Original
article
Anti-in
fl
ammatory
and
anti-nociceptive
effects
of
strontium
ranelate
on
the
zymosan-induced
temporomandibular
joint
in
fl
ammatory
hypernociception
in
rats
depend
on
TNF-
a
inhibition
Sheila
Moreira
Alves
a,
Susana
Capistrano
Abreu
a,
Jonas
Cavalcante
Lemos
a,
Francisco
Isaac
Fernandes
Gomes
a,
Shirley
Moreira
Alves
a,
Danielle
Rocha
do
Val
b,
Raul
Sousa
Freitas
a,
Karuza
Maria
Alves
Pereira
a,
Vicente
de
Paulo
Teixeira
Pinto
a,
Gerly
Anne
de
Castro
Brito
c,
Mirna
Marques
Bezerra
a,
Gerardo
Cristino-Filho
a,
Hellíada
Vasconcelos
Chaves
a,*
aFederalUniversityofCeará,AvenidaComandanteMaurocélioRochaPontes,100Derby,CEP:62.042-280,Sobral,Ceará,Brazil
bNortheastBiotechnologyNetwork(Renorbio),FederalUniversityofPernambuco,Av.Prof.MoraesRego,1235CidadeUniversitária,CEP:50670-901,Recife,
Pernambuco,Brazil
cDepartmentofMorphology,FederalUniversityofCeará,RuaDelmirodeFarias,Porangabussu,CEP:60440-261,Fortaleza,Ceará,Brazil
ARTICLE INFO
Articlehistory:
Received2July2016
Receivedinrevisedform24January2017 Accepted10March2017
Availableonline12March2017
Keywords:
Temporomandibularjoint Arthritis
Strontiumranelate TNF-a
ABSTRACT
Background: Temporomandibular joint (TMJ) disorders show inflammatory components, heavily impacting on quality of life. Strontium ranelate has previously shown anti-inflammatory and antinociceptiveeffectsonotherexperimentalinflammatorypainmodels.Thus,weaimtoinvestigate thestrontiumranelateefficacyinreducingthezymosan-inducedinflammatoryhypernociceptioninthe TMJofratsbyevaluatingtheTNF-a,IL-1b,andhemeoxygenase-1(HO-1)involvement.
Methods:Wistarratsweretreatedwithstrontiumranelate(0.5,5or50mg/kg,peros)1hbeforezymosan injection(iart).MechanicalthresholdwasassessedbyVonFreytestandsynoviallavagewascollectedfor leukocyte countingand myeloperoxidase measurement, jointtissue and trigeminal ganglionwere excisedforhistopathologicalanalysis(H&E)andTNF-a/IL-1blevelsdosage(ELISA).Moreover,ratswere pre-treated with ZnPP-IX (3mg/kg, sc), a specific HO-1 inhibitor, before strontium ranelate administration (0.5mg/kg,peros),andEvansBlue(5mg/kg,iv)wasadministered toassessplasma extravasation.Pre-treatmentwithindomethacin(5mg/kg,sc)wasusedaspositivecontrolwhilethe shamgroupreceived0.9%sterilesaline(perosandiart).
Results:Strontiumranelatedidnotreduceleukocytecounting,myeloperoxidaseactivity,EvansBlue extravasation,IL-1blevels,andTNF-a/IL-1bimmunolabeling;butitincreasedthenociceptivethreshold andreducedTNF-alevels.Additionally,HO-1inhibitiondidnotchangethestrontiumranelateeffects. Conclusion:StrontiumranelatemayachieveitsantinociceptiveeffectsthroughthereductionofTNF-a
levelsinthetrigeminalganglion,butnotsuppressingIL-1bexpressionnorinducingtheHO-1pathway. ©2017InstituteofPharmacology,PolishAcademyofSciences.PublishedbyElsevierSp.zo.o.Allrights reserved.
Introduction
Thepathogenesisoftemporomandibularjoint(TMJ)disorders stillremainsunclearevenwiththeremarkableprogressalready madetowardstheelucidationoftheirpathogenesis.Experimental models that allow the investigation of the inflammatory pain relatedtothesedisordersareofgreatclinicalrelevanceandour
group developed an experimental model of zymosan-induced
arthritisinrats.Zymosanisayeastcell-derivedpolysaccharidethat *Correspondingauthor.
E-mailaddresses:eishyla2010@hotmail.com(S.M.Alves),
suzanact@hotmail.com(S.C.Abreu),jonas_cavalcante_@hotmail.com(J.C.Lemos), isaac.odonto@hotmail.com(F.I.F.Gomes),shirley_sma31@yahoo.com.br (S.M.Alves),danielleval@hotmail.com(D.R.doVal),raul.sf2@gmail.com (R.S.Freitas),karuzaalves@yahoo.com.br(K.M.A.Pereira),pintovicente@gmail.com (V.dePauloTeixeiraPinto),gerlybrito@hotmail.com(G.A.deCastroBrito), mirnabrayner@gmail.com(M.M.Bezerra),gerardocristino@uol.com.br (G.Cristino-Filho),helliadachaves@yahoo.com.br(H.V.Chaves).
http://dx.doi.org/10.1016/j.pharep.2017.03.007
1734-1140/©2017InstituteofPharmacology,PolishAcademyofSciences.PublishedbyElsevierSp.zo.o.Allrightsreserved. –
ContentslistsavailableatScienceDirect
Pharmacological
Reports
producesasevereanderosivesynovitisalongwithinflammatory paininanimalmodelsofkneearthritis[1–3].
Inflammatorystimulicausemechanicalhypernociceptionbya definedsequentialreleaseofcytokineswithininflamedjointsby manycellssuchastumornecrosisfactor-
a
(TNF-a
)and interleu-kin-1b
[4]. TNF-a
and IL-1b
arehighly expressedin thejoints affected by TMJ disorders. Many studies showed considerable levelsofbothinthesynovialfluidofpatientssufferingfromthis condition [5,6]. Furthermore, studies demonstrated that heme oxygenase–1(HO-1)exertsanantioxidantroleanditsinduction wouldleadtonegativefeedbackforcellactivationandproduction ofinflammatorymediators[7–9].Strontiumranelate(Sran){5-[bis(carboxy-methyl)
amino]-2-carboxy-4-cyano-3-thiophen-acetic acid distrontium salt} is a compoundwithtwostablestrontiumatomsand ranelicacid. It affectstheboneturnoveranditisanorallyactivetreatmentwhich
decreasestherisk ofvertebraland hipfracturesinosteoporotic women [10,11]. Although its mechanism of action is not fully understood,this drugmaypossessanalgesiceffects[12].Recent studiesshowedthatstrontiumranelatehadaprotectiveeffectin experimental osteoarthritis [13] and it promoted analgesia in arthriticrats,whichwasassociatedtoinhibitionofthereleaseof inflammatorycytokinesintoinflamedjoints[14].
Thus,thepresentstudyattemptstoinvestigatetheunexplored anti-nociceptive and anti-inflammatory effects of strontium ranelateonthezymosan-inducedinflammatoryhypernociception intheTMJofratsbyevaluatingtheIL-1
b
andTNF-a
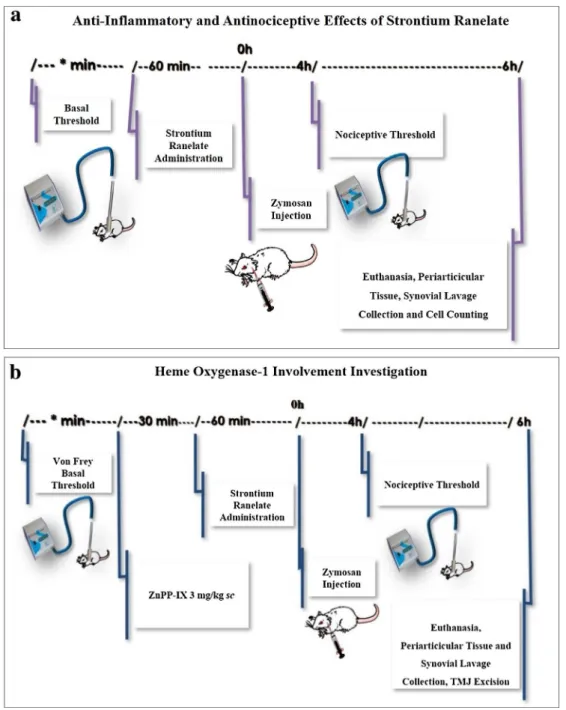
levelsafter strontium ranelate treatment. Further, we aim to determine whetherstrontiumranelateeffectsonthisexperimentalmodelof arthritiswoulddependonHO-1pathwayintegrityasourprevious results showedthatHO-1pathwayinhibitionisassociatedwith increasedinflammatoryresponses[8].Fig.1.ExperimentaldesigntoassessstrontiumranelateeffectsontheTMJarthritis.(a)Evaluationoftheanti-inflammatoryandantinociceptiveeffectsofstrontiumranelate onthezymosan-inducedmechanicalallodyniaintherattemporomandibularjoint.(b)Investigationoftheinvolvementofhemeoxygenase-1intheanti-inflammatoryand antinociceptiveeffectsofstrontiumranelateonthezymosan-inducedmechanicalallodyniaintherattemporomandibularjoint.
Materialsandmethods
Animals
MaleWistarrats(n=6pergroup;160–220g)werehousedin standardplasticcages,theyhadaccesstofoodandwateradlibitum andweremaintainedinatemperature-controlledroom(232C) with a 12/12-h light-dark cycle. This study was conducted in accordancewiththelocalInstitutionalAnimalCareandwiththe approvalofthelocalethicalcommittee(registrationnumber54/ 12)andwedesignedittoreduceanimalsufferingandthenumber ofanimals.
Zymosan-inducedinflammatoryhypernociception
To induce the inflammatory hypernociception, rats were
anesthetized with inhaled isoflurane (4%) and received intra-articular(iart) injectionof zymosan (2mg;40
m
L)dissolved in sterilesaline into theleft TMJ using a 30-gauge needle. Sham animalsreceivedsalinesolution(peros)beforezymosanorsaline solutioninjections.TheTMJskinwasshaved,thepostero-inferior borderof the zygomaticarch was located,and the needle was inserted in a position inferior to this point until the needle contactedthecondyle,whichwasverifiedbythemovementofthe mandibleandthepunctureoftheneedleintothejointspacewas confirmedbythelossofresistance. Gentleaspiration ruledout intravascular injection, after which zymosan or salinesolution wereinjected.Aspreviouslyshownbyourgroup[1]the zymosan-induced inflammatory hypernociception is maximal at 4h of arthritiswhilepolymorphonuclearcellinfluxpeaksafter6h.Thus, weused thesetime points toassess thefollowing parameters: headwithdrawalthreshold,totalcellcounting,and myeloperox-idaseactivity.Mechanicalthresholdevaluation
Inflammatoryhypernociceptionin theTMJ was evaluatedby measuringthethresholdofforceneededtobeappliedtotheTMJ regionuntil the head withdrawaloccurred. The measurements wereperformedbyablindedexaminerwhousedadigitaldevice (Insight, Brazil) that consisted of a rigid filament linkedto an electronicdevice–automaticVonFreyanesthesiometer,whichin turn measures the response threshold in grams (g) when the filament isappliedtothesurfaceofthetestedregion[15].The facialareastobetestedaroundtheTMJwereshavedbeforethe experimentalprocedureandtheanimalswereplacedinindividual plastic cages 45min before the tests. The animals underwent conditioningsessionsinthetestingroomfor4consecutivedays. Ondayfive,thebasalforcethresholdvaluewasrecordedthree timesbeforeand4haftertheintra-articularinjectionsofeither
zymosan or vehicle. Then, we measured the inflammatory
hypernociception of the zymosan-group animals and treated controls. The applied mechanical stimuli were innocuous in controlanimals.
Pharmacologicalmodulation
Strontium ranelate (PROTOS1 2g, Les Laboratoires Servier Industry,45 520Gidy, France) (0.5,5 or 50mg/kg, per os) was administeredonehourpriortozymosaninjection(iart).Foodwas removed1hbeforethetreatmentinordertoavoidanychangesin thepharmacokineticprofileofstrontiumranelate(Fig.1a).Data validationwasachievedbyusingapositivecontrolgroupthatwas pre-treatedwithindomethacin(5mg/kg,sc)1hbeforezymosan injection.Shamgroupreceived(perosandiart)0.9%sterilesaline.
ToanalyzethepossibleeffectofHO-1pathwayonanti-nociceptive and anti-inflammatory efficacy of strontium ranelate, animals were pre-treated (sc) with ZnPP IX (3mg/kg), a specific HO-1 inhibitor,followedbyaninjection(peros)ofstrontiumranelate (0.5mg/kg) 30min later (Fig. 1b). After 1h, intra-articular zymosan-injectionwasperformedandatthe4thhour, inflamma-toryhypernociceptionintheTMJwasevaluated.
SynovialLavageCollection,CellCountingandMyeloperoxidase ActivityAssessment
Six hours after zymosan injections, the rats weresacrificed underanesthesiaandexsanguinated.Thesuperficialtissueswere dissectedandtheTMJcavitywaswashedtwotimestocollectthe synovial fluid by the pumping and aspiration technique using 0.05mLofEDTA(1.77mg EDTA/1mLPBS).Thetotal numberof whitecellsinthesynoviallavagewascountedusingaNeubauer
chamber. MPO activity assay measurement was described by
Bradleyetal.anditwasconductedonthecollectedsynoviallavage whichwascentrifugedat4500rpmfor12minat4C.MPOactivity was assayedbymeasuringthechangein absorbanceat450nm using o-dianisidine dihydrochloride and 1% hydrogenperoxide. TheresultsarereportedastheMPOunits/jointfluidandaunitof MPOactivitywasdefinedastheconversionofa
m
molofhydrogen peroxidetowaterin1minat22C.Evansblueextravasationassay
Strontium ranelate(0.5mg/kg, peros) was administered 1h prior to zymosan injection. Thirty minutes before euthanasia, Evans Blue (5mg/kg, iv) was then injected to assess plasma extravasation. After its excision, the periarticular tissue was weighed,immersedintoformamide solution(1mL), andplaced intowaterbath(60C)overnight.Theresultingsupernatantwas
collected and the absorbance was read at 620nm using a
spectrophotometer.The concentrationwas determinedby com-parisonwithastandardcurveofEvansbluedyeintheextraction solution and the amount of Evans blue dye (
m
g) was then calculatedpermLofexudate[16].Histopathologicalanalysis
The TMJ was excised six hours after the induction of the inflammatoryhypernociception.Thespecimenswerefixedin10% neutralbufferedformalinfor24h,demineralizedin10%EDTAfor 7days,embeddedinparaffin,andsectionedalongthelongaxisof the TMJ. Sectionsof 5
m
m, includingthe condyle,the articular cartilage, the articular disc, the synovial membrane, the peri-articulartissue,andtheskeletalmusclewereevaluatedunderlight microscopy (400). The specimens were prepared for routinehematoxylin-eosin(H&E)stainingandhistologicalanalysis con-sidereda0–4scorebasedonthefollowingparameters:cellinflux intothesynovialmembrane,cellinfluxintotheconnectivetissue andtheskeletalmuscleoftheperiarticulartissue, andsynovial membranethickness.
Immunohistochemistry
Immunohistochemistryfor TNF-
a
and IL-1b
was performed usingthestreptavidin-biotin(LabeledStreptavidinBiotin –LSAB) method in formalin-fixed, paraffin-embedded tissue sections (5m
m thickness), mounted on glass slides prepared with an organosilane-basedadhesive(3-aminopropyltriethoxysilane, Sig-maChemicalCoJ,StLouis,MO,USA).Thesectionsunderwent2 bathsinxylolfortenminuteseachone.Theywereafterimmersedthree timesinto alcohol at 100%concentration,and washedin distilledwater.
Antigenrecoverywasperformedwithcitrate(pH6.0;30min; 99C).Afterreturningtoambienttemperature,thesectionswere immersedintoa3%hydrogenperoxideblockingsolutionfor10min. Thesectionswerethenincubatedovernight(4C)withaprimary rabbitanti-TNF-
a
and anti- IL-1b
antibody (ABCAMJ, England, UK),atthedilutionof1:200,andwashedwithPBSsolution.ThesampleswereincubatedwiththesecondaryantibodyLSAB Kit for 10min at ambient temperature. Next, incubation was performedinachromogensolutionpreparedwith3,30 diamino-benzidine(DAB) (DAKOJ, Carpentaria,CA,USA),for 10minina
dark chamber. Afterwards, the specimens were washed into
running water and then into distilled water. Counter-staining wasperformedwithhematoxylin,andafterwardsthespecimens weredehydratedinalcoholanddiaphanizedinxylol.Finally,they weremountedonglassslides.Thenegativecontrolsectionswere performed excluding the application of the primary antibody.
The parameter of positivity for the immunohistochemical
marking of the antigen in all the specimens included in the sampleconsistedofthecellsthatexhibitedbrownstainingintheir cytoplasmirrespectiveoftheintensityoftheimmunomarking.
TNF-
a
andIL-1b
ELISAassaysTheTMJ tissueand thetrigeminalganglionwereexcised6h afterthezymosan-injectioninratsandwerehomogenizedina solutionof RIPALysis BufferSystem (SantaCruz Biotechnology, USA).Thesampleswerecentrifugedat10000rpmfor15minat 4C.Thesupernatantswerestoredat80Cforposterioranalysisto evaluatetheproteinlevelsofTNF-
a
andIL-1b
intheTMJtissueand thetrigeminalganglion.Thecytokinelevelswerequantifiedbythe following kits:TNF-a
–RatTNF-alpha/TNFSF1AQuantikineELISA Kit (R&DSystems, catalog number RTA00); and IL-1b
–RatIL-1 beta/IL-1F2QuantikineELISAKit(R&DSystems,catalognumber DY501).Theabsorbancewasmeasuredat450nm.IL-1b
andTNF-a
concentrationswereexpressedaspg/mL.Statisticalanalysis
ThedataarepresentedasthemeanSEMormedianswhere
appropriate. Differencesbetween means were compared using one-way ANOVA followed by theBonferroni test. The Kruskal-WallistestfollowedbyDunn’stestwasusedtocomparemedians. Avalueofp<0.05indicatedsignificantdifferences.
Fig.2.Effectsofstrontiumranelateonthezymosan-inducedTMJinflammatoryhypernociception.(a)Headwithdrawalthresholdinstrontiumranelate-treatedrats(b) Leukocytecountinginstrontiumranelate-treatedrats(c)MPOactivityfromTMJsynoviallavageinstrontiumranelate-treatedrats.(d)Plasmaextravasationinstrontium ranelate-treatedrats.DataareexpressedasthemeanSEMof6miceforeachgroup;*p<0.05indicatesasignificantdifferencefromtheshamgroup,+p<0.05indicatesa
significantdifferencefromthezymosangroup(ANOVA,Bonferroni).
Results
Effectsofstrontiumranelateonthezymosan-inducedinflammatory hypernociception(Fig.2)
Theintra-articularinjectionofzymosancausedinflammatory hypernociceptionthatisobservedasadecreaseinthemechanical thresholdofheadwithdrawal(Fig.2a).Itresultedinasignificant increaseinthenumberofpolymorphonuclearcells(Fig.2b)which inturnwascertifiedbytheincreaseofMPOactivityintheTMJ synoviallavageafterzymosaninjection(Fig.2c). Thesechanges were followed by plasma extravasation into the TMJ after 6h
(Fig. 2d). Sham animals showed no significant changes in
withdrawalthreshold,polymorphonuclearcells count,and MPO activity(Fig. 2 a, b, c). Strontium ranelate(0.5, 5 or 50mg/kg) injected (per os) 1h prior to zymosan injection significantly (p<0.05)increasedthenociceptivethreshold(Fig.2a).However, strontiumranelatefailedtodecreasethenumberof polymorpho-nuclearcells(Fig.2b),MPOactivity(Fig.2c),andEvansbluedye extravasationinthesynoviallavage(Fig.2d).
EffectsofzincprotoporphyrinIX(ZnPPIX)onthestrontiumranelate efficacy(Fig.3)
ToinvestigatetheroleofHO-1activityintheantinociceptive effectofstrontium ranelate,theanimalswerepre-treated with ZnPPIX (3mg/kg; sc), a specific HO-1 inhibitor. The effects of strontiumranelate(0.5mg/kg)onthezymosan-inducedinfl am-matoryhypernociception(Fig.3)werenotchangedinthepresence ofZnPP-IX(3mg/kg).
JointtissueandtrigeminalganglionTNF-
a
andIL-1b
ELISAassays (Fig.4)Theintra-articularinjectionofzymosanresultedinasignificant increaseinTNF-
a
(Fig.4aand4b)andIL-1b
(Fig.4candd)levelsin bothjointtissue andtrigeminalganglionafter.Albeitstrontium ranelatetreatmentwasnotabletosignificantlyreduceIL-1b
levelswhen compared with the zymosan group (Fig. 4c and 4d),
strontiumranelatereducedTNF-
a
levelsinbothjointtissueand trigeminalganglion(Fig.4aand4b).Histopathologicalanalysis(Fig.5)
Inflammatory cell influx was observed into the synovial
membrane(Fig.5b)6hafterzymosan-injectioncomparedwith the sham group (Fig. 5a). The predominant cell types were neutrophils,whichcharacterizedacuteinflammation.Edemawas alsoobservedinthesynovium(Fig.5b).Table1showsthescores attributed to TMJ histopathological analysis and compares the
values between the sham and zymosan groups. A significant
(p<0.05)increaseintheinflammatoryparameterswasobserved inthezymosangroup.Table1alsoshowsthescoresattributedto the TMJ histopathological analysis and compares the values betweenthezymosan andstrontium ranelate(0.5,5 or 50mg/ kg)groups.Strontiumranelate(0.5,5or50mg/kg)didnotreduce theinflammatoryparameters.Figs.5cand5dshowtheTMJofrats pre-treatedwithstrontiumranelate(0.5mg/kg,peros).
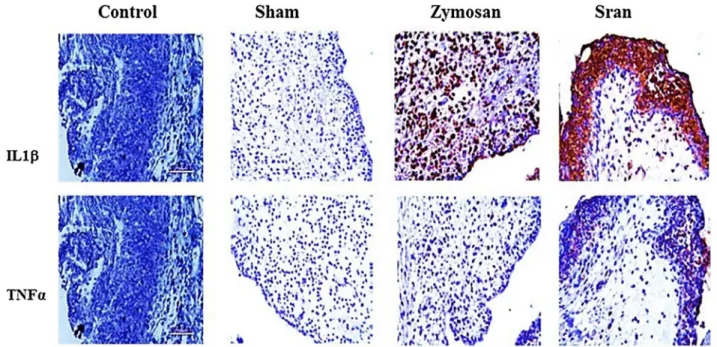
Immunohistochemicalanalysis(Fig.6)
TheimmunohistochemicalanalysisofTNF-
a
andIL-1b
showed increasedimmunolabelingforbothTNF-a
andIL-1b
in synovio-cytesand neutrophlisafterzymosanchallengethatwas charac-terizedbybrown-coloredcellsinthesynovialmembrane(Fig.6). Thesynovialcellsinthesynovialmembraneofthezymosanand strontiumranelate-treatedanimalsalsoshowedbothTNF-a
and IL-1b
expression (Fig. 6). However, in the conjunctive tissue, strontiumranelate(0.5mg/kg)treatmentreducedTNF-a
expres-sion.Thenegativecontrolgroupsectionsconsistedof zymosan-inducedTMJinflammatoryhypernociceptionthatwerenottreated with anti-TNF-a
or anti-IL-1b
antibody. None of the negative controlsshowedTNF-a
orIL-1b
immunoreactivity.Discussion
Wedemonstratedthattheeffectsofstrontiumranelateonthe zymosan-inducedTMJinflammatoryhypernociceptioninratsmay occurviaTNF-
a
suppressionaswellasitsmechanismofactionin this disease model is IL-1b
/HO-1 independent. Experimental animalmodelsofTMJinflammatoryhypernociceptionhavebeen usedtostudyinflammatoryconditionsandweperformedthefirst demonstration of TMJ arthritis induced by zymosan, throughwhich we showed that zymosan caused a time-dependent
leucocyte migration, plasma extravasation, mechanical hyper-nociception,andneutrophilaccumulation[1].Thisshowsthatthe zymosan-induced TMJ arthritis is a reproducible experimental modelthatcanbeusedtoexplorethemechanismsunderlyingTMJ inflammationandpotentialtherapies.
Strontiumranelatewasoriginallydesignedtotreat osteoporo-sis[17–21],butitmayexerteffectsonosteoarthritisandithasled
topositiveoutcomesinaphaseIIIclinicalstudy[13,22].Albeitthe mechanismofactionofstrontiumranelateisnotfullyunderstood, itappearstostimulatethedifferentiationofosteoblastsbyeliciting thecalciumsensor receptor,inhibitingosteoclastdifferentiation byinhibitingRANKL production,andincreasingosteoprotegerin (OPG)activity[23,24].
In addition, patients treated with strontium ranelate had a greaterreductioninthetotalscoreandpainsubscorecompared withtheplacebogroup[25].Sinceosteoporosisandosteoarthritis areassociatedwithavarietyofsymptoms,includingpain,itcould behypothesizedthatstrontiumranelatemayalsobeeffectivein reducingthetemporomandibularjointinflammatory hypernoci-ception.Ourresultsdemonstratedthattheintra-articularzymosan injectiondiminishedthemechanicalnociceptivethreshold,which Fig.3. EffectofzincprotoporphyrinIX(ZnPPIX),aspecificHO-1inhibitor,onthe
strontiumranelateefficacyonzymosan-inducedTMJinflammatory hypernocicep-tion.DataareexpressedasthemeanSEMof6ratsforeachgroup;*p<0.05
indicates a significant difference from the sham group,+p<0.05 indicates a significantdifferencefromthezymosangroup(ANOVA,Bonferroni).
in turn was increased by the strontium ranelate treatment. However, the inflammatory parameters cell influx and MPO activitywerenotreduced afterstrontiumranelatetreatment.
Evansblueextravasationmeasurementintothesynoviallavagedid notchangefollowingthedrugadministration.TheTMJ histopath-ological analysisafter zymosan injection showed inflammatory cellinfluxintothesynovialmembrane,periarticular tissue,and musculoskeletal tissue associated with thickness of synovial membrane, being the drug treatment unable to reverse these findingstoa normalstatus. Likewise,itwas demonstratedthat strontiumranelatetreatmenthadnoparticulareffectonsynovitis indogs[13].
Studies correlate the HO-1 activity with oxidative damage inhibitionandreductioninproinflammatorycytokinesproduction [7].Itwasreportedthatthisenzymehadantinociceptiveeffectson acetic acid-evoked nociception and positive outcomes after inductionofHO-1inazymosan-inducedairpouchinflammation model[8,26].Consideringthesedata,weevaluatedthe involve-mentofHO-1inthestrontiumranelateantinociceptiveeffectsand weobservedthattheywerenotchangedafterthepre-treatment withZnPP-IX,suggestingthatHO-1activityisnotinvolvedinits antinociceptiveeffects.
Manycelltypesproducecytokinesinresponsetoavarietyof stimuli,whichisalinkbetweencellularinjuryandthe develop-ment of localsigns and symptoms of inflammation. There is a cascadeofreleaseofcytokineslinkinginjuriesandthereleaseof
nociceptive mediators in rats: a concept that allows us to understand why the inhibition of cytokines causes analgesia [27].ManystudieshavedemonstratedthecontributionofTNF-
a
to inflammatoryhyperalgesiaandtheclinicalsuccessoftheanti-TNF-a
therapyof rheumatoid arthritis alsoexemplifies this concept [4,28]. During the inflammatory response, TNF-a
is the first released cytokine and IL-1b
is a potent pleiotropic mediator involvedininflammatoryresponses[29].Hence,TNF-a
andIL-1b
arerecognizedcontributorstothepathogenesisofjointdiseases, leadingtosynovialfibroblasthyperplasiaandtothedestructionof theextracellularmatrix[30,31].In the present study, the zymosan injection resulted in a significantincrease in both TNF-
a
and IL-1b
levelssothatour findingsareinaccordancewithotherones,suggestingthatTNF-a
is asdrivingcytokineofthenociceptiveprocess.TNF-a
playsa crucial role in the development of inflammatory hyperalgesia duringtheinflammatoryresponseinrats,beinghighlyexpressed in the synovial fluid of patients with TMJ disorders [5–7]. Additionally,wealsodemonstratedthattreatmentwithstrontium ranelatereducedTNF-a
levelsinbothjointtissueandtrigeminal ganglion.TNF-
a
is thefirst cytokinereleased duringan inflammatory response, triggering the release of IL-1b
known to activate inflammatoryanddegradativepathwaysinsynovialcells.Studies suggested high IL-1b
levels in the synovial fluid of patients suffering from TMJ disorders [5,29,32]. Nunes et al. [14] Fig.4.JointtissueandtrigeminalganglionTNF-a (a/b)andIL-1b(c/d)levelsfromstudyratseithersubjectedornottoonzymosan-inducedTMJinflammatory hypernociceptionandassayedonthe6thhourpostchallenge.DataareexpressedasthemeanSEMof6ratsforeachgroup;*p<0.05indicatesasignificantdifferencefromtheshamgroup(ANOVA,Bonferroni).
investigatedtheanti-inflammatoryactivityofstrontiumranelate inthearticularincapacitationtest,inthepaw-pressuretest,andin the anterior cruciate ligament transection model. Unlike our protocol,theanimalsreceivedstrontiumranelateathigherdoses: 30–300mg/kg per os. They find that strontium ranelate dose-dependentlyinhibitedjointpaininbothtypesofarthritismodels, butitdidnotaltercellinfluxwhichisasimilarresultobtained here.Contrarytothem,ourfindings suggestthatthestrontium ranelate treatment is not capable of reducing IL-1
b
levels comparedwiththezymosangroup.Thismightberelatedtothedifferencesinthedesignperformed byNunesetal.[14].Thestrontiumranelatedoseadministeredin theirprotocolisfarhigher thanthe oneweused, which could potentiate the analgesic effects of this drug. Other essential difference is the experimental model of diseases that could
culminateindistinctresultsasdifferentdiseasemodelsmayimply incontrastingpathogenesismechanisms,thus,leadingtodifferent results.AspointedoutbyNunesetal.[14],naloxoneabolishedthe strontiumranelateanalgesiceffectwhichisvaluableinformation onthestrontiumranelateunspecificmechanismofaction.
Furthermore,the immunohistochemicalanalysis showed in-creasedTNF-
a
and IL-1b
immunolabeling in the synovialcells after the intra-articular zymosan injection. Albeit strontium ranelate slightly reduced the TNF-a
immunolabeling in the conjunctive tissue, it was not able to diminish the IL-1b
immunolabeling one. Contrary to this result, in the synovial membraneofdogsundergoingsectioningoftheanteriorcruciate ligament,thegeneticexpressionofIL-1b
wassignificantlyreduced bystrontiumranelatetreatmentatthedosesof50or75mg/kgperday for 16 weeks [13]. A possible explanation for these
Fig.5. Photomicrographsofthehistopathologicalanalysisoftemporomandibularjoints(TMJ).(a)shamgroupTMJ(100);(b)zymosan2mggroup(400)showing
inflammatorycellinfluxinthesynovialmembrane;(c)and(d)TMJofratspretreated(peros)withstrontiumranelate(0.5mg/kg)andinjected(i.art.)withzymosan2mg(100 and400x,respectively).C:condyle;AC:articularcartilage;AD:articulardisc;SM:synovialmembrane;PAT:periarticulartissue.Hematoxylinandeosin(H&E)staining.
Table1
Histopathologicalanalysisbyhematoxylin-eosinstaining(HE)ofthetemporomandibularjointofratsafterintra-articularzymosaninjectionandstrontiumranelate(Sran) treatment.
Groups Cellinfluxinthe synovialmembrane
Periarticularcellinflux Cellinfluxinthe Musculartissue
Sham 1(0–1) 1(0–2) 0(0–0)
Zy 4(2–4)* 3(3–4)* 2.5(2–4)*
Indo 2(1–2)** 2(1–2)** 1.5(1–2)**
Sran0.5 3.5(2–4) 3.5(3–4) 3.5(2–4)
Sran5 3(2–3) 3.5(3–4) 3.5(2–4)
Sran50 3(2–4) 4(2–4) 4(2–4)
*p<0.05versusSham;**p<0.05versusZymosan(Kruskal-Wallis,Dunn’s).
contradictory results could be the different animal model of diseasealongwiththeadoptedposology,leadingtocontradictory resultsfromwhatweobtained.
Therefore, albeit the mechanisms of action through which strontiumranelateexertsantinociceptiveeffectsremainrelatively elusive,thisstudyprovidesnovelinformationonitseffectsonthe zymosan-inducedTMJ inflammatory hypernociceptionas stron-tiumranelateprimarilysuppressedTNF-
a
levelsandreducedthe nociceptivethreshold.Theinflammatorystimuliortissueinjuries stimulatetherelease of characteristiccytokine cascades,which ultimatelytriggertherelease of final mediatorsresponsiblefor inflammatorypain.Thesefinalmediators,suchasprostanoidsor sympathetic amines, act directly on the nociceptors to cause hypernociception,which resultsfromtheloweringofthreshold duetomodulationofspecificvoltage-dependentsodiumchannels. As reported here,strontium ranelate could decrease hyper-nociceptionthresholdsbyreducingTNF-a
levelsinthe periartic-ulartissuesandtrigeminalganglion.Giventheimportanceofthesestructures to the temporomandibular joint pain onset and
progression,we suggest thatTNF-
a
functionsasa target point forstrontiumranelate.Thissuggeststhatstrontiumranelatemight beapotentialcandidatefor thetreatmentof TMJpainthrough TNF-a
inhibition and more studies of longer duration are necessarytovalidate theuse ofstrontium ranelatein thepain management.Conflictofinterests
The authors declare that they have no conflict of interest regardingthepublicationofthisstudy.
Funding
Thisworkwassupported byBrazilian grantsfromFundação CearensedeApoioao DesenvolvimentoCientíficoeTecnológico (FUNCAP), Conselho Nacional de Desenvolvimento Científico e Tecnológico(CNPq),CoordenaçãodeAperfeiçoamentodePessoal
deNívelSuperior(CAPES),andInstitutodeBiomedicinado Semi-ÁridoBrasileiro(INCT).
Acknowledgment
The authorsthank AdalbertoNascimentode LimaJúnior for technicalassistance.
References
[1]ChavesHV, RibeiroRA, SouzaAM,Silva AAR,GomesAS,Vale ML,etal. Experimentalmodelofzymosan-inducedarthritisintherat
temporomandibularjoint:roleofnitricoxideandneutrophils.JBiomed Biotechnol2011;200:1–2011.
[2]Bezerra MM, Brain SD, Girão VCC, Greenacre S, Keeble J, Rocha FAC. Neutrophils-derivedperoxynitritecontributestoacutehyperalgesiaand cellinfluxinzymosanarthritis.NaunynSchmiedebergsArchPharmacol 2007;374:265–73.
[3]GegoutP,GilletP,ChevrierD,GuingampC,TerlainB,NetterP.Characterization ofzymosan-inducedarthritisintherat:effectsonjointinflammationand cartilagemetabolism.LifeSci1994;55:321–6.
[4]CunhaFQ,PooleS,LorenzettiBB,FerreiraSH.Thepivotalroleoftumour necrosisfactoralphainthedevelopmentofinflammatoryhyperalgesia.BrJ Pharmacol1992;107:660–4.
[5]AkutsuM,OguraN,Ito K,KawashimaM,KishidaT,KondohT. Effectsof interleukin-1bandtumornecrosisfactor-aonmacrophageinflammatory protein-31productioninsynovialfibroblast-likecellsfromhuman temporomandibularjoints.JOralPatholMed2013;42:491–8.
[6]Gulen H, Ataoglu H, Haliloglu S, Isik K. Proinflammatorycytokines in temporomandibularjointsynovialfluidbeforeandafterarthrocentesis. OralSurgOralMedOralPatholOralRadiolEndod2009;107:1–4.
[7]AlcarazMJ,FernándezP,GuillénMI.Anti-inflammatoryactionsoftheheme oxygenase-apathway.CurrPharmDes2003;9:2541–51.
[8]GrangeiroNMG,AguiarJA,ChavesHV,SilvaAAR,LimaV,BenevidesNMB,etal. Hemeoxygenase/carbonmonoxide-biliverdinpathwaymaybeinvolvedinthe antinociceptiveactivityofetoricoxib,aselectiveCOX-2inhibitor.Pharmacol Rep2011;63:112–9.
[9]VanderleiESO,AraújoIWF,QuinderéALG,PontesBP,EloyYRG,RodriguesJAG, etal.TheinvolvementoftheHO-1pathwayintheanti-inflammatoryactionof asulfatedpolysaccharideisolatedfromtheredseaweedGracilariabirdiae. InflammRes2011;60:1121–30.
[10]CianferottiL,D’AstaF,BrandiLM.Areviewonstrontiumranelatelong-term antifractureefficacyinthetreatmentofpostmenopausalosteoporosis.Her AdvMusculoskelDis2013;0:1–13.
[11]ReginsterJ,BadurskiJ,BellamyN,BensenW,ChapurlatR,ChevalierX.Efficacy andsafetyofstrontiumranelateinthetreatmentofkneeosteoarthritis: Fig.6.RepresentativeimmunohistochemistryofTMJtissuesforIL-1b(upperpanel),andTNF-a(lowerpanel)fromratsatthesixthhourafterzymosaninjection.Theupper panelshowsincreasedIL-1bimmunolabelingofsynoviocytesandneutrophlisafterzymosan-challenge(400).ThebottompanelshowsincreasedTNF-aimmunolabelingof
synoviocytesandneutrophlis afterzymosan-challenge(400).Control: negativecontrol(sectionsin theabsenceofanti-IL-1band anti-TNF-a antibody);Sham:
resultsofadouble-blind,randomised,placebo-controlledtrial.AnnRheum Dis2013;72:179–86.
[12]KarsdalMA,Bay-JensenAC,LoriesRJ,AbramsonS,SpectorT,PastoureauP, etal.Thecouplingofboneandcartilageturnoverinosteoarthritis: opportunitiesforboneantiresorptivesandanabolicsaspotentialtreatments? AnnRheumDis2014;73(2):336–48.
[13]PelletierJP,KapoorM,FahmiH.Strontiumranelatereducestheprogressionof experimentaldogosteoarthritisbyinhibitingtheexpressionofkeyproteases incartilageandofIL-1betainthesynovium.AnnRheumDis2013;72:250–7. [14]NunesRM,MartinsMR,SilvaJuniorFS,MeloLeiteAC,GirãoVC,CunhaFQ,etal. Strontiumranelateanalgesiainarthritismodelsisassociatedtodecreased cytokinereleaseandopioid-dependentmechanisms.InflammRes 2015;64:781–7.
[15]Denadai-SouzaA,CamargoL,de L,RibelaMT,KeebleJE,CostaSK,etal. ParticipationofperipheraltachykininNKreceptorsinthecarrageenan– inducedinflammationoftherattemporomandibularjoint.EurJPain 2010;13:812–9.
[16]KwanCL, HuJW,SessleBJ.Neuroplasticeffectsofneonatalcapsaicin on neuronsinadultrattrigeminalnucleusprincipalisandsubnucleusoralis.J Neurophysiol1996;75:210–98.
[17]AmmannP,ShenV,RobinB,MaurasY,BonjourJP,RizzoliR.Strontiumranelate improvesboneresistancebyincreasingbonemassandimprovingarchitecture inintactfemalerats.JBoneMinerRes2004;19:2012–20.
[18]BuehlerJ,ChappuisP,SaffarJL,TsouderosY,VigneryA.Strontiumranelate inhibitsboneresorptionwhilemaintainingboneformationinalveolarbonein monkeys.Bone2001;29:176–9.
[19]DelannoyP,BazotD,MarieP.Long-termtreatmentwithstrontiumranelate increasesvertebralbonemasswithoutdeleteriouseffectinmice.Metabolism 2002;51:906–11.
[20]ParkSY,KimYH,KimEK,RyuEY,LeeSJ.Hemeoxygenase-asignalsareinvolved inpreferentialinhibitionofpro-inflammatorycytokinereleasebysurfactinin cellsactivatedwithporphyromonasgingivalislipopolysaccharide.ChemBiol Interact2010;3:437–45.
[21]ReginsterJ,DeroisyR,NeuprezA,HiligsmannA,ZegelsB,BruyereO.Strontium ranelate:newdataonfracturepreventionandmechanismsofaction.Cur OsteoporosRep2009;7:96–102.
[22]Alexandersen P, Karsdal MA, Qvist P. Strontium ranelate reduces the urinarylevelofcartilagedegradationbiomarkerCTX-IIinpostmenopausal women.Bone2007;40:218–22.
[23]AtkinsGJ,WelldonKJ,HalboutP.Strontiumranelatetreatmentofhuman primaryosteoblastspromotesanosteocyte-likephenotypewhileelicitingan osteoprotegerinresponse.OsteoporosInt2009;20:653–64.
[24]CoulombeJ,FaureH,RobinB.Invitroeffectsofstrontiumranelateonthe extracellularcalcium-sensingreceptor.BiochemBiophysResCommun 2004;323:1184–90.
[25]BruyereO,DelferriereD,RouxC. Effectsofstrontiumranelateonspinal osteoarthritisprogression.AnnRheumDis2008;67:335–9.
[26]VicenteAM,Guillen MI,HabibA,AlcarazMJ.Beneficialeffects ofheme oxygenase-aup-regulationinthedevelopmentofexperimentalinflammation inducedbyzymosan.JPharmacolExpTher2003;307:1030–7.
[27]CunhaTM,VerriWA.JrSilvaJS,PooleS,CunhaFQ:Acascadeofcytokines mediatesmechanicalinflammatoryhyperalgesiainmice.ProcNatlAcadSciU SA2005;102(5):1755–60.
[28]RankinEC,ChoyEH,KassimosD,KingsleyGH,SopwithAM,InsenbergDA,etal. Thetherapeuticeffectsofanengineeredhumananti-tumournecrosisfactor alphaantibody(CDP571)inrheumatoidarthritis.Rheumatol1995;34:334–42. [29]CunhaTM,VerriWA,SilvaJrJS,PooleS,CunhaFQ.Acascadeofcytokines mediatesmechanicalinflammatoryhyperalgesiainmice.ProcNatlAcadSciU SA2005;102(5):1755–60.
[30]KayJ,CalabreseL.Theroleofinterleukin-ainthepathogenesisofrheumatoid arthritis.Rheumatology2004;43:2–9.
[31]FieldM.Tumournecrosisfactorpolymorphismsinrheumaticdiseases.QJ Med2001;94:237–46.
[32]KubotaE, KubotaT, Matsumoto J,Shibata T,Murakami K.Synovialfluid cytokinesandproteinasesasmarkersoftemporomandibularjointdisease.J OralMaxillofacSurg1998;56:192–8.