w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Metabolic
profile
and

-glucuronidase
inhibitory
property
of
three
species
of
Swertia
Swagata
Karak,
Gargi
Nag,
Bratati
De
∗PhytochemistryandPharmacognosyResearchLaboratory,DepartmentofBotany,UniversityofCalcutta,Kolkata,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received29February2016 Accepted18July2016 Availableonline3October2016
Keywords: Swertia
-Glucuronidase Xanthones Hepatoprotective
a
b
s
t
r
a
c
t
-Glucuronidaseinhibitorsaresuggestedaspotentialhepatoprotectiveagents.Swertiachirayita(Roxb.)
Buch.-Ham.exC.B.Clarke,Gentianaceae,isknownforitshepatoprotectiveandanti-hepatotoxic
activ-ityinAyurvedicsystemofmedicineforages.ThisplantissubstitutedbyotherspecieslikeS.decussata
NimmoexC.B.ClarkeandS.bimaculata(Siebold&Zucc.)Hook.f.&ThomsonexC.B.Clarke.Theaimofthe
studywastocomparemetaboliteprofileand-glucuronidaseinhibitoryactivityofthesethreeimportant
speciesofSwertiaandtoidentifytheactiveconstituents.S.chirayita(IC50210.97g/ml)andS.decussata
(IC50269.7g/ml)showed-glucuronidaseinhibitoryactivitysignificantlyhigherthanthatofsilymarin,
theknowninhibitoroftheenzyme.TheactivityofS.bimaculatawaslow.Themetabolitespresentinthe
threespecieswereanalyzedbyHPLCandGC-MSbasedmetabolomicsapproach.Fiveaminoacids,twenty
oneorganicacids,oneinorganicacid,eightfattyacids,twentyonephenolsincludingxanthones,eight
sugars,sevensugaralcohols,fiveterpenoidsandamarogentinwereidentified.Activitiesofthe
xan-thonesmangiferin(IC5016.06g/ml),swerchirin(IC50162.84g/ml),decussatin(IC50195.11g/ml),
1-hydroxy-3,5,8-trimethoxyxanthone(IC50245.97g/ml),bellidifolin(IC50390.26g/ml)were
signifi-cantlyhigherthanthatofsilymarin(IC50794.62g/ml).Quinicacid(IC502.91mg/ml),O-acetylsalicylic
acid(IC5048.4mg/ml),citricacid(IC501.77mg/ml),d-malicacid(IC5014.82mg/ml)andsuccinicacid
(IC5038.86mg/ml)alsoinhibitedtheenzyme-glucuronidase.Thefindingssuggestthatconstituents,
inadditiontothexanthones,probablyalsocontributetothebioactivityofdifferentSwertiaspeciesby
synergisticeffect.Furtherinvivostudyisrequiredtosupporttheclaim.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Liverdiseasehasbecomeamajorhealthissueglobally(Byass,
2014). The liver is a vital organ that is involved in
mainte-nance of metabolic functions and helps in the detoxification
processbycountering severalexogenousand endogenous
chal-lenges(Kshirsagaretal.,2011).Glucuronidationisamajorpathway
ofphase IIxenobiotic biotransformation(deGraafet al.,2002).
Conjugationoftoxinswithglucuronicaciddeactivatespotentially
damagingcompoundsandsubsequentlyeliminatesthemfromthe
body.However,thisprocessbecomeslimitedbytherateof
deglu-curonidationby-glucuronidase.Hydrolysis oftheglucuronide
moietycanbecarriedoutby-glucuronidasepresentinmostof
thetissues,inendocrineandreproductiveorgans(Dutton,1980).
∗ Correspondingauthor.
E-mail:bdbot@caluniv.ac.in(B.De).
Liverdamagecausesanincreaseinthelevelof-glucuronidasein
blood(Pinedaetal.,1959),andlivercancercouldberelatedtothis
enzyme(MillsandSmith,1951).
-Glucuronidaseinhibitorsreducethecarcinogenicpotentialof
toxiccompoundsnormallyexcretedinbileafterglucuronidation
(Walaszeketal.,1984).Duetothiscorrelation,-glucuronidase
inhibitorsaresuggestedaspotentialhepatoprotectiveagents(Shim
et al., 2000). Certain hepatoprotective plant extracts and their
constituentsare knowntoinhibittheenzyme, -glucuronidase
(JoshiandSanmugapriya,2007).Silymarin(amixtureof
flavono-lignans),thecommercialplantderived-glucuronidaseinhibitor
(Kimetal.,1994),isusedtotreatliverdisordersandalsocertain
cancers(Dixitetal.,2007).Butithaspoorbioavailability(Dixitetal.,
2007).Silymarinhascertainotherlimitationsrelatedto
gastroin-testinaltractlikebloating,dyspepsia,nausea,irregularstooland
diarrhoea.Italsoproducedpruritus,headache,exanthema,malaise,
asthenia,andvertigo(PradhanandGirish,2006).Hence,searchfor
glucuronidaseinhibitorycompoundsfrommedicinallyimportant
traditionalplantsthatareearlierreportedtobehepatoprotective
isnecessary.
http://dx.doi.org/10.1016/j.bjp.2016.07.007
PlantsofthegenusSwertia,Gentianaceae,arewellrecognizedin
literatureasimportantmedicinalherbhavinganarrayof
biologi-calandtherapeuticproperties(Negietal.,2011).Hepatoprotective
andanti-hepatotoxicactivityofSwertiasp.havealreadybeen
estab-lishedinAyurvedicmedicalsystemandvalidatedscientificallyin
animalsystem(Mukherjeeetal.,1997;Karanetal.,1999;Reen
etal.,2001).Swertiachirayita(Roxb.)Buch.-Hamex C.B.Clarke,
consideredtobethemostimportantspeciesofSwertiareported
fromIndia,foritsmedicinalproperties,hasbeenconsideredas
crit-icallyendangeredplant(Pantetal.,2000;JoshiandDhawan,2005;
Bhargavaetal.,2009).Thisplantissubstitutedbyotherspecies likeS.decussataNimmoexC.B.ClarkeandS.bimaculata(Siebold&
Zucc.)Hook.f.&ThomsonexC.B.Clarke(Chopraetal.,1956;Phoboo
etal., 2010).Metabolitessuchas terpenoids,flavonoids, iridoid
glycosidesandxanthonesareconsideredasactiveconstituentsof
Swertiasp.,xanthonesbeingthemainactivesecondarymetabolite
(Brahmacharietal.,2004;Nagetal.,2015).
In a previous study antioxidant, anti-glycosidase and
anti-acetylcholinesterase properties of S. chirayita and the two
substituteswerereportedfromthelaboratory(Nagetal.,2015).
Although the hepatoprotective property of S. chirayita is well
known,themodeofactionforhepatoprotectionhasnotyetbeen
studied.Theactive principlesfor hepatoprotection arealso not
known.-Glucuronidaseinhibitorypropertiesoftheextractsof
theseplantswouldfurthervalidatetheirhepatoprotective
prop-erty.So,theaimofthestudywastocomparemetaboliteprofile
and-glucuronidaseinhibitoryactivityofthreeimportantspecies
ofSwertiai.e.S.chirayita,S.decussataandS.bimaculatainorderto
identifytheactiveconstituents.
Materialsandmethods
Plantmaterial
LeafyshootsofthreespeciesofSwertia,Gentianaceae,namely
Swertiachirayita(Roxb.) Buch.-HamexC.B.Clarke (Voucherno.
Bot332S-1),S.bimaculata(Siebold&Zucc.)Hook.f.&Thomson
exC.B.Clarke(VoucherNo.Bot332S-2)werecollectedfrom
Dar-jeelingHimalayas.Thethird speciesS.decussata NimmoexC.B.
Clarke(VoucherNo.Bot332S-3)wascollectedfromtheWestern
Ghats,India.VoucherspecimensareavailableintheDepartment
ofBotany,UniversityofCalcutta.ThetwonamesS.chirayitaand
S.decussataareunresolvedasperIPNI(InternationalPlantNames Index).
Chemicalsandreagents
-Glucuronidase (ex. bovine liver), 4-nitrophenyl--d
-glucuronide; methoxyamine hydrochloride, N-methyl-N
-(trimethylsilyl)trifluoroacetamidewith1%trimethylchlorosilane
(MSTFA),adonitoland FAME(Fatty Acid MethylEster) markers
were obtainedfrom Sigma–Aldrich (St. Louis, MO, USA); HPLC
gradeacetonitrile,formicacid,water,methanol,chloroformand
pyridinefromMerckSpecialitiesPrivateLimited(Mumbai,India).
Six standard compounds: mangiferin, amarogentin, bellidifolin,
swerchirin/methylbellidifolin, decussatin and 1-hydroxy-3, 5,
8-trimethoxyxanthonewereavailableinthelaboratory.
Samplepreparation
MethanolicextractsoftheleafyshootsofSwertiasp.were
pre-paredbyrefluxingdried,groundmaterialswithmethanolfor5h.
Foreachplantmaterial,thefiltrate,afterextraction,was
evapo-ratedtodrynessunderreducedpressure.Differentconcentrations
ofthemethanolicextractandthatofreferencecompoundswere
usedforstudyingtheenzymeinhibitionactivityinvitroaswellas
forHPLCandGC/MSanalysis.
Assayforˇ-glucuronidaseinhibition
-Glucuronidaseinhibition assaywascarried outas perthe
methodofKimetal.(1999)withmodification.Inbrief,100lof
-glucuronidase(986.4units/mlin0.1Mphosphatebuffer,pH7.0)
and340loftestsolution/referencestandardofvarious
concentra-tionsin0.1Mphosphatebuffer(pH7.0)werepre-incubatedat37◦C
for15min.Followingthepre-incubation,60lofp-nitrophenyl-
-d-glucuronide(3.15mg/mlin0.1Mphosphatebuffer,pH7.0)was
addedandincubatedat37◦Cfor50min.Thecolourdevelopedwas
readat405nminspectrophotometer.Controlsweredevoidoftest
samples.Thepercentinhibitionwascalculatedasfollows:
Inhibition (%)=
ControlOD−SampleODControlOD
×100.HPLCanalysis
TheHPLCanalysiswasperformedonanAgilent1260(Agilent
Technologies,USA)HPLCsystemconsistingofaquaternarypump,
acolumntemperaturecontrollerandadiode-arraydetector(DAD).
The analytical column (Agilent Eclipse plus C18, 100×4.6mm,
3.5m)wasusedfortheanalysis.Themobilephasewascomposed
ofsolventA(acetonitrile)andsolventB(0.1%formicacidaqueous,
v/v).Thelineargradientprogrammefollowedwas:10%Aat0min,
30%Aat20min,60%Aat35minand80%Aat45min(Duetal.,
2012).Theflowratewas0.7ml/min.20laliquotswereinjected.
UVspectraofthepeakswererecordedfrom190–400nmovera
rangeof8differentUVwavelengths(210,214,230,250,254,260,
273,and280nmrespectively).
GC/MSanalysis
GC–MSanalysiswasperformedusingAgilent7890AGC
[soft-waredriverversion4.01(054)]equippedwith5795CinertMSD
withTripleAxisDetector.Thecolumnusedforquantification
anal-ysiswasHP-5MScapillary column[AgilentJ&W;GC Columns
(USA)]of dimensions30m×0.25mm×0.25m. Themethodof
Kind et al. (2009) was followed after modification (Das et al., 2016).Theanalysiswasperformedunderthefollowingoven
tem-peratureprogramme:ovenramp60◦C(1minhold),to325◦Cat
10◦C/min,heldfor10minbeforecool-downproducingaruntime
of37.5min.Theinjectiontemperaturewassetat250◦C,theMSD
transferlineat290◦Candtheionsourceat230◦C. Heliumwas
usedasthecarriergas(flowrate0.723ml/min;carrierlinear
veloc-ity 31.141cm/s). Thedried crude extract wasderivatized using
methoxyaminehydrochlorideandMSTFAtoincreasethe
volatil-ityofthemetabolites.AmixtureofinternalRetentionIndex(RI)
markers(methylestersofC8,C10,C12,C14,C16,C18,C20,C22,
C24andC26linearchainlengthfattyacids)(2l)wasaddedto
each sample. Derivatizedsamples wereinjected viasplit mode
(splitratio10:1)ontothecolumn.Massspectraranging30–500m/z
wererecorded.Automatedmassspectraldeconvolutionand
iden-tificationsystem(AMDIS)wasusedtodeconvoluteand identify
chromatographicpeaks.Themetaboliteswereidentifiedby
com-paringthefragmentationpatternsofthemassspectra,retention
times(RT)and retentionindices(RI)withentriesofmass
spec-tra,RTandRIinAgilentGC-MSMetabolomicsRTLLibrary(2008)
(AgilentTechnologies,USA).Therelativeresponseratiosofallthe
metaboliteswerecalculatedafternormalizingthepeakareasofthe
metabolitesbyextractdryweightandthepeakareaoftheinternal
Statisticalanalysis
Each experiment was repeated four–five times. Percentage
inhibitionin activityispresentedasmean±standarddeviation.
Regressionequationswerepreparedfromtheconcentrationsofthe
extractsandpercentageinhibitionofenzymeactivity.IC50
(concen-trationofsamplerequiredtoinhibit50%enzymeactivity)values
werecalculatedfromtheseregressionequations.Thedifferences
inactivitywerecalculatedbyTukey’sandBonferroni’stests.
Resultsanddiscussions
The entire plant of S. chirayita is used in traditional
sys-temof medicine(Joshi andDhawan, 2005).During thepresent
studyleafyshoots couldbecollected. Somethanolicextractsof
theleafy shoots of three species of Swertiawere tested for 
-glucuronidaseinhibitoryactivity.Concentrationrequiredfor50%
inhibitionofenzymeactivity(IC50value)forsilymarinwasdetected
tobe794.62±10.01g/ml.So, initially,-glucuronidase
inhibi-tionactivitiesofallthreespeciesweremeasuredat500g/ml.It
wasobservedthatS.bimaculatahadlowestactivityatthetested
concentration(Fig.1).TheactivitiesofS.chirayitaandS.
decus-satawerehigherwithnosignificantdifferencesinactivitybetween
them. Sothe IC50 values of the two species S. chirayita and S.
decussataweredetermined.Theextractsinhibitedtheenzymein
adosedependentmanner.ItwasobservedthatS.chirayitahad
lowerIC50valuebetweenthetwospeciesindicatingstronger
activ-ity (Fig. 2). IC50 values of both the extracts were significantly
0
S. chirayita S. decussata S. bimaculata
Concentration (500 µg/ml)
% inhibition
98.19 ± 0.77 95.95 ± 1.36
30.89 ± 4.02
20 40 60 80 100 120
Fig.1.Comparisonof-glucuronidaseinhibitoryactivityofSwertiasp.
0.00
S. chirayita
210.97 ± 0.04
IC
50
v
alue (
µ
g/ml
±
sd)
269.70 ± 2.11
794.62 ± 10.01
S. decussata Silymarin
100.00 200.00 300.00 400.00 500.00 600.00 700.00 800.00 900.00
Fig.2.Comparisonofenzymeactivity.
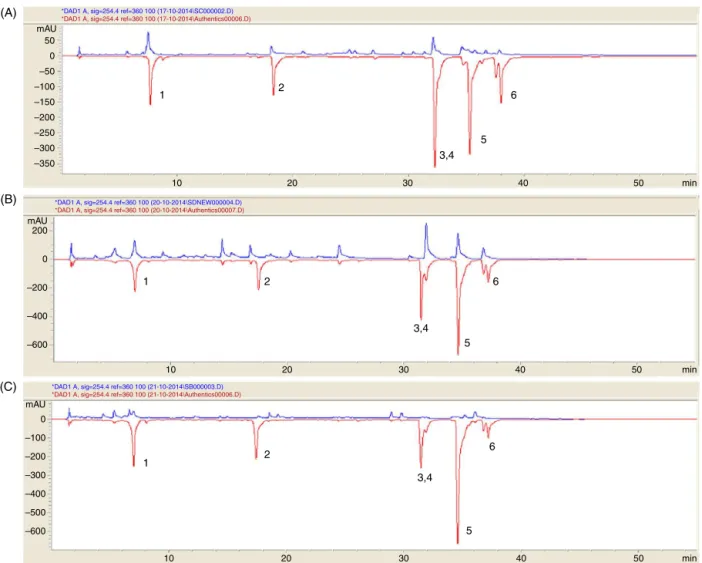
mAU 50 0 –50 –100 –150 (A)
(B)
(C) –200 –250 –300 –350
10
1 2
3,4 5
6
6
5 3,4 2
1
1 2
3,4
5 6
20 30 40 50 min
10 20 30 40 50 min
10 –600
–600 –400 –200 0 200
–500 –400 –300 –200 –100 0 mAU mAU
*DAD1 A, sig=254.4 ref=360 100 (21-10-2014\SB000003.D) *DAD1 A, sig=254.4 ref=360 100 (21-10-2014\Authentics00006.D)
*DAD1 A, sig=254.4 ref=360 100 (20-10-2014\SDNEW000004.D) *DAD1 A, sig=254.4 ref=360 100 (20-10-2014\Authentics00007.D) *DAD1 A, sig=254.4 ref=360 100 (17-10-2014\SC000002.D) *DAD1 A, sig=254.4 ref=360 100 (17-10-2014\Authentics00006.D)
20 30 40 50 min
Table1
ComparisonofHPLCidentifiedmetabolitesamongthreespeciesofSwertiainelutionorder.
Standard/referencecompounds S.chirayita S.decussata S.bimaculata
Relativeresponseratiopergextract
Mangiferin 1.084±0.39 0.758±0.17 0.209±0.02
Amarogentin 0.376±0.07 – 0.040±0.01
Bellidifolin+1-Hydroxy-3,5,8-trimethoxyxanthone 0.661±0.22 1.463±0.31 0.043±0.01
Decussatin 0.159±0.02 1.130±0.21 0.066±0.01
Swerchirin 0.233±0.06 0.452±0.17 –
100.00 80.00 60.00 40.00 20.00 0.00
0.00
0 100 200 300 400 100.00 200.00 300.00
Concentration (µg/ml)
Concentration (µg/ml)
Bellidifolin
Decussatin Mangiferin
1-hydroxy-3.5.8-trimethoxy xanthone
y=0.2004x+0.8437
R2=0.9448
y=0.499x−31 174
R2=0.9879
y=0.3225x−75 669
R2=0.9597
y=0.2868x−5.9433
R2=0.9876 y=–0.1502x2+9.2781x– 60 279
R2=0.9827
Swerchirin
Concentration (µg/ml)
Concentration (µg/ml)
Concentration (µg/ml)
% inhibition
100.00 80.00 60.00 40.00 20.00 0.00
% inhibition
120.00
80.00 100.00
60.00 40.00 20.00 0.00 0
0 10 20 30 40
100 200 300
90 80 70 60 50 40 30 20 10
80.00 70.00 60.00 50.00 40.00 30.00 20.00 10.00 0.00
0 100 200 300 400 0
% inhibition
% inhibition
% inhibition
400.00 500.00 600.00
Fig.4. -Glucuronidaseinhibitionbythexanthones.
lower than that of silymarin. The bioactivity of a plant is due tothephytoconstituentspresent in it.For a comparativestudy ofthemetaboliteprofilein thethree species,identificationand semiquantitativeanalysesofthemetaboliteswereperformedby HPLC withphotodiode array detectionand GC–MSfollowing a metabolomicsapproach.HPLCprofileofthethreespeciesof
Swer-tia(Fig.3) showedthe confirmedpresence of three xanthones
(swerchirin,decussatin,mangiferin)andtheiridoidamarogentin.
Bellidifolinand1-hydroxy-3,5,8-trimethoxyxanthonecouldnotbe
separatedfromeach otherbyHPLCastheirretentiontime(RT)
andabsorbanceweresame.Semiquantitativecomparisonofthe
normalizedpeakarearevealedthatmangiferinwaspresentin
max-imumconcentrationinS.chirayita,followedbyS.decussataandS.
bimaculata.AmarogentinandswerchirinwerenotdetectedinS. decussataandS.bimaculatarespectively.Decussatinwasfoundto
beinmaximumamountinS.decussata.Acomparativeaccountof
theHPLCidentifiedmetaboliteshasbeenrepresentedinTable1.
GC–MSbasedmetabolomicsapproachhelpedinidentificationof
72compoundsfromthemethanolextractofthreespeciesof
Swer-tia.Fiveaminoacids,twentyoneorganicacids,oneinorganicacid,
eightfattyacids,sixteenphenols,eightsugars,sevensugaralcohols,
fiveterpenoidsandoneotherorganiccompound(porphine)were
identified.Asemiquantitativecomparison,basedontherelative
responseratiopergextract,oftheidentifiedmetaboliteshasbeen
representedinTable2.S.chirayitapresentedmaximumnumberof
metabolitesfollowedbyS.bimaculataandS.decussatarespectively.
Five xanthones and the iridoid amarogentin, isolated from
S. chirayita previously (Nag et al., 2015), were tested for their
-glucuronidaseinhibitory property. The activitiesof the
com-pounds were compared with that of silymarin. The activities
of the xanthones tested were proportional to their
concentra-tions (Fig. 4). All the xanthones showed inhibitory activities
significantly higher than that of the commercial drug (Fig. 5).
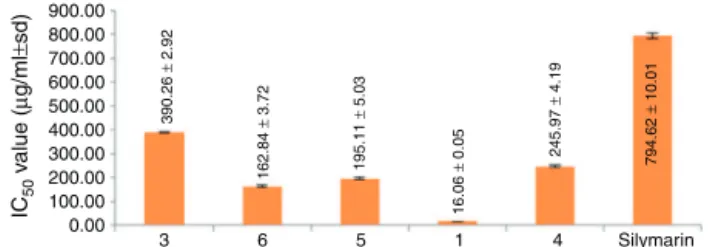
0.00
3 6
IC
50
v
alue (
µ
g/ml
±
sd)
5 1 4 Silymarin
390.26
±
2.92
162.84
±
3.72
195.11
±
5.03
16.06
±
0.05 245.97
±
4.19
794.62
±
10.01
100.00 200.00 300.00 400.00 500.00 600.00 700.00 800.00 900.00
Table2
ComparativemetabolicprofileofthreespeciesofSwertiausingGC/MS.
Metabolites Relativeresponseratiopergextract
Swertiachirayita Swertiadecussata Swertiabimaculata
Aminoacids
N-Ethylglycine 1.13±1.09 – –
N-Acetyl-l-glutamicacid 15.73±2.85 – 21.72±9.41
l-Glutamicacid(dehydrated) 14.43±1.50 1.57±0.71b –
l-Pyroglutamicacid – – 17.12±3.46
Allantoin 37.14±8.73 – –
Organicacids
l-(+)Lacticacid 28.97±2.06 25.03±7.83 137.05±14.21a
Glycolicacid 6.83±0.31 2.74±3.95 12.21±0.83a
Oxalicacid 0.98±0.31 – 4.00±2.27a
Malonicacid 3.82±0.91 – 2.88±1.66
Maleicacid 2.59±0.29 2.21±0.45 1.75±1.00
Succinicacid 86.36±2.13 1.62±0.08b 98.39±7.51a
Glycericacid 90.34±2.72 4.77±0.82b 67.42±3.79b
Fumaricacid 103.78±7.32 7.43±1.57b 18.97±3.81b
Citraconicacid 1.03±0.22 – 0.34±0.25b
Citramalicacid 3.79±0.92 – –
Mandelicacid 1.05±0.38 – 0.84±0.19
d-Malicacid 705.76±25.78 6.08±0.58b 296.55
±44.42b
Adipicacid – – 0.76±0.66
Citricacid 19.33±4.44 – 17.68±11.19
Gluconicacidlactone 144.13±14.48 41.30±5.64b 76.96±8.32b
Gluconicacid 15.29±7.38 – –
2-Isopropylmalicacid 1.51±0.19 – –
3-Hydroxy-3-methylglutaricacid(dicrotalicacid) 3.29±0.13 – –
Ribonicacid-gamma-lactone 22.24±35.46 – –
4-Guanidinobutyricacid 0.93±0.30 – –
Nicotinicacid 2.04±0.87 – 2.74±0.81
Inorganicacid
Phosphoricacid 14.97±2.36 3.99±0.45b 5.66±1.15b
Fattyacids
Lauricacid 1.26±0.13 1.44±0.73 6.16±2.69a
Behenicacid 8.71±1.06 – 14.63±3.04a
Myristicacid – – 6.79±1.82
Palmiticacid 109.52±11.08 94.42±15.02 247.32±10.48a
Linoleicacid 26.08±5.82 3.69±2.31b 27.39±7.70c
Oleicacid 32.02±9.41 4.93±3.89b 20.48
±16.95
Stearicacid 64.63±7.34 64.87±10.13 116.75±13.21a
Arachidicacid 4.13±0.99 – 20.27±4.24a
Phenols
Benzoicacid – – 1.97±0.22
O-Acetylsalicylicacid – – 3.60±0.35
4-Hydroxybenzoicacid 9.44±0.72 – 4.75±0.12b
2,3-Dihydroxybenzoicacid 41.22±0.14 22.78±1.75b 112.23±2.24a
4-Hydroxy-3-methoxybenzoicacid 56.08±1.65 1.48±0.40b 47.02±0.72b
Gentisicacid – 3.58±1.76 33.83±6.61c
Shikimicacid 1.47±0.21 – –
3,4-Dihydroxybenzoicacid 43.59±16.66 – 8.83±0.67b
Quinicacid 2.06±0.33 5.29±2.96 26.20±0.77a
Coniferylalcohol 6.90±1.95 – 22.27±9.60a
4-Hydroxycinnamicacid – 11.30±5.60 –
Ferulicacid – 7.76±6.08 –
Sinapylalcohol – – 3.46±1.96
Caffeicacid 0.86±0.09 1.44±0.72 –
Neohesperidin 0.95±0.34 –
Isoquercitrin 59.38±8.10 – –
Sugars
Methyl--d-galactopyranoside 105.99±1.29 26.71±14.48b 45.95
±40.50b
Sucrose 1482.49±114.56 27.04±4.29b 1682.28
±1055.76c
Lactose 46.34±43.84 – –
d-(+)-Trehalose 248.92±66.71 22.69±7.72b 86.93±15.65b
Raffinose 2.46±1.91 – 6.39±5.06
Melezitose 3.70±1.99 3.19±0.28 4.55±3.22
d-(+)-Melezitose 1.98±0.83 86.62±100.91 –
Adenosine 9.66±7.90 – 6.95±1.24
Sugaralcohols
Glycerol 108.61±1.07 58.94±1.09b 563.47
±10.97a
Glycerol1-phosphate 2.98±0.26 – –
D-Threitol 58.63±4.42 7.61±1.60b 4.60
±0.93b
Table2(Continued)
Metabolites Relativeresponseratiopergextract
Swertiachirayita Swertiadecussata Swertiabimaculata
d-Mannitol 533.42±19.80 12.45±1.06b 66.75±1.01b
d-Sorbitol 199.74±2.90 115.11±18.17b 323.37
±47.73a
Galactinol 37.99±6.29 – –
Terpenoids
Phytol – – 3.44±0.66
Loganin – 105.87±12.81c 34.05
±7.11
Palatinitol 28.05±15.45 – 8.68±4.87
Stigmasterol 39.26±5.79 3.15±2.76b 42.25±7.60
Lanosterol – – 35.94±7.56
Macrocycleorganiccompound
Porphine 6.41±4.38 – –
–,notdetected.
aSignificantlyhigherthanS.chirayita.
b SignificantlylowerthanS.chirayita.
c SignificantlyhigherbetweenS.decussataandS.bimaculata.
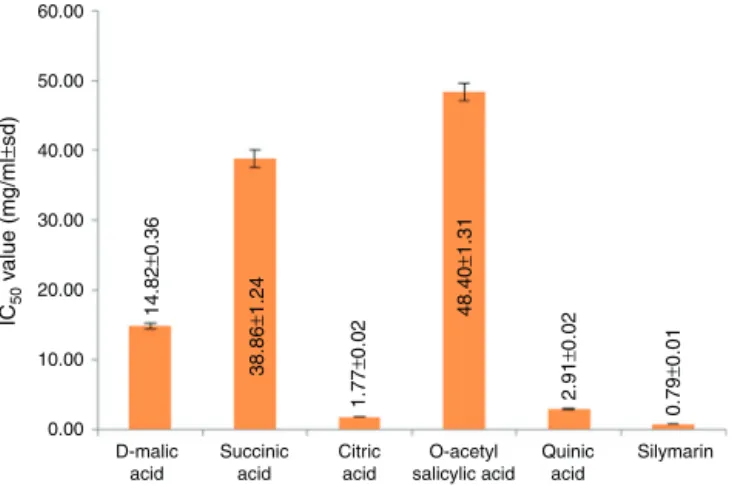
60.00
50.00
40.00
30.00
20.00
10.00
0.00 D-malic
acid
Succinic acid
IC
50
v
a
lue (mg/ml
±
sd)
14.82
±
0.36
38.86
±
1.24
1.77
±
0.02
48.40
±
1.31
2.91
±
0.02
0.79
±
0.01
Citric acid
O-acetyl salicylic acid
Quinic acid
Silymarin
Fig.6.Comparisonof-glucuronidaseinhibitoryactivityoforganicandphenolic acidswithrespecttosilymarin.
Mangiferinshowedthebest-glucuronidaseinhibitionwithan
IC50valueof16.06±0.05g/mlor0.038mMfollowedby
swer-chirin(IC50 162.84±3.72g/ml or 0.565mM), decussatin(IC50
195.11±5.03g/ml or 0.646mM), 1-hydroxy-3,5,8-trimethoxy
xanthone(IC50245.97±4.19g/mlor0.814mM)andbellidifolin
(IC50390.26±2.92g/mlor1.424mM).However,thebitteriridoid
compound,amarogentindidnotshowanyenzymeinhibitory
activ-ity.Outof72metabolitesidentifiedbyGC–MS,ninecompounds,
availableinthelaboratory,weretestedfortheir-glucuronidase
inhibitory activity. These were succinic acid, d-malic acid,
cit-ricacid,O-acetylsalicylicacid,4-hydroxybenzoicacid,quinicacid,
4-hydroxycinnamicacid,sucroseandglycerol.4-Hydroxybenzoic
acid,4-hydroxycinnamicacid,sucroseandglyceroldidnothaveany
-glucuronidase inhibitory activity. Remaining five compounds
inhibited-glucuronidaseinadose-dependentmanner.
Compari-sonoftheiractivitieswithrespecttosilymarinhadbeenillustrated
in Fig.6.Among these, citric acid (IC50 1.77±0.02mg/ml) and
quinicacid(IC502.91±0.02mg/ml)showedactivitycloseto
sily-marin(IC500.79±0.01mg/ml).
Xanthoneshad already beenreported topossess a range of
pharmacologicalactions(Peresetal.,2000).S.chirayita,S.
decus-sata and S. bimaculata are considered to be a natural source
oftetraoxygenatedxanthones(Pereset al.,2000).Mangiferin, a
xanthone-C-glycoside,had previously beenreported to possess
antioxidant(Nagetal.,2015);anti-diabeticandantitumour
activ-itiesto namea few (Suryawanshiet al., 2006).Bellidifolin and
swerchirinhadbeenfoundtobepotenthypoglycemicagent(Bajpai
etal.,1991;Basnetetal.,1995).Inaddition,swerchirin,hadalso
beenreportedtobehepatoprotective(Hajimehdipooretal.,2006)
onparacetamol-inducedhepatotoxicity inmice models.Several
studiesthathadbeencarriedouttoadvocatethehepatoprotective
andanti-hepatotoxicpropertyofthisgenuscreditsthisattribute
tothexanthonecontentpresentinextractoftheplant.Thefive
xanthonesthathadbeenconsideredinourstudyshowedgood
-glucuronidaseinhibition incomparisontothecommercialdrug,
silymarin,therebyproposingapossiblemechanismof
hepatopro-tectiveactionvia-glucuronidaseinhibition.Thesefindingshad
not been reported earlier in literature. The present study also
revealsthatsomeorganicacidsviz.,succinicacid,d-malicacid,citric
acidandphenoliccompoundsviz.,O-acetylsalicylicacidandquinic
acidhave-glucuronidaseinhibitoryproperties.Citricacidwas
reportedearliertoreducelipopolysaccharideinducedliverinjury
andoxidativestress(Abdel-Salametal.,2014).So,-glucuronidase
inhibitionpropertyoftheconstituentspresentinSwertiasp.maybe
amechanismforhepatoprotectiveactivityoftheseplants.Further
invivostudyisrequiredinthisregard.
Conclusion
Theproperties of three Swertiasp. e.g. S.chirayita, S.
decus-sataandS.bimaculatatoinhibit-glucuronidase,amechanismfor
hepatoprotection,wereassessedandthecontributoryconstituents
hadbeenidentified.Severalxanthoneswereidentifiedtobemajor
componentstohavesignificantlyhigher-glucuronidase
inhibi-tionpropertiesthanthatofsilymarin.Metabolitesotherthanthe
xanthones,probablyalsocontributetothebioactivityofdifferent
Swertiaspeciesbysynergisticeffect.Thepresentfindingssuggest
that-glucuronidaseinhibitionmaybeoneofthemechanismsfor
thehepatoprotectivepropertyofSwertiasp.Furtherinvivostudy
isrequiredtosupporttheclaim.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thatnoexperimentswereperformedonhumansoranimalsduring
thestudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
Authors’contributions
SKperformedextraction, chromatographicanalysis,
bioactiv-ity studies and preparation of the manuscript. GN performed
extractionandsomeanalysis.BDprovidedidea,projectplanand
subsequentlypreparationofthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsacknowledgefinancialsupportfromDepartmentof
ScienceandTechnology(GovernmentofWestBengal),Department
ofScienceandTechnologyFISTProgramme(GovernmentofIndia),
UniversityGrantsCommission.
References
Abdel-Salam,O.M.E.,Youness,E.R.,Mohammed,N.A.,Morsy,S.M.Y.,Omara,E.A., Sleem,A.A.,2014.Citricacideffectsonbrainandliveroxidativestressin lipopolysaccharide-treatedmice.J.Med.Food17,588–598.
Bajpai,M.B.,Asthana,R.K.,Sharma,N.K.,Chatterjee,S.K.,Mukherjee,S.K.,1991. HypoglycemiceffectofswerchirinfromthehexanefractionofSwertiachirayita. PlantaMed.57,102–104.
Basnet,P.,Kadota,S.,Shimizu,M.,Takata,Y.,Kobayashi,M.,Namba,T.,1995. Bellidifolinstimulatesglucoseuptakeinrat1fibroblastsandameliorates hyper-glycemiainstreptozotocin(STZ)-induceddiabeticrats.PlantaMed.61,402–405. Bhargava,S.,Rao,P.S.,Bhargava,P.,Shukla,S.,2009.AntipyreticpotentialofSwertia
chirataBuchHam.rootextract.Sci.Pharm.77,617–623.
Brahmachari,G.,Mondal,S.,Gangopadhyay,A.,Gorai,D.,Mukhopadhyay,B.,Saha,S., Brahmachari,A.K.,2004.Swertia(Gentianaceae):chemicalandpharmacological aspects.Chem.Biodivers.1,1627–1651.
Byass,P.,2014.Theglobalburdenofliverdisease:achallengeformethodsandfor publichealth.BMCMed.,http://dx.doi.org/10.1186/s12916-014-0159-5. Chopra,R.N.,Nayar,S.L.,Chopra,I.C.,1956.GlossaryofIndianMedicinalPlants.CSIR,
NewDelhi,pp.237.
Das,S.,Dutta,M.,Chaudhury,K.,De,B.,2016.Metabolomicandchemometricstudy ofAchrassapotaL.fruitextractsforidentificationofmetabolitescontributing totheinhibitionof␣-amylaseand␣-glucosidase.Eur.FoodRes.Technol.242, 733–743.
deGraaf,M.,Boven,E.,Scheeren,H.W.,Haisma,H.J.,Pinedo,H.M.,2002. Beta-glucuronidase-mediateddrugrelease.Curr.Pharm.Des.8,1391–1403. Dixit,N.,Baboota,S.,Kohli,K.,Ahmad,S.,Ali,J.,2007.Silymarin:areviewof
pharmacologicalaspectsandbioavailabilityenhancementapproaches.Indian J.Pharmacol.39,172–179.
Du,X.G.,Wang,W.,Zhang,Q.Y.,Cheng,J.,Avula,B.,Khan,I.A.,Guo,D.A.,2012. IdentificationofxanthonesfromSwertiapuniceausinghigh-performanceliquid chromatographycoupledwithelectrosprayionizationtandemmass spectrom-etry.RapidCommun.MassSpectrom.26,2913–2923.
Dutton,G.J.,1980.GlucuronidationofDrugsandOtherCompounds.CRCPress,Boca Raton,FL.
Hajimehdipoor,H.,Sadeghi,Z.,Elmi,S.,Elmi,A.,Ghazi-Khansari,M.,Amanzadeh,Y., Sadat-Ebrahimi,S.E.,2006.ProtectiveeffectsofSwertialongifoliaBoiss.andits activecompound,swerchirin,onparacetamol-inducedhepatotoxicityinmice. J.Pharm.Pharmacol.58,277–280.
Joshi,C.S.,Sanmugapriya,E.,2007.-Glucuronidaseinhibitoryeffectofphenolic constituentsfromPhyllanthusamarus.Pharm.Biol.45,363–365.
Joshi, P., Dhawan, V., 2005. Swertia chirayita – an overview. Curr. Sci. 89, 635–640.
Karan,M.,Vasisht,K.,Handa,S.S.,1999.AntihepatotoxicactivityofSwertiachirata
onparacetamolandgalactosamineinducedhepatotoxicityinrats.Phytother. Res.13,95–101.
Kim,D.H.,Jin,Y.H.,Park,J.B.,Kobashi,K.,1994.Silymarinanditscomponentsare inhibitorsof-glucuronidase.Biol.Pharm.Bull.17,443–445.
Kim,D.H.,Shim,S.B.,Kim,N.J.,Jang,I.S.,1999.-Glucuronidaseinhibitory activ-ityandhepatoprotectiveeffectofGanodermalucidum.Biol.Pharm.Bull.22, 162–164.
Kind,T.,Wohlgemuth,G.,Lee,D.Y.,Lu,Y.,Palazoglu,M.,Shahbaz,S.,Fiehn,O.,2009. FiehnLib–massspectralandretentionindexlibrariesformetabolomicsbased onquadrupoleandtime-of-flightgaschromatography/massspectrometry.Anal. Chem.81(24),10038–10048.
Kshirsagar,A.D.,Mohite,R.,Aggrawal,A.S.,Suralkar,U.R.,2011.Hepatoprotective medicinalplantsofAyurveda–areview.AsianJ.Pharm.Clin.Res.4,1–8. Mills,G.T.,Smith,E.E.B.,1951.The-glucuronidaseactivityofchemicallyinduced
rathepatoma.Science114,690–692.
Mukherjee,S.,Sur,A.,Maiti,B.R.,1997.HepatoprotectiveeffectofSwertiachirataon rat.IndianJ.Exp.Biol.35,384–388.
Nag,G.,Das,S.,Das,S.,Mandal,S.,De,B.,2015.Antioxidant,anti-acetylcholinesterase andanti-glycosidasepropertiesofthreespeciesofSwertia,theirxanthonesand amarogentin.Acomparativestudy.Pharmacogn.J.7,117–123.
Negi,J.S.,Singh,P.,Rawat,B.,2011.Chemicalconstituentsandbiologicalimportance ofSwertia.Areview.Curr.Res.Chem.3,1–15.
Pant,N.,Jain,D.C.,Bhakuni,R.S.,2000.PhytochemicalsfromgenusSwertiaandtheir biologicalactivities.IndianJ.Chem.39B,565–586.
Peres,V.,Nagem,T.J.,Oliveira,F.F.,2000.Tetraoxygenatednaturallyoccurring xan-thones.Phytochemistry55,683–710.
Phoboo,S.,Bhowmik,P.C.,Jha,P.K.,Shetty,K.,2010.Anti-diabeticpotentialofcrude extractsofmedicinalplantsusedassubstitutesforSwertiachirayitausinginvitro
assays.Bot.Orient.:J.PlantSci.7,48–55.
Pineda,E.P.,Goldbarg,J.A.,Banks,B.M.,Rutenburg,A.M.,1959.Thesignificanceof serum-glucuronidaseactivityinpatientswithliverdisease.Apreliminary report.Gastroenterology36,202–213.
Pradhan, S.C., Girish, C.,2006. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinicalmedicine. Indian J. Med.Res. 124, 491–504.
Reen,R.K.,Karan,M.,Singh,K.,Karan,V.,Johri,R.K.,Singh,J.,2001.Screeningof var-iousSwertiaspeciesextractsinprimarymonolayerculturesofrathepatocytes againstcarbontetrachloride-andparacetamol-inducedtoxicity.J. Ethnophar-macol.75,239–247.
Shim,S.B.,Kim,N.J.,Kim,D.H.,2000.-Glucuronidaseinhibitoryactivityand hep-atoprotectiveeffectof18--glycyrrhetinicacidfromtherhizomesofGlycyrrhiza uralensis.PlantaMed.66,40–43.
Suryawanshi,S.,Mehrotra,N.,Asthana,R.K.,Gupta,R.C.,2006.Liquid chromatogra-phy/tandemmassspectrometricstudyandanalysisofxanthoneandsecoiridoid glycosidecompositionofSwertiachirata,apotentantidiabetic.RapidCommun. MassSpectrom.20,3761–3768.
Walaszek, Z., Hanausek-Walaszek, M., Webb, T.E., 1984. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumorigenesis by