w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Evaluation
of
cytotoxic
activity
of
protein
extracts
from
the
leaves
of
Morinda
pubescens
on
human
cancer
cell
lines
Anvy
Susan
Thomas,
Rupachandra
Saravanakumar
∗,
Pratiksha
V.
Gupta
DepartmentofBiotechnology,SchoolofBioengineering,SRMUniversity,Kattankulathur,Kancheepuram,TamilNadu,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10September2015 Accepted8August2016
Availableonline15September2016
Keywords: Cytotoxicactivity FTIRanalysis Morindapubescens MTTassay Proteinextraction
a
b
s
t
r
a
c
t
Biologicallyactiveproteinsisolatedfromplantspeciescanbeusedintraditionalmedicineasprolific
resourcesfornewdrugsMorindapubescensSm.,Rubiaceae,isapromisingmedicinalplantwhichiswidely
usedinfolkmedicinetotreatfeverduetoprimarycomplex,ulcerandglandularswellings.Inthisstudy,
proteinswereextractedfromtheleavesofM.pubescens,andprecipitatedwithammoniumsulphate
atvarioussaturationconcentrationsrangingfrom20to80%.Theprecipitatedproteinsampleobtained
with80%saturationwasfurtherpurifiedusingultrafiltrationmembrane(<10kDa).SDS-PAGEanalysis
identifiedthepresenceofcrudeandultrafilteredproteinbands.FTIRspectrumoftheultrafilteredprotein
fractionsdepictedthepresenceofhydroxylandcarbonylgroupsofproteins.Theultrafilteredproteins
exhibitedincreasedcytotoxicactivityonA549cellsattheconcentrationsrangingfrom15to100g/ml.
About98%cellviabilitywasalsoobservedinVerocellstreatedwiththemaximumconcentrationof
100g/mlofultrafilteredproteinextract.DNAfragmentationwasobservedinA549cellstreatedwith
10g/mlofultrafilteredproteins,indicatingtheonsetofapoptosis.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Bioactive proteins and peptides from plant sources exhibit differentactivities,suchasantimicrobial,antioxidant, antithrom-botic, antihypertensive, hypocholesterolemic, hypoglycemic, immunomodulatory,opioid,andantiproliferativeactivities.These activities can affect the condition of major body systems, like cardiovascular,digestive,endocrine,immuneandnervoussystem (Ledesmaetal.,2009).Plantspossessinganticanceractivitywere foundinViolaceae,Rubiaceae,FabaceaeandCucurbitaceae fam-ilies (Gerlach and Mondal,2007).Macrocyclic proteins, suchas circulinsA(32.8kDa)andB(31.5kDa),wereisolatedfromChassalia parvifolia and cyclopsychotride from Psycho trialongipes which belongtoRubiaceaefamily.Thesecyclicproteinsshowcytotoxic activity,antiHIVand hemolyticactivity(Gustafson etal., 1994; Witherupetal.,1994).Anticancerousbyproductsarederivedfrom Morindacitrifolia(noni)fruit(A549humanlungcarcinomacells) (Jang,2012)andbromelain,aproteinfoundinseveralmembers of Rubiaceae family are reported to have anti-tumor activity (MarshallandGolden,2012).
MorindapubescensSm.,commonlyknownasAalisaspeciesof floweringplantofthefamilyRubiaceae,nativetoSouthernAsia.
∗ Correspondingauthor.
E-mail:rupachandra.s@ktr.srmuniv.ac.in(R.Saravanakumar).
ThebarkofM.pubescens,isusefulintreatingeczema,feverdue toprimarycomplex,ulcerandglandularswellings,whileleaves are useful for digestivedisorders and venereal diseases (Nivas etal.,2011).Thepreliminaryphytochemicalstudyofthemethanol extractsofleafandstembarkofM.pubescens,exhibited antimicro-bialandantioxidantproperties(Muruganetal.,2012).Theaimof thisstudyistoisolateandpurifycytotoxicproteinsfromtheleaves ofM.pubescens.
Materialsandmethods
Plantmaterialandreagents
TheleavesofMorindapubescensSm.,Rubiaceae,werecollected andauthentified(PARC/2012/1384)byDr.P.Jayaraman,Directorof PlantAnatomyResearchCentre,Chennai.Allbuffersandchemicals usedwereofanalyticalgrade.HumancancercelllinesuchasA549 (adenocarcinomichumanalveolarbasalepithelialcells)andVero celllines(Africangreenmonkeykidneycells)werepurchasedfrom NCCS,Pune.
Proteinextraction
LeavesofM.pubescenswerewashedwithdistilledwaterand shadedried.Thedriedleavesweregroundtofinepowder.About 5gofpowderedleafsamplewasextractedwith50mlofextraction
http://dx.doi.org/10.1016/j.bjp.2016.08.003
buffer(Ribeiroetal.,2007)consistingof10mMNa2HPO4,15mM NaH2PO4,10mMKCl,2mMEDTA(pH7.0)andkeptinconstant stir-ringfor3hat4◦C.Thenthecontentswerefilteredandcentrifuged at5000×gfor20min.Thecrudesupernatantwaslyophilizedand
storedforfurtheruse.Thecrudesupernatant(lyophilized)was fur-thertreatedwithammoniumsulphateforprecipitationofproteins withvarioussaturationlimitfrom20to80%.Theconcentrations ofproteinspresentintheprecipitatedsampleswereestimatedby Bradfordassay(Bradford,1976).
Ultrafiltrationofproteinextracts
The precipitated proteins obtained using 80% saturation of ammoniumsulphatefromtheseedsofM.pubescenswere frac-tionatedusingultrafiltrationmembrane(10kDacut-offmembrane, Amicon).Theconcentrated filteredsolutioncontainingproteins smallerthantheporesizewhichislessthan10kDa,waslyophilized andstoredat4◦Cforfurtheruse(Ketnawa,2011).
SDS-PAGEanalysis
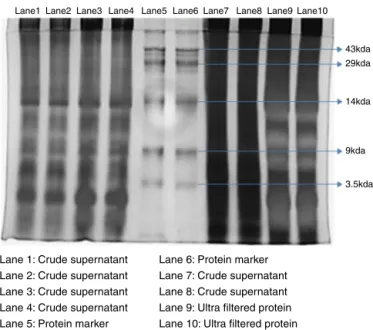
Sodiumdodecylsulfatepolyacrylamidegelelectrophoresisis awidelyusedtechniquetoseparateproteinsaccordingtotheir electrophoreticmobility(Mahmoodetal.,2012).About40lof thecrudesupernatantwasloadedintoLanes1–4,7,8and40lof theultrafilteredproteinsamplewasloadedintoLanes9,10.Lanes 5,6representstheproteinmarkerofsizerangingfrom3.5kDato 43kDa.Thegelwaskeptunderelectrophoreticrunfor2hat100V andtheproteinbandswereidentified.
FTIRanalysis
Identificationoffunctionalgroupsoftheultrafilteredproteins isolatedfromtheleavesofM.pubescenswasperformedusing Shi-madzuFourier transforminfraredspectrophotometer (Surewicz andMantsch,1988)Ultrafilteredproteinswerehomogenizedwith potassiumbromidetoobtainapellet.Thepelletwasscannedin theinfraredabsorptionregionbetween400and4000cm−1witha resolutionof4cm−1(Widjanarkoetal.,2011).
Cytotoxicactivityofproteinextracts
The ultrafiltered protein fractions were tested for cytotoxic activity against the selected cancer cell lines using MTT assay (Pascariuetal.,2011).Differentconcentrationsofultrafiltered pro-teinsrangingfrom2to100g/mlwereaddedtoeachwellof96 wellplates.Thecellswereculturedin96-wellplates(2×105cells
perwell)inDMEMsupplementedwith10%FBSfor24h.After24h thecellswereobservedunderphasecontrastmicroscopeand mor-phologyofcellswereobserved.Themediumcontainingpositive controlandtestsampleswereremoved.MTT(50l)dyewasadded tothewellscontaining200loffreshmedium.Thecelllineswere incubatedinCO2incubatorfor4h.After4hofincubation,medium containingdyewasremovedand200lofDMSOwasaddedto dis-solvetheformazancrystal.Theabsorbancewasrecordedat570nm andthepercentageofcellviabilitywascalculatedusingthe for-mula:
%ofcellviability=
At Ac×100
whereAt,absorbanceoftreatedcell;andAc,absorbanceofcontrol (untreatedcells).
DNAfragmentationassay
DNAfragmentationanalysis(Kalininaetal.,2002)wascarried outtoevaluatethemechanismofcelldeathinA549cancercell linetreatedwith10g/ml, ofultrafiltered proteinextractfrom the leaves of M. pubescens and incubated at −20◦C overnight.
Cells were freezed and thawed three times for detachment of cellsfromtheflasks.Cells(300l)fromtheflaskswastakenin aneppendorfand addedwith800lofproteinaseKbufferwas added. About4l of proteinaseK was added and keptfor 1h incubation at56◦C in a water bath.Afterincubation, 700lof phenol:chloroform:isoamylalchohol(25:24:1)and 100lof5M sodiumacetatewereaddedtothemixture.Themixturewas cen-trifugedfor15minat5000×g,4◦C.Thesupernatantwasadded
with200lofisopropylalcoholandincubatedat−20◦Cfor1h. Afterincubation,thecontentswerecentrifugedagainat5000×g,
4◦C for15min.Thesupernatantwasdiscardedand 1mlof70% ethanolwasaddedtothepelletwhichwascentrifugedfor15min at 6000×g, 4◦C. The supernatant was again discarded and air
dried.Nucleasefreewater(20l)wasaddedandstoredat−20◦C.
Agarosegelelectrophoresis(0.8%)wasperformedat100V.
Statisticalanalysis
The experiments werecarried out in triplicates. The results werecalculatedasmeanalongwithstandarderrorvalues. Statisti-calsignificancewascalculatedusingone-wayanalysisofvariance (ANOVA).Avalueofp<0.05wasconsideredasstatistically signifi-cant.
Resultsanddiscussion
Plant-basedproductsincludingproteinsand smallmolecular compoundshavebeensuggestedasthefavorabledrugsfor can-certreatmentinregardtomanyadverseeffectsexertedbycurrent cancertreatments,namelychemotherapyand radiationtherapy (Ledesmaetal.,2005).Theconcentrationofproteinspresentinthe crudesupernatant(1mg/ml),80%precipitatedsample(0.8mg/ml) and ultrafiltrate (0.5mg/ml) wereestimated by Bradfordassay. Similarstudieshavebeenperformedbyextractingbioactive pro-teinsfromvariousplantsources(Mauryaetal.,2011;Kumarand Santhi,2012).SDS-PAGEgel(12%)showedthepresenceofprotein
Lane1Lane2Lane3 Lane4 Lane5 Lane6Lane7 Lane8Lane9Lane10
43kda 29kda
14kda
9kda
3.5kda
Lane 1: Crude supernatant Lane 2: Crude supernatant Lane 3: Crude supernatant Lane 4: Crude supernatant Lane 5: Protein marker
Lane 6: Protein marker Lane 7: Crude supernatant Lane 8: Crude supernatant Lane 9: Ultra filtered protein Lane 10: Ultra filtered protein
54.6
52
50
48
46
44
42
40
38
36
34
32
30
27.5
4000.0 3000
MP
MP 001 7201 4000.00 400.00 27.52 53.30 4.00 %T 4 1.00
REF 4000 34.94 2000 41.90 600
3353.97 27.52 2359.95 30.64 2341.43 32.94 1631.26 37.19 1403.00 39.55 1295.63 39.36 1036.60 34.36 909.81 45.45 668.03 41.80 597.22 43.68
pK
3353.97 2359.95 2341.43
1631.26 1403.00
1295.63
1036.60 668.03 909.81
597.22
2000 1500
cm-1
%T
1000 400.0
Fig.2. FTIRspectralanalysisofultrafilteredproteinextractofMorindapubescens.
bandsofultrafilteredprotein(lanes:9,10)andcrudesupernatant (lanes:1–4,7,8).Lanes5,6depictsthebandsofproteinmarker rangingfromthesizeof3.5–43kDa(Fig.1).
TheFTIRspectrumoftheultrafilteredproteinfractions(Fig.2) detectedthepeaksat 1631.26cm−1 and3353.97cm−1 showing the presence of carbonyl (C O) and hydroxyl (OH) stretching vibrations. Further FTIR analysis of ultrafiltered protein frac-tionsalsodepictedIRabsorptionbandsat2359.95,2341.43and
Fig.3.Photomicrographs(40×)ofuntreatedVerocells(Control).
1403.00cm−1 indicating the presence of aminoacids, peptides and proteinscontainingN H bonds.Characteristicbandsfound intheinfraredspectraofproteinsandpolypeptidesinclude the Amide I and Amide II. These arise from the amidebonds that linktheaminoacids.TheabsorptionassociatedwiththeAmideI bandleadstostretchingvibrationsoftheC Obondoftheamide, absorptionassociatedwiththeAmideIIbandleadsprimarilyto
bending vibrations of the N H bond. Since both the C O and
theN Hbondsareinvolvedinthehydrogenbondingthattakes placebetweenthedifferentelementsofsecondarystructure,the locationsofboththeAmideIandAmideIIbandsaresensitiveto thesecondarystructurecontentofaprotein(SusiandByler,1983; BylerandSusi,1986).Similarstudiesweredoneonsoya,wheat, cornetc.,whereproteinwereisolatedwhichwerefoundoutusing FTIRstudies(Thanietal.,2010).
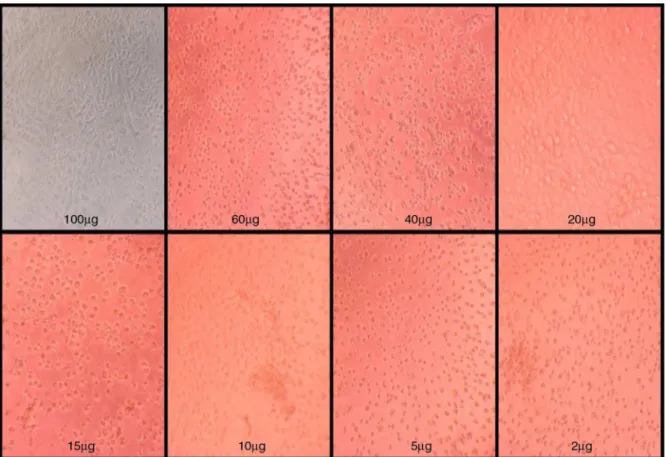
Fig.4.Photomicrographs(40×)ofVerocellstreatedwithdifferentconcentrationsofultrafilteredproteinextractrangingfrom2to100g/ml,isolatedfromtheleavesof
120
100
80
60
40
20
0
0 2 5 10 15
Concentration of ultrafiltrate protein extract (µg/ml) Vero cell lines
Vero cell lines
% cell viability
20 40 60 100
Fig.5. PercentageofcellviabilityofVerocelllinestreatedwithdifferent concen-trationsofultrafilteredproteinextractrangingfrom2to100g/ml,isolatedfrom theleavesofMorindapubescens.
ThecytotoxicactivityofultrafilteredproteinsfromleavesofM. pubescensSm.,onnormalVerocellsandA549cancercellswere measuredby MTT colorimetric assay.Untreated Verocell lines were used as control (Fig.3).Vero cell lines treated with dif-ferentconcentrationsofultrafilteredproteinsrangingfrom2to 100g/ml,showedincreasedcellviabilityof98%(Figs.4and5). AnincreasedpercentageofcellviabilityofA549cellswasnoted attheminimumconcentrationsof2–5g/mlofultrafiltered pro-teinextract(Figs.6–8).Cellviability(50%)ofA549cells treated with10g/mlofultrafilteredproteinextractwasalsoexamined (Figs. 6–8).Furtherit wasobservedthatthere wasadecreased percentageofcellviabilityofA549cells treatedwithincreasing
Fig.6.Photomicrographs(40×)ofuntreatedA549cells(Control).
concentrationsofultrafilteredproteinextractrangingfrom15to 100g/ml (Figs. 6–8).Previous studieswere reported on anti-cancerpropertyof proteinsextracted fromGynuraprocumbens, Asteraceae,onbreastcancercellline, MDA-MB-231,atanEC50 valueof3.8g/ml(Ngetal.,1992).Similarstudieswerecarriedout fortheanticancerpropertyofBidensalbaprotein-extractagainst humancolorectalcancer(SW480)cells whichdepictedmarked DNAdamagesandapoptosis-relatedcellularmorphologies(Hew etal.,2013).
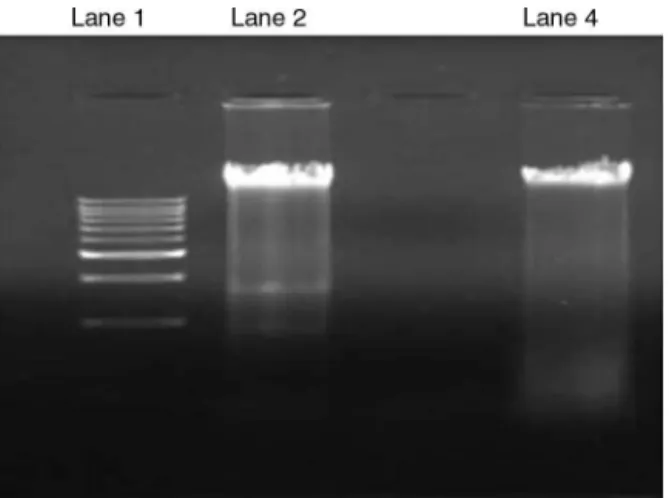
FurtherDNAfragmentationassaywasperformedbyagarose gel electrophoresis. A DNA ladder characteristic of apoptosis was observed in A549 cells treated with 10g/ml of ultrafil-teredproteins(Lane2,Fig.9).However,theDNAextractedfrom theuntreatedVerocells,appearedasdistinctbandshowingthe
Fig.7. Photomicrographs(40×)ofA549cellstreatedwithdifferentconcentrationsofultrafilteredproteinextractrangingfrom2to100g/ml,isolatedfromtheleavesof
120 100 80 60 40 20 0
0 2 5 10 15
Concentration of ultrafiltrate protein extract (µg/ml) A549 cell lines
A549 cell lines
% cell viability
20 40 60 100
Fig.8.PercentageofcellviabilityofA549celllinestreatedwithdifferent concen-trationsofultrafilteredproteinextractrangingfrom2to100g/ml,isolatedfrom theleavesofMorindapubescens.
Fig.9.AgarosegeldepictingDNAfragmentationofA549cellsshowing,Lane1: Marker(M)–1kbpladder;Lane2:A549cellstreatedwith10g/mlofultrafiltered proteinextract;Lane4:UntreatedVerocelllines.
viabilityofVerocells(Lane4,Fig.9).Apoptosis,akindofcellular deathischaracterizedbytheearlyactivationofendogenous pro-teases,cellshrinkageandDNAfragmentation(Nagata,2000).The nuclearDNAofapoptoticcellsshowsacharacteristicladdering pat-ternofoligonucleosomalfragments(Nagataetal.,2003).Similar observationswerenotedwiththehumancolorectalcancerSW480 cellsand monocyticleukemiaTHP-1cells treatedwiththe pro-teinextractsofCalvatialilacina,PleurotusostreatusandVolvariella volvacea.TheviabilitiesofTHP-1andSW480cellsdecreasedina concentration-dependentmannerafter24hoftreatmentwiththe proteinextractsoftheselectedplants.Apoptoticanalysisrevealed thatthepercentageofSW480cellsintheSubG1phase(amarkerof apoptosis)wasincreasedupontreatmentwithprotein-extractsof PleurotusostreatusandVolvariellavolvacea,indicatingthat oligonu-cleosomalDNAfragmentationexistedconcomitantlywithcellular death(Ongetal.,2008;Jin-Yietal.,2011).
Inconclusiontheultrafilteredproteinsextractedfromtheleaves ofM. pubescensexhibitedsignificantcytotoxicactivityonA549 cellsattheIC50concentrationof10g/ml.Besides,theultrafiltered proteinfractiondidnotshowanyinhibitoryeffectsonthe pro-liferationofVerocells.Further,DNAfragmentationwasobserved inA549cellstreatedwithultrafilteredproteins,thereby indicat-ingtheonsetofapoptotic celldeath. Thus,theresultsobtained in this study suggest that the ultrafiltered proteins (10kDa) with apoptosis-inducing activity, isolated from the leaves of
M. pubescens can act as potential anticancer agents in cancer chemotherapy.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
ASTcontributedtobiologicalstudiesandlaboratorywork.SR andPVGcontributedtocriticalreadingofthemanuscript.PVG con-tributedtoplantcollection.SRdesignedthestudyandsupervised thelaboratorywork.Alltheauthorshavereadthefinalmanuscript andapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Bradford,M.,1976.Arapidandsensitivemethodforthequantitationof micro-gramquantitiesofproteinutilizingtheprincipleofprotein-dyebinding.Anal. Biochem.72,248–254.
Byler,D.M.,Susi,H.,1986.Examinationofthesecondarystructureofproteinsby deconvolvedFTIRspectra.Biopolymer25,469–487.
Gerlach,S.L.,Mondal,D.,2007.Thebountifulbiologicalactivitiesofcyclotides.CYS 3,169–177.
Gustafson,K.R.,SowderII,R.C.,Henderson,L.E.,Parsons,I.C.,Kashman,Y.,Cardellina II,J.H.,McMahon,J.B.,Buckheit,R.W.,Pannell,L.K.,Boyd,M.R.,1994.CirculinsA andB:novelHIVinhibitorymacrocyclicpeptidesfromthetropicaltreeChassalia
parvifoliaK.Schum.J.Am.Chem.Soc.116,9337–9338.
Hew, C., Khoo, B.Y., Gam, L.H., 2013. The anti-cancer property of pro-teins extracted from Gynura procumbens (Lour.) Merr. PLOS ONE, http://dx.doi.org/10.1371/journal.pone.0068524.
Jang,B.C.,2012.ThefruitjuiceofMorindacitrifoliaL.(noni)downregulates hif-1␣proteinexpressionthroughinhibitionofpkb,erk-1/2,jnk-1 ands6in manganese-stimulatedA549humanlungcancercells.Int.J.Mol.Med.29, 499–504.
Jin-Yi,W.,Chi-Hung,C.,Wen-Huei,C.,King-Thom,C.,Yi-Wen,L.,Fung-Jou,L., Ching-Hsein,C.,2011.Anti-cancereffectsofproteinextractsfromCalvatialilacina, PleurotusostreatusandVolvariellavolvacea.Evid.BasedComplement.Altern. Med.,http://dx.doi.org/10.1093/ecam/neq057.
Kalinina,T.S.,Bannova,A.V.,Dygalo,N.N.,2002.QuantitativeevaluationofDNA fragmentation.Bull.Exp.Biol.Med.134,554–556.
Ketnawa,S.,2011.Extractionofbromelainfrompineapplepeels.FoodSci.Technol. Int.17,395–402.
Kumar,D.J.,Santhi,R.J.,2012.Antioxidantandcytotoxiceffectsofhexaneextractof
MorindapubescensSm.leavesinhumanlivercancercellline.AsianPac.J.Trop.
Med.5,62–66.
Ledesma,H.C.,Hsieh,C.,deLumen,B.O.,2005.Chemopreventivepropertiesof pep-tidelunasin:areview.ProteinPept.Lett.20,424–432.
Ledesma,H.B.,Hsieh,C.,DeLumen,B.O.,2009.Lunasin,anovelseedpeptidefor cancerprevention.Peptides30,426–430.
Mahmood,A.,Raja,G.K.,Mahmood,T.,Gulfraz,M.,Khanum,A.,2012.Isolationand characterizationofantimicrobialactivityconferringcomponent(s)fromseeds ofbittergourd(MomordicacharantiaL.).J.Med.PlantsRes.6,566–573. Marshall,S.J.,Golden,K.D.,2012.CharacterizationofbromelainfromMorinda
citri-foliaL.,(noni).JSR4,445–456.
Maurya,D.K.,Nandakumar,N.,Devasagayam,T.P.A.,2011.Anticancerpropertyof gallicacidinA549,ahumanlungadenocarcinomacellline,andpossible mech-anisms.J.Clin.Biochem.Nutr.48,85–90.
Murugan,M.,Mohan,V.R.,Thamodharan,V.,2012.Phytochemicalscreeningand antibacterialactivityofGymnemasylvestreR.Br.,andMorindapubescensSm.JAPS 2,73–76.
Nagata,S.,2000.ApoptoticDNAfragmentation.Exp.CellRes.256,12–18. Nagata,S.,Nagase,H.,Kawane,K.,Mukae,N.,Fukuyama,H.,2003.Degradationof
Ng,T.B.,Chan,W.Y.,Yeung,H.W.,1992.Proteinswithabortifacient,ribosome inactivating, immunomodulatory, antitumor and anti-aids activities from Cucurbitaceaeplants.Gen.Pharmacol.23,579–590.
Nivas,D.,Gaikwad,D.K.,Chavan,P.D.,2011.Antioxidant potentialofMorinda
pubescensSm.fruits.J.Pharm.Res.4,829–831.
Ong,P.L.,Weng,B.C.,Lu,F.J.,Lin,M.L.,Chang,T.T.,Hung,R.P.,Chen,C.H.,2008. Theanticancereffectofprotein-extractfromBidensalbainhuman colorec-talcarcinomaSW480cellsviathereactiveoxidativespecies-andglutathione depletion-dependentapoptosis.FoodChem.Toxicol.46,1535–1547. Pascariu,M.,Nevoie,A.,Jitaru,D.,Carasevici,E.,Luchian,T.,2011.Theevaluation
ofbiologicaleffectofcytotoxicpeptidesontumorcelllines.Dig.J.Nanomater. Biostruct.7,79–84.
Ribeiro,S.F.F.,Carvalho,AndréO.,Cunha,M.,Rodrigues,R.,Cruz,L.P.,Melo,V.M.M., Vasconcelos,I.M.,Melo,E.J.T.,Gomes,V.M.,2007.Isolationandcharacterization ofnovelpeptidesfromchillipepperseeds.Toxicon50,600–611.
Surewicz,W.K.,Mantsch,H.H.,1988.Newinsightintoproteinsecondary struc-turefromresolution-enhancedinfraredspectra.Biochim.Biophys.Acta952, 115–130.
Susi,H.,Byler,D.M.,1983.ProteinstructurebyFouriertransforminfrared spec-troscopy:secondderivativespectra.Biochem.Biophys.Res.Commum.115, 391–397.
Thani,W.,Vallisuta,O.,Siripong,P.,Ruangwises,N.,2010.Anti-proliferativeand antioxidativeactivitiesofThainoni/Yor(MorindacitrifoliaL.).SoutheastAsianJ. Trop.Med.PublicHealth41,482–489.
Widjanarko,S.B.,Nugroho,A.,Estiasih,T.,2011.Functionalinteractioncomponents ofproteinisolateandglucomannaninfoodbarsbyFTIRandSEMstudies.Afr.J. FoodSci.5,12–21.