UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Differentiation and genetic variability in cork oak populations
(Quercus suber L.)
Joana Seabra Pulido Neves da Costa
MESTRADO EM BIOLOGIA HUMANA E AMBIENTE
Lisboa 2011
UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Differentiation and genetic variability in cork oak populations
(Quercus suber L.)
Joana Seabra Pulido Neves da Costa
Dissertação orientada por:
Prof. Doutor Octávio Fernando de Sousa Salgueiro Godinho Paulo Doutora Dora Cristina Vicente Batista Lyon de Castro
MESTRADO EM BIOLOGIA HUMANA E AMBIENTE
Lisboa 2011
III
Nota prévia
A presente tese de mestrado encontra-se escrita na língua Inglesa uma vez que esta é considerada a língua científica universal. Por esta razão, o conhecimento e treino da sua escrita apresentam uma importância considerável para quem tenciona seguir uma carreira em investigação científica em Biologia. Com a escrita da tese em Inglês pretende-se também acelerar o processo de elaboração dos manuscritos e subsequentes publicações científicas.
As referências bibliográficas foram elaboradas segundo os parâmetros da revista científica internacional, “Trends in Ecology and Evolution” (www.cell.com/trends/ecology-evolution/authors). Esta é uma das revistas mais relevantes na área em que esta tese foi desenvolvida e possui um sistema de citações cómodo para a leitura de textos de revisão científica. Adicionando o seu elevado factor de impacto na sociedade científica, pareceu apropriada a escolha desta revista como referência para a apresentação da bibliografia.
O estudo elaborado nesta tese foi desenvolvido no âmbito do projecto PTDC/AGR-GLP/104966/2008, “Avaliação dos recursos genéticos e genómicos do sobreiro: bases para uma gestão prospectiva”, financiado pela Fundação para a Ciência e Tecnologia (FCT).
IV
Foreword
The present master thesis is written in English. This is considered as the universal scientific language and, therefore, is of the upmost importance the practice of its writing and grammar for those who intend to follow a career in Biology and scientific investigation. Also, the writing of the present thesis in the English language allows to accelerate the process of submission of the manuscripts for further publication.
The bibliographic references were elaborated following the parameters of the international scientific journal “Trends in Ecology and Evolution” (www.cell.com/trends/ecology-evolution/authors). This is one of the most relevant journals in the area where this thesis was developed, with an elevated impact factor in the scientific society. Also it possesses a confortable citations system for the reading long texts.
This study is part of the project PTDC/AGR-GLP/104966/2008, “Avaliação dos recursos genéticos e genómicos do sobreiro: bases para uma gestão prospectiva”, funded by Fundação para a Ciência e Tecnologia (FCT).
V
Agradecimentos
No terminar desta tese surge a necessidade de agradecer a todos aqueles que de alguma forma a tornaram possível.
O primeiro agradecimento é devido aos meus orientadores, Octávio Paulo e Dora Batista. Ao Professor Octávio pelo incentivo e voto de confiança que depositou em mim desde o início. À Dora pela proposta do tema de mestrado e pelo despertar do meu interesse pelas plantas.
À Professora Deodália Dias pelas oportunidades que me proporcionou e pelo apoio incondicional quando os problemas fogem ao nosso controlo e não dependem de nós.
Agradeço em particular à Professora Helena Almeida do Instituto Superior de Agronomia pelo acesso à Herdade Monta da Fava de onde vieram algumas das populações de sobreiro mais importantes para o desenvolvimento deste trabalho. A todos os que directa ou indirectamente foram importantes para a recolha das amostras.
Ao CoBiG2 pelo grupo que se formou e pelos bons momentos. Ao Francisco Pina-Martins e à Vera Nunes por me terem criado nos momentos iniciais da minha vida de laboratório. À Sofia Seabra pela sua calma natural e amizade. Ao Eduardo Marabuto pelo seu bom humor, muito necessário em tempos difíceis. À Sara Ema, por incrível que te possa parecer acho que stressas mais que eu e isso é uma ajuda enorme, assim como estares comigo até ao dia da entrega… mesmo de moletas. Ao Diogo Silva pelo partilhar de alguns momentos difíceis com os orientadores a afins. À Catarina Dourado, Ana Sofia, Patrícia Brás, Renata Martins, Inês Modesto e Bruno Vieira que me foram ajudando com as usuais dificuldades de uma tese. Aos restantes membros do CoBiG2, assim como a antigos membros e às mais recentes aquisições, muito obrigada!
À Rita Oliveira e Raquel Vaz, não fazem parte do CoBiG2, mas fazem parte da família e merecem o devido reconhecimento e agradecimento pelo que “aturaram” da minha parte. Um agradecimento especial a quatro pessoas que devem ter sofrido muito comigo. Seriam precisas páginas de agradecimentos, mas como não o posso fazer fica a intenção. À Catarina Dourado um agradecimento em particular. Foi um longo caminho e fica o agradecimento pelo carinho, apoio e amizade. Ao Eduardo Marabuto pelos valiosos comentários, acima de tudo na Introdução. Tens razão em muitas coisas mas há que fazer compromissos. À Sofia Seabra pelas importantíssimas correcções, seria muito mais difícil sem ti. Ao Bruno Vieira pelas
VI
horas infinitas que me ouviu queixar da vida em geral, da tese em particular. Sempre com muito amor e carinho! Obrigada aos quatro!
À Diana Martins. Não estás sempre comigo mas estás sempre a pensar em mim e tens timings impecáveis para quando preciso mais de ti.
E ao Pai, Mãe e Avós. Apesar de estarem no fim desta lista foram provavelmente as pessoas que mais contribuíram para que esta tese pudesse ser concluída. Claramente não estaria cá sem o apoio precioso da minha família.
VII
Resumo
O ano 2011 foi designado como “O Ano Internacional das Florestas” pela Assembleia Geral das Nações Unidas, na tentativa de despertar o interesse público e promover a sustentabilidade da gestão e conservação florestal para o benefício das gerações futuras. Estimativas da FAO (Food and Agriculture Organization) para o ano de 2010 demonstraram que 31% da superfície terrestre ainda está coberta por florestas e que as árvores correspondem a 90% da biomassa terrestre, compreendendo um total de 60.000 a 100.000
taxa. Contudo, certas alterações induzidas pelo Homem, principalmente a desflorestação e as
alterações climáticas elevaram o número de espécies ameaçadas de extinção para 10%.
Nos últimos tempos as espécies florestais têm sido bastante usadas em estudos de genética populacional e evolutiva, assim como em estudos genómicos. As principais razões são as características particulares que estes modelos não-clássicos apresentam, visto resultarem de milhões de anos de divergência e diversificação, e assim apresentarem impressionantes níveis de diversidade morfológica, divergência evolutiva e diversidade ecológica. Apesar de o impacto que as alterações globais vão ter sobre estas espécies depender grandemente da sua capacidade de reacção e da dos seus ecossistemas, os estudos genéticos permitem-nos, até certo ponto, prever as consequências evolutivas das alterações uma vez que nos possibilitam aumentar o conhecimento da biodiversidade e evolução destas espécies.
O conceito de “Filogeografia” foi apresentado por Avise et al. em 1987, e durante os últimos 25 anos teve um grande impacto na investigação, particularmente em animais. Nas plantas os resultados produzidos não têm sido tão explícitos, principalmente devido à falta de variabilidade genética aplicável à análise filogeográfica. Tem sido consideravelmente difícil encontrar um marcador genético em plantas com um poder de resolução semelhante ao DNA mitocondrial animal. No entanto a filogeografia em plantas tem-se desenvolvido bastante, principalmente nos últimos anos, com o crescimento do uso de marcadores moleculares nucleares e com a recolha de informação de fragmentos maiores do genoma cloroplastidial.
O género Quercus (carvalhos) (Fagaceae) é um dos grupos mais importantes de angióspermicas lenhosas no hemisfério norte, nomeadamente em relação à diversidade de espécies, dominância ecológica e valor económico. O género é bastante antigo considerando que o fóssil mais antigo encontrado pertence ao Oligoceno (34-23 milhões de anos). Os
VIII
carvalhos são os membros dominantes de uma grande variedade de habitats e pensa-se que existam 500-600 espécies na Terra.
O sobreiro (Quercus suber L.) representa umas das espécies arbóreas mais importantes da região Oeste do Mediterrâneo, tanto económica como ecologicamente, onde define espaços florestais abertos (criados e mantidos pelo Homem) conhecidos em Portugal como “montados”. A área de distribuição do sobreiro, apesar de descontínua, vai desde a costa Atlântica do Norte de África e Península Ibérica até às regiões sudoeste de Itália, incluindo as ilhas Mediterrânicas Sicília e Sardenha, assim como as zonas costeiras do Mediterrâneo da Argélia e Tunísia. As florestas de sobreiro cobrem uma área total de cerca de 2,2 milhões de hectares, de onde são extraídas 340.000 toneladas/ano de cortiça. As maiores extensões de área coberta estão localizadas em Portugal com cerca de 700.000 hectares, correspondendo a 21% da área florestal Portuguesa e 30% da área mundial de produção de cortiça. O sobreiro tem sido usado desde a Antiguidade para a produção de cortiça e este produto natural apresenta um grande valor económico. As maiores ameaças contudo, são enfrentadas pelas populações naturais e marginais, que muitas vezes são pequenas e se encontram dispersas e em habitats restritos. Muitas destas populações podem estar em risco de desaparecer, principalmente devido à falta de regeneração.
Devido ao seu valor económico e também porque os espaços florestais de sobro são reservatórios de biodiversidade e abrigo para uma grande variedade de espécies ameaçadas de extinção, estas populações representam material importante para estudos genéticos que possam servir de base ao delineamento de programas de conservação. Assim sendo, é necessário fortalecer e aumentar o conhecimento da organização espacial da variação genética da espécie, para assim se poder tomar decisões conscientes e informadas sobre a conservação dos recursos genéticos.
Os estudos filogeográficos em Quercus suber têm sido pouco aprofundados e alguns até inconclusivos. Isto leva a que não haja uma boa compreensão da história evolutiva da espécie, muito provavelmente devido ao número limitado de áreas amostradas ou o baixo conteúdo informativo dos marcadores usados. Por exemplo, nos estudos que envolveram populações Portuguesas, foram feitas inferências com base numa amostragem deficiente de Portugal, e sendo esta uma das regiões mais relevantes na história presente e passada do sobreiro, é necessária uma maior cobertura da área de distribuição, incluindo algumas zonas referidas como potenciais zonas de refúgios glaciais para outras espécies. Por outro lado, uma
IX
vez que a maioria dos estudos filogeográficos são suportados por dados derivados do DNA cloroplastidial (cpDNA) (PCR-RFLPs e SSRs), deve considerar-se se outras abordagens moleculares ou marcadores genéticos, que evoluam a taxas mais rápidas que o cpDNA não indicariam um cenário evolutivo diferente. Esta tese de mestrado propõe uma abordagem diferente dos estudos anteriores, complementando dados obtidos a partir de DNA cloroplastidial e nuclear. Esta abordagem nunca foi aplicada ao sobreiro, e espera-se que possa adicionar informação filogeográfica relevante. Mais especificamente os objectivos deste trabalho foram: 1. Inferir a história evolutiva e os padrões demográficos de Quercus
suber; 2. Explorar os padrões de hibridação e introgressão do sobreiro com outras espécies de Quercus; 3. Avaliar os níveis de diversidade e diferenciação entre e dentro de algumas
populações chave de sobreiro.
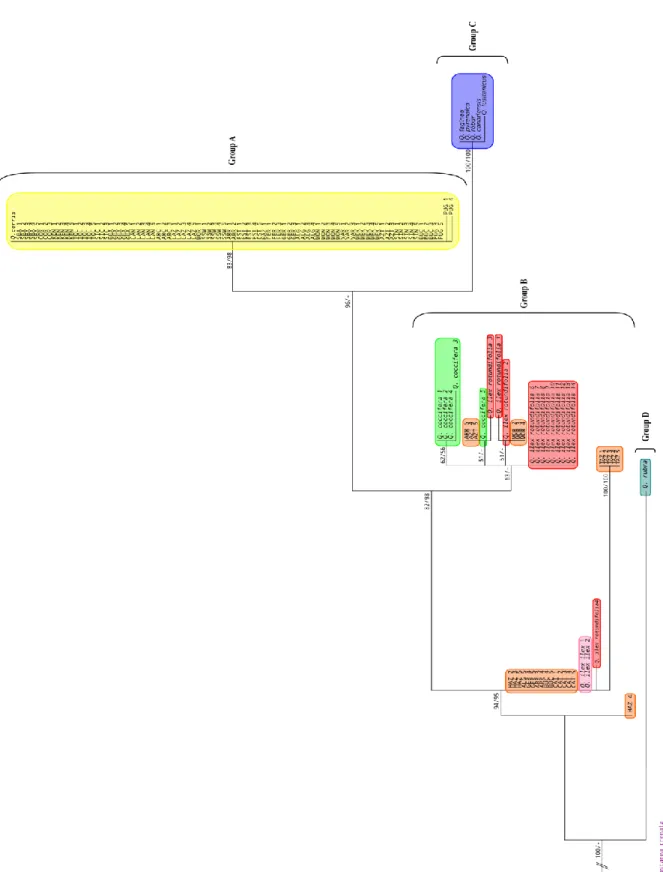
A sequenciação de vários fragmentos permitiu inferir alguns detalhes sobre a história evolutiva da espécie. O tradicional cpDNA foi seleccionado para sequenciação de 3 regiões inter-génicas (TrnL-F, TrnS-PsbC e TrnH-PsbA), num total de 148 amostras provenientes de 26 populações. No entanto, e porque inferências filogeográficas baseadas num único tipo de marcador não-recombinante pode dar informações erróneas sobre a história evolutiva da espécie, o genoma nuclear (nuDNA) também foi explorado com a sequenciação de um gene candidato potencialmente envolvido no stress osmótico (EST 2T13), em 104 amostras provenientes das mesmas 26 populações. Para ambos os conjuntos de dados foram detectadas duas linhagens presentes em sobreiro. Uma linhagem, a “linhagem pura”, parece praticamente exclusiva do sobreiro e divide-se em três sub-linhagens possivelmente resultantes de três zonas de refúgio, sendo uma predominante na zona Oeste do Mediterrâneo, e as outras duas na zona Este do Mediterrâneo. A outra linhagem aparece associada a
Quercus ilex (azinheira) e Quercus coccifera (carrasco) e foi apelidada de “linhagem introgredida”. Esta linhagem parece resultar de vários fenómenos de hibridação e
introgressão com Quercus ilex. A análise combinada das sequências do cpDNA e nuDNA sugere que esta introgressão aconteceu em ambos os sentidos entre as duas espécies, assim como sugere que estes eventos foram frequentes e consecutivos durante um período de tempo.
Finalmente, microssatélites nucleares, derivados de ESTs (Expressed Sequence Tags) (EST-SSRs) e anónimos (nu(EST-SSRs), permitiram obter uma perspectiva dos padrões de diversidade genética e estrutura populacional do sobreiro. Numa primeira fase foi possível estabelecer os EST-SSRs como marcadores válidos no sobreiro, contrariando a ideia de que os EST-SSRs
X
tendem a ser pouco polimórficos. Posteriormente, uma análise combinada destes dois marcadores (5 EST-SSRs e 3 nuSSRs) em 379 indivíduos provenientes de 13 populações detectou uma diversidade genética relativamente baixa, mas altamente significativa. Apesar de não ter sido detectada estrutura populacional nas populações Portuguesas, aparecendo em conjunto num grupo populacional, verifica-se uma tendência para considerar a Catalunha (Espanha) como uma das populações mais diferenciadas.
No geral os objectivos do trabalho foram cumpridos, esclarecendo alguns pontos da filogeografia e história evolutiva do sobreiro. A introdução dos novos marcadores moleculares foi claramente informativa, revelando novos aspectos inesperados acerca dos padrões genéticos da espécie e assim o gerar de hipóteses explicativas completamente novas em sobreiro.
Palavras-Chave
XI
Abstract
Cork oak (Quercus suber L.) is one of the most important tree species, economically but also ecologically, in the Western Mediterranean region. Consequently there is an enormous interest in understanding the evolutionary history and current population structure in cork oak. Although some details on the genetic divergence of cork oak populations have been uncovered, it is most probable that a different and complementary analysis of chloroplastidial and nuclear DNA markers (cpDNA and nuDNA) can bring additional phylogeographical relevant information. So far, no one has attempted the molecular approach proposed in the present study for cork oak by combining cpDNA and nuDNA sequence variation and also anonymous nuclear microsatellites (nuSSRs) and EST-derived (Expressed Sequence Tags) (EST-SSRs) polymorphism data to infer phylogeographical patterns and history, possible glacial refuges, diversity levels and geographic structure.
A genetic survey was conducted sampling populations throughout the entire distribution range of the species. Genetic diversity was monitored at 8 nuclear microsatellite loci (3 EST-SSRs and 5 nuEST-SSRs) in 379 individuals derived from 13 populations, and at 4 DNA sequences (3 cpDNA intergenic spacer regions and 1 osmotic-stress related candidate gene) in 148 samples from 26 populations.
DNA sequences, of both cpDNA and nuDNA, confirmed two main lineages of cork oak haplotypes, the first named as pure lineage (mostly exclusive of cork oak but also shared with
Q. cerris) and the second as introgressed lineage (shared with Q. ilex and Q. coccifera).
However, sequences of the cpDNA show the complexity of the introgressed lineage, apparently indicating that these events of hybridization and introgression may have happened frequently and consecutively over a period of time. The theory of cork oak refugia over the last glaciations was also revisited (over the pure lineage of the cpDNA haplotypes) and three major haplotypes were detected, reflecting three possible refuge areas. Finally, with the microsatellite data, population differentiation was low but rather significant and the geographic subdivisions that could be defined isolated the Portuguese populations in one cluster, further characterizing the Catalonia (Spain) population as possibly the most differentiated population.
Key words
XIII
List of abbreviations
AFLP – Amplified Fragment Length Polymorphisms
BA – Bayesian analysis
BLAST – Basic Local Alignment Search Tool bp – base pairs
BP – Before Present
CBOL – The Consortium for the Barcode of Life
COI or Cox1 – cytochrome c oxidase I
cpDNA – Chloroplastidial DNA ESTs – Expressed Sequence Tags
EST-SSRs – EST-derived SSRs
FAO – Food and Agricultural Organization (of the United Nations)
ITS – Internal Transcriber Spacer
kb – Kilobases
MCMC – Markov Chain Monte Carlo
MP – Maximum Parsimony
mtDNA – Mitochondrial DNA
nuDNA – Nuclear DNA
nuSSRs – nuclear SSRs
PCR - Polymerase chain reaction
RAPDs – Random Amplified Polymorphic DNA
rDNA – Ribosomal DNA
RFLP – Restriction Fragment Length Polymorphism
sncDNA – Single-nuclear copy DNA SNPs – Single Nucleotide Polymorphisms
XV
Table of Contents
1. Introduction ... 17
Thesis Main Goals ... 18
1.1 An emblematic tree: Quercus suber L. ... 20
1.1.1 General aspects on cork oak and the “montado” ... 20
1.1.2 Taxonomic classification and phylogenetic studies ... 22
1.1.2.1 Barcoding in oak phylogenetics ... 24
1.1.3 Geographical distribution ... 25
1.1.4 Evolutionary history – Origin, glacial refugia and post-glacial recolonization ... 27
1.1.5 Genetic diversity studies ... 30
1.1.6 Hybridization and cytoplasmatic introgression ... 35
1.2 Molecular markers in phylogeography ... 37
1.2.1 Mitochondrial DNA (mtDNA) ... 37
1.2.2 Chloroplastidial DNA (cpDNA) ... 38
1.2.3 Nuclear DNA (nuDNA) ... 39
1.2.4 Simple Sequence Repeats (SSRs) ... 40
1.2.5 Expressed Sequence Tags (ESTs) ... 41
2. Materials and Methods ... 43
2.1 Sampling and DNA extraction ... 43
2.2 DNA sequencing ... 44
2.3 Microsatellite genotyping ... 47
2.4 Phylogenetic and phylogeographic analysis ... 48
2.5 Selective neutrality tests and demographic history ... 49
2.6 Genetic diversity and population differentiation ... 50
2.7 Genetic structure of populations ... 51
3. Results ... 53
3.1 Sequencing of chloroplast and nuclear DNA fragments ... 53
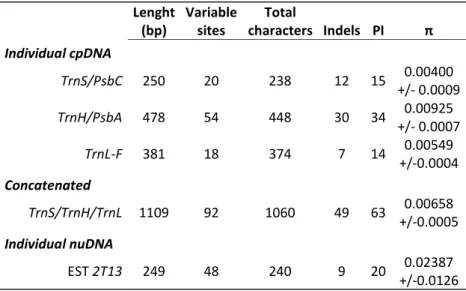
3.1.1 cpDNA and nuDNA diversity levels ... 53
3.1.2 Differentiation patterns ... 54
3.1.3 Mismatch distribution and neutrality tests ... 62
3.2 Microsatellite analysis... 64
3.2.1 Genetic diversity values ... 64
XVI
3.2.3 Population structure ... 68
4. Discussion ... 73
4.1 Differentiation and demographic patterns ... 73
4.2 Hybridization and introgression ... 75
4.3 Genetic diversity and population structure ... 79
5. Final Remarks ... 82 6. Bibliographic References ... 84 Supporting Information ... 95 Supporting Information 1 ... 96 Supporting Information 2 ... 97 Supporting Information 3 ... 99 Supporting Information 4 ... 101 Supporting Information 5 ... 105 Supporting Information 6 ... 109 Supporting Information 7 ... 110 Supporting Information 8 ... 111 Supporting Information 9 ... 115
17
1. Introduction
The year of 2011 has been designated as „The International Year of Forests‟ by the United Nations General Assembly, in an attempt to raise awareness and strengthen a more sustainable forest management and conservation of all types of forests for the benefit of current and future generations. Estimates by the Food and Agriculture Organization (of the United Nations) (FAO), in the year of 2010, demonstrated that 31% of the Earth‟s terrestrial surface is still covered by forests, and that trees correspond to 90% of Earth‟s biomass [1]. Some estimates of the global tree species richness state that there are 60,000 to 100,000 taxa, and that forests harbour the majority of the world‟s terrestrial biodiversity [2]. However, the ongoing deforestation and other human-induced global changes (such as climate and land use) brought the number of the world‟s tree species threatened with extinction close to 10% [3,4] and, although the overall rate of deforestation remains alarmingly high (estimated at 9.4 million hectares per year in the late 1990s), this rate is surprisingly slowing down [5].
In recent years forest trees have been gaining much attention as a non-classical model for several types of studies. For purposes of population and evolutionary genetic and genomic studies, they are particularly interesting since forest trees result of millions of years of lineage divergence and diversification and present amazing levels of diversity in morphology, adaptation, and ecology [6,7]. Although, in the end, the impact of global changes in forest trees will depend to great extent on the reaction of these trees and their ecosystems, genetic studies open the possibility of predicting the evolutionary consequences of the future global changes by increasing the knowledge on tree biodiversity and evolution [8]. For that purpose phylogeographic genetic studies seem to be an important step in understanding these processes.
Avise et al. presented the concept of “Phylogeography” in 1987 [9], and during the past 25 years or so phylogeography has had a major impact on research, particularly in animal species. In plants, however, the produced results have not been so explicit. One of the major problems has been a lack of useful genetic variation applicable to the phylogeographic analysis. It is quite difficult to find genetic markers in plants with a resolving power comparable to animal mitochondrial DNA (mtDNA) [7,10]. Nonetheless, plant
18
phylogeography has come a long way over the last few years with the availability of nuclear markers and with the collection of data from larger sections of chloroplastidial genome [11].
The genus Quercus (oaks) of the Fagaceae family is one of the most important groups of woody angiosperms in the northern hemisphere in terms of species diversity, ecological dominance, and economic value. The genus is quite old, since the oldest unequivocal oak fossils belong to the Oligocene, which ranges from 34 to 23 million years before present. Oaks are dominant members of a wide variety of habitats, and somewhat 500-600 species exist on earth [12,13].
The Quercus suber L. (commonly known as cork oak) is among the most important tree species (economically and ecologically) in the Western Mediterranean region, from where it is endemic, defining unique open woods (created and maintained by man) known in Portugal as “montados” ” and in Spain as “dehesas”. Quercus suber has been mostly used to produce cork and this natural product has a great economic value. The biggest threats, however, are faced by the marginal natural populations, often growing in small and scattered stands and in restricted habitats that are at risk of disappearing, mainly due to a lack of regeneration [14]. Due to the species economic value and also because cork oak woodlands are renowned reservoirs of biodiversity, home to a variety of threatened and endangered species, and crucial to avoid soil erosion, Q. suber populations represent valuable material for genetic studies as well as gene conservation programs. With that purpose, a greater knowledge about the spatial organization of genetic variation within the species is necessary to allow decisions to be made about tree breeding and the conservation of genetic resources.
Thesis Main Goals
Although previous studies have already addressed population genetics in Quercus suber, there is still a void in the current understanding of the evolutionary history of the species, mostly due to the limited number of geographical areas sampled or to low marker informative content. In particular, Portuguese populations have been poorly represented in those studies, and being Portugal one of the most relevant regions in the recent and past history of cork oak, a more complete range of cork oak distribution and differentiation should be covered,
19
including some areas referred as potential glacial refuges for other species. On the other hand, since the majority of the previous phylogeographical inferences are supported by data from chloroplastidial markers [Restriction Fragment Length Polymorphism (RFLP) and microsatellites (SSRs)], it should be raised the question whether other molecular approaches or genetic markers evolving at faster rates than chloroplastidial DNA (cpDNA), would provide a different microevolutionary scenario. Therefore, the main objectives of this work were to:
1. Infer the evolutionary history and demographic patterns of Quercus suber;
2. Assess the hybridization and introgression patterns by other Quercus species;
3. Evaluate the diversity and differentiation levels among and within some key cork oak populations.
To achieve these goals populations from the entire Mediterranean distribution of the species were analysed using different approaches with several molecular markers. A multi-locus sequencing approach was applied to infer the evolutionary history of the species and its relationships and also introgression patterns with other Quercus species. The traditional cpDNA was selected for sequencing of several fragments. Additionally, and because phylogeographic inferences based on a single non-recombining marker can be misleading, the nuclear genome was also explored with the sequencing of one candidate gene. Finally, Expressed sequence tag (EST) derived SSRs (EST-SSRs) and anonymous nuclear SSRs (nuSSRs) were also used with the intention of providing a perspective of patterns of genetic diversity and population structure.
20
Figure 1.1: Quercus suber L. – Cork
oak‟s natural population in Serra da Estrela, Portugal
1.1 An emblematic tree: Quercus suber L.
1.1.1 General aspects on cork oak and the “montado”
Cork oak (Quercus suber Linné, 1753) is an emblematic Mediterranean evergreen sclerophyllous tree. It is a slow growing, extremely long-lived tree, reaching about 20 meters height, with massive branches forming a round crown (Fig. 1.1). It is a diploid (2n=24), monoecious (both male and female reproductive organs in one individual) species with a protandrous system (anthers mature before carpels) to ensure cross-pollination. Plant propagation in natural populations occurs by seed (acorn) dispersal and subsequent germination (sexual reproduction), which is called natural regeneration. Cork oaks natural regeneration is mostly assured by wind and animals, as with those of most oak species [15-17].
Cork oak, along with holm oak (Quercus ilex L., 1753), are the two main evergreen oak species in the western part of the Mediterranean Basin [17]. These two species, particularly in the Iberia Peninsula are mostly present as semi-natural stands known as “montados”, which are open woods with a delicate and particular ecosystem, created and maintained by man.
The montado semi-natural landscape is valued because it represents a viable land use still preserving a rich biodiversity at all levels from insects and flora to top predators such as the Iberian Imperial Eagle (Aquila adalberti) or the Iberian Lynx (Lynx pardinus), the world‟s most endangered cat and their mutual prey species, the rabbit (Oryctolagus cuniculus).
They also represent an important economical resource, but with the exception of central Spain, holm oak forests can be regarded as rare cases of woodlands that have undergone very little or no silvicultural management. Cork oak management is, however, at a different level, since its high economical importance is associated not only with harvesting of acorns, but
21
also of cork. The thick and soft bark of cork oak is used to produce the familiar cork which is the main product responsible for the important economical role of this partly domesticated species. Trees are first stripped of cork, from the lower portion of the trunk at about 14 years of age and subsequently every 9-12 years, and can live through this process for 100 to 500 years without any apparent effect on tree physiology. Acorns are eaten by birds and they are highly valued as fattening fodder for domestic Iberian pigs. Since ancient times, cork oak has been favoured, and sometimes widely spread, by preferably using acorns from trees producing good quality cork [15,18-20]. Therefore, Q. suber is widely cultivated within its natural range, but according to Carrión et al. [21],without human activities, cork oak would never develop pure stands in the Iberian Peninsula, and would form mixed forests with other sclerophyllous and deciduous oaks, and with Pinus pinaster.
Cork oak forests cover 2,2 million hectares worldwide, from where 340,000 tons/year of cork are extracted (F. Simões de Matos, PhD thesis, INETI Lisbon, 2007). The largest stands, covering about 700,000 ha are located in Portugal and correspond to 21% of the forest area in Portugal and to 30% of the world‟s cork producing area. Currently, cork industry represents 3% of all Portuguese exportations. Cork stoppers for wine bottles are the most representative product of this industry, responsible for 70% of the exportations (see: http://www.amorim.com/cor_glob_cortica.php). In spite of the economic importance of this renewable material, there is still much to discover about both the biological and the genetic mechanisms involved in its formation. Human intervention through extensive plantations and systematic clear-cutting in forests with the objective of empirically selecting varieties with higher quality levels of cork is supposed to have strongly contributed to the genetic homogenization of Q. suber populations in the Iberian Peninsula [22].
Cork oak plantations are very important for the economy and play an important social and environmental role that has to be taken into consideration as the unparalleled decline occurring in the Iberian Peninsula and in Morocco is threatening the entire ecosystem [22]. Although the marginal and natural populations of cork oak are possibly the most endangered, Iberian cork oak montados are also currently threatened and in decline due to multiple factors. The main factor contributing to this decline is the occurrence of very severe drought periods over several consecutive years [18]. The lack of natural regeneration (mainly due to overgrazing and insolation, particularly in North Africa) is one of the most important factors and so stand sustainability cannot rely exclusively on the decreasing resprouting ability of
22
aged and decaying adult trees [23,24]. In Portugal and Spain another contributing factor to this decline is the occurrence of ink disease, a root disease caused by the soil born pathogen
Phytophthora cinnamomi. Moreover, the increasing use of synthetic stoppers in wine bottles
replacing the traditional cork is an additional factor that in conjunction with the above stated threatens this ecosystem at medium term [18,22].
Holm oak montados are also endangered for some of these and a number of other reasons. Thus, the admired sustainability of montados is jeopardized, and these formations may become „fossil forests‟ [23]. In this sense, the outlook is more favourable for Q. ilex which is a more euryecious species than Q. suber (whose presence is limited by cold, drought and soil type). In recent years, there has been increasing recognition of the important contribution made by these species to the preservation of seminatural habitats and landscapes in Europe [18,23,24]. Several studies on the regeneration of Mediterranean forest have been published, and some of them are centred on Q. ilex and Q. suber [24,25]. However, these works have focused mainly on ecological aspects of regeneration, silviculture and land use [23], without addressing the genetic bases of montado regeneration or the populations‟ diversity with consequences on adaptation, which is now an immediate priority to allow informed decisions for conservation of genetic resources.
1.1.2 Taxonomic classification and phylogenetic studies
Several proposals for Quercus taxonomy based on morphology have been presented [12,26], however these classifications have always been surrounded by controversy mainly due to a generalised intraspecific morphological variation that may be produced by hybridization and adaptation to ecological changes in the environment [27], especially abundant in oaks. As a result, classifications have been all but straightforward and especially at the subgenus level, still uncertain. The taxonomic scheme proposed by Schwarz in 1964 [26] is possibly the most accepted for the classification of cork oak, and appears to be the most suitable in describing the systematics of European oaks [19,28,29]. According to the Flora Europaea [17], and that same taxonomic scheme, the genus Quercus is divided in four subgenera (or subsections), as follows:
23 Order Fagales Family Fagaceae Genus Quercus Subgenus Cerris Quercus Sclerophyllodrys Erythrobalanus
Quercus suber belongs to the family Fagaceae, genus Quercus and subgenus (or subsection)
Cerris (Spach) Oersted.
Quercus comprises 500-600 species, of which 350–500 species are distributed throughout the
Northern Hemisphere [12,13,30]. They are conspicuous members of the temperate deciduous forests of North America, Europe, Asia, as well as the evergreen Mediterranean maquis. A smaller number of oak species (30–35) are evergreen and grow mainly in south-western Asia, western North America and around the Mediterranean Basin. In the Mediterranean area, only four evergreen oak species have been identified. These include Quercus alnifolia Poech. (golden oak) endemic to Cyprus and Quercus suber L. (cork oak) distributed exclusively in the western part of the Mediterranean Basin. The third species is the holly oak which is a complex including Quercus coccifera L. and Quercus calliprinos Webb. Allozyme studies suggest that holly oak should perhaps be considered as a single species (Q. coccifera L.) with subspecies coccifera and calliprinos [19]. The fourth Mediterranean oak species, Quercus ilex L. (holm oak), shows two morphological types, rotundifolia and ilex type [18,19,26], which are sometimes regarded as distinct species.
According to Schwarz [26], Q. ilex and Q. coccifera (including subsp. calliprinos) belong to subgenus Sclerophyllodrys (O. Schwartz) whereas Q. suber and Q. alnifolia relate to subgenus Cerris (Spach). This classification was also supported by RFLP analysis of the nuclear ribosomal DNA (rDNA) 18S and 25S and spacer regions [28] and chloroplastidial DNA [30] and by nuclear DNA (nuDNA) Internal Transcriber Spacer (ITS) sequences [27,30], however Q. alnifolia was not included in these studies. Moreover, from the study of Manos et al. [30], evidence was obtained that the two groups of Mediterranean oaks (subg. Sclerophyllodrys and subg. Cerris sensu Schwarz; ”Ilex group” and “Cerris group” sensu
24
Nixon) are monophyletic, as reported previously by Nixon [29]. More recently, they constitute a larger group (the Eurasian Cerris group) which includes all the European and Asiatic evergreen oak species analysed [27,30]. When considering the subg. Cerris, several systematic studies support that Quercus cerris and Quercus crenata are the most closely related species to Q. suber [27,29-31].
1.1.2.1 Barcoding in oak phylogenetics
Tree species share several attributes, such as longevity, complex reproductive strategies, great potential for local adaptation, and slow mutation and speciation rates [2], that makes barcoding of forest trees a captivating issue from both speculative and practical points of view. “DNA Barcoding” is a molecular approach to identify the species to which any living organism belongs by the use of a standardised gene region of the genome (or several loci used together as a complementary unit). Ideally, the barcode system would be an universal and valuable resource that would allow fast and unequivocal species identification and taxon characterization at any life stage of the specimen and from minimal tissue samples (http://www.barcoding.si.edu) [29,32,33]. Besides taxonomy, a widespread application of barcoding would be a powerful research complement for molecular ecology, phylogenetics, and population genetics [34].
The success of a DNA sequence as a species identification tool - the barcode - depends on the prerequisite of existence of unique substitutions that distinguish among closely related species, and ease of application across a broad range of taxa. A portion of the mitochondrial cytochrome c oxidase I (COI or cox1) gene sequence is currently being used as a universal barcode in certain groups of animals, fungi, diatoms, and red algae. However, COI has proved to be unsuitable in land plants, mainly because of the low nucleotide substitution rates of the plant mitochondrial genome [7,35,36]. The nuclear and plastid plant genomes therefore offer the best expectation of yielding a suitable sequence (or pool of sequences) for DNA barcoding, i.e., a sequence(s) that will be variable enough to differentiate species, but at the same time still stable enough at a lower taxonomic level as to have low infraspecific variability [33,35]. The difficulty in finding a single-locus for barcode in plants suggested a multilocus approach, focusing on the chloroplast genome as the most promising strategy for barcoding plant species. Therefore a pool of loci has been recently considered, with the
25
greatest interest turned to seven candidates: rpoB, rpoC1 and rbcL as three easy-to-align coding regions, a section of matK as a rapidly evolving coding region, and trnH-psbA,
atpF-atpH, and psbK-psbI for being three rapidly evolving intergenic spacers [36,37]. Based on the
relative ease of amplification, sequencing, multialignment, and on the amount of variation displayed, many research groups have proposed different combinations of these loci [32,36-39]. However, in 2009, the CBOL (The Consortium for the Barcode of Life) Plant Working Group stated the combination of rbcL and matK as the most convenient in terms of universality, sequence quality and discrimination power. Nevertheless, it is still argued that regardless of the regions adopted for barcoding, some species will always be better resolved with the use of other regions [29,36,40]. Such an example is the oaks, which represent an obstacle to the idea of barcode in plants.
A recent attempt of barcoding in the Italian wild dendroflora, with the use of four plastid regions (trnH-psbA, rbcL, rpoC1, matK), revealed that the genus Quercus is noncompliant to barcoding (0% discrimination success) [29], a probable consequence of factors like low variation rate at the plastid genome level and hybridization. Nonetheless, it appears that the main obstacle to barcoding success in difficult genera, such as Quercus, cannot simply be overcome by adding additional plastid DNA data. Nuclear DNA may offer some advantages due to higher mutation rates and modes of inheritance. Discrimination of the same set of oak species was already obtained by means of internal transcribed spacer region of ribosomal DNA (ITS) sequence variation [27], and it even supports the recognition of the subgenus Schlerophyllodrys, Cerris, and Quercus, as proposed by Schwarz [26]. The rapidly evolving ITS may thus represent a useful supplementary barcode in difficult genera, although not without completely overcoming extant problems, namely the paralogy and other factors associated with the complex concerted evolution of this highly repeated part of the nuclear genome, which still requires further refinement of current protocols [7,35].
1.1.3 Geographical distribution
The Mediterranean evergreen Quercus species are a group with overlapping habitats. In the Western Mediterranean Basin, holm oak, cork oak and holly-oak are the dominant broadleaved species. These three species are sympatric in many areas, but some differences in their ecological requirements produce distinct responses to environmental conditions and
26
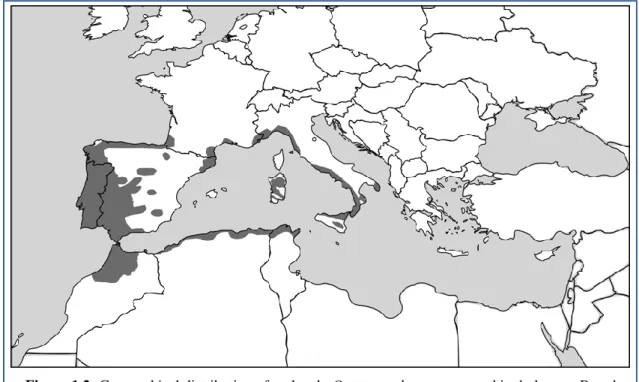
Figure 1.2: Geographical distribution of cork oak, Quercus suber, represented in dark grey. Based
on Magri et al. [16]
hence different evolutionary histories as interestingly confirmed by several studies showing differences in their genetic variation patterns at both nuclear and cytoplasmic levels [18,19,30,41,42].
Q. suber has quite a narrow geographical range when compared to the other main evergreen
Mediterranean oak species, mainly due to its ecological restrictions. The modern distribution of cork oak, rather discontinuous, ranges from the Atlantic coasts of North Africa and Iberian Peninsula to the southeastern regions of Italy, and includes the main western Mediterranean islands of Sicily and Sardinia as well as the coastal belts of Algeria and Tunisia, Provence (France) and Catalonia (Spain) [16,43] (Fig. 1.2).
As opposed to holm oak which shows a great ecological amplitude, cork oak is restricted to hot (>4ºC – 5ºC mean temperature for the coldest month) variants of the humid and sub-humid Mediterranean areas with at least 450 mm mean annual rainfall [18,20].
27
In Europe there are, theoretically, low winter temperatures that appear to set the geographic distribution limits and most cork oak stands are located in areas below 800 meters in altitude, since cork oak leaves are less tolerant to frost and to drought than those of the more widespread holm oak. In addition, whereas holm oak is indifferent to soil types, cork oak usually grows in acidic soils on granite, schist, or sandy substrates and it avoids limestone and other carbonated substrates. Cork oak distribution is therefore more shifted to the west and more patchy than that of holm oak (sensu latu) which constitutes a continuum from Turkey to Portugal, including all the larger Mediterranean islands [17,18,20,43]. In spite of this, within its geographical range, cork oak shows high levels of morphological and phenological variability, albeit most of this diversity is considered to be result of past introgressive hybridization with other sympatric species [15,44,45]. Nowadays, in their common distribution area, cork and holm oaks often grow together and the local occurrence of morphologically intermediate trees has been reported [18,27].
1.1.4 Evolutionary history – Origin, glacial refugia and post-glacial recolonization
Several hypotheses have been advanced concerning the evolutionary history of cork oak as well as the geographical location of its centre of origin; however the details of its differentiation processes are still largely unknown.
It was originally suggested that Q. suber may have originated in the Iberian Peninsula where the species has its current main range (Fig. 1.2). This hypothesis was based on geobotanical studies and on allozyme variation from the whole cork oak range, which revealed a substantially higher genetic diversity in the Iberian populations as compared with those from North Africa, Italy and France [18,27]. Paleoecological data indicate that both cork and holm oak species have been present in south Europe since the end of the Tertiary period. Also, two fossil records of cork oak from Miocene age were found in Portugal and two belonging to the Pliocene were recovered in Tunisia and Galicia (Spain). Therefore it seems plausible an early Cenozoic origin for Q. suber in Iberia and subsequently, at the end of the Miocene, the colonization of North Africa from the Gibraltar strait [16,18,43].
Alternatively, according to fossil records of other oak species of subgenus Cerris, dating to the Tertiary and found in the Balkanic Peninsula, it has also been considered that Q. suber
28
might have appeared first in more eastern countries (either in the Balkanic Peninsula or, alternatively, in the Middle Eastern-Peri-Caucasian area), in common to the whole Cerris group. It has been suggested that the species expanded westward during the late Miocene and was widespread throughout the Mediterranean Basin during the Pliocene, where it survived thanks to the lack of climatic constraints, but going extinct in the eastern part of its distribution area [27,43]. Data from PCR–RFLPs over cpDNA fragments seems to constitute additional evidence to support an eastern origin for cork oak [43].
Glacial and periglacial environments have had a significant effect on the modern vegetation of Europe. It is widely accepted that the climatic oscillations that occurred during the Quaternary (i.e., over the past 1.8 million years) are one of the most crucial determinants of the current distribution of biota in temperate latitudes. The spatial patterns of several tree species throughout the European continent are the long-term result of late glacial and post-glacial migration from refugial populations that were able to withstand the severe climatic conditions of Pleistocene stadials [46-49]. With few exceptions [8,50,51], during the coldest periods of the last full glacial epoch (37,000 – 16,000 years BP – before present) the locations postulated for glacial refugia of most European woody angiosperms have been south of the parallel 40º N, which runs from central Portugal to Sardinia, Calabria and northern Greece. This is considered to be the boundary between polar aridity and warmer climates during part of the Quaternary. The theory that southern Europe (particularly the three southern peninsulas - Balkan, Italian and Iberian) and the Near East provided appropriate conditions for refugia of temperate tree taxa is based on a number of assumptions relating to the full-glacial environments of those regions and their ability to supply the necessary conditions for growth [52,53].
The original refugial model idea implied that „forests‟ could have survived in these southern locations during the cold stages of the Quaternary. However, extensive populations of trees have never been detected. Instead, the traditional palaeogeographical models (although inferred from a scarce palynological evidence) suggest a small number of refugia - the “few
southern refugia” hypothesis [52,53]. Temperate tree taxa possibly survived in small pockets
of microenvironmentally favourable locations where usually only a few tree taxa are detected and in low concentrations. Aridity was probably a significant limiting factor for tree growth. Assuming the hypothesis of “few southern refugia”, common patterns of post-glacial colonization for temperate European tree species are defined and expected, with high
29
diversity levels in southern Europe and decreasing northwards [54-57]. Some of the main European trees species have been analysed using molecular markers, including for example several Quercus species and Abies alba (European silver fir), and the resulting patterns of diversity correspond to the expected ones [58,59].
However, more complete palaeobotanical data sets [50,53], palaeoclimatic modeling [60] and genetic research [52] are starting to question the paradigm of “few southern refugia” in southern Europe (and in particular in the three southern peninsulas) during full-glaciations. Increasing evidence indicates that during the last full-glacial period populations of coniferous and some deciduous trees grew much further north and east than previously assumed [53]. In addition, new palaeoclimatic simulations suggest that full-glacial conditions in central and eastern Europe were not nearly as severe as previously anticipated [60,61]. While some refugia for Mediterranean trees were previously identified in the Iberia Peninsula, López de Heredia et al. (2007) results based on cpDNA PCR-RFLPs and a review of paleobotanical data support the presence of multiple refugia for the evergreen oaks within the Iberian Peninsula (e.g. Cantabric mountain ranges, south-eastern Spain or even central Spain) during, at least, the last glacial period [52]. Under the “multiple refugia” hypothesis, tree species that nowadays are present in the north and central Europe would have recolonized these areas from populations located in the north of the Iberian Peninsula. Moreover, these populations would have been barriers preventing expansion from southern refugia. If that was the case, cpDNA data should show complex patterns of spatial distribution that would have resulted from the generation of multiple secondary contact zones [8].
For the last glacial and postglacial periods, results from palynological data indicate the occurrence of cork oak in south-western Iberia since the Late Glacial period (17,000-12,000 years BP) and in North Africa since the early Postglacial (approximately 8,500 years BP) [18,27]. It is accepted that during the Quaternary glaciations, cork oak may have survived in scattered refugia which possessed favourable microclimate conditions, and from which postglacial colonization occurred over recent millennia. Palynological [21] and molecular data [43,52] indicate a glacial refugia in south-western Iberia that expanded northwards in the absence of mountain barriers and which was favoured by the existence of siliceous substrates. It is also possible that the extensive introgression of Q. suber with Q. ilex may also indicate several potential refugia in eastern Iberia [52]. RFLP analysis of the whole cpDNA show a phylogeographical pattern of three groups corresponding to potential glacial refuges in Italy,
30
North Africa and Iberian Peninsula [43], from which, after the last glaciations, Q. suber may have begun migrating northward to the southern part of France. However no fossil record supports the molecular data for the Italian and North African refuge.
Reliable scientific evidence is lacking to confirm the presence of Q. suber in more northern and eastern European countries. The Tertiary and Quaternary remains (megafossils and pollen) found in several European countries did not allow taxonomic identification at the species level and could be attributable to any Mediterranean oak species of the Cerris group [43,62]. In fact, Q. suber is more thermophilous and has stricter soil requirements than many other Quercus species, thus a bigger reduction of this species‟ range during glacial times is expected to have happened. However, the uncertainty of palynological discrimination and the lower cpDNA variation itself could bias the identification of glacial refugia for Q. suber [52].
1.1.5 Genetic diversity studies
As cork oak is predominantly allogamous, i. e. favouring cross-fertilization, with a life span of up to 500 years or more and having a low replacement rate, it can be expected that at least in some places, and mostly for selectively neutral characters, selection over time may have resulted in reduced genetic differentiation both among trees of the same population and between populations. However, a differentiation among populations has been detected around the Mediterranean Basin by investigating both chloroplast and mitochondrial DNA (which are maternally inherited in oaks, as demonstrated by Dumolin et al. [63]), as well as allozyme variation [16,18,19,67]. Isozyme variation in the genus Quercus also shows that genetic variability is high and similar to that found in conifers [15,65].One of the main causes for the high polymorphism found in cork-oak, as well as in holm-oak, may be attributable to the physiological plasticity of the species, which allows them to adapt to variable and unpredictable climatic conditions, characteristic of the Mediterranean climate. High levels of diversity within populations are observed; conversely, low inter-population variability indicates that most of the total genetic diversity in the species is found within rather than among populations [15,18,19,23]. According to Elena-Rosselló & Cabrera [15], more than 83% of the total diversity in this species is found within populations, and the decline of kinship estimates with distance suggests that isolation by distance has led to this structure. The results obtained for Q. suber contrast with those found for most temperate forest species,
31
for which a generally weak and narrower within-population structure is the trend [23]. In cork oak, gene flow between populations was estimated as more than one migrant per generation (F. Simões de Matos, PhD thesis, INETI Lisbon, 2007) and is theoretically enough to prevent genetic drift from causing local genetic differentiation and therefore population divergence, under the Wright‟s Island Model [66].
PCR-RFLPs over specific cpDNA fragments illustrate a complex pattern of variation in the evergreen oaks [19,41]. Jiménez et al. [41] detected three very distinct lineages of cpDNA haplotypes, two of them being present in cork oak. One of the lineages, the “suber” lineage, is specific to cork oak populations and may be considered as the original and most widely distributed lineage in this species. The partial geographical distribution of this lineage was reported by López de Heredia et al. [64], from peninsular Italy, Sardinia, Sicily, Corsica, northern Africa and the island of Minorca. Cork oak populations from the Spanish mainland and from the island of Majorca were characterized by another maternal lineage also shared with Q. ilex and Q. coccifera, the “ilex-coccifera I” lineage [41,64]. This fact was interpreted as the result of multiple and mainly unidirectional cytoplasmic introgression of Q. suber by
Q. ilex.
RFLP analysis over the whole chloroplastidial DNA was used by Lumaret et al. [43] for the first time in Q. suber to analyse the phylogeographical variation over the whole species range (Fig. 1.3). The chlorotypes showed a clear phylogeographical pattern of three groups corresponding to potential glacial refuges in Italy, North Africa and Iberian Peninsula. The most ancestral and recent groups were observed in populations located in the eastern and western parts of the species range, respectively. Unrelated chlorotypes of an “ilex” cpDNA lineage were also identified in specific western populations [43]. From the cpDNA variants of „ilex‟ lineage recovered through interspecific introgression, additional successive cpDNA changes may have occurred in Q. suber, and so two distinct cpDNA lineages in cork oak were predicted. A particular chlorotype S1, observed predominantly in continental Italy and in Sicily, was identified by Lumaret et al. [43] in a few populations from Sardinia, and from Corsica which also shared a rare chlorotype S7 with Tunisia. This situation possibly reflects the occurrence of rare natural events of long-distance dispersal from several geographical sources located in the closest areas to those islands. Moreover, the possibility of an intentional acorn transport by people for economic purposes cannot be ruled out and its impact on the geographical patterns of cork oak genetic variation should not be
32
underestimated [43]. López-de-Heredia et al. [64] also proposed the possibility of long-distance dispersal events to explain the sharing of a rare chlorotype by cork oak populations located in Minorca and in Sardinia.
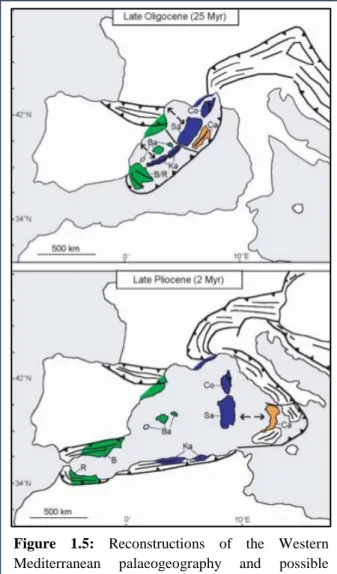
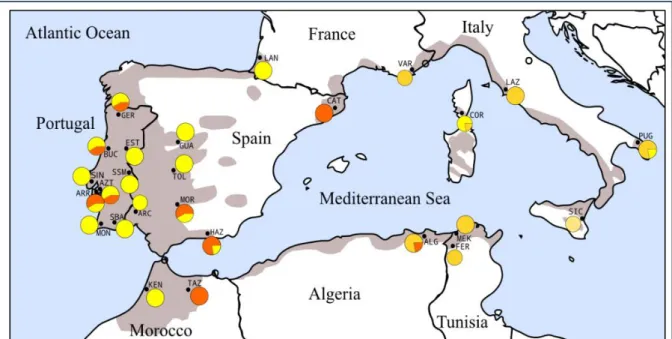
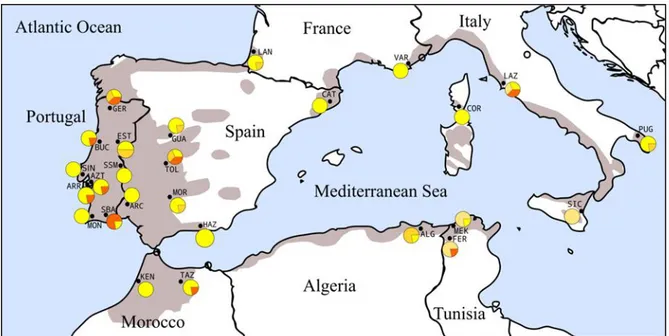
Using cpDNA microsatellites, Magri et al. [16] analysed cork oak populations throughout the species distribution range and found a high geographical structure characterized by five distinct haplotypes (Fig. 1.4). It was assumed that H3 (north Africa-Sardinia-Corsica-Provence) and H4 (Portugal-western Spain-southwest France-northern Morocco) were the ancestral Q. suber haplotypes, with H1, H2 (Italy) and H5 (scattered populations) originating through ancient or recent introgression with Q. cerris (H1 and H2) and Q. ilex (H5). Also, the cpDNA SSR data combined with paleobotanical and geodynamics models demonstrated that cork oak populations have possibly experienced a genetic drift geographically consistent with the Oligocene and Miocene break-up events of the European–Iberian continental margins and persisted in some of the separate microplates that are currently found in Tunisia, Sardinia,
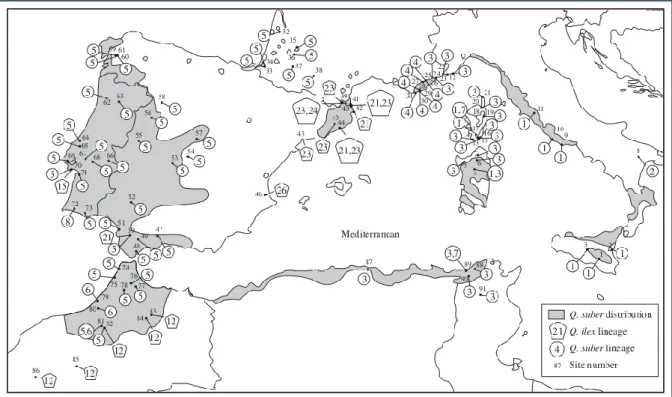
Figure 1.3: Geographical distribution of the eight and six chlorotypes of the „suber‟ and „ilex‟ lineages
identified in Q. suber populations by Lumaret et al. [43]. Chlorotypes were scored by RFLP variation over the whole cpDNA molecule. The identity of sampled populations and cpDNA chlorotypes assayed through RFLP as well as affiliation to the „suber‟ or „ilex‟ cpDNA lineages are indicated in the Figure. Source:
33
Figure 1.4: Distribution of cpDNA haplotypes found by Magri et al. [16] with cpDNA SSRs and
phylogenetic reconstruction of the relationships between haplotypes. The black circle in the network indicates a hypothesized mutation, which is required to connect existing haplotypes within the network
with maximum parsimony. The grey area corresponds to the current distribution of Q. suber Source: Magri et al. [16].
Corsica, and Provence [16] (Fig. 1.5). All these events seemed to have occurred without detectable cpDNA modifications for a time span of over 15 million years.
The modern history of Quercus suber is closely related to human activity over the use of its cork. For this reason, humans have been considered responsible for a reduction in genetic variation in some stands of cork oak, as well as for hybridization with congeners [67]. Other cultivated tree species in the Mediterranean area display a similar low geographical structure in genetic variation, arguing for a multidirectional diffusion because of human activity. For example, in Castanea sativa, the low geographical structure of the chloroplast genetic diversity may be explained by the effect of a strong human impact [67,68]. However the geographical distribution of the cork oak haplotypes found by Magri et al. [16] does not appear to be related to cultivation. In fact, fossil pollen and wood records suggest that cork oak was distributed in approximately the same areas as today even before the Neolithic.
34
Figure 1.5: Reconstructions of the Western
Mediterranean palaeogeography and possible location of Quercus suber haplotypes found by Magri et al. [16] (colours as in Fig. 1.4). Continental microterranes rifted off the European-Iberian continental margin: Rif (R), Betic range (B), Balearics (Ba), Kabylies (Ka), Corsica (Co), Sardinia (Sa), Calabria (Ca). Source: Magri et al. [16].
Another possible hypothesis to explain these results is postglacial population expansion from the potential glacial refuges in Italy, North Africa and Iberian Peninsula [43].
Some studies have also assessed the genetic variability of cork oak populations in Portugal. Coelho et al. [22] used AFLP markers and reported low levels of differentiation among cork oak populations. The reasons pointed out are owed to the outcrossing characteristic of the species, long distance anemophilous pollination and eventual secondary acorn dispersal by animals, leading to extensive gene flow and an increased homogeneity of allele frequencies between populations [22,45]. The values of population differentiation reported by Coelho et al. [22] (FST =0.0172)
are below the average of 0.07–0.09 expected for long-lived, wind-pollinated woody species. These results are similar to those found by Simões de Matos (F. Simões de Matos, PhD thesis, INETI Lisbon, 2007) with nuclear SSRs (FST=0.02), confirming
the absence of population structure. This pattern of genetic differentiation within Portuguese cork oak stands, some located over a distance of 700 km, may be explained by anthropogenic pressure in addition to a constant gene flow. This study shows that 90% of the polymorphic markers identified in cork oak genotypes are uniformly distributed through the populations of Algarve, Alentejo and Trás-os-Montes regions.
35
1.1.6 Hybridization and cytoplasmatic introgression
Capture of unexpected chloroplast haplotypes by hybridization and introgression has been proposed as the most likely explanation for the sharing of cytoplasmic genes both in deciduous and evergreen oaks [69] as well as in others [41,70]. Q. suber was reported to hybridize with several species of the evergreen oak group, particularly, with holm oak [17,45] this being regarded as one factor contributing to the increase of genetic diversity in cork oak [22]. Q. suber and Q. ilex possess overlapping geographical distributions [17], and hybridization occurs in nature, although it is not a frequent event [45]. Nevertheless, these species are not very closely related, as shown from both cytoplasmic and nuclear genetic analyses [19,27,69] and belong to subgenera Cerris and Schlerophyllodrys, respectively [26], although the more recent classification includes both species within the same Eurasian Cerris group [30].
The two most easily recognizable oak hybrids are Quercus x crenata (Q. cerris x Q. suber) and Quercus x morisii (Q. suber x Q. ilex) [27]. It must be noted that these relatively rare hybrids (0.3%) are found only when both parental species co-occur. Mature hybrid individuals are easily recognized due to intermediate morphological traits between the two parental oaks [27], but seedlings and even juvenile trees show very similar morphological traits so that, in mixed stands, species identification is usually very difficult or even impossible until the adult stage [71]. Asymmetric hybridization has been confirmed by Boavida et al. [45], upon the description of post-pollination barriers in Q. suber to interspecific crosses with Q. ilex, Q. coccifera, Q. faginea and Q. robur. The cross between
Q. ilex and Q. suber shows evidence of unidirectional compatibility and a higher success rate
was reported in the interspecific crosses in which Q. suber acts as pollen donor rather than as female parent due to a differential growth in the pollen tubes of both species [45]. Also, since both species are protandrous and Q. ilex flowers earlier, early cork oak male flowers can pollinate late holm oak female flowers, the reverse not usually occurring.
By analysing polymorphism at allozyme loci and DNA markers for which alleles are distinct in the two species growing in separate areas (diagnostic markers), evidence was obtained for the occurrence of hybrids and genetic introgression (backcrosses between hybrids and parental species) between sympatric holm oak (female) and cork oak (male) in several locations [18,43,71]. Further evidence was advanced that, in initial hybridization and in backcrosses, Q. ilex is predominantly, but not exclusively, the maternal species. This
36
interpretation is supported by the discovery of “ilex-coccifera I” haplotypes (chlorotype shared by Q. ilex and Q. coccifera) in Q. suber individuals, and the absence of the opposite situation, that is no Q. suber haplotypes within the Q. ilex pool [41].
The effect of hybridization and introgression in Q. suber cpDNA can produce the total replacement of the Q. suber chlorotype by the “ilex-coccifera I” lineage (chlorotype shared by Q. ilex and Q. coccifera) [41,64]. This situation is common in eastern Spain, where siliceous soils are scarce and the effective population size is lower than in the continuous forests from western Iberia. It has been suggested, on the basis of the differences between Q.
ilex and Q. suber chlorotypes found in sympatric populations, that hybridization and
introgression in these populations may be ancient [43]. Therefore, as reported by López de Heredia et al [52] it cannot be ruled out that in the eastern range of the species some populations withstood the glacial conditions by hybridizing with Q. ilex. For instance, a particular chlorotype found by these authors (named “c66”) is predominant in all Q. suber populations from Catalonia (north-eastern Spain), being very rare in Q. ilex.
The absence of cork oak populations possessing „ilex‟ chlorotypes in the eastern Mediterranean range of the species was reported both by López-de-Heredia et al. [64] and by Lumaret et al. [43]. However in a Corsican population, one of the 50 cork oak individuals scored for cpDNA RFLPs was shown to possess an „ilex‟ chlorotype [43], suggesting that cytoplasmic introgression of Q. suber by Q. ilex does occur in the eastern range although apparently much less commonly. A substantial number of trees showing intermediate morphology between both species have been observed in south-eastern continental Italy [27], in Sardinia and Provence [42], also possessing predominantly an „ilex‟ chlorotype and for many of them a hybrid origin was confirmed on the basis of nuclear interspecific diagnostic markers. So, interspecific hybridization is likely to have happened quite frequently in the eastern part of the range of Q. suber as well [43].