UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
Cystoscopic-guided laser ablation of an intramural ectopic ureter
in a dog – clinical case
Dissertação de Mestrado Integrado em Medicina Veterinária
Rita Manuel Henriques Duarte Pereira
Orientador: Professor Doutor Luís Miguel Viana Maltez da Costa
2
UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
Cystoscopic-guided laser ablation of an intramural ectopic ureter
in a dog – clinical case
Dissertação de Mestrado Integrado em Medicina Veterinária
Rita Manuel Henriques Duarte Pereira
Orientador: Professor Doutor Luís Miguel Viana Maltez da Costa
Composição do júri:
Professor Doutor José Eduardo Teixeira Pereira Professor Doutor Luís Miguel Viana Maltez da Costa
Professora Doutora Maria da Conceição Medeiros Castro Fontes
iii Dissertação apresentada à Escola de Ciências Agrárias e Veterinárias - Departamento de Ciências Veterinárias - da Universidade de Trás-os-Montes e Alto Douro, como requisito para a obtenção do grau de Mestre em Medicina Veterinária.
iv Acknowledgements
I would like to thank the University of Trás-os-Montes e Alto Douro (UTAD), personified by its Rector Magnificus, Professor António Augusto Fontaínhas Fernandes, for providing the possibility of writing this master’s thesis.
To my mentor, Professor Luís Maltez da Costa, thank you for accepting this dissertation’s orientation and for all the dedication and availability shown during its elaboration.
To my parents, that always taught me the value of hard work and its importance on the existence of every living being. A big thank you for all the transmitted knowledge that directly contributed to the development of this master’s thesis.
To my brother, one of the biggest reasons for my desire to succeed. To the rest of my family, for all the support given through these 6 years.
To Maria Andrade, Inês Fontes, Zé Diogo, Ana Pereira, Aniana Correia e Catarina Nascimento, who walked with me side by side from the beginning of this journey until the very end, making it a truly unforgettable travel through college life.
To Sara, for the expertise in playing the role of sister that I chose.
v Resumo
A ectopia ureteral é uma anomalia congénita na qual um ou ambos os ureteres terminam distalmente ao trígono vesical resultando em incontinência urinária. Os ureteres ectópicos dividem-se em duas grandes classes: os extramurais, que tem um percurso extravesical e terminam na uretra na maioria dos casos; e os intramurais, que geralmente entram na bexiga ao nível anatomicamente correto, mas tunelizam na submucosa da parede da bexiga, terminando também maioritariamente na uretra. A ectopia ureteral é a causa mais comum de incontinência urinária em cães, embora seja uma doença de ocorrência rara. É tipicamente diagnosticada em animais jovens e é mais frequente em fêmeas que em machos. Existem algumas raças predispostas para esta doença, incluindo o Husky Siberiano, Caniche, West Highland White Terrier, Golden Retriever, Bulldog Inglês, Newfoundland, Skye Terrier e Fox Terrier.
O diagnóstico definitivo é alcançado através de métodos imagiológicos, em integração com os dados recolhidos na apresentação clínica e história pregressa do animal. O tratamento de eleição desta doença é cirúrgico, havendo várias técnicas passiveis de serem realizadas: neoureterostomia, reimplantação ureteral, nefroureterectomia ou cistoscopia e ablação por laser. A cistoscopia e ablação por laser é uma técnica recente, realizada pela primeira vez em cães em 2006, que se destaca por ser minimamente invasiva e de rápida execução. As taxas de sucesso obtidas até ao presente não variam significativamente consoante a técnica utilizada. Frequentemente, os animais com ectopia ureteral necessitam de terapia médica pós-operatória que muitas vezes não é suficiente para alcançar a continência urinária, havendo necessidade de recorrer a outras técnicas ainda em desenvolvimento.
O caso clínico apresentado nesta dissertação foi recebido no Hospital Veterinário da Universidade do Tennessee onde foi diagnosticada uma ectopia ureteral a um Golden Retriever de quatro meses de idade através de tomografia computorizada e instituída a resolução cirúrgica através de cistoscopia e ablação por laser. O animal permanecia com episódios de incontinência urinária, seis meses após a cirurgia e sob tratamento médico com fenilpropanolamina, pelo que foi aconselhada a colocação de um constritor uretral. Os resultados obtidos neste caso clínico estão em conformidade com a bibliografia atual.
vi Abstract
Ureteral ectopia is a congenital anomaly in which one or both ureters end past the bladder trigone, leading to urinary incontinence. Ectopic ureters can be divided in two categories: extramural that totally bypasses the bladder, entering the urethra most of the times; and intramural that usually enter the bladder in the right anatomical position, but then tunnel through the bladder wall and terminate also most frequently in the urethra. Ureteral ectopia is the most common cause of urinary incontinence in dogs, even though it is a rare condition. It is typically diagnosed in young animals and more frequently in female than male dogs. There are some breeds considered predisposed to this condition such as Siberian Husky, Toy Poodle, West Highland White Terrier, Golden Retriever, English Bulldogs, Newfoundland, Skye Terrier and Fox Terrier.
The definitive diagnosis is achieved associating imaging techniques to information gathered through the clinical presentation and past history of the patient. The treatment of choice for this condition is surgical and different techniques can be performed: neoureterostomy, ureteral re-implantation, nephroureterectomy and cystoscopic-guided laser ablation. Cystoscopic-guided laser ablation is a recent technique, firstly performed in dogs in 2006 that stands out for being minimally invasive and of rapid execution. Success rates obtained until today, do not vary significantly depending on the chosen technique. Patients with ureteral ectopia often need post-surgical medical therapy which sometimes is not enough to achieve urinary continence, hence the need to perform other developing techniques.
The clinical case presented in this dissertation was received at University of Tennessee Veterinary Teaching Hospital, in which a four-month-old Golden Retriever was diagnosed with ureteral ectopia using computed tomography. Further surgical resolution with cystoscopic-guided laser ablation was established. Six months after surgery, the patient still had episodes of urinary incontinence and whereby an urethral artificial occluder placement was advised. The results obtained in this clinical case match what is shown in the current literature.
vii
Table of contents Page
Acknowledgements iv
Resumo v
Abstract vi
I. Introduction 1
II. Literature Review 3
1. Definition 3
2. Classification 5
3. Embryology 6
4. Pathophysiology and predisposition 6
5. Diagnosis 8
5.1. Signalment and medical history 8
5.2. Clinical signs 9
5.3. Physical examination 9
5.4. Laboratory findings 10
6. Differential diagnosis 10
7. Complementary diagnostic tests 11
7.1. Radiology 11 7.1.1. Intravenous urography 11 7.1.2. Cystography 13 7.1.3. Retrograde urethrography 15 7.1.4. Retrograde vaginourethrography 15 7.2. Ultrasonography 16 7.3. Uroendoscopy 17 7.4. Computed Tomography 20 7.5. Urodynamic testing 22 8. Treatment 23 8.1. Medical treatment 23 8.2. Surgical treatment 23 8.2.1. Pre-surgical preparation 24 8.2.2. Anesthesia 24 8.2.3. Surgical anatomy 24
viii
8.2.4. Surgical approach 25
8.2.5. Surgical techniques 25
8.2.5.1. Neoureterostomy 25
8.2.5.2. Ureteral re-implantation (ureteroneocystostomy) 29
8.2.5.3. Nephro-ureterectomy 31
8.2.5.4. Cystoscopic-guided laser ablation (CLA) 32
8.2.6. Post-surgical care 34
9. Comparison of techniques 35
10. Prognosis 36
III. Clinical Case 37
1. Signalment and presenting complaint 37
2. Past pertinent history 37
3. Physical exam 37
4. Complementary diagnostic tests 37
4.1. Serum biochemical profile 37
4.2. Urinalysis 38
4.3. Urine culture and sensitivity tests 38
4.4. Contrast CT 38 5. Problem list 41 6. Definitive diagnosis 41 7. Treatment decisions 41 8. Outcome 43 IV. Discussion 45 V. References 49 VI. Appendix 53
ix List of figures
Figure 1 – Normal entrance of the ureter in the bladder.
Figure 2 - Schematic drawing of the male and female lower urinary tract and possible ectopic openings of the ureters.
Figure 3 – Different types of ectopic ureters. Figure 4 – Squematic innervation of the bladder. Figure 5 - Perivulvar dermatitis due to urine scalding. Figure 6 – IVU with negative contrast cystography. Figure 7 – NCC lateral view.
Figure 8 – PCC and IVU showing bilateral, dilated extramural EU and its pathway past the Bladder Trigone.
Figure 9 – DCC associated to IVU. Figure 10 – RVU associated to IVU.
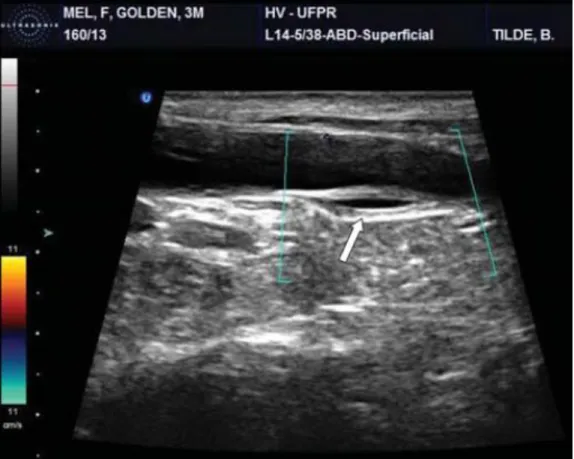
Figure 11 - Ultrasonographic appearance of an EU.
Figure 12 – Cystoscopic image of the vaginal vestibule and external urethral meatus in a female dog.
Figure 13 – Cystoscopic image of a mesonephric remnant in a female dog.
Figure 14 – Small blind-ended fenestrations in the external urethral meatus that might be mistaken for EU.
x Figure 16 – CT image showing a longitudinal section of the left ureter and transversal section of the right ureter.
Figure 17 – Squematic representation of an urethral pressure profile.
Figure 18 – Squematic representation of the anatomy of the female urinary apparatus, ventral view.
Figure 19 – Squematic representation of neoureterostomy.
Figure 20 – Intra-operative images of a neoureterostomy with ligation technique. Figure 21 – Diagram of a neoureterostomy and resection technique.
Figure 22 – Final appearance of the bladder after neostomy and resection and closure of the DUS.
Figure 23 – Squematic representation of the ureteral re-implantation technique. Figure 24 – Ureteral re-implantation technique.
Figure 25 – Squematic representation of nephroureterectomy.
Figure 26 – Cystocopic images of a right intramural EU in a female dog. Figure 27 – CT image showing the normal appearance of both kidneys. Figure 28 – CT image of the bladder and ureters.
Figure 29 – CT image of the right EU tunneling along the dorsolateral bladder wall. Figure 30 – CT image showing an EU filled with contrast dorsal to the urethra. Figure 31– Cystoscopic images acquired during cystoscopic-guided laser ablation. Figure 32 – Cystoscopic-guided laser ablation.
xi List of tables
Table 1 – Different views and timings to be followed after intravenous injection of contrast in IVU.
Table 2 – Comparison between open abdominal techniques and CLA. Table 3 – Results of the urinalysis.
Table 4 – Results of the serum chemistry panel. Table 5 – Results of the urine culture.
List of abbreviations and acronyms CLA – Cystoscopic-guided laser ablation CT – Computed tomography
DCC – Double contrast cystography EU – Ectopic ureter
UE – Ureteral ectopia
IVU – Intravenous urography
NCC – Negative contrast cystography
NSAIDs – Non-steroid anti-inflammatory drugs PCC – Positive contrast cystography
RU – Retrograde urethrography UI – Urinary incontinence
USMI – Urethral sphincter mechanism incompetence UTI – Urinary tract infections
PO – Per Os
1 I. Introduction
The technique presented in this thesis was performed by Dr. Joseph Bartges at University of Tennessee (UT), Knoxville, United Stated where I spent 3 months doing an externship. I chose UT because of its stupendous facilities, bright and skillful veterinarians and extremely helpful staff. All animals referred to UT are fully evaluated before surgery and the post-operative control period is also conducted there. All patients are discharged with written instructions for the required care and medication. The referral cases are then transferred back to the referring veterinarian. This way of working contributed to a big learning experience both veterinary and socially wise. During the externship I had the opportunity of working in different services including Dermatology, Internal Medicine, Physical Therapy, Orthopedic Surgery, Soft Tissue Surgery and Anesthesia. Soft Tissue Surgery has always been my biggest area of interest and cystoscopy and laser ablation of an ectopic ureter turned out to be a fascinating technique. Ureteral ectopia is particularly interesting for me because it is a very challenging condition that often has an unsuccessful endpoint. There is a strong need of investigation and innovation in order to find new and more successful techniques and possibly achieve better outcomes. These limitations are not only frustrating for the veterinarian community but also to owners for which managing this condition with all the problems associated to it can end up being unbearable. Writing this dissertation intends to gather as much information as possible regarding ectopic ureters and present a recent technique that can contribute to achieve better post-operative continence rates and better outcomes for the animals that suffer from this condition.
3 II. Literature review
1. Definition
Ureters are tubular structures located in the retroperitoneum that arise from the renal hilus to enter the dorsolateral caudal aspect of the bladder in a characteristic J-shaped bent cranially after a short intramural course (MacPhail, 2012; Mayhew & Berent, 2013).
Ureteral ectopia (UE) is a congenital defect in which the location and/or anatomy of the distal ureter is abnormal (Davidson & Westropp, 2014; Mathews, 2011; Mayhew, Lee, Gregory, & Brockman, 2006). This anomaly may be present in one or both ureters and cause them to empty past the bladder trigone (outside the bladder), leading to continuous or
4 Figure 2 - Schematic drawing of the male (left) and female (right) lower urinary tract and possible ectopic
openings of the ureters (Adapted from M. A. McLoughlin & Chew, 2012).
intermittent urinary incontinence (UI) (Ho, Troy, & Waldron, 2011; MacPhail, 2012; Mathews, 2011; M. a McLoughlin & Chew, 2000; North et al., 2010).
In females, ectopic ureters (EU) open most commonly in the urethra but can also open in the vagina, oviduct or uterus (Cannizzo et al., 2003; Ho et al., 2011; MacPhail, 2012) whereas in males the vas deferens, seminal vesicles and urethra have been reported as possible terminations (Ho et al., 2011).
The endings of the ureters may present as blind-end to single, double or trough openings (MacPhail, 2012; Taney, Moore, Carro, & Spencer, 2003). They are often associated with other congenital abnormalities such as hydroureter, urethral sphincter mechanism incompetence (USMI), bladder hypoplasia, small, absent or misshapen kidneys, ureteroceles and vestibulovaginal anomalies (Davidson & Westropp, 2014; MacPhail, 2012; Mathews, 2011; Smith, Radlinsky, & Rawlings, 2010).
According to Mason, LK, et al. (1990), referenced by (M. a McLoughlin & Chew, 2000), in a radiographic evaluation of 18 dogs with UE, 94% were diagnosed with concurrent abnormalities of the urinary tract being hydroureter the most common. Ureteroceles have rarely been reported in veterinary literature (Anders et al., 2012).
5 2. Classification
EU can be classified as intramural or extramural (Davidson & Westropp, 2014; Ho et al., 2011; MacPhail, 2012; Mathews, 2011; Mayhew & Berent, 2013; Mcloughlin & Bjorling, 2003). An intramural EU usually enters the bladder wall where it should open but instead it creates a submucosal tunnel that extends along the bladder wall, opening in the bladder neck, urethra or vagina (Mayhew et al., 2006; M. a McLoughlin & Chew, 2000; Taney et al., 2003). Troughs, fenestration and branching may also be seen (Ho et al., 2011; Mathews, 2011; M. a McLoughlin & Chew, 2000). Extramural EU run outside the bladder, bypassing it and entering the bladder neck, urethra, vagina or uterus (Ho et al., 2011; Taney et al., 2003).
Figure 3 – Different types of ectopic ureters: A – intramural; B – extramural; C – double ureteral opening; D
6 Because intramural EU appear to create a tunnel that acts like a valve, intermittent obstruction to the urine outflow from the ureter occurs leading to an increased pressure and consequent ureteral dilation (M. a McLoughlin & Chew, 2000). This is the reason why ureteral dilation, being the most frequent abnormality secondary to ureter displacement, is most commonly associated with intramural UE (Anders et al., 2012; M. a McLoughlin & Chew, 2000).
3. Embryology
A defect in the embryologic development and migration of the metanephric duct (which originates the ureter later in growth) is believed to be the cause of UE (A. C. Berent, 2011; Davidson & Westropp, 2014; Ho et al., 2011; Mayhew & Berent, 2013). Normally, the right and left ureteral paths run along the dorsal urethra until they reach the bladder trigone, where they open (Davidson & Westropp, 2014). If the ureteral bud develops in a higher position, the migration distance to reach the urogenital sinus (bladder later in growth) is longer and thus the period of migration within the bladder is shorter, causing the ureteral orifice to open more distally than normal (in the bladder neck or even more distal) (Kuwayama, Miyazaki, & Ichikawa, 2002; M. a McLoughlin & Chew, 2000).
It has also been hypothesized that failure in apoptosis of the common excretory duct during ureter transposition is responsible for the ectopic openings that occur along the path of ureteral migration (Davidson & Westropp, 2014; North et al., 2010). Many congenital anomalies of the kidney and urinary tract are due to ectopia of the initial ureter. It has even been suggested that, because often times different abnormalities appear together, they might share pathogenic mechanisms and genetic causes (Ho et al., 2011; Kuwayama et al., 2002).
4. Pathophysiology and predisposition
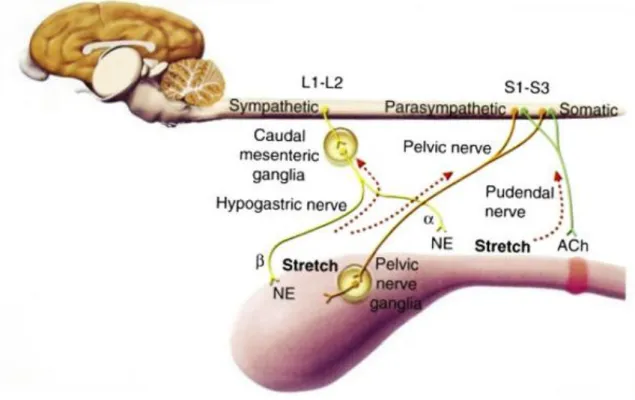
Normal micturition involves a passive phase (storage of urine) and an active phase (voiding of urine). The detrusor smooth muscle composes the body of the bladder and its fibers continue to the proximal urethra to form the internal urethral sphincter. The storage phase is regulated by the sympathetic nervous system that leads to relaxation of the detrusor muscle, while the bladder is filled with urine, and to the contraction of the internal urethral sphincter, avoiding leakage. When the stretch receptors of the detrusor muscle are activated due to bladder fullness, the voiding phase takes place. This phase is regulated by the
7 parasympathetic nervous system and results in contraction of the detrusor muscle and relaxation of the internal urethral sphincter, leading to the act of micturition. Voluntary relaxation of external urethral sphincter also occurs in this phase.
The inappropriate passage of urine during the storage phase is called UI. It can be divided in neurogenic causes (detrusal-urethral dyssynergia, dysautonomia) and non-neurogenic causes (overflow incontinence, urethral sphincter mechanism incontinence, UE). In dogs with EU, UI occurs because there is a flow of urine distal to the internal urethral sphincter. However, in some cases of UE, a normal voiding pattern can be observed (Adams & Syme, 2009; Grauer, 2009).
Even though it is a rare condition, EU are the most common cause of UI in young animals (Anders et al., 2012; Davidson & Westropp, 2014; Ho et al., 2011). Intramural EU are more common in the canine species than the extramural type, which is considered rare (Davidson & Westropp, 2014; Ho et al., 2011; MacPhail, 2012; Mathews, 2011; Mayhew & Berent, 2013; Mcloughlin & Bjorling, 2003). A prevalence of more than 95% has been reported regarding intramural UE (A. C. Berent et al., 2012). Bilateral UE is also reported more frequently than unilateral (MacPhail, 2012; M. A. McLoughlin & Chew, 2012).
8 Although it can occur in male dogs, this condition is much more commonly reported in females (A. C. Berent, 2011; MacPhail, 2012; M. a McLoughlin & Chew, 2000; North et al., 2010; Samii et al., 2004; Steffey & Brockman, 2004; Taney et al., 2003). There may be either a true gender predisposition or EU are being underdiagnosed in males due to lack of clinical signs (Anders et al., 2012). It is believed to exist a clinical masking since males have longer urethras and a strong external urethral sphincter which allows retrograde filling of the bladder to happen preventing them from becoming incontinent (Anders et al., 2012; Davidson & Westropp, 2014; MacPhail, 2012; Mcloughlin & Bjorling, 2003; Thomas & Yool, 2010). For this reason, the prevalence of UE in male dogs might be underestimated and this condition should always be considered as a differential diagnosis in continent male dogs with recurrent urinary tract infections (UTI), renal agenesis, hydroureter or hydronephrosis (A. C. Berent, Mayhew, & Porat-mosenco, 2008). Female dogs with more than 20 kg may have a higher lifetime risk of developing UI than those with less than 20 kg (A. C. Berent et al., 2012).
Some breeds seem to be predisposed to UE such as Siberian Husky, Toy Poodle, West Highland White Terrier, Golden Retriever, English Bulldogs, Newfoundland, Skye Terrier and Fox Terrier as they present more frequently (Ho et al., 2011; MacPhail, 2012; Mathews, 2011; Mayhew & Berent, 2013; M. a McLoughlin & Chew, 2000; North et al., 2010). According to Johnston et al., (1977), referenced in Ho et al., (2011) the lack of reported EU cases in mixed-breed dogs and its incidence in certain purebreeds suggests that familial genetics is highly related to this congenital process. Its high prevalence in Labrador Retriever and Golden Retriever populations also supports this theory (Anders et al., 2012).
Neutering dogs with EUs might be a predisposing factor to UI since the removal of the gonads leads to a reduction in the urethral closure pressure (Reichler et al., 2012).
5. Diagnosis
5.1 Signalment and medical history
Usually, female dogs present with UI at younger age than male dogs. Median age at surgery in male dogs is 3-4 years according to latest reports (Reichler et al., 2012) while most female patients are under one year of age when diagnosed, being the mean age of presentation 10 months (Ho et al., 2011; MacPhail, 2012) Hannah W. Velzen, 2013). However, it has been
9 reported a case of delayed-onset of UI in a 32-month-old female Newfoundland (Thomas & Yool, 2010). Older animals with delayed-onset of UI must be evaluated for EU, since this type of clinical presentation has been reported before, suggesting that it may be more common than the current literature shows (Thomas & Yool, 2010). The reason why female dogs can initially be continent is not yet understood (Thomas & Yool, 2010). It is believed that older male dogs with undiagnosed EUs become incontinent later in life due to reduced urethral sphincter tone (Thomas & Yool, 2010). Animals with UE are usually incontinent since birth or weaning and may have had recurrent urinary infections (A. C. Berent et al., 2012).
5.2 Clinical signs
The typical clinical presentation of dogs with UE is related to continuous or intermittent UI (Ho et al., 2011; Mathews, 2011; Mcloughlin & Bjorling, 2003; North et al., 2010). It usually begins at birth or weaning, but many dogs develop incontinence after a period of continence or only in certain positions (A. C. Berent et al., 2012). However, a high prevalence of continent dogs with intramural EUs has been reported in one study (North et al., 2010), which shows that normal voiding patterns can be observed (M. a McLoughlin & Chew, 2000). For this reason it is important to consider UE as a differential diagnosis in continent animals with hydroureter or hydronephrosis (North et al., 2010; Steffey & Brockman, 2004). Pollakiuria, stranguria and hematuria can be present due to urinary infection (Adams & Syme, 2009; Grauer, 2009; MacPhail, 2012). Nocturia can sometimes be the only clinical sign (Davidson & Westropp, 2014).
5.3 Physical examination
Most of the times, the physical examination is within normal limits (M. a McLoughlin & Chew, 2000). Perivulvar or prepucial dermatitis secondary to urine scalding can be present as well as moist or urine-stained hair on the same regions (Davidson & Westropp, 2014; M. a McLoughlin & Chew, 2000; Mcloughlin & Bjorling, 2003).
10 5.4 Laboratory findings
A complete blood cell count, biochemical profile, urinalysis and urine culture with sensitivity tests must be performed firstly in order to assess the patient’s systemic condition and particularly renal function. The urine must be obtained by cystocentesis even though it can be difficult due to continuous UI (Davidson & Westropp, 2014; M. a McLoughlin & Chew, 2000). If this is not accomplished, urine from the bladder or the ureter must be obtained during surgery (M. a McLoughlin & Chew, 2000). Usually all the blood work is within normal limits unless the renal function is diminished (M. a McLoughlin & Chew, 2000). A study showed that 24 out of 33 dogs had positive urine culture before surgery and E. Coli was the predominant isolated organism (Ho et al., 2011).
6. Differential diagnosis
Functional and structural abnormalities such as urinary tract infection, cystic or urethral calculi, neurogenic disorders, primary USMI, endocrine abnormalities, renal dysfunction, vestibulovaginal abnormalities and neoplasia must be taken in consideration (Hoelzler & Lidbetter, 2004; M. a McLoughlin & Chew, 2000).
11 7. Complementary diagnostic tests
7.1 Radiology
There are several radiologic techniques to assess UE being some of them outdated nowadays. They can be used individually but often times a combination of techniques is performed in order to achieve more accurate and reliable information and possibly a definitive diagnosis.
7.1.1 Intravenous urography
A normal ureter cannot be visualized with simple radiography (Rozear & Tidwell, 2003). For this reason, contrast radiography is used to evaluate the upper and lower urinary tract regarding organ location, size and morphology as well as location of the ureteral openings and presence of unilateral or bilateral UE (M. a McLoughlin & Chew, 2000). Normal ureters appear as radiopaque lines connecting the kidneys to the bladder in an intravenous urography (IVU) (Grauer, 2009).
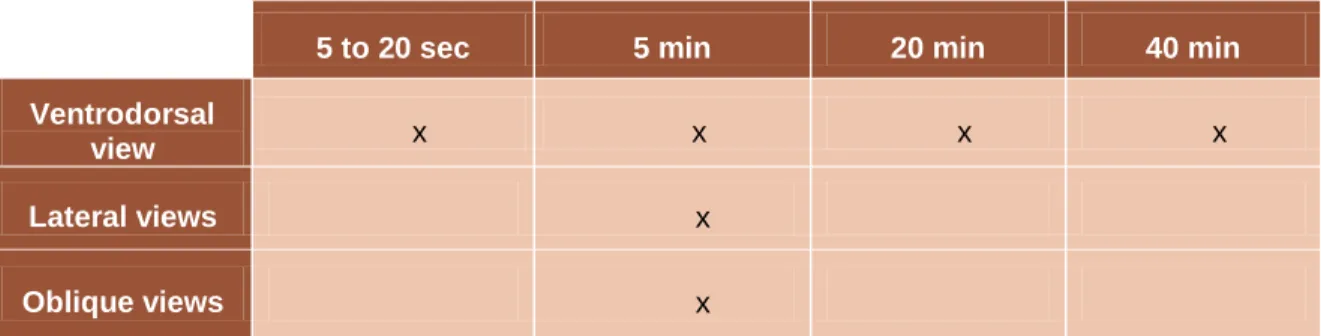
A dog that undergoes IVU needs to be fasted for at least 24 hours and enemas must be done 2 hours before the procedure. Factors like fecal matter in the colon, bladder not fully distended, hypoplastic or pyelonephritic kidneys that decrease the excretion of contrast or cause accumulation of contrast in the bladder may obscure the exact opening of the ureters (Rozear & Tidwell, 2003; Taney et al., 2003). An evaluation of the hydration status is very important and if the animal is dehydrated IVU should not be performed. A plain radiograph must be taken to verify the effectiveness of the enemas. After that, the iodinated contrast solution is injected intravenously as a bolus injection. To evaluate all the urinary tract, after the injection of the contrast material, several abdominal radiographs must be taken as follows:
5 to 20 sec 5 min 20 min 40 min
Ventrodorsal
view x x x x
Lateral views x
Oblique views x
Table 1 – Different views and timings to be followed after intravenous injection of contrast in IVU (Adapted
12 Lateral, ventrodorsal and oblique views are important to determine the segment of nondilated ureter in the pelvic region (M. a McLoughlin & Chew, 2000). If the diameter of the ureter is greater than 0.09 times the length of the second lumbar vertebrae it is considered to be dilated (M. a McLoughlin & Chew, 2000).
The accuracy of IVU is increased if associated to pneumocystography since it decreases the number of misdiagnosis caused by superimposition of pelvic structures (Barthez, 1998; Cannizzo et al., 2003; Taney et al., 2003). It also greatly helps in the identification of the ureteral junction (P. Mantis, D. Brockman, 2006). The pneumocystogram must be performed prior to the injection of the intravenous contrast (P. Mantis, D. Brockman, 2006). Fluoroscopy and vaginourethrography are also recommended in association with IVU in order to improve evaluation of the ureterovesicular junction (Barthez, 1998). In one study, IVU allowed the diagnosis of UE in 12 out of 17 dogs. The diagnosis was facilitated by the use of digital fluoroscopy (Samii et al., 2004).
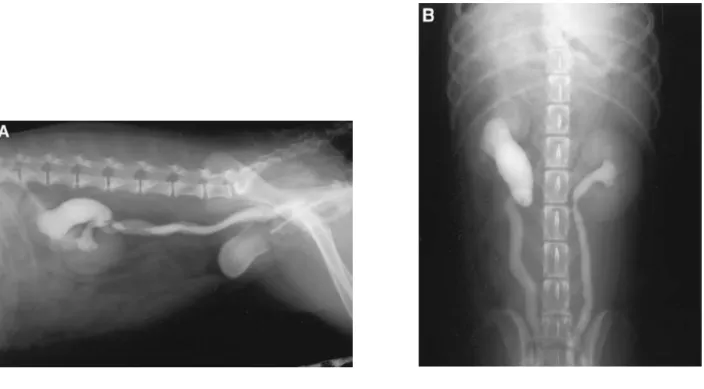
Limitations of IVU include the evaluation of fenestrations that allow part of the contrast to enter the bladder proximally while there are other distal abnormalities that can be missed (M. A. McLoughlin & Chew, 2012). This technique in combination with retrograde urethrography was the primary imaging method in perioperative assessment of dogs with UE for a long period of time but even so, this condition often went undiagnosed (Samii et al., Figure 6 – IVU with negative contrast cystography (NCC). Lateral (A) and ventrodorsal (B) views revealing
13 2004). It is considered today that IVU cannot consistently detect EUs and ureteral terminations even when combined with other radiographic techniques (Cannizzo et al., 2003).
7.1.2 Cystography
Cystography is a technique that allows a radiologic study of the bladder. There are three types of cystography: negative contrast cystography (NCC), also known as pneumocystography; positive contrast cystography (PCC) and double contrast cystography (DCC) (Bartges, 2011). NCC is a cheap, easy way to evaluate the location of the bladder and possibly detect stones or masses. Room air, oxygen, nitrous oxide and carbon dioxide can be used as negative contrast agents. Carbon dioxide is the safest choice because it is more soluble in water decreasing the possibility of gas embolism which is the most significant complication of this technique. The injection of this gas will lead to mild distension of the bladder. Severe distension must be avoided since it can cause rupture or mask mild mucosal irregularities (Bartges, 2011).
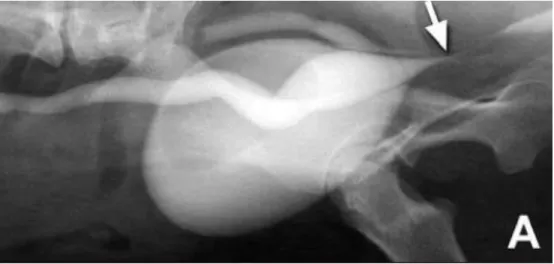
PCC is an excellent mean to detect mural lesions such as infiltrative neoplasia. For this technique, the bladder is filled with iodinated contrast medium, a positive contrast. This is the best method to detect ureteroceles sometimes associated to EUs (Bartges, 2011). Also, by increasing intravesicular pressure with contrast solution we can cause a backflow of contrast Figure 7 – NCC lateral view. A soft tissue opacity can be seen in the dorsal region of the bladder (Adapted
14 Figure 9 – DCC associated to IVU revealing an apparently normal entrance of the ureter in the bladder
trigone (arrow). The presence of a fenestration allowed a portion of contrast to enter the bladder in the correct place while the EU continued distally (Adapted from Cannizzo et al., 2003).
into the terminal portion of the ureters allowing a better image of the ureteral junction (Taney et al., 2003).
The method of choice to evaluate the integrity of the bladder wall as well as its thickness and intraluminal fillings is DCC. In this procedure, a small amount of positive contrast is administered after distending the bladder with negative contrast . The animal should then be rotated in order to coat the entire mucosal surface (Bartges, 2011).
Figure 8 – PCC and IVU showing bilateral, dilated extramural EU and its pathway past the bladder trigone
15 For all of the techniques mentioned above, the animal needs to be sedated or anesthetized and have the urethra catheterized (Bartges, 2011).
7.1.3 Retrograde urethrography (RU)
This technique allows an evaluation of the urethra which is a common anatomical region for EU’s openings. For this procedure, the bladder should be distended to provide a better visualization of the proximal urethra. The positive contrast is then administered in the urethra and the radiographs must be taken while the last couple of milliliters of contrast are being injected (Bartges, 2011). Because urethral catheterization is required to this procedure, the value of this diagnostic can be reduced since the catheter can obscure the ureteral orifices in the urethral area (M. a McLoughlin & Chew, 2000).
One study reported a 47% success rate in diagnosing UE using RU (Samii et al., 2004). In many dogs without ureteral dilation in which contrast backflow was not observed, this diagnostic produced false negative results. In one other study this technique was used in three dogs and only lead to a definitive diagnosis in one (Anders et al., 2012).
7.1.4 Retrograde vaginourethrography (RVU)
This technique allows the visualization of the vestibule and vagina as well as full urethral length (Bartges, 2011). It does not require catheterization of the urethra and can be very helpful in evaluating the ectopic ureteral orifices located in the observed areas as well as other abnormalities that lead to lower urinary tract signs such as vestibulovaginal stenosis and vaginal septa (Bartges, 2011; M. a McLoughlin & Chew, 2000). A balloon-tip catheter must be placed within the vestibule, caudal to the urethral orifice in which the contrast medium will be injected. The vulva can be clamped with forceps to prevent leakage of contrast during the exam. Lateral and ventrodorsal views must be obtained in the end of the injection of contrast (Bartges, 2011).
16 Figure 10 – RVU and IVU. The arrow points at an EU entering the urethra (Adapted from P. Mantis, D.
Brockman, 2006).
All radiographic techniques may be compromised by obscured visualization of the bladder trigone caused by overlying pelvic structures (Samii et al., 2004).
7.2 Ultrasonography
Ultrasound is a good method for diagnosing EUs, identifying concurrent abnormalities and suggesting the need for other diagnostic tests such as IVU (Smith et al., 2010). It is very useful to evaluate the entire urinary system since it allows visualization of the complete urinary tract.
A normal ureter is not visible on ultrasound (Bartges, 2011; Rozear & Tidwell, 2003). The visualization of ureteral jets is an indicator of normalcy but it can also be seen in cases of EU with multiple openings, not excluding the possibility of a distal ectopic opening. An EU is visualized as a nonvascular structure filled with fluid and with an hyperechoic wall passing dorsal to the bladder or clearly entering the proximal urethra. Easily seen ureteral walls and its pulsatile motion are indicators of UE. Visualization of the bladder neck and proximal urethra can be difficult due to pubic bone impedance. Ultrasonographic appearance of the kidneys, bladder, ureters and urethra can be altered by infectious and inflammatory processes often times present in animals with UE (Davidson & Westropp, 2014).
17 Documented benefits of this technique include being a less invasive procedure, avoiding general anesthesia and limiting radiation exposure when compared to computed tomography (CT) (Novellas, Stone, Pratschke, & Hammond, 2013). It has been referred as the most sensitive, specific and accurate imaging method. Identification of the side of UE is a limitation of this technique as it is the limited acoustic window available in bladders with intrapelvic location (P. Mantis, D. Brockman, 2006).
7.3 Uroendoscopy
Uroendoscopy has been reported as superior to other imaging studies in achieving definitive diagnosis of EU and determining its morphology, as well as detecting other urogenital congenital abnormalities (Davidson & Westropp, 2014; Ho et al., 2011; North et al., 2010). Urachal diverticuli (that can occur with EU) can be identified with cystoscopy (Davidson & Westropp, 2014). It allows the operator to control bladder distension and observe the ureteral opening’s morphology and position (A. C. Berent, 2011; North et al., 2010). That is why it has become a popular way of diagnosing and characterizing EUs in the
18 Figure 12 – Cystoscopic image of the vaginal vestibule (dorsal) and external urethral meatus (ventral) in a
female dog (Bartges, 2011).
last few years (M. a McLoughlin & Chew, 2000; Smith et al., 2010). This technique significantly improved the identification of EUs and the study of its morphology, especially extramural ureters (Cannizzo et al., 2003). It is a very reliable method for diagnosing UE and in a 25 dog study, the agreement between uroendoscopic and surgical findings was 100% (Cannizzo et al., 2003).
There are two main types of endoscopes: rigid and flexible. Usually, rigid endoscopes are used in females and flexible in males. The rigid endoscope used in females provides superior imaging regarding image clarity and amount of illumination of the visual field than the flexible endoscope used in males which can lead to a decrease in its diagnostic utility (Anders et al., 2012). Uroendoscopy can be performed in adult dogs, puppies larger than 3 kg, adult female cats and male cats after perineal urethrostomy (M. a McLoughlin & Chew, 2000).
For this procedure, animals need to be anesthetized and placed in sternal, dorsal or lateral recumbency. The cystoscope is introduced in the vaginal vestibule where it is possible to see the vaginal orifice dorsally and the urethral orifice ventrally. A wide band of tissue called the mesonephric remnant is often seen across the vaginal opening in animals with UE. Some blin-ended fenestrations may be seen in a normal canine external urethral meatus and they should not be mistaken for EU. Moving the cystoscope cranially and ventrally, the urethra is entered and it can be evaluated in all its length. Normal urethral mucosa is smooth and light pink, and its length varies between normal dogs. As the bladder trigone is reached, the ureteral openings can be seen in normal dogs and they present as two C-shaped structures facing each other as mirror images (Bartges, 2011). Visualization of intermittent, pulsating urine jets is possible if the ureters are patent (M. a McLoughlin & Chew, 2000). A normal bladder has a light pink mucosa with a fine vascular pattern (Bartges, 2011).
19 Figure 13 – Cystoscopic image of a mesonephric remnant in a female dog (Adapted from Bartges & Polzin,
2011).
Figure 14 – Small blind-ended fenestrations in the external urethral meatus that might be mistaken for EU
20 EUs can be encountered anywhere from the vestibule to the urethrovesicular junction and are sometimes large enough to be catheterized (Bartges, 2011). Submucosal tunnels of intramural ureters are not always visible because fluid insufflation leads to an increase in urethral pressure causing the tunnel to collapse, a problem the operator can overcome by frequently repositioning the endoscope (M. a McLoughlin & Chew, 2000).
A benefit of this technique is the minor invasion required (Smith et al., 2010). Some risks are ureteral and urethral tearing that can lead to uroperitoneum, bleeding and recannulization (Smith et al., 2010). Limitations include the requirement of a high level of operator expertise and the inability to thoroughly evaluate the upper urinary tract (Samii et al., 2004).
7.4 Computed Tomography
CT of the urinary tract follows the same principals as conventional radiography where X-rays and contrast medium are used to acquire image. However, CT provides superior contrast resolution and anatomical detail which makes it a more specific and sensitive diagnostic test (Bartges, 2011). The fact that pelvic superimposition does not occur with this technique, allows a more detailed evaluation of the anatomy (Anders et al., 2012; Samii et al., 2004). It is possible to evaluate the location, size and morphology of the ureters as well as the location of the openings and identification of other urinary tract anomalies (Novellas et al., 2013; Samii et al., 2004).
It has been reported that colonic content and bladder volume do not interfere with the correct identification of the ureters. Therefore, aside from general anesthesia, minimal or no Figure 15 – Cystoscopic image of the ureters in their normal anatomical position: the bladder trigone
21 preparation is required for patients that undergo CT to evaluate ureteral morphology (Bartges, 2011; Barthez, 1998; Rozear & Tidwell, 2003).
Normal ureters should have a uniform appearance in all its length on contrast CT. They should be less than 1-2 mm in diameter and have normal peristaltic activity (Bartges, 2011). This peristaltic activity can lead to asymmetric opacification of the ureters which can be differentiated from strictures and other causes of obstruction by reacquiring images of the same region (Bartges, 2011; Barthez, 1998; Rozear & Tidwell, 2003). Often times EUs have structural and functional abnormalities than can compromise its contractility which leads to even more consistent ureteral opacification (Barthez, 1998). Even when not opacified by contrast medium, it is possible to identify the ureters due to the contrast provided by the surrounding fat tissue (Barthez, 1998; Rozear & Tidwell, 2003). Using contrast CT the complete course of a ureter and its termination can be defined. If the ureter can be followed caudal to the bladder trigone, the diagnosis of UE is achieved. Ureteral intramural tunneling is present if the ureter apposes the bladder for more than 9 mm in length. Dorsal and sagital planes can help define the complete path of the ureters (Bartges, 2011).
Figure 16 – CT image showing a longitudinal section of the left ureter and transversal section of the right
ureter. Both the ureters and the left kidney are normal and enhanced with contrast medium (Adapted from Bartges & Polzin, 2011).
22 This technique along with uroendoscopy has been referred as the most useful diagnostic for EU identification (A. C. Berent, 2011; A. C. Berent et al., 2012; Cannizzo et al., 2003; Samii et al., 2004). The results of one study showed that CT is as successful documenting EU in dogs as it is in humans, diagnosing 16 out of 17 dogs with this condition. For this reason, it is considered the diagnostic imaging modality of choice to diagnose UE (Samii et al., 2004).
7.5 Urodynamic testing
USMI is often associated with UE (Hoelzler & Lidbetter, 2004). Urethral pressure profile is a test used to evaluate the functional capability of the urethra in patients with UI and it is a record of the intralumenal pressure exerted by the urethral wall in all its extension (M. a McLoughlin & Chew, 2000). Even though it cannot be used to diagnose UE, this diagnostic can provide information regarding urethral tone and some degree of prediction of postsurgical continence (Davidson & Westropp, 2014; Hoelzler & Lidbetter, 2004; M. a McLoughlin & Chew, 2000). It is also a good method for identifying concurrent abnormalities of the bladder and urethra (M. a McLoughlin & Chew, 2000). In a 9 dog study (Lane IF et al., 1995), referenced by M. a McLoughlin & Chew, 2000, 89% of dogs with EU were reported as having concurrent functional abnormalities of the bladder or urethra after urodynamic evaluation was done. The same study showed a questionable urethral function in 78% of the dogs. For this reason, and even though it does not have a diagnostic value, this test should be performed in patients with UI.
Figure 17 – Squematic representation of an urethral pressure profile. The animal is catheterized with a urethral
pressure profile catheter and fluid is injected into the bladder (blue arrow) while resistance to infusion is measured in cm H2O at the catheter tip (multiple white arrows). IVP – intravesicular pressure; BUP – begin urethral pressure; MUP
23 Due to the lack of equipment and formal training in most practices and the interference of inflammation (often present in animals with EUs) and urethral spasm in this test, the practicality of this technique is considered low (MacPhail, 2012).
8. Treatment
8.1 Medical treatment
As mentioned before, USMI is very often present in animals with UE, especially females (Davidson & Westropp, 2014; MacPhail, 2012). Medical management of UE can be attempted to control UI and it may be beneficial in cases where USMI is present. α-adrenergic drugs such as phenylpropanolamine (1.0-1.5 mg/kg orally every 8-12 hours) may be used to increase urethral tone. Estrogen compounds as diethylstilboestrol (0.02 mg/kg orally daily, then tapered to 1 to 3 times weekly or 2 mg orally daily then tapered) increase sensitivity of α-adrenegic receptors in the urethra and have proven to be effective as well (Davidson & Westropp, 2014). The combination of these two drugs can be more effective when the single agent therapy is not working (Davidson & Westropp, 2014). α-adrenegic drugs are more effective when treating acquired incontinence rather than the congenital form (Reichler et al., 2012).
However, surgery is the treatment of choice for UE and medical treatment should always be seen as an adjunctive therapy to maintain urinary continence after surgery in these cases. One study reported 7 out of 17 dogs that responded to medical treatment after surgery improving their continence (Mayhew et al., 2006).
8.2 Surgical treatment
Surgery is the treatment of choice to correct UE and whatever technique is performed, the goal is to create a new stoma in the bladder trigone in order to improve urinary continence and decrease the incidence of UTI (Mayhew et al., 2006). If untreated, EUs can evolve to hydroureters, hydronephrosis, bacterial cystitis and pyelonephritis and irreversible renal damage can occur (Davidson & Westropp, 2014).
24 8.2.1 Pre-surgical preparation
Hydroelectrolytic and acid-base abnormalities should be corrected before surgery (MacPhail, 2012). According to the results of the urine culture and sensitivity tests, an appropriate antibiotic must be administered (A. C. Berent et al., 2012; MacPhail, 2012).
8.2.2 Anesthesia
Unless there is renal compromise, there is no need for a specific anesthetic protocol (MacPhail, 2012). If there is some degree of renal damage, alfa 2 agonists and acepromazine should be avoided as they lead to severe hypotension (MacPhail, 2012). Epidural administration of analgesics is an excellent strategy for intraoperative and postoperative pain management. However for epidurals, local anesthetics should be avoided in hypotensive patients and morphine must be used carefully since it may cause bladder atony and urine retention (Bartges, 2011).
8.2.3 Surgical anatomy
In an open abdomen, most of the urinary apparatus can be seen and explored. The kidneys are located in the retroperitoneal space lateral to the aorta and vena cava. They are involved by a fibrous capsule and held in place by subperitoneal connective tissue. The cranial pole of the right kidney is located at the level of the 13th rib and the left kidney can be found approximately half a kidney length caudal to the right one. In the medial border of the kidneys, in the hilus, the renal artery and vein and the ureter can be observed. The ureters leave the renal pelvis and extend along the sublumbar muscles towards the dorsal surface of the bladder where they enter. The ureters form the base limits of the bladder trigone in which the urethra is included. Blood supply to the kidneys is provided from the renal artery that normally bifurcates in dorsal and ventral branch, although variations are common. Blood supply to the ureter is provided from a branch of the renal artery (cranial ureteral artery) and a branch of the prostatic or vaginal artery (caudal ureteral artery) (MacPhail, 2012; Mathews, 2011; Mcloughlin & Bjorling, 2003).
25 8.2.4 Surgical approach
To evaluate the lower urinary tract using an open abdominal technique, a caudal ventral midline celiotomy extending to the pubis is required. A medial ventral cystotomy and urethrotomy are performed to expose the bladder and urethral lumen. Stay sutures are placed to facilitate manipulation and exploration of the region. If the ectopic ureteral openings are located in the terminal aspect of the urethra or vagina, a pubic osteotomy might be necessary to expose these areas. (Mcloughlin & Bjorling, 2003).
8.2.5 Surgical techniques
8.2.5.1 Neoureterostomy
Neoureterostomy is one of the techniques used to correct intramural EU. Identification of the course of the ureter and its ectopic opening is required and can be
Figure 18 – Squematic representation of the anatomy of the female urinary apparatus, ventral view (Adapted
26 achieved by three different methods: retrograde ureteral catheterization using cystoscopy, intra-operative direct visualization or intra-operative distension of the ureter by digitally occluding both the urethra and the EU (Ho et al., 2011). After identification and evaluation of the EU, a new stoma is created in the bladder trigone, (the right anatomic region for ureteral openings) by incising the bladder mucosa into the ureteral lumen. The ureteral mucosa is then sutured to the bladder mucosa using monofilament sutures in a simple interrupted pattern (Ho et al., 2011; MacPhail, 2012; Mathews, 2011; Mayhew et al., 2006). The distal ureteral segment (DUS) is then occluded using the ligation technique in which circumferential sutures are placed through the bladder wall, around a urinary catheter and back out (Ho et al., 2011; MacPhail, 2012; Mathews, 2011; Mayhew et al., 2006). These sutures should not enter the bladder lumen (MacPhail, 2012; Mathews, 2011).
Figure 19 – (A) Incision of the bladder mucosa into the ureteral lumen. (B) Suture of the bladder
mucosa to the ureteral mucosa. (C) Catheterization of the DUS. (D) Ligation of the DUS placing circumferential sutures around the catheter and beneath the bladder mucosa (Adapted from Fossum, 2012).
27 Figure 21 – Diagram of a neoureterostomy and resection technique. (A) The DUS has been catheterized
and excised. (B) The urethral/trigonal defect has been closed distally to the new stoma (Adapted from Hoelzler & Lidbetter, 2004)
Figure 20 – (A) Neostomy orifices. (B) Catheterization of the DUS for further ligation and the neostomy to
verify its patency (Adapted from Maria et al., 2013).
Alternatively, the DUS can be excised using the resection technique (neoureterostomy with bladder neck reconstruction) in which the ureteral segment is ligated proximally near the new stoma and the distal tissue dissected and resected (Ho et al., 2011; MacPhail, 2012; Mathews, 2011; Mayhew et al., 2006). The created submucosal defect is then closed with absorbable suture material (Mayhew et al., 2006). Some theoretical advantages of this technique include restoration of the functional anatomy of the urethral sphincter mechanism, decreased risk of recannulization and improvement in urinary continence as well as decreased incidence of UTI (Mayhew et al., 2006).
28 It was hypothesized that leaving the intravesical portion of the DUS would result in a higher prevalence of cystitis and recannulization so they performed a modified version of neoureterostomy in which they ligate the EU distal to the new stoma with two absorbable sutures and then dissect and remove the portion of ureter between the most proximal ligature and the neostomy. The success rate of this modified technique was higher than previous results after complete resection of the distal end of the EU (Reichler et al., 2012).
Some possible complications of using the ligation technique include the inability to correct the interference of the DUS with the urethral sphincter mechanism, the possibility of recannulization of the distal segment perpetuating UI and the risk of reflux into the distal segment promoting recurrent UTI (Maria et al., 2013; Mayhew et al., 2006). The resection technique is considered very demanding and there is a greater risk of hemorrhage and damage to the urethra (Mayhew et al., 2006).
One study showed that there is no difference in clinical outcome regarding UI and UTI incidence using neoureterostomy with resection of the DUS or neoureterostomy with ligation of the DUS. However, it has been hypothesized that resection of the ureteral distal segment may damage the urethral sphincter due to edema, inflammation and possible fibrosis raising questions about the actual benefits of resection over ligation (Mayhew et al., 2006).
Figure 22 – Final appearance of the bladder after neostomy and resection and closure of the DUS (Adapted
29 Figure 23 – Ureteral re-implantation. (A) Ureter is ligated and transected. The circle represents the place of the new
stoma. (B) The forcep is pushed through the bladder and grasps for the ureteral stay suture. (C) A longitudinal incision is made in the ureter to increase the area of suturing (spatulation). (D) Simple interrupted sutures are placed between the bladder and ureteral mucosa. (Adapted from McLoughlin & Chew, 2000)
8.2.5.2 Ureteral re-implantation (end-to-side neoureterocystostomy)
This technique is usually performed in extramural EU. Using this technique, the ureter is ligated as distally as possible and transected to be later re-implanted in an avascular area of the bladder trigone (Ho et al., 2011; Reichler et al., 2012). A stay suture is placed in the ureter. In an intravesicular ureteroneocystotomy, a ventral cystotomy is performed and stay sutures are placed along the edges of the incision. A 4-5 mm ellipse of bladder mucosa can be excised at the proper site of the ureteroneocystotomy and a mosquito hemostatic forceps is pushed from the lumen and through the bladder wall. The forceps grasp for the ureteral stay suture and pull it through the bladder wall and into the bladder lumen. After verifying that it is not twisted and removing the periureteral fat on its distal end, the ureter is spatulated (Bartges, 2011). An anastomosis is performed apposing the ureteral and bladder mucosa in a simple interrupted pattern, using absorbable monofilament sutures (Ho et al., 2011; Mathews, 2011). The placement of too many sutures must be avoided to prevent mucosal edema and scarring that can occlude the ureteral orifice (Hoelzler & Lidbetter, 2004).
30 A variation of the previous technique is the extravesicular ureteroneocystotomy in which instead of performing a cystotomy to further create the new stoma, a partial thickness incision is made through the apex of the bladder to expose the mucosa. The ureter is spatulated and an incision with the same length as the spatulated ureter is made in the bladder mucosa. Single interrupted sutures are placed between the ureteral and bladder mucosa at the cranial and caudal ends of the incision. After verifying the patency of the re-implanted ureter, the anastomotic suture can be finished with a simple interrupted pattern. The seromuscular bladder incision is partially and carefully closed to keep the ureter unobstructed (Bartges, 2011).
Stricture formation at the implantation site and vesicoureteric reflux are the most common complications associated with this technique (Ho et al., 2011). Hydroureter, cystitis, anastomotic dehiscense and loss of normal ureteric peristalsis are other reported complications (Hoelzler & Lidbetter, 2004; Maria et al., 2013). Uroperitoneum has been reported in a cat due to leakage around the re-implantation site (Steffey & Brockman, 2004). According to Holt et al. (1995) referenced in Smith et al., 2008, 8% of the animals that underwent ureteral re-implantation suffered from hydronephrosis in the post-operative period.
Figure 24 – (A) Location of the extramural EU (white arrows). (B) Double ligature of the distal ureter
(white arrow). (C) Re-implanted ureters (white arrows), ligated distal portion of the ureters (black arrows) and position of the urethra (blue arrow) after ureteral re-implantation. (Adapted from Maria et al., 2013)
31 8.2.5.3 Nephroureterectomy
If the kidney associated with the EU is severely damaged, a nephroureterectomy should be performed. If there is the need to perform this technique, function of the contralateral kidney should be evaluated (Ho et al., 2011; Mathews, 2011; Smith et al., 2010). Only if the contralateral kidney is functioning normally should this be considered an option (Hoelzler & Lidbetter, 2004). To perform a nephroureterectomy, the kidney should be detached from the retroperitoneum and reflected medially (Mayhew & Berent, 2013). Renal artery and vein must be identified, dissected, double or triple ligated and sectioned (Ho et al., 2011; Mayhew & Berent, 2013). The ureter should then be dissected and ligated as distally and close to the bladder as possible and resected together with the kidney (Ho et al., 2011; Mayhew & Berent, 2013).
Figure 25 – Nephroureterectomy. (A) Ventral midline celiotomy is performed to enter the abdominal
cavity. (B) The kidney is detached from the retroperitoneum and reflected medially. (C) Renal artery and vein are ligated. (D) Renal artery and vein are transected. (E) The ureter is ligated and transected as distally as possible. (Adapted from Tobias & Johnston, 2011)
32 8.2.5.4 Cystoscopic-guided laser ablation (CLA)
Lately, cystoscopic identification of intramural EUs and laser ablation has become a popular technique within the veterinary community (Smith et al., 2010). This technique was first described in 2006 by Dr. Timothy McCarthy in an 8-week-old female Labrador Retriever (McCarthy, 2006).
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation and it is a light transmitting device that produces a beam of intense, small and nearly non-divergent monochromatic radiation. When focused in a close range, they produce immense heat and power (Bartges, 2011). The variety of equipment that can be used for this procedure includes diode laser, Holmium:yttrium-aluminium-garnet laser (Holmium-YAG) and radiofrequency surgical device (A. C. Berent, 2011; Davidson & Westropp, 2014). Dr. McCarthy performed laser ablation of an intramural EU for the first time using a diode laser but, nowadays, YAG laser fiber is used most frequently (Bartges, 2011). Holmium-YAG is one of the newest and safest lasers and its active medium is a crystal of yttrium, aluminium and garnet (YAG) doped with holmium (chromium and thulium). Its beam falls near the infrared portion of the electromagnetic spectrum (Bartges, 2011). Diode laser works at a wavelength of 980 nm and has both coagulation and cutting properties while the Hol:YAG laser works at a wavelength of 2100 nm in a pulsed fashion and it is best for fragmentation of stones even though it can be used to ablate tissue. To avoid excessive bleeding using the Hol:YAG laser, direct contact with the tissue should be avoided keeping the laser 0.1 to 0.5 mm away from the tissue to be ablated providing coagulation instead of cut (A. C. Berent et al., 2012).
For this procedure, the patient must be anesthetized and can be positioned in dorsal, sternal or right lateral recumbency. A rigid cystourethroscope (1.9-4.0 mm of diameter) with passive flow of saline 0.9% is used in females (A. C. Berent et al., 2012; Davidson & Westropp, 2014; Smith et al., 2010). For male dogs, a 5-7.5 fr flexible endoscope may be used (Bartges, 2011). Before the use of the laser fiber, a thorough inspection of the lower urinary tract and reproductive tract must be performed in order to find other possible concurrent anomalies such as vestibulovaginal septal remnants and hydroureters (Smith et al., 2010). In one study, vestibulovaginal septal remnants were present in 93% of female dogs with EU (Burdick, Berent, Weisse, & Langston, 2014). These are believed to cause dorsal traction to the urethral meatus contributing to UI and so they must be removed (A. C. Berent, 2011;
33 Davidson & Westropp, 2014; Smith et al., 2010). After the normal and EUs are identified, a 4-french open-ended ureteral catheter or guide wire is introduced in the ectopic opening in order to protect the urethral wall from the laser (A. C. Berent et al., 2012; Davidson & Westropp, 2014; Smith et al., 2010). Then, the laser fiber is inserted in the working channel of the cystoscope and used to transect the free (medial) wall of the EU starting at the distal orifice and continuing cranially carefully into the bladder trigone, placing the previously ectopic orifice in its normal anatomic location (A. C. Berent, 2011; A. C. Berent et al., 2012; Davidson & Westropp, 2014; Smith et al., 2010). When the laser is being used in contact mode, an anodized metal guide wire is preferable to a propylene catheter to reduce tissue injury from laser reflection (Smith et al., 2010).
Figure 26 – Cystocopic images of a right intramural EU in a female dog. (A) The urethra (black arrow) and
the ectopic ureteral orifice (black arrowhead). (B) The ectopic ureteral opening is catheterized (white arrow). Notice the urethral orifice (black arrow). (C) The laser fiber is placed at the ectopic ureteral opening (red arrow). Notice the catheter protecting the lateral ureteral wall. (D) The laser cuts the medial wall of the ureter (red arrow) while the catheter protects the lateral ureteral wall (white arrow). (E) Ureteral catheter after the laser ablation is done (white arrow) and urethrovesicular junction (black arrow). (F) The neoureteral orifice with the catheter inside its lumen (white arrow). Notice the opening of the right ureter is even with the opening of the left ureter (yellow arrow). (Adapted from Berent et al., 2012)
34 Cystoscopic-guided laser ablation is a minimally invasive procedure and involves a very low complication rate (Smith et al, 2010). It provides a diagnosis and simultaneously the possibility of performing the surgery necessary to correct the problem, avoiding the risks and complications of an open abdominal surgery (A. C. Berent et al., 2012). Some benefits of this technique include quicker recovery time and elimination of the possibility of recannulization of the DUS (Bartges, 2011; Davidson & Westropp, 2014). It is also a faster and less expensive procedure than an open abdominal surgery (Bartges, 2011). This technique also allows intraoperative injections of collagen to shorten the urethral diameter in dogs that have an excessively large urethra (A. C. Berent et al., 2012; Smith et al., 2010). However, this procedure is not indicated since leakage from the incised urethral mucosa into the urethral lumen can occur (Smith et al., 2010).
The need for specialized laser ablation equipment and a trained surgeon to perform the technique are holding back factors for most of the general veterinary practices (Smith et al., 2010). This technique is not indicated for extramural UE, even though this type is considered rare (A. C. Berent et al., 2008). Complications include post-operative hematuria and are not related to the type of laser used (A. C. Berent et al., 2012). If the laser ablation is continued too far cranially, perforation and uroabdomen can occur (Mathews, 2011). In one study, a case was reported to have developed hydronephrosis after laser ablation of EU. It is suspected that the inflammation caused by the laser lead to ureteral stricture and further hydronephrosis (Smith et al., 2010). Vulvovaginal inflammation has been reported and can be managed with NSAIDs or narcotics (Davidson & Westropp, 2014).
8.2.6 Post-surgical care
After cystoscopy and laser ablation the patient is usually sent home on the same day or following morning and analgesic medication is sent to be used if needed. NSAIDs such as Meloxicam (0.1 mg/kg PO q 24h for 2 to 3 days) can be administered to dogs without renal compromise whereas Tramadol (2 to 5 mg/kg PO q 8h for 24h) is a good option for dogs with renal damage (A. C. Berent et al., 2012). A six week follow-up examination with imaging must be performed to evaluate the surgical outcome and possible complications (A. C. Berent et al., 2008).
In the postoperative period of an open abdominal surgery (neoureterostomy, ureteral re-implantation or nephroureterectomy) the patient should be monitored for signs of
35 obstruction or leakage. An urinary catheter can be maintained for 3 to 4 days if urethral obstruction occurs due to swelling. If renal impairment is present, the animal should also be monitored for signs of renal failure (MacPhail, 2012). Because hemorrhage is expected after these surgical procedures and clots can obstruct the lower urinary tract, patients must be kept on fluids for 12-36 hours after surgery. Pain management is required for several days (Bartges, 2011).
Appropriate antibiotic therapy is essential whatever technique is used and should be based on antibiotic sensitivity results (M. a McLoughlin & Chew, 2000).
9. Comparison of techniques
The clinical outcome using laser ablation is similar or better to that obtained using open abdominal techniques such as neoureterostomy and ureter re-implantation (A. C. Berent et al., 2012; Davidson & Westropp, 2014; Smith et al., 2010). Complications associated were proved to be minor and easily managed (Smith et al., 2010). The following table points out some of the advantages and disadvantages of CLA vs open abdominal surgery:
Open abdominal surgery CLA
Advantages
Allows better visualization of the surgical field;
Inspection of all the urinary tract can be performed;
Possibility to explore the rest of the abdominal cavity;
Performed with regular surgical material.
Minimally invasive and faster procedure (45 min aprox.) (A. C. Berent et al., 2012);
Quicker recovery;
Less expensive;
Less painful postoperative period;
Decreased time under anesthesia;
Allows diagnosis and treatment during the same anesthetic episode.
Disadvantages
Very painful postoperative period;
Need for more postoperative medication;
Need to perform pubic osteotomy to access all the urethra;
Hospitalization required.
Expensive material required for the technique;
Need for surgeon expertise;
Inability to explore the upper urinary tract.