D3 1-alpha-hydroxylase, which catalyzes the conversion of the inactive provitamin D3 hormone into its active form (1,25-dihydroxyvitamin D3). This process induces the expression of the cathelicidin gene and the killing of intracellular M. tuberculosis (Liu et al., 2006), establishing LL-37 as a main modulator of mycobacterial killing (Sonawane et al., 2011).
For these reasons, several studies have addressed the ability of LL-37 to inhibit mycobacteria growth and control TB (Rivas-Santiago et al., 2013; Santos et al., 2014; Sonawane et al., 2011). LL-37 involvement in TB treatment was explored in a clinical trial for adults with active TB, using two synergistic inducers of LL-37 expression, 4-phenylbutyrate and 1,25-dihydroxyvitamin D3 (Mily et al., 2015).
Aside from its direct antimicrobial effects, LL-37 has also shown a marked role in altering macrophage metabolism and phenotypes, an immunomodulatory activity that can direct the immune response to kill mycobacteria. However, these effects have not been conclusive, since the LL-37 has been reported also with diverging effects, namely pro-inflammatory (van der Does et al., 2010) and anti-inflammatory (Brown et al., 2011). These different results may be due to the different cell types (human or mouse) used in the two studies. In addition, the interaction between LL-37 and macrophages can have different outcomes depending on the precise orchestration of events at each time point.
1.3.3 LLKKK18, a LL37 derivative peptide
Recently, new AMPs have been synthesized aiming to overcome some of 37 limitations. LL-37 is very sensitive to proteolytic degradation, decreasing its half-life, requiring the use of higher concentrations, which potentially results in cytotoxicity (Afacan et al., 2012).
In general, antimicrobial peptide activities depend on structural and physicochemical parameters, such as cationicity, hydrophobicity and amphipathicity meaning that, by modification of these parameters, the range of activities and its potency can be manipulated (Nagaoka et al., 2002). Since human proteases cannot degrade amino acid peptides, a derivate peptide from LL-37 was synthesized, named D5 (a D-enantiomer with a few altered amino acids) which increases its half-life and decreases self-association (Z. Jiang et al., 2011).
Another derivative peptide from LL-37 was also developed – LLKKK18. This peptide is a synthetic truncated variant from LL-37, with 14 and 5 residues less at N- and C-terminus, respectively, resulting in a shorter peptide (Figure 3). In the amino acid sequence, the polar uncharged residues glutamine (Q), asparagine (N) and negatively charged aspartic acid (D) are substituted by positively charged lysine (K) and overall increasing its net charge (Figure 3; Mohanty et al., 2013; Sonawane et al., 2011). Other truncation variants from LL-37 on N- and C-terminus result in decreased antimicrobial activity, suggesting that these regions contain residues that are fundamental for its activity (Sonawane et al., 2011). However, in LLKKK18 the substitution with three positively charged lysine residues seems to result in higher affinity for the negatively charged bacterial membrane due to the increased hydrophobicity and cationicity (Yount et al., 2006).
Figure 3. Comparison of helical wheel projections of the α-helical peptide LL-37 and its truncated derivative LLKKK18, evidencing the hydrophobic and hydrophilic regions, with improved hydrophobicity and cationicity of LLKKK18. Underlined is the LL37 amino acid sequence that gave rise to LLKKK18. Adapted from Nagaoka et al. (2005).
As we have seen so far, LL-37 and LLKKK18 specific structure – small size, cationic and amphipathic – allows them to interact with cell membranes, leading to its rupture. The derivative peptide LLKKK18 has recently become of greater interest due to its stronger antimicrobial activity, relatively to LL-37. LLKK18 antimicrobial activity extends to mycobacteria, as demonstrated in in vitro and in vivo studies (Silva et al., 2016; Sonawane et al., 2011), showing higher efficiency in mycobacterial killing comparatively to LL-37 (Sonawane et al., 2011). Such activities make LLKKK18 a good candidate as an antimicrobial agent in TB treatment. Ideally, the use of natural occurring antimicrobial peptides or synthetic derivatives could dispense the use of common antibiotics, while also reducing the risk of resistance acquisition.
LLKKK18 peptide has not been so extensively characterized as LL-37. Before further studies regarding its antimicrobial activities, it is also imperative to study its effects in target cells that can affect infection resolution, namely its influence in macrophage immune response, metabolism and phenotype.
While LLKKK18 antimicrobial activity has been improved relatively to LL-37, its cytotoxicity remains a limitation. The higher hydrophobicity of LLKKK18 allows a stronger interaction with lipid membranes. Cell membranes associated with cholesterol, as found in mammals, are protected from this effect. However, the presence of higher concentrations of these peptides can lead to some toxicity, cell rupture (Vandamme et al., 2012) and increased hemolytic activity (Ciornei et al., 2005).
LL-37 LLKKK18
Peptide Sequence Net charge
LL-37 LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES +6
To overcome this problem, these antimicrobial peptides should be targeted only to infected macrophages, decreasing the potential damage to surrounding cells. The use of a drug delivery system able to achieve the targeted delivery of the peptide would largely increase the potential application of LLKKK18 to TB therapies. In TB, the localization of mycobacteria, entrapped in the phagosome, within the macrophage, inside the granuloma, represents a great challenge (Gutsmann, 2016). Therefore, the use of a drug delivery system could protect LLKKK18 from proteolytic degradation and enhance the delivery to target cells.
1.4 Drug delivery systems
The emergence of new drugs with high potential in various fields can sometimes be limited due to toxicity and pharmacokinetic issues. The development of new drug delivery systems may allow to increase drug efficacy by reducing its toxicity, increasing its stability, and contributing to drug distribution to target organs (Sivaram et al., 2015). The ideal delivery system should: guarantee the release of the drug within the therapeutic window and only to target cells; be nontoxic and non-immunogenic; be biodegradable or easily excreted; be stable upon storage and with reduced production costs (Gaspar et al., 2008).
Different types of drug carriers have been reported, including micelles, hydrogels, nanogels and polymeric-drug conjugates, with the potential to improve patient compliance and appliance (Pedrosa et al., 2014; Silva et al., 2015).
Hydrogels have been actively investigated for its physicochemical and biological characteristics. Hydrogels are hydrophilic networks of polymers with ability to swell retaining a large amount of water in its structure, while allowing drug loading and stimuli responsive release (Ahmed, 2015; Sivaram et al., 2015). Nanogels, hydrogels in the nanoscale, are suited for the systemic delivery in the blood stream (Sivaram et al., 2015). These nanogels can be made of ionic or non-ionic amphiphilic polymer chains that, to minimize the thermodynamic energy, organize to expose the hydrophilic regions on its surface while the hydrophobic segments aggregate in its core, forming stable and well-defined aggregates (Sharma et al., 2016). Drugs can be loaded in the nanogels, and by electrostatic and/or hydrophobic interactions, become physically entrapped in the polymer matrix. Nanogels as drug delivery systems offer a great deal of advantages, such as high biocompatibility, a high drug loading capacity and controlled release, together with protection from in vivo degradation of bioactive molecules (Sivaram et al., 2015). TB treatment efficacy depends on the targeting of M. tuberculosis, that is mainly localized inside infected macrophages. For that, a delivery system able to distinguish infected from non-infected
macrophages would be ideal. Sladek and Rysanek (2009) identified that the membrane receptor CD44 is highly expressed in activated macrophages, in comparison to non-activated macrophages, during the inflammatory response. Therefore, this cell marker can be used to distinguish between infected and non-infected macrophages and has been explored previously (Silva et al., 2016).
1.4.1 CD44 receptor: a target for drug delivery
CD44 is a widely expressed glycoprotein on the surface of many mammalian cells including leukocytes, involved in cell–cell and cell–matrix interactions, cell migration and lymphocyte activation (Sherman et al., 1994). CD44 expression is also associated with stem cells, tumorigenesis and the metastatic phenotype (Zoller, 2011). Hyaluronic acid (HA) is the main ligand of CD44 receptor (Mattheolabakis et al., 2015).
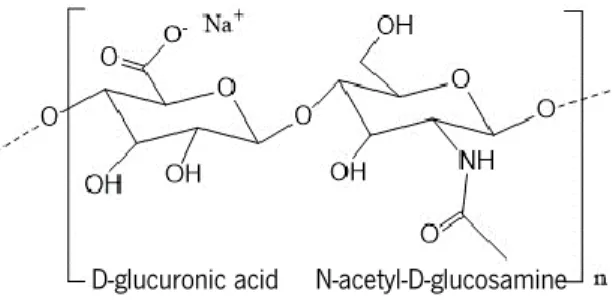
HA, a polymer of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine (Figure 4), is a major component of the extracellular matrix (D. Jiang et al., 2011). At physiological pH, HA is a polyanion and highly hydrophilic polymer, with a high water retention ability, important for the viscoelasticity of liquid connective tissues and control of tissue hydration (Cowman and Matsuoka, 2005).
Figure 4. Schematic representation of sodium hyaluronate, a hyaluronic acid (HA) salt. Repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine are on the basis of HA structure. Adapted from Pedrosa et al. (2014).
HA recognition by the CD44 receptor may result in internalization and eventual degradation of HA inside the target cell by hyaluronidases or in the presence of reactive oxygen species (ROS) (Knudson et al., 2002; Mattheolabakis et al., 2015). This endocytic pathway for HA internalization can be explored for the delivery of HA-based drug delivery systems (Mattheolabakis et al., 2015). Delivery systems based on HA are of great interest due to their biocompatibility; biodegradability; high drug loading ability and intrinsic targeting ability for the CD44 receptor. Their carboxylic groups are, in addition, easy to modify (Mero and Campisi, 2014).
Several strategies might be used in the targeting of CD44-expressing cells using HA, including the conjugation of the drug to HA (Montagner et al., 2013), surface modification of nanoparticles with HA (Wang et al., 2016) and synthesis of self-assembling HA nanogels (Choi et al., 2010; Pedrosa et al., 2014).
1.4.2 HA self-assembling nanogels for TB therapy
Taking advantage of the easier modification of HA, a novel drug delivery system has been developed (Pedrosa et al., 2014). This system is based on the grafting of a hydrophobic molecule to a HA backbone, resulting in an amphipathic conjugate that self-assembles in aqueous solutions into a nanogel, with high stability (Pedrosa et al., 2014).
The use of such delivery system presents numerous advantages for the loading of antimicrobial peptides, particularly LLKKK18. Since HA is a negatively charged polysaccharide, the positively charged and amphipathic LLKKK18 can become entrapped, upon self-assembling of the nanogel. Through encapsulation, LLKKK18 becomes protected from proteolytic degradation in the blood stream and bodily fluids, reducing peptide loss. Since HA is naturally recognized by CD44 receptors, the nanogel can be internalized by phagocytosis, directing the peptide-loaded nanogel, preferentially to infected macrophages that overexpress the receptor. Such targeting allows the use of higher dosages of LLKKK18 with reduced cytotoxic effects to the surrounding healthy tissues (Silva et al., 2016).
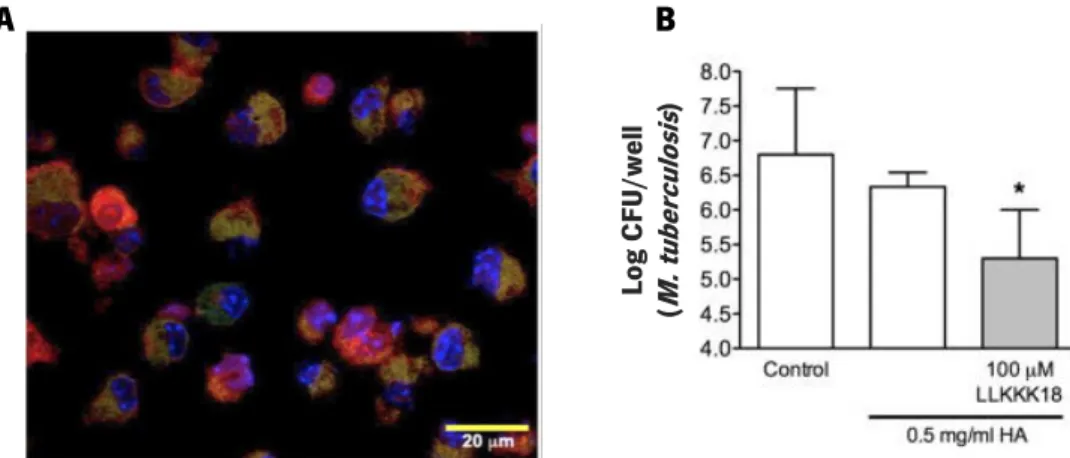
The investigation of this HA-based nanogel as a delivery system for the controlled released of LLKKK18 in TB infected mice was already performed by Silva et al. (2016). This research had impressive results, namely with the delivery of LLKKK18-loaded HA nanogel and internalization by macrophages, absence of cytotoxic effects in macrophages and, importantly, a significant anti-mycobacterial effect (Figure 5; Silva et al., 2016). By using this strategy, the researchers succeeded to reduce the mycobacterial burden in infected macrophages, making LLKKK18 encapsulation into an HA-based nanogel a promising strategy in TB treatment. The release of peptide-loaded nanogels intracellularly, where mycobacteria are mainly localized, enhances the peptide activity and reduces possible cytotoxic effects (Figure 5). The use of this system should be further explored for TB therapies given the countless advantages.
Figure 5. Results obtained by Silva et al. (2016) showing the effects of the LLKKK18-loaded nanogels. Merged image from confocal fluorescence microscopy allowed to confirm that macrophages (nuclei stained with DAPI, blue) internalized the TAMRA tagged-peptide-loaded nanogels (red) and that these nanogels co-localize with mycobacteria expressing GFP (green) (A). This co-localization may favor the direct mycobactericidal effect observed in M. tuberculosis-infected BMMΦ, incubated with peptide-loaded nanogels, after 4 days of treatment, the number of mycobacteria CFUs present in macrophages was significantly reduced (B). Adapted from Silva et al. (2016).
Despite the demonstrated direct mycobactericidal effects of LLKKK18, little is known about other effects that can be involved in elimination of mycobacteria by macrophages. The effects of this peptide on macrophage metabolism or phenotype have yet to be clarified since these parameters can affect macrophage activities and their ability to deal with infection with intracellular pathogens (Benoit et al., 2008). Since macrophages are central cells involved in TB infection (Guirado et al., 2013), further research on the effects of LLKKK18 in different signaling pathways involved in M. tuberculosis recognition by macrophages and following response to this pathogen is needed to clarify its potential as an adjuvant in TB therapy. B A Lo g C FU /w el l (M . tuberc ul os is )
2. R
ATIONAL ANDA
IMSThe current panorama of TB worldwide, associated with the need for more effective treatments without resistance acquisition from mycobacteria has paved the way for the search of new therapies. The use of natural compounds as is the case of the antimicrobial peptides, herein described, seems to be a promising strategy; particularly the peptide LL-37 and its derivative LLKKK18 have shown ability to kill mycobacteria (Sonawane et al., 2011). Although a significant anti-mycobacterial effect has been demonstrated, the precise action mechanisms of LLKKK18, when interacting with macrophages, is not completely elucidated. In that sense, the aims of this Master Thesis are centered on:
a) Production and characterization of an amphiphilic HA-based nanogel as a delivery system for LLKKK18.
b) Reduction of the cytotoxic effects of LLKKK18 and improvement of intracellular delivery by loading in the HA-based nanogel.
c) Evaluation of the effects of the LLKKK18-loaded nanogels on Toll-like receptors (TLRs) signaling pathways usually activated when macrophages are exposed to M. tuberculosis, by analysis of the cytokine profile exhibited.
3. M
ATERIALS ANDM
ETHODS3.1 Materials
Sodium hyaluronate 4.7 kDa (401 g/mol per disaccharide unit) was purchased from Lifecore Biomedical (Minnesota, EUA). Hexadecylamine, 1-ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC), Dimethyl sulfoxide (DMSO), N-hydroxysuccinimide (NHS), tetrabutylammonium fluoride (TBA-F) trihydrate, LPS from Escherichia coli (O111:B4) were obtained from Sigma Aldrich (Barcelona, Spain). AG 50W cationic exchange resin was purchased from Bio-Rad. The synthetic antimicrobial peptide LLKKK18 was obtained from Schafer-N (Copenhagen, Denmark). The synthetic triacylated lipopeptide Pam3CSK4 and E. coli K12 double-stranded genomic DNA were obtained from InvivoGen (Toulouse, France). IL-1β, IL-6 and IL-10 and TNF-α mouse ELISA kits were obtained from ThermoFisher (Massachusetts, EUA). Phorbol 12-myristate 13-acetate (PMA), Roswell Park Memorial Institute (RPMI) 1640 and Dulbecco's Modified Eagle Medium (DMEM) liquid cell culture media, both with stable glutamine, fetal bovine serum (FBS), Trypsin (1:250)/EDTA (0.25 %/0.02 %), penicillin/streptomycin liquid (10000 U/mL /10000 g/mL) were all purchased from Merk (Darmstadt, Germany).
3.2 Methods
The study of macrophage’s response to the antimicrobial peptide LLKKK18, loaded into a HA-based nanogel, involved a sequence of steps to obtain further information on the action mechanisms of this peptide. This project involved, initially, the synthesis of the HA-based nanogels and elucidation of LLKKK18 loading ability followed by characterization of the LLKKK18-loaded nanogels. Dosage optimization, to obtain the peptide-loaded nanogel formulation used for following assays was performed to minimize potential cytotoxic effects. The study of nanogel internalization was also important to confirm the LLKKK18 intracellular delivery. Finally, we studied the effects of the formulations on macrophage’s response to different TLR ligands by downstream analysis of cytokine secretion.
3.2.1 HA-based nanogel synthesis
The synthesis of the amphiphilic hyaluronic acid (HA) conjugate, that self-assembles into a nanogel in aqueous solution, was performed by grafting hexadecylamine (Hexa) to a HA backbone based on a
react with the carboxylic group from HA originating, in a simple reaction, the amphiphilic conjugate (Gonçalves et al., 2007). This process has the advantage of being more economical than other hydrophobic molecules used previously (Silva et al., 2016). To obtain the amphiphilic conjugate, it is firstly required to dissolve HA in DMSO. The sodium ions of sodium hyaluronate were exchanged by the lipophilic tetrabutylammonium (TBA+) cation, changing the solubility of HA and enabling its dissolution in DMSO. Tetrabutylammonium can be easily removed at the end of the reaction by dialysis against a NaCl solution (Dantas-Santos et al., 2012).
For the ion exchange, 1 g of cationic exchange resin (AG 50W) was initially saturated with TBA+ that was previously dissolved in 12,5 mL ultrapure water (Milli-Q®) (3,45 g tetrabutylammonium fluoride in 12.5 mL). The mixture was incubated for 1 h at room temperature, under mild agitation allowing the cationic resin to become impregnated with TBA+ ions. After that period, the resin was elutriated using a 0.45 µm filters (mixed cellulose ester) and washed with ultrapure water. The washed resin was then mixed with a solution of 1 % sodium hyaluronate (25 mL) and the ion exchange occurred for 2 h, at room temperature, under agitation. By the end of this step, the sodium ion of the carboxylic group of sodium hyaluronate was exchanged with TBA+ rendering HA soluble in DMSO (Figure 6). The resin was removed by centrifugation at 1000 g for 2 minutes, and supernatants carefully collected, and frozen at -80 ºC, followed by freeze-drying for 8 days.
Figure 6. Schematic representation of the ion exchange step which renders HA soluble in DMSO. The sodium ions (Na+) interacting with the carboxylic groups of HA were exchanged by the lipophilic tetrabutylammonium (TBA+) increasing HA solubility in DMSO. Adapted from Pedrosa et al. (2014).
Ion exchange with TBA+
The resulting HA-TBA (315 mg), was crosslinked with the hydrophobic Hexa through amide bond formation by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) chemistry (Grabarek and Gergely, 1990). For the synthesis of these conjugates, 1 % (w/v) HA-TBA was dissolved in DMSO. EDC (97 mg) and NHS (58 mg) were then added and agitated together on a rotary mixer, until dissolved. Hexa (18.4 mg) was then added, in an amount corresponding to 15 % of the number of moles of HA-TBA. The mixture was stirred for 24 h at room temperature to allow amide bond formation between HA and Hexa (Figure 7). Conjugation of these two molecules allows the self-assembling in aqueous environment into nanogels, with hydrophilic HA closer to the surface and Hexa hydrophobic chains gathered in nanogel’s core. The final suspension was dialyzed using a 1000 Da cut-off membrane, against a NaCl 150 mM solution, for 3 days, to remove TBA+ ions and for additional 2 days against distilled water to further remove excess NaCl (Figure 7). The resulting HA-Hexa amphiphilic conjugate was freeze-dried for 5 days and the final material stored at room temperature until use.
Figure 7. Representative illustration of the synthesis of the amphiphilic HA-Hexa nanogels. HA-TBA is hydrophobically modified through amide formation with hexadecylamine in the presence of EDC and NHS, resulting in amphiphilic conjugates. Subsequent dialysis against NaCl removes TBA+ ions and dialysis against distilled water removes excess Na+, completing the synthesis of the amphiphilic conjugate. Adapted from Pedrosa et al. (2014).
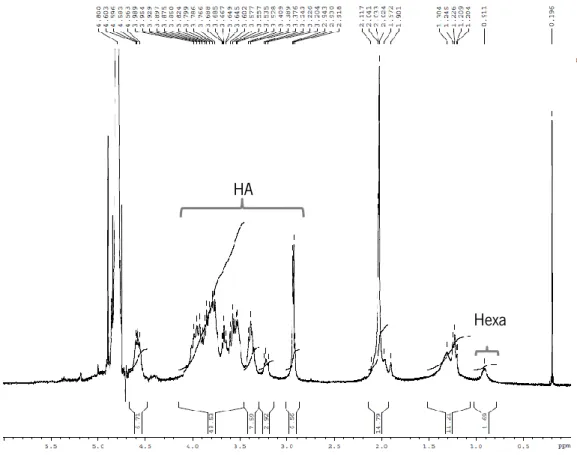
The crosslinking was confirmed by 1H NMR spectroscopy, dissolving HA-Hexa in D
2O (7 mg/mL). Hexadecylamine
From the 1H NMR spectrum, the degree of substitution (DS) which corresponds to the number of HEXA molecules per 100 disaccharide units of HA, was calculated based on the following formula:
DS = number of H
+from HA
number of H+from HEXA ×
∫ peak area HEXA ∫ peak area HA
3.2.2 LLKKK18 encapsulation in the HA-Hexa nanogel
For the use of HA-Hexa nanogel as a carrier for LLKKK18 antimicrobial peptide it was fundamental to characterize the LLKKK18 encapsulation efficiency. This was determined by dissolving the lyophilized HA-Hexa (1 mg/mL), in phosphate buffered saline (PBS) containing 25, 50, 100 or 200 µM LLKKK18, to analyze if a different loading concentration of peptide could influence its encapsulation. The mixtures were left under mild rotation for 24 h, allowing nanogel assembling and peptide encapsulation (Silva et al., 2016). In each nanogel suspension, non-encapsulated peptide was separated from the peptide-loaded nanogel using Amicon® Ultra-centrifugal filter units (Millipore, MWCO = 100 kDa) by centrifugation at 2000 rpm for 2 min. The initial volume on the concentrated retained solution, containing the peptide-loaded nanogel, and on the filtrated solution with free peptide, was then restored. Encapsulation efficiency was determined by Waddell’s method, as previously described by Wolf (1983), for protein quantification in the retained and filtrated solutions, to determine the percentage (%) of peptide encapsulation. This method is based on peptide bond’s absorption of far ultraviolet radiation, being a simple and sensitive method for protein quantification that does not depend on amino acid composition or peptide structure (Waddell, 1956). In this method, absorbance of the samples at 225 nm is subtracted to the absorbance at 215 nm and the difference is multiplied by the constant factor 0.144 to obtain the protein concentration (mg/mL).
Encapsulation efficiency (EE) (%) was calculated based on the following formula (Zhang and Feng, 2006). By determining the encapsulation efficiency, LLKKK18 initial concentration can be adjusted to obtain the desired final concentration loaded in the HA-Hexa nanogels in the following assays.
3.2.3 Nanogel characterization
Although HA is well known to be biocompatible (Jansen et al., 2004), nanomaterials based on this polymer can behave differently. Their biological activity may depend on a series of physicochemical characteristics, like size, charge, shape and surface modifications (Deng et al., 2009). For these reasons physical nanogel characterization is also fundamental to assure that the nanogel has the desired properties, with and without the loading peptide.
The hydrodynamic diameter of the nanogels was analyzed by Dynamic Light Scattering (DLS), as previously described by Pedrosa et al. (2014). DLS is a useful technique that allows to analyze nanometric particles based on their Brownian motion, that is directly influenced by size, since the larger the nanoparticle the slower the Brownian motion (Stetefeld et al., 2016). Particle’s superficial charge influences electrostatic interaction with the dispersion media and results in a layer of counter ions on its surface and generates a Zeta potential. When an electrical field is applied, the particle moves towards the opposing electrical charge and this electrophoretic mobility can be used to determine Zeta potential by Henry’s equation (Delgado et al., 2007).
For size distribution analysis, nanogels were prepared in distilled water, PBS or Roswell Park Memorial Institute (RPMI) culture media (without phenol red or growth factors) to clarify if different dispersion media, with different ionic strength, could influence nanogel assembling. For Zeta potential analysis, nanogels were only prepared in distilled water. To a 1 mg/mL nanogel solution, LLKKK18 was added and agitated together, on a rotary mixer, for 24 h. Peptide-loaded nanogels were prepared to obtain the final concentration of 50 µM LLKKK18, taking in consideration its encapsulation efficiency of approximately 70 %. For the peptide-loaded nanogels, free peptide was again removed using the Amicon ® Ultra-centrifugal filter units and the volume of the resulting retained sample reconstituted. Right before analysis, empty or LLKKK18-loaded nanogels were finally filtered through a 0.20 µm PES syringe filter, as they would if added to the cells. The samples were analyzed using a folded capillary cell, in a Zetasizer NANO ZS (Malvern Instruments Limited, UK), with a He-Ne gas laser (633 nm) and a detector angle of 173°. For each formulation, the z-average diameter, polydispersity index (PdI) and Zeta potential values were determined.
3.2.4 Cell culture conditions
macrophages (BMMΦ) were used. While animal and human in vivo studies are a more accurate representation of the biological response and interaction with different organ systems, these studies are associated with ethical and financial constraints, increasing the relevance of in vitro studies (Chanput et al., 2014). Mouse or human cell lines are a useful in vitro tool for the study of basic cellular responses, functions and signaling pathways, with the additional advantage of being easy to maintain in culture, with a homogenous genetic background that minimizes variability (Chanput et al., 2014).
By using these cell models, we expected to be able to compare the macrophage’s responses to the peptide in mice and human cell lines, and to additionally observe any differences between a cell line and a primary culture of macrophage’s response. As a control we used, in this study, a fibroblast cell line (L929 cells) to evaluate the cytotoxicity in a non-macrophage cell type. Culture conditions for each cell model, were performed as described below.
L929 fibroblasts
L929 cells are a mouse fibroblastic cell line, frequently used in standard cytotoxicity and biocompatibility studies (Faria et al., 2009). Cells were cultured in RPMI media, supplemented with 10 % fetal bovine serum (FBS), and 1 % Penicillin:Streptomycin, in an humidified incubator (37 ºC, 5 % CO2). Subcultures were performed by trypsinization. For the assays, L929 cells were seeded in 96-well plates, at a density of 2 x 105 cells/mL and incubated overnight, for cell adhesion, before any treatment.
RAW 264.7 macrophages
RAW 264.7 cells are a murine macrophage cell line, frequently used in inflammation studies or other responses to pharmacological agents, because these cells are easy to maintain in culture and show high reproducibility in response to immune cell activators (Tweedie et al., 2009). RAW 264.7 cells were kindly offered by Professor Paula Sampaio from the Biology Department, University of Minho. The vial containing the frozen cells was slowly thawed, and the cells immediately dispersed in high glucose Dulbecco's Modified Eagle Medium (DMEM) complete media, supplemented with 10 % FBS. The cell culture was maintained in a humidified incubator (37 ºC, 5 % CO2), for 3 days until subculture. Cells were subcultured by gentle scrapping, and transferring to new culture flasks with fresh medium, every 2-3 days and for no longer than 25 passages. Cells were usually maintained until 80 % confluence, and for each assay, scrapped and seeded in 96-well plates, at a density of 5 x 105 cells/mL. Before any treatment the cells were left to adhere overnight.
THP-1 monocytes
THP-1 is a human leukemia monocytic cell line, easy to differentiate in macrophages and widely used in the study of the immune response (Daigneault et al., 2010). THP-1 cell vials were kindly offered by Doctor Joana Gaifem from Life and Health Sciences Research Institute, University of Minho. Frozen cells were thawed and cultured using RPMI complete media (RPMI supplemented with 10 % FBS and 1 % Penicillin:Streptomycin), in a humidified incubator (37 ºC, 5 % CO2). These cells grow in suspension in the culture media, and the close contact between cells is key for their adequate growth. The cell density was controlled by subculturing every 2-3 days to prevent cell density from exceeding 1 x 106 cells/mL. For subculture, the cell suspension was centrifuged at 160 g for 5 min, and the cells resuspended in fresh complete RPMI media, and transferred to new culture flasks at final cell density of 2 x 105 cells/mL, until new subculture.
Differentiation of THP-1 monocytes into macrophages was performed by induction with phorbol 12-myristate 13-acetate (PMA) (Tsuchiya et al., 1982). PMA stimulation induces cell adherence to culture plates, cell’s morphology changes to a flat amoeboid shape, increased expression of the classical macrophage markers, a higher phagocytic activity and lower proliferation capacity, as described by Daigneault et al. (2010).
To obtain these THP-1 derived macrophages, cells were seeded in 96-well plates, at a density of 1 x 105 cells/mL, and incubated with 100 nM PMA, for 24 h. After this period, the culture media was removed and replaced with fresh complete culture media, without PMA, for additional 48 h, to allow cell recovery before any treatment was applied.
BMMΦ cells
Bone marrow-derived macrophages (BMMΦ) provide a useful model for the study of primary cell function, and are commonly regarded as model for the study of resident macrophage’s behavior (Manzanero, 2012). Differentiation of bone marrow cells into macrophages is a simple and inexpensive process, that allows to obtain a homogenous population of macrophages in just a few days. The growth factor most commonly used to obtain macrophages in vitro is the colony stimulating factor 1 (CSF-1), also known as macrophage colony-stimulating factor (M-CSF) (Manzanero, 2012). An inexpensive method to obtain CSF-1, for bone marrow differentiation, takes advantage of L929 fibroblasts, which are capable of secreting high amounts of this growth factor (Boltz-Nitulescu et al., 1987; Stanley et al., 1978). Thus, in vitro starting with bone marrow cells by using the