Iranian Journal of Basic Medical Sciences
ijbms.mums.ac.ir
MiR‐
suppresses the proliferation and invasion of gastric
cancer cells by targeting RhoC
Wenhua Zhou ,Chi Zhang , (ao Jiang , Zhiwei Zhang , Liming Xie , Xiusheng (e *
Cancer Research )nstitute, University of South China, Key Laboratory of Cancer Cellular and Molecular Pathology of (unan Provincial University, (engyang, People's Republic of China
Function Laboratory Center, Medical School, University of South China, (engyang, (unan, People s Republic of China
Center for Gastric Cancer Research of (uman Province, The First Affiliated (ospital, University of South China, (engyang, (unan, People's Republic of China
A R T ) C L E ) N F O A B S T R A C T
Article type:
Original article Objective(smetastasis. (owever, their effect and prognostic value in gastric cancer is still poorly known. ):MiRNAs have been proposed to be key regulators of tumorigenesis, progression and
Materials and Methods: Gastric cancer cell lines were cultured. Tissue samples obtained from
gastric cancer patients were used for quantitative real‐time PCR qRT‐PCR analysis. The tissue microarrays TMAs consisted of cases of gastric carcinoma that were used for In situ
hybridisation )S( . Lentivirus plasmids were co‐transfected into FT cells. Cell migration was examined using wound‐healing assays. Statistical analyses were performed using SPSS . software.
Results: )n this study, we found that the expression levels of miR‐ were strongly down‐regulated in
gastric cancer and were associated with clinical stage and the presence of lymph node metastases. Moreover, miR‐ might independently predict OS and RFS in gastric cancer. We further found that up‐regulation of miR‐ inhibited the proliferation and metastasis of gastric cancer cells, invitro and invivo. )n addition, miR‐ directly targeted RhoC, which resulted in a marked reduction of the
expression of mRNA and protein. This effect, in turn, led to a decreased ability of growth, invasion and metastasis in gastric cancer cells.
Conclusion: Taken together, our findings demonstrate that miR‐ is important for gastric cancer
initiation and progression and holds promise as a prognostic biomarker to predict survival and relapse in gastric cancer. )t is also a potential therapeutic tool to improve clinical outcomes in this disease.
Article history:
Received: Jan , Accepted: Aug ,
Keywords:
Gastric cancer miR‐ Proliferation )nvasion
Prognostic significance
►Please cite this article as:
ZhouW, ZhangCh, Jiang(, ZhangZh, XieL, (e X. MiR‐ suppresses the proliferation and invasion of gastric cancer cells by targeting RhoC. )ran J Basic Med Sci ; : ‐ .
Introduction
Gastric cancer is one of the most common malignancies and is the second leading cause of cancer mortality worldwide . Nearly half of gastric cancer occurs in China with an overall ‐year survival rate of approximately % , most of which are diagnosed in advanced stages losing the opportunity for radical surgery. Lack of early detection and limited treatment options contribute to its bad prognosis. Therefore, identification of novel mediators of invasion and metastasis, in addition to novel biomarkers of gastric cancer progression, is crucial to improve patient outcome , .
MiRNAs are – nucleotides in length and regulate gene expression by imperfect base‐pairing with complementary sequences located mainly. (ence, miRNAs represent one of the major regulatory families of genes in eukaryotic cells, and work by inducing translational repression and transcript degradation . MiRNAs have been reported
to be significantly involved in tumorigenesis and progression, acting as either oncogenes or tumor suppressors ‐ . Emerging studies have reported that a group of miRNAs is commonly dysregulated in gastric cancer. For example, miR‐ , miR‐ a, miR‐ b, miR‐ , miR‐ , miR‐ and miR‐ b/c ‐ were reported to be always down‐regulated, while miR‐ a, miR‐ a, miR‐ , miR‐ a, miR‐ b and miR‐ b ‐ were over‐expressed. Since these specific miRNAs regulate different target genes which are involved in various signaling pathways and biological processes, gastric cancer could be complicated diseases with multiple genes being dysregulated .
Recently, Ueno et al reported that miR‐ was
significantly down‐regulated in bladder cancer as shown by miRNA microarray analysis and decreased cell motility and migration ability . miR‐ induction during carcinogenesis blocks metastatic settlement of colon cancer cells in the liver .
*Corresponding author: Xiusheng (e. Cancer Research )nstitute, University of South China, West Changsheng Road, (engyang, , People s Republic
Thus, we investigated the effect of miR‐ on the carcinogenesis and progression of gastric cancer and their prognostic significance.
)n the present study, we found that miR‐ expression is down‐regulated in stomach tumour specimens, as well as gastric cell lines, by quantitative RT‐PCR analysis. miR‐ expression was detected by in situ hybridisation on tissue microarrays, and the association between miR‐ levels and clinicopathologic factors and prognosis were analysed. Our results indicated that decreased miR‐ correlates with advanced clinical stage, lymph node metastases and poor clinical outcomes. Additionally, we observed that miR‐ suppresses gastric cancer growth and metastasis, invitro.
Materials
and
Methods
Cellculture
The gastric epithelial cell line GES‐ was purchased from Beijing )nstitute for Cancer Research Beijing, China . The gastric cancer cell lines SGC‐ , (GC‐ , AGS MKN‐ , MGC‐ , BGC‐ and MKN‐ were obtained from the American Type Culture Collection ATCC, Rockville, MD . These cells were maintained at
°C in an atmosphere of % CO in RPM)‐
medium supplemented with % foetal bovine serum, penicillin and streptomycin Gibco BRL, NY, USA . All transfections were performed using Lipofectamine
)nvitrogen, Carlsbad, USA .
Clinicalsamples
All tissue samples used in the present study were collected from (unan Provincial Tumour (ospital Changsha, (unan, China . Written informed consent was obtained from all the participants . The study was approved by the Ethics Committee of the University of South China (ealth Authority. Collection and use of tissues followed the procedures that are in accordance with the ethical standards as formulated in the (elsinki Declaration. Tissue samples obtained from gastric cancer patients were used for quantitative real‐time PCR qRT‐PCR analysis. Resected cancerous tissues Tumour and paired matched normal gastric tissues Normal were immediately cut and stored in RNAlater Ambion . The tissue microarrays TMAs consisted of cases of gastric carcinoma. All data, including age, sex, histological grade, tumour size, invasion depth, and lymph node metastasis were obtained from clinical and pathological records.
QuantitativeRT‐PCRanalysis(qRT‐PCR)
Total RNA was extracted from cells or tissues using Trizol reagent )nvitrogen, Carlsbad, CA, USA , and RT reactions were performed using miR‐ special prime. The specific stem–loop RT primers for
miR‐ were purchased from Ribobio co., Ltd Guangzhou, China . Real‐time PCR was
performed using a standard protocol from the SYBR Green PCR kit Toyobo, Osaka, Japan . Fold change was determined as ‐ΔΔCt. The Ct is the fractional cycle number at which the fluorescence of each sample passes the fixed threshold. The ΔCt was calculated by subtracting the Ct of snRNA U from the Ct of the miRNA of internal control. The ΔΔCt was calculated by subtracting the ΔCt of the reference sample paired non‐tumorous tissue for surgical samples from the ΔCt of each sample. U snRNA primer, '‐ATTGGAACGATACAGAGAAGATT‐ ' and
'‐GGAACGCTTCACGAATTTG‐ '.
Insituhybridisation
Tissue microarray slides were deparaffinised and rehydrated. The miR‐ miRCURYTM LNA custom detection probe Exiqon, Vedbaek, Denmark
was used for in situ hybridization )S( . The sequence ‐ enhanced with LNA was
TCAGGAACTGCCTTTCTCTCCA with digoxigenin D)G at the and ends. (ybridization, washing, and scanning were carried out according to the manuals and protocols provided by the Exiqon life science department. The intensity of staining was scored from to , while the extent of staining was scored from to %. Relative expression was calculated by multiplying the two scores. The slides were analysed by two independent pathologists.
Overallsurvival(OS)anddisease‐freesurvival(DFS)
DFS was defined as the interval between surgery and the date of diagnosis of the first recurrence or the date of the last follow‐up. OS was calculated from diagnosis to the date of death for any causes, while patients who were alive were censored at the date of last follow‐up visit.
Lentivirusproductionandinfection
Lentivirus plasmids were co‐transfected into FT cells )nvitrogen with pLP , pLP , and pLP/VSVG )nvitrogen , and virus containing supernatants were prepared according to manufacturer s instructions. As for lentivirus infection, cells were incubated with virus‐ containing supernatants in the presence of ug/ml polybrene. )nfected cells were selected in the presence of ug/ml puromycin to generate two paired stable monoclonal cell lines a stable cell line expressing miR‐
, MKN‐ ‐miR‐ and control stable cell line, MKN‐ ‐control . For infection of GFP‐expressing viruses for miRNA expression, flow cytometry analyses FacsCalibur, Becton Dickinson were performed to make sure that % of cells were infected.
Cellmigrationandinvasionassays
Table1. Analysis of the correlation between expression of miR‐ in primary gastric cancer and its clinicopathological parameters
Viable Cases miR‐
low high P‐ value
Age years
< .
Gender Male
Female .
(istological grade a
well and moderate
Poor and Other .
T stage T ‐T
T ‐T .
TNM stage )‐))
)))‐)V .
Lymph node metastasis Present
Absent .
a Well differentiated adenocarcinoma Well , Moderately differentiated adenocarcinoma Moderate , Poorly differentiated adenocarcinoma
Poor , Other histological type Other
photographs were taken using an inverted microscope Olympus, Tokyo, Japan at hr. As for cell invasion assay, cells were seeded onto the basement membrane matrix present in the insert of a ‐well culture plate EC matrix, Chemicon, Temecula, CA and fetal bovine serum was added to the lower chamber as a chemo‐attractant. After hr, the non‐invading cells and EC matrix were gently removed with a cotton swab. )nvasive cells which located on the lower side of the chamber were stained with Crystal Violet, counted and imaged.
Westernblotanalysis
Protein concentration in the lysates was measured with the Protein BCA Assay Kit Bio‐Rad , and mg of protein mixed with × SDS loading buffer was loaded per lane. The proteins in the lysates were separated by % SDS‐PAGE and transferred to polyvinylidene difluoride membranes Millipore . Next, the membranes were incubated for hr at °C with an
antiserum containing antibodies against Rhoc, )GF R, FZD and β‐actin purchased from Cell signaling Technology. A peroxidase‐conjugated secondary antibody and ECL western blotting detection reagents were used to visualise the target proteins ECL New England Biolabs . Then, proteins were quantified with a Bio )mage )ntelligent Quantifier ‐D Version . . ,
Nihon‐Bio)mage Ltd. . An anti‐β‐actin antibody was used as a protein loading control.
Statisticalanalysis
Data were expressed as the mean ± standard error of the mean SEM from at least three independent experiments. Comparisons between groups were done by t‐test and x test. All differences were statistically significant at the level of P . . Statistical analyses
were performed using SPSS . software.
Results
miR‐493isdown‐regulatedingastriccancer
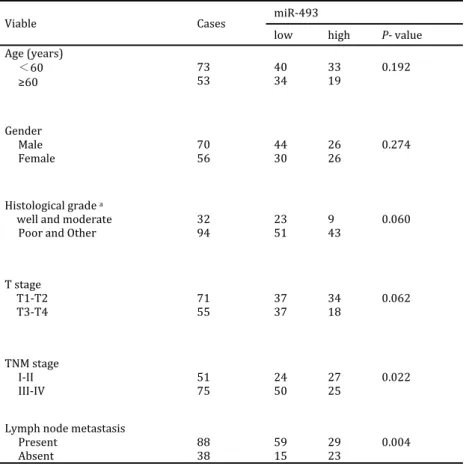
First, a set of human gastric cancer cell lines was analyzed to assess the expression profile of miR‐ in gastric cancer using qRT‐PCR Figure A . Compared with the non‐malignant gastric cell line GES‐ , seven gastric cancer cell lines showed reduced miR‐ expression, especially the MKN‐ cells. We also compared miRNA‐ expression levels in a series of pairs of gastric cancer tissues and their matched adjacent tissues. Among the patients with gastric cancer, approximately %
P< . , out of patients of tumours revealed a
Figure1. Analysis of the correlation between the expression of
miR‐ in primary gastric cancer and its clinicopathological parameters
miR‐ expression level was frequently down‐regulated in human gastric cancer. A Relative expression of miR‐ in seven cell lines derived from gastric cancer and one non‐malignant gastric cell line GES‐ was determined by qRT‐PCR. The error bars represent the standard deviations SD from triplicates of one representative experiment. *P< . and **P< . . B miR‐
expression was detected in gastric cancer patients by qRT‐PCR
Decreased miR‐493 correlates with advanced
clinical stage, lymph node metastases and poor
clinicaloutcomes
To further verify the results concerning the biological role of miR‐ in gastric cancer, we employed in situ hybridisation to evaluate miR‐ levels in gastric tumour tissues in a tissue microarray TMA . Of note, the miR‐ level inversely correlated with clinical stage and lymph node metastasis P= . and P= . , respectively
Table. . (owever, neither miR‐ levels in gastric cancer patients correlated with age, gender, tumour size, cell differentiation or invasion depth. Our results suggest that miR‐ could play critical roles in carcinogenesis and progression of gastric cancer. To further analyze the significance of miR‐
in terms of clinical prognosis, Kaplan‐Meier survival analysis was performed using patient overall survival and relapse‐free survival. The results demonstrated that patients with low miR‐ expression had shorter mean months of OS
P= . Figure A and RFS P= . Figure
B than did patients with high miR‐ expression.
MiR‐493inhibitedgastriccancercellproliferation
andinvasioninvitro
Because miR‐ levels were down‐regulated in gastric cancer and were associated with the clinical stage, lymph node metastasis and clinical outcome, we evaluated the effect of miR‐ overexpression ongastriccancercellphenotype.Since MKN‐ with
Figure2. Survival curves of OS A and DFS B according to miR‐
expression. Low miR‐ expression was correlated with worse outcome
relatively low basal expression of miR‐ Figure A , stable ectopic overexpression cell subsets MKN‐
/miR‐ and their paired control cells were constructed. qRT‐PCR analysis showed that the transfection were successful data not shown . We determined that overexpression of miR‐ in MKN‐ cells markedly attenuated cell proliferation. The proliferation assay showed that ectopic expression of miR‐ in MKN‐ markedly attenuated cell proliferation compared with control cells Figure A . Moreover, the expression of miR‐ significantly inhibited the capacity of cells for invasion Figures B and C . These data demonstrate miR‐ functions as a tumour suppressor in gastric cancer.
Identification of miR‐493 target genes and their
effect on proliferation and invasion of gastric
cancercells
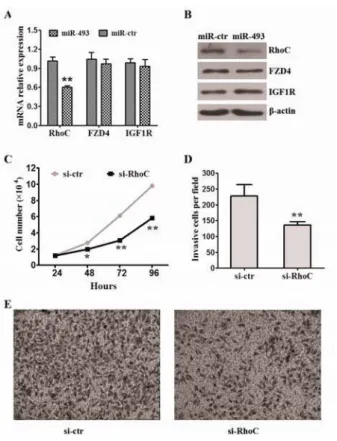
On the basis of the observation showing that miR‐ affects cell proliferation and invasion, we searched for target genes of miR‐ related to migration. Among these candidate target genes, three known genes Rhoc, FZD and )GF R which had been proved as target genes of miR‐ , attracted our attention. To verify if miR‐ directly targets and inhibits aforementioned genes, we firstly checked the target genes expression level of mRNA and protein in MKN‐ cells infected with miR‐ or mock lentivirus. The results indicated that miR‐
Figure 3. MiR‐ inhibited cell proliferation and invasion in
gastric cancer. A The growth of MKN‐ cells infected with miR‐ lentivirus or control lentivirus was assayed. Data represent the means±SEM from three independent experiments. *P< . ,
**P< . . B The invasion assay of MKN‐ cells infected with
miR‐ lentivirus or control lentivirus. MiR‐ lentivirus inhibited the cells invation. Data represent the means±SEM from three independent experiments. *P< . . C Representative
images of the assays are shown. Original magnification: ×
Discussion
Until now, a handful of studies have identified specific miRNAs involved in human tumorigenesis and tumor progression , , . Therefore, we believe more effort should be made, not only towards the identification of relevant miRNAs but also to identify the specific mechanisms through which they accomplish their specific functions, particularly with regard to the oncogenesis of different types of tumors , , . )n this study, we used qRT‐PCR and )S( to show that miR‐ was frequently down‐regulated in gastric cancer, and % out of of the gastric cancer had . ‐fold reduced expression of miR‐ as compared to their corresponding non‐tumorous tissues. )ntriguingly, we found that lower expression of miR‐ tended to have more advanced TNM stage stage )/)) vs. stage )))/)V, P= . , suggesting that low
expression of miR‐ is associated with gastric cancer progression. Kaplan‐Meier survival analyses revealed that patients whose primary tumours displayed low expression of miR‐ had a shorter OS and RFS in gastric cancer. Further studies showed that overexpression of miR‐ suppressed gastric cancer cell proliferation and invasion, invitro. The
data from the current study suggests that miR‐ is important for gastric cancer initiation and progression and that down‐regulated miR‐ contributes to lymph node‐metastasis and tumor progression in gastric cancer patients.
As the next step, we explored the possible targets of miR‐ in lung cancer cells. Rhoc, FZD and
Figure4. MiR‐ inhibited the capability of invasion of gastric
cancer invitro by targeting Rhoc. A miR‐ strongly reduced
the expression of Rhoc but not FZD and )GF R in mRNA level. The error bars represent the standard deviations SD from triplicates of one representative experiment. **P< . . B miR‐
strongly reduced the expression of RhoC but not FZD and )GF R in protein level. C Knockdown of Rhoc inhibited the cells proliferation. Data represent the means±SEM from three independent experiments. *P< . , **P< . . D Knockdown of
Rhoc inhibited the cells invasion. Data represent the means±SEM from three independent experiments. *P< . . E Representative
images of the assays are shown. Original magnification: ×
and gastric cancer . RhoC deletion did not affect breast cancer growth in a mouse model, but metastasis was specifically impaired . )nterestingly, we found that knockdown Rhoc partly inhibited the proliferation and invasion of MKN‐ cells.
)n summary, we observed down‐regulation of miR‐ in gastric cancer cells and tissues. We further found that miR‐ as an important tumor suppressive miRNA which inhibits cell proliferation and invasion by blocking RhoC in gastric cancer. Our findings demonstrated that miR‐ is important for gastric initiation and progression and could be considered as a potential therapeutic to suppress gastric cancer invasion.
Conclusion
Our findings demonstrated that miR‐ is important for gastric cancer initiation and progression and holds promise as a prognostic biomarker to predict survival and relapse in gastric cancer. )t is also a potential therapeutic tool to improve clinical outcomes in this disease.
Acknowledgment
This work was supported by funds from the National Natural Science Foundation of China
, . .
References
. (artgrink ((, Jansen EP, van Grieken NC, van de
Velde CJ. Gastric cancer. Lancet ; : ‐ .
. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the
world. J Clin Oncol ; : ‐ .
. Tang (, Kong Y, Guo J, Tang Y, Xie X, Yang L, etal. Diallyl disulfide suppresses proliferation and induces apoptosis in human gastric cancer through Wnt‐
signaling pathway by up‐regulation of miR‐ b and
miR‐ . Cancer Lett ; : ‐ .
. Tan Z, Jiang (, Wu Y, Xie L, Dai W, Tang (, etal. miR‐ is an independent prognosis factor and suppresses tumor metastasis in gastric cancer. Mol
Cell Biochem ; : ‐ .
. German MA, Pillay M, Jeong D(, (etawal A, Luo S, Janardhanan P, et al. Global identification of microRNA‐target RNA pairs by parallel analysis of
RNA ends. Nat Biotechnol ; : ‐ .
. Gu Y, Cheng Y, Song Y, Zhang Z, Deng M, Wang C, et
al. MicroRNA‐ suppresses tumor growth, invasion
and metastasis of lung cancer by regulating E F .
PLoS One ; :e .
. Cheng CJ, Slack FJ. The duality of oncomiR addiction in the maintenance and treatment of
cancer. Cancer J ; : ‐ .
. Tang (, Deng M, Tang Y, Xie X, Guo J, Kong Y, etal.
miR‐ b and miR‐ c as Prognostic Factors and
Mediators of Gastric Cancer Cell Progression. Clin
Cancer Res ; : ‐ .
. Zheng B, Liang L, Wang C, (uang S, Cao X, Zha R, et al. MicroRNA‐ a suppresses tumor cell invasion and metastasis by downregulating ROCK in gastric
cancer. Clin Cancer Res ; : ‐ .
. Liu D, Xia P, Diao D, Cheng Y, Zhang (, Yuan D, et al. MiRNA‐ suppresses the growth of gastric
cancer cells invitro. J Biomed Res ; : ‐ .
. (e XP, Shao Y, Li XL, Xu W, Chen GS, Sun ((, etal.
Downregulation of miR‐ in gastric cancer
correlates with cyclooxygenase‐ overexpression and
tumor growth. FEBS J ; : ‐ .
. Zhang L, Liu X, Jin (, Guo X, Xia L, Chen Z, etal. miR‐ inhibits gastric cancer proliferation in part
by repressing cyclinD . Cancer Lett ; : ‐
.
. Qin S, Ai F, Ji WF, Rao W, Zhang (C, Yao WJ. miR‐ a Promotes Cell Growth and Tumorigenesis through Targeting SOCS in Gastric Cancer. Asian Pac
J Cancer Prev ; : ‐ .
. Wang M, Li C, Yu B, Su L, Li J, Ju J, et al.
Overexpressed miR‐ a promotes cell proliferation
and invasion by targeting RUNX in gastric cancer. J
Gastroenterol ; : ‐ .
. Wu W, Takanashi M, Borjigin N, Ohno S), Fujita K, (oshino S, et al. MicroRNA‐ a modulates STAT activity through negative regulation of P)AS during
gastric adenocarcinogenesis. Br J Cancer ;
: ‐ .
. Yang ZX, Lu CY, Yang YL, Dou KF, Tao KS. MicroRNA‐ b expression in gastric adenocarcinoma and its effect on the proliferation of gastric cancer cells. Mol
Med Rep ; : ‐ .
. Zhi Q, Guo X, Guo L, Zhang R, Jiang J, Ji J, etal.
Oncogenic miR‐ is an important molecular target
in gastric cancer. Anticancer Agents Med Chem ;
: ‐ .
. Guo JX, Tao QS, Lou PR, Chen XC, Chen J, Yuan JB.
miR‐ b as a potential molecular target for
anticancer therapy of gastric neoplasms. Asian Pac J
Cancer Prev ; : ‐ .
. Ueno K, (irata (, Majid S, Yamamura S, Shahryari V, Tabatabai ZL, etal. Tumor suppressor microRNA‐ decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC
and FZD . Mol Cancer Ther ; : ‐ .
. Okamoto K, )shiguro T, Midorikawa Y, Ohata (, )zumiya M, Tsuchiya N, etal. miR‐ induction during carcinogenesis blocks metastatic settlement of colon
cancer cells in liver. EMBO J ; : ‐ .
. Gao J, Li L, Wu M, Liu M, Xie X, Guo J, etal. MiR‐ a inhibits proliferation and migration of breast
cancer through repression of MCL‐ . PLoS One ;
:e .
. Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y, Tong L, etal. Metadherin confers chemoresistance of cervical cancer cells by inducing autophagy and activating
ERK/NF‐kappaB pathway. Tumour Biol ;
: ‐ .
. Zhu K, Dai Z, Pan Q, Wang Z, Yang G(, Yu L, etal. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial‐
mesenchymal transition. Clin Cancer Res ;
: ‐ .
metastasis via activation of RhoA and RhoC. J Biol
Chem ; : ‐ .
. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim
Biophys Acta ; : ‐ .
. )koma T, Takahashi T, Nagano S, Li YM, Ohno Y, AndoK,etal. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res
; : ‐ .
. Shida A, Fujioka S, Takahashi N, Aoki (, Okamoto
T, Mitsumori N, etal. Reduced expression of Rho GDP dissociation inhibitor mRNA is associated with lymph node metastasis in gastric carcinoma. Oncol
Lett ; : ‐ .
. (akem A, Sanchez‐Sweatman O, You‐Ten A, Duncan G, Wakeham A, Khokha R, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev