ContentslistsavailableatSciVerseScienceDirect
Phytomedicine
j o ur na l h o me p a g e :w w w . e l s e v i e r . d e / p h y m e d
Effect
of
subinihibitory
and
inhibitory
concentrations
of
Plectranthus
amboinicus
(Lour.)
Spreng
essential
oil
on
Klebsiella
pneumoniae
Thially
Braga
Gonc¸
alves
a,b,∗,
Milena
Aguiar
Braga
a,
Francisco
F.M.
de
Oliveira
a,b,
Gilvandete
M.P.
Santiago
c,d,
Cibele
B.M.
Carvalho
b,e,
Paula
Brito
e
Cabral
a,b,
Thiago
de
Melo
Santiago
f,
Jeanlex
S.
Sousa
f,
Eduardo
Bedê
Barros
f,
Ronaldo
Ferreira
do
Nascimento
g,
Aparecida
T.
Nagao-Dias
a,baDepartamentodeAnálisesClínicaseToxicológicas,FaculdadedeFarmácia,OdontologiaeEnfermagem,UniversidadeFederalCeará,RuaCapitãoFranciscoPedro1210,
CEP60430-370,Fortaleza,Ceará,Brazil
bCursodePós-Graduac¸ãoemMicrobiologiaMédica,FaculdadedeMedicina,UniversidadeFederaldoCeará,RuaMonsenhorFurtadoS/N,CEP60430-270,Fortaleza,Ceará,Brazil
cDepartamentodeFarmácia,FaculdadedeFarmácia,OdontologiaeEnfermagem,UniversidadeFederaldoCeará,RuaCapitãoFranciscoPedro1210,CEP60430-370,Fortaleza,
Ceará,Brazil
dCursodePós-Graduac¸ãoemQuímica,CentrodeCiências,UniversidadeFederaldoCeará,CxPostal12.200,CEP60021-970,Fortaleza,Ceará,Brazil
eDepartamentodePatologiaeMedicinaLegal,FaculdadedeMedicina,UniversidadeFederaldoCeará,RuaMonsenhorFurtadoS/N,CEP60430-270,Fortaleza,Ceará,Brazil
fDepartamentodeFísica,CentrodeCiências,UniversidadeFederaldoCeará,CEP60451-970,Fortaleza,Ceará,Brazil
gDepartamentodeQuímicaAnalíticaeFísico-Química,UniversidadeFederaldoCeará,CampusdoPici,Bloco940,Fortaleza,Ceará,Brazil
a
r
t
i
c
l
e
i
n
f
o
Keywords:
Antibioticmultiresistance Antimicrobialactivity Klebsiellapneumoniae Plectranthusamboinicus
a
b
s
t
r
a
c
t
Weevaluatedtheantimicrobialactivityandsomemechanismsusedbysubinhibitoryandinhibitory concentrationsoftheessentialoil,obtainedfromleavesofPlectranthusamboinicus,againstastandard strainofKlebsiellapneumoniaeand5multiresistantclinicalisolatesofthebacteria.Theminimalinhibitory concentration(MIC)andminimalbactericidalconcentration(MBC),therateofkillandthepHsensitivity oftheessentialoilweredeterminedbymicrodilutiontestsperformedin96-wellplates.Subinhibitory andinhibitoryconcentrationsoftheessentialoilweretestedinordertocheckitsactiononK.pneumoniae membranepermeability,capsuleexpression,ureaseactivityandcellmorphology.TheMICandMBCof theessentialoilwere0.09±0.01%.Acompleteinhibitionofthebacterialgrowthwasobservedafter2h ofincubationwithtwicetheMICoftheessentialoil.AbetterMICwasfoundwhenneutraloralkalinepH brothwasused.Alterationinmembranepermeabilitywasfoundbytheincreaseofcrystalvioletuptake whenthebacteriawereincubatedwithtwicetheMIClevelsoftheessentialoil.Theureaseactivitycould bepreventedwhenallthesubinhibitoryconcentrationsweretestedincomparisontotheuntreated group(p<0.001).Alterationofthebacterialmorphologybesidesinhibitionofthecapsuleexpression wasverifiedbyatomicforcemicroscopy,andAnthony’sstainmethod,respectively.Ourdataallowusto concludethattheessentialoilofP.amboinicuscanbeagoodcandidateforfutureresearch.
© 2012 Elsevier GmbH. All rights reserved.
Introduction
Sincetheintroductionofantimicrobialagents,therehasbeen aselectivepressureofmicroorganisms, primarilycaused bythe indiscriminate useof antibiotics and chemotherapy,which has resultedinthedevelopmentofresistantspecies(Peres-Botaetal. 2003;Pittet2005).Oneofthemostseriouspublichealthproblems facedinthelastdecadeshasbeentheaggravationof antimicro-bialresistanceinbacterialpopulations,especially innosocomial
∗Correspondingauthorat:DepartamentodeAnálisesClínicaseToxicológicas, FaculdadedeFarmácia,OdontologiaeEnfermagem,UniversidadeFederalCeará, RuaCapitãoFranciscoPedro1210,CEP60430-370,Fortaleza,Ceará,Brazil. Tel.:+558533668270;fax:+558533668292.
E-mailaddress:thially@gmail.com(T.B.Gonc¸alves).
outbreaks(Oliveiraetal.2006).Animportantaspectwhich fre-quently results in antibioticresistance resides on the bacterial enzymeproduction,suchas,beta-lactamases.Thebeta-lactamases ofclinicalinterestareextendedspectrumbeta-lactamase(ESBL), carbapenemases (KPC),metalobetalactamase (MBL) and class C beta-lactamase(AmpC).K.pneumoniaeESBLiscapableof inacti-vatingthirdgenerationcephalosporinsandmonobactams,except carbapenemsandareinhibitedbybeta-lactamaseinhibitorssuch asclavulanate,sulbactamandtazobactam.TheenzymeKPCwas firstlydescribedintheU.S.in2001andwasfurtheridentifiedin othercountriesaroundtheworld.Thisenzymehastheabilityto hydrolyzecephalosporinsandaztreonam(PattersonandBonomo 2005;Bertrandetal.2006).
ManyplantsareusedinBrazilintheformofcrudeextracts, infusionsfortreatmentofvariousconditions,includingbacterial
0944-7113/$–seefrontmatter© 2012 Elsevier GmbH. All rights reserved.
19 (2012) 962–968 963
andfungalinfections(Gurgeletal.2009).Essentialoilshavelong beenused due totheir many therapeutic properties, including antiviral,antispasmodic,analgesic,antimicrobial,woundhealing, expectorant,soothing,antisepticairway,larvicide, antihelmintic and antiinflammatoryactivities(Costa et al.2010; Oyedeji and Afolayan2006).
ThegenusPlectranthusinvolvesabout300speciesoccurring nat-urallyinAfrica,AsiaandAustralia(Lukhobaetal.2006).Speciesof thisgenusexhibitbiosyntheticsesquiterpenes,diterpenesand phe-noliccompounds,somewithprovenrelevantbiologicalproperties (Lukhobaetal.2006).
Plectranthusamboinicus(Lour.)Spreng(Labiatae),alsoknownas
ColeusamboinicusLour.,Coleusaromaticus,Plectranthusaromaticus
(Benth)(Murthyetal.2009),andpopularlyknownas“malvarisco”, isanaromaticperennialherb cultivatedallover theNorth and NortheastregionsofBrazil(Matos2000).Itiswidelyusedin Brazil-ianfolkmedicinefortherapeuticpurposesagainstcolds,asthma, constipation,headache,cough,feveranddigestivediseases(Morais etal.2005).
Thispresent workproposedtoinvestigate theantimicrobial activityoftheessentialoilagainstK.pneumoniaestandardstrains andmultiresistantclinicalisolates,andalsotoevaluatethe abil-ityofsubinhibitoryandinhibitoryconcentrationsofP.amboinicus
essentialoilinalteringthemembranepermeability,thecapsule expressionandtheureaseactivityofK.pneumoniae,itsrateofkill anditscapabilityongrowingindifferentpHconditions.
Materialsandmethods
Plantmaterial
LeavesofPlectranthusamboinicus(Lour.)Sprengwerecollected fromtheHorto de Plantas MedicinaisProfesssor FranciscoJosé de AbreuMatos (Fortaleza, Ceará, Brazil). A voucher specimen (EAC40080)has been deposited at the Herbário Prisco Bezerra (EAC),UniversidadeFederaldoCeará,Brazil.
Extractionoftheessentialoil
ThefreshleavesofP.amboinicusweresubjectedto hydrodistilla-tioninaClevenger-typeapparatusfor2htoaffordpaleyellowoil. Theisolatedoil,afterdryingoveranhydroussodiumsulfateand filtration,wasstoredinsealedglassvialsandmaintainedunder refrigerationuntilfurtheranalysis.Theyield(w/w)wascalculated basedonthefreshweightoftheleaves.
Theessentialoilwasobtainedinthreedifferentperiodsand ana-lyzedbygaschromatography(GC)andGC/MSinordertocompare thechemicalconstituentsfromeachextraction.Theessentialoils werenamedS1,S2andS3.
Chemicalanalysisoftheessentialoil
Gas chromatography (GC) analysis was performed on a Shimadzu GC-17A gas chromatograph equipped with a flame
ionizationdetectorusinganon-polarDB-5fusedsilicacapillary column(30m×0.25mm×0.25mfilmthickness).Hydrogenwas usedascarriergasataflowrateof1ml/minand30psiinlet pres-sure;splitratio1:30.Thecolumntemperaturewasprogrammed from35◦Cto180◦Catarateof4◦C/min,thenheatedatarateof
17◦C/minto280◦Candheldisothermalfor10min;bothinjector
anddetectortemperatureswere250◦C.
TheGC/MSanalysiswascarriedoutonaHewlett-PackardModel 5971GC/MSinstrument,usinganon-polarDB-5fusedsilica capil-larycolumn(30m×0.25mm×0.1mfilmthickness);carriergas helium,flowrate1ml/minandwithsplitmode.Theinjectorand detectortemperatureswere250◦Cand200◦C,respectively.The
column temperature was programmed from 35◦C to 180◦C at
4◦C/minandthen180to250◦Cat10◦C/min.Massspectrawere
recordedfrom30to450m/z.
Individualcomponentswereidentifiedbymatchingtheir70eV massspectrawiththoseofthespectrometerdatabaseusingthe WileyL-builtlibraryandtwoothercomputerlibraries.Mass spec-trometry(MS)searchesusingretentionindicesasapre-selection routine,aswellasbyvisualcomparisonofthefragmentation pat-ternswiththosereportedin theliterature(Alencar etal.1990; Adams2007).
Microbialstrains
TheKlebsiellapneumoniae(ATCC700603)wasobtainedfrom the Instituto Nacional de Controle de Qualidade em Saúde (INCQS),InstitutoOswaldoCruz,RiodeJaneiro,Brazil.Fiveclinical isolates were obtained from the Hospital Universitário Wal-ter Cantídio(Fortaleza, Ceará,Brazil). Theclinical isolateswere analyzed by standard methods for identification of Enterobac-teriaceae (Koneman 2008). Susceptibility profile was tested by disc-diffusionagar(CLSI2009)withthefollowingdrugs:amikacin, ampicillin,ampicillin+sulbactam,cephalothin,cefoxitin,cefpime, caeftazidime, ceftriaxone, gentamicin, imipenem, meropenem, piperacillin,quinolones,andsulfazotrimticarcillin+clavulanicacid (Table1).
Determinationoftheminimalinhibitoryconcentrationand minimalbactericidalconcentration
To determinethe minimal inhibitoryconcentration we per-formed microdilution tests (CLSI 2009) in 96-well microplates (Costar,USA).Eachwellcontained100lMueller-HintonInfusion (MHI)broth,10lofinoculum(1.5×108CFU/ml)and100lofthe essentialoil,whichrangedfrom0.009%to1.25%(v/v)inDMSO3%. Theinoculumbacterialconcentrationwasdeterminedby adjust-ingitsturbidity(at625nm)to0.5McFarlandscale.Theplateswere coveredandincubatedfor24hat37◦C.Thelowestconcentrationof
thetestsampleinwhichnogrowthcouldbevisualizedwasdefined astheMIC.Weusedciprofloxacin(SigmaChemicalCo.,St.Louis, MO,USA), rangingfrom0.125to32g/ml,asthepositive con-trolanddimethylsulfoxide(Merck,Darmstadt,Germany),ranging from0.039to10%(v/v),asthenegativecontrol.Aftertheincubation
Table1
TableofpatternofdrugresistanceinK.pneumoniaeclinicalisolates:CE,clinicalspecimen;URO,uroculture;ATR,trachealaspirate;BLO,blood;NT,nottested;S,sensitive;R, resistant;I,intermediate;AMI,amikacin;AMP,ampicillin;ASB,ampicillin+sulbactam;ATM,aztreonam;CEP,cephalothin;FM,cefepime;CTX,cefotaxime;CFO,cefoxitin; CAZ,ceftazidime;CEN,ceftriaxone;CIP,ciprofloxacin;COL,colistin;ERT,ertapenem;GEN,gentamicin;IPM,imipenem;MER,meropenem;PPT,piperacillin+tazobactam; TIG,tigecycline.
Clinicalisolates AMI AMP/ASB/CEF ATM/ERT/IPM/MER COM/CFO/CAZ/PPT CTX CEN CIPRO COL GEN TIG
K2(uro) nt R R R R nt S S S S
K5(blood) S R R R R nt R S S R
K8(uro) nt R S R nt R S nt R R
K12(uro) I R R R R nt R S S S
964 19 (2012) 962–968
period,10lofsterile0.05%resazurin (7-hydroxy-3H-phenoxazin-3-one-10-oxide)(Sigma,USA)inaqueoussolutionwereaddedto theplates.After2hofincubation,thereadingsweredone.Acolor changecaused bythereduction ofresazurintoresorfurin indi-catedpresence(pinkcolor)orabsence(bluecolor)ofcellviability (Palominoetal.2002).Theminimalbactericidalconcentrationwas determinedfortheessentialoilbysubculturing5lfromeachtube (MIC,2and4×MIC)ontoBHIagar.TheMBCwasdefinedasthe lowestconcentrationoftheessentialoilthatcouldinhibit99%of thebacterialgrowth.Thetestswererunintriplicatewiththethree samples(S1–S3)oftheessentialoil.
pHsensitivityassay
TheeffectofpHonantibacterialactivityofP.amboinicus essen-tialoil (S1) and ciprofloxacin against K. pneumoniae was done accordingtotheproceduredescribedbyDevietal.(2010).Cultures ofthestandardstrainweregrownonMuller-Hintonbroth previ-ouslypreparedandcalibratedtodifferentpHranges(from5.5to 9.0).TheMICwasdetermined.
Determinationofrateofkill
Onemilliliterofthestandardstrainwasadjustedtothe concen-trationof1.5×108CFU/mlandincubatedat35◦Cfor2h,beforeits
additiontotubescontaining0.16%(whichrepresentedtwotimes theMIC).Controltubeswerealsopreparedwithouttheessential oil(S1).ThetubesTestandControlwereincubatedat37◦Cand
aliquotsof10lwerecollectedatintervalsof0,2,4,6,8and24h aftertheadditionoftheplantsamples.Serialdilutionswereplated ontoMacConkeyagartodeterminethenumberofviablecellswhich wasexpressedaslogofcolonyformingunitsperml(logCFU/mL). Thebactericidal(≥3-log-unitreductioninlog10CFU/ml)and bac-teriostaticactivities(<3-log-unitreductioninlog10CFU/ml)were determined(Devietal.2010).
Crystalvioletassay
Thealterationinmembranepermeabilitywasdetectedby crys-talvioletassayaccordingtotheproceduredescribedinliterature (Devietal.2010).Briefly,suspensionsofthebacteriawere pre-paredinBrainHeartInfusion(BHI)broth.Cellswereharvestedat 4500×gfor5minat4◦C.Thecellswerewashedtwiceand
resus-pendedinPBSat pH7.4.Theessentialoil(S1)of P.amboinicus
(testedatMICandtwicetheMIC),theantibiotics(testedatMIC), besidesethylenediaminetetraaceticacidorEDTA(positivecontrol) andphosphatebufferedsalineorPBS(negativecontrol)wereadded tothecellsuspensionsandincubatedfor30minat37◦C(alltests
weredoneinquintuplicate).Thecellswereharvestedat9300×g
for5min.Afterthisprocedure,thecellswereresuspendedinPBS containing0.010mgofcrystalviolet.Thecellsuspensionwasthen incubatedfor10minat37◦Candcentrifugedat13400×gfor2h
andtheOD590ofthesupernatantwasmeasured.
Effectofsub-MICandMIClevelsofP.amboinicusessentialoilon ureaseactivity
Bacterial urease activity was determined by the procedure describedbyDerakhshanetal.(2008).FiveclinicalisolatesofK. pneumoniaeandtheATCCstandardweregrownovernightinurea brothcontainingsub-MICandMIClevelsoftheessentialoil(S1) (1/8to1/1×MIC).Untreatedcellswereusedasnegativecontrol.All tubeswereincubatedfor24hat37◦C.Next,thesuspensionswere
centrifugedat5000×gfor3minandthesupernatantswere recov-ered.Color intensitywasmeasuredat560nmusingauniversal
microplatereader(Biotek,China).Thesampleswererunin tripli-cate.
Effectofsub-MICandMIClevelsofP.amboinicusessentialoilon bacterialcapsuleexpression
TheK.pneumoniaecapsulewasstainedbytheAnthony’scapsule stain,which procedurewasdescribed inliterature(Derakhshan etal.2008).Briefly,fiveclinicalisolatesofthebacteriaandtheATCC standardstrainweregrowninmilkbroth(9.5gpowdermilk/l)for 24hinthepresence ofdifferentconcentrationsoftheessential oil(S1)(1/8to1/1×MIC).Smearswerepreparedonglassslides andallowedtoairdry.Thereafter,theslideswerecoveredwith1% crystalvioletfor2minandrinsedgentlywitha20%copper sul-fatesolution.Thebacterialcells(nottreatedcontrol)wouldappear purplishwhilethecapsuleswouldappeartransparentunderanoil immersionlensatopticalmicroscopy.
Atomicforcemicroscopy
Changesin themorphology of K.pneumoniaecaused by the essential oil of P. amboinicus were analyzed by atomic force microscopy(AFM)(BragaandRicci1998).Suspensionsofcultures wereincubatedinthepresenceofdifferentconcentrationsofthe essential oil (S1) (1/8 to1/1×MIC)for 18hat 37◦C. The cells
werewashedandcentrifugedat4500×gfor5minat4◦C,washed
twiceandresuspendedinPBS.Avolumeof10lofthe suspen-sionwasplacedonaglassslideanddriedinairfor15min.Then thesamplewasexaminedbya MultimodeAtomicForce Micro-scope NanoscopeIII-a (Digital Instruments, SantaBarbara, CA). Scanswereperformedinairandamplitudeimageswereacquired byintermittentcontactmodeusingcrystallinesiliconcantilevers (Veeco-probes)withaspringconstantofapproximately40N/m, resonancefrequencyof242.38kHz andtipradiusof15nm.The amplitudeimagescanbeusedtobetterevidencecellbordersand theirshape.CulturesofnottreatedK.pneumoniaeweretakenas controls.
Statisticalanalysis
TheeffectofvariousconcentrationsofP.amboinicusessentialoil ontheK.pneumoniaeureaseactivityandontheuptakeofcrystal violetbythebacteriawereanalyzedbyone-wayanalysisof vari-ance(ANOVA)andTukey–Kramermultiplecomparisonstests.The statisticalsignificancewasconsideredatp-valueof0.05.
Results
Chemicalanalysisoftheessentialoilanddeterminationofthe minimalinhibitoryconcentrationandminimalbactericidal concentration
ThreedifferentsamplesofessentialoilfromtheleavesofP. amboinicuswereanalyzedbyGCandCG/MS.Table2showsthe chemicalcompositionofthethreesamplesoftheessentialoil. Car-vacrol(90–98%)isthemajorconstituentidentifiedinallsamples. Thesamplesofessentialoilobtainedindifferentperiodshavefew differencesintheirchemicalconstitutions.
Alltestedstrains(clinicalandthestandard)weresusceptibleto theessentialoil(MICmean±standarderror,thatis,0.09±0.01%,
19 (2012) 962–968 965
Table2
ChemicalcompositionofessentialoilfromleavesofP.amboinicus.
Compound RI RT S1 S2 S3
4-Terpineol 1177 16.80 1.34 – –
Carvacrol 1298 21.39 90.55 95.39 98.03
-Caryophyllene 1417 25.46 3.09 – 0.01
␣-Bergamotene 1433 25.97 1.78 – –
Caryophylleneoxide 1583 30.97 1.36 – 0.20
p-Cimene 1024 8.28 – – 1.65
␥-Terpinene 1059 9.55 – – 0.01
␣-trans-Bergamotene 1434 25.79 – – 0.02
␣-Caryophyllene 1454 25.49 – – 0.02
Totalidentified – – 98.12 95.39 99.94
RI:relativeretentionindex;RT:retentiontime;S:sampleofessentialoilofP.amboinicus.
Table3
EffectofpHonantibacterialactivityofP.amboinicusessentialoilagainstKlebsiella pneumoniae.
pH Essentialoil Cipro(g/ml)
% g/ml
5.5 0.08 700.00 4.0
6.0 0.08 700.00 4.0
6.5 0.08 700.00 4.0
7.0 0.08 700.00 2.0
7.5 0.04 350.00 2.0
8.0 0.04 350.00 1.0
8.5 0.04 350.00 1.0
9.0 0.04 350.00 1.0
Determinationofratekill
TreatmentofK.pneumoniaewith0.16%ofessentialoil,which representedtwotimestheMIC,ofessentialoilwasenoughtokill allthebacteriaafter2hofincubationcomparedtocontrolandto ciprofloxacin.
pHsensitivityassay
WithrespecttothepHsensitivityassay,abetterMICwasfound when neutral or alkaline pHbrothwas used (Table 3).Similar resultswerefoundwhenciprofloxacinwastested.
Crystalvioletassay
Theuptakeof crystalviolet wasconsideredtobesignificant whentwicetheMICofessentialoilwasused(Table4),compared tothenottreatedgroup(p<0.01)ortociprofloxacin(p<0.01)and toEDTA(p<0.05).EDTAisachelatingagentwhichincreasesthe membranepermeabilityofthebacterialcells.
Effectofsub-MICandMIClevelsofP.amboinicusessentialoilon ureaseactivity
Alltheconcentrationsoftheessentialoiltestedcould signifi-cantlyreducetheureaseactivity,comparedtothenottreatedgroup (p<0.001),asshowninFig.1.ThedashedlineinFig.1isthebest exponentialfitofthedata.
Effectofsub-MIClevelsofP.amboinicusessentialoilonbacterial capsule
AsshowninFig.2,smallercolonieswithreducedexpression ofcapsulescouldbefoundwhenthebacteriaweretreatedwith 1/8×MICoftheessentialoilcomparedtothenottreatedcontrols (Fig.2aandb).However,whenthebacteriaweretreatedwith1/4
Fig.1. Effects ofsub-inhibitory(1/8 to 1/2)andinhibitory concentrationsof P.amboinicus essential oil onKlebsiella pneumoniae urease activity. Itshows the absorbance averaged over five samples vs the MIC. The dashed line is the best exponential decay fitting of the experimental data {optical den-sity=0.552+0.423exp(−MIC/0.251)}.Thisexpressionindicatesthatforsufficient highvaluesofMICtheabsorbancepresentsasaturationvalue,near0.552.
orhighersubinhibitoryconcentrationsoftheessentialoilalmost nobacteriacouldbefound,onlycellulardebris(Fig.2c–e).
Atomicforcemicroscopy
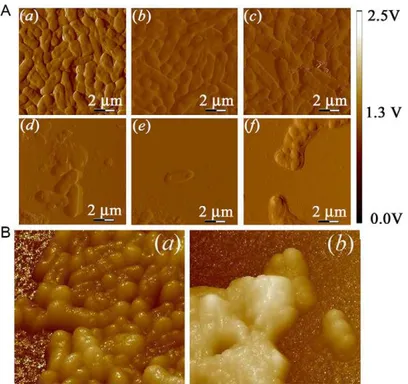
Accordingtotheatomicforcemicroscopy(Fig.3AandB),the morphologyofuntreatedK.pneumoniaewassmoothandregular withanintactcellmembraneandindividualbacteriawerereadily distinguishable.Fig.3Ashowsafter24hoftreatmentwith1/8and 1/4×MICessentialoil(Fig.3AbandAc),itwasobservedan increas-ing loss of the membrane integrity with a consequent altered morphologyofthebacteria.Finally,aseverecelldamagewas veri-fiedwhen1/2×MICandtheinhibitoryconcentrations(1×MICand 2×MIC)wereused(Fig.3Ad,AeandAf).Fig.3Bshowsthethree dimensionalaspectofthenottreatedbacteriacomparedtothose treatedwithtwicetheMICoftheP.amboinicusessentialoil.
Discussion
966 19 (2012) 962–968
Table4
CrystalvioletuptakebyKlebsiellapneumoniaepreviouslyincubatedwithPlectranthusamboinicusessentialoil.TheresultswereexpressedbymeanofOD590values±standard error.Thesamplesweretestedinquintuplicate.
Groups PBS EDTA CIPRO Essentialoil
MIC 2×MIC
xAbs±EM 0.412–0.013 0.383–0.019 0.412–0.037 0.315–0.029 0.254–0.016 CI95 0.371–0.454 0.329–0.437 0.308–0.517 0.234–0.396 0.209–0.299
p N.S. N.S. N.S. N.S. <0.01(vsPBSandCIPRO)<0.05(vsEDTA)
Fig.2.Effectsofsub-inhibitory(1/8to1/2),inhibitoryandtwicetheinhibitoryconcentrationsofP.amboinicusessentialoilonKlebsiellapneumoniaecapsuleexpression.The bacteriawerestainedwiththeAnthonycapsularstainingmethod.Thecolonymorphologyandcapsulevisualizationwasdoneunderlightmicroscopy(magnification1000× with3×opticalzoom).
Carvacrolisamonoterpenephenolwhichisknowntobeeither bactericidal or bacteriostatic agent, depending on the concen-trationused(Nostroetal.2004).Previousstudiesreportedthat carvacrolhadthepropertyofdestroyingthecellularmembraneof
Escherichiacoli,Salmonellatyphimurium,Shigellaflexneri(Dorman andDeans2000).
P.amboinicusessentialoilpresentsbactericidaleffectwhena concentrationoftwicetheMICisaddedtoK.pneumoniae suspen-sion.After2hofincubation,thereisacompletelossofbacterial viability.The datashown is relevantby thefact K.pneumoniae
isoneofthemainpathogensassociatedwithnosocomial infec-tions;also,asthepHsensitivitytestshowed,theessentialoilwas
19 (2012) 962–968 967
moreactivein higherpHranges.These dataarevaluable, once
K.pneumoniaecangrowinthesmallintestine,wherepancreatic juiceandbilemaketheenvironmentpHhighlyalkaline(Koneman 2008).
Klebsiellapneumoniaeisanonmotileentericorganismthat fer-mentslactose,decarboxylateslysine,hydrolyzesurea,andforms largemucoidcolonies,duetothepresenceofcapsule(Koneman 2008).ThecapsuleofK.pneumoniaeconsistsofthickanddense bundles of fine fibers, which contributes to its high virulence capacity.
Generally, subinhibitory concentrations of an essential oil, althoughnotabletokillbacteria,canmodifythearchitectureof theiroutermostsurface and may interferewith somebacterial functions(Derakhshanetal.2008).In ourstudy,whenK. pneu-moniaewastested in thepresence of1/8 MICof P.amboinicus
essentialoil,analterationofitsmorphologybesidesreductionof capsuleexpressionwasfound.Whenhighersubinhibitory concen-trations(1/4and1/2)oftheessentialoilweretested,almostno bacteriacouldbevisualized,but onlycellulardebris. Thiscould meanthatsubinhibitoryconcentrationof1/8×MICcouldreduce thebacterialcapacity ofcapsule expression.Besidesthis obser-vation,alterationinmembranepermeabilitywasdetectedbythe increaseofcrystalvioletuptakewhenthebacteriawereincubated withtwicetheMIClevelsofP.amboinicusessentialoil.Lossof mem-braneintegrityandleakageofintracellularcomponentscouldbe demonstratedbytheatomicforcemicroscopy,when1/2×MIC lev-elsoftheessentialoilwasused,andacompletedestructionofthe cellsattheinhibitoryconcentrations(MICand2×MIC)oftheplant sample.
Manymicroorganismsneedtouseureaseinordertoobtain nitrogenfortheirgrowth(Maroncleetal.2006).Ureasecatalyzes thehydrolysisofureatoammoniaandcarbamateandhasbeen foundto beimplicated in kidneyand bladder stone formation and to the pathogenesis of several diseases. Urease inhibitors are potential alternatives for the prevention of K. pneumoniae
fromcolonizingthegastrointestinaltract,whereisplentyofurea (Koneman2008;Hostacká2000;Maroncleetal.2006).Imipenem andofloxacin,donotaffecttheenzymeactivity(Hostacká2000). Our data showed a strong inhibition of urease activity by the
P.amboinicusessentialoilinallconcentrationstestedcompared to the not treated bacteria (p<0.001). Although 1/8 MIC was only able to reduce 15.7% of the urease activity, 1/4×MIC to 1 MIC reduce from 30.5% to 42%, respectively. These percent-ages of the enzyme inhibition were superior to -lactam and aminoglycoside actions (20% of inhibition), and equivalent or superior toacetohydroxamic acid(30% of inhibition), which is frequentlyusedtoinhibitureaseactivity(Maroncleetal.2006; Raufetal.2011).Ontheotherhand,ithasbeenrecentlyshown novelbarbituricandthiobarbituricacid-derivedsulfonamideswith ability to inhibit more than 88% of the urease activity when the drugs were tested at 500M concentrations (Rauf et al. 2011).
Conclusion
The results of our data clearly demonstrated that essential oilofPlectranthus amboinicuspossessesapotentialantibacterial activityagainst Klebsiella pneumoniae. Subinhibitory concentra-tions of the essential oil could affect the capsule expression, alterationsinmembraneintegrity,inhibitionoftheurease activ-ity.Atbactericidalconcentrations,acompletedestructionofthe bacteriawasfound.Ourdataallowustoconcludethatthe essen-tial oil of P. amboinicus could be a good candidate for future research.
Conflictofinterest
Noconflicttodisclose.
Acknowledgements
ThisresearchwasfinanciallysupportedbytheCNPq(Processes 579437/2008-6and554970/2010-4)andFUNCAP/PPSUS(Process 09100057-2).
References
Adams,R.P.,2007.IdentificationofEssentialOilComponentsbyGas Chromatogra-phy/MassSpectrometry.AlluredPublishingCorporation,CarolStream,IL. Alencar,J.W.,Craveiro,A.A.,Matos,F.J.A.,Machado,M.I.L.,1990.Kovatsindices
sim-ulationinessentialoilsanalysis.QuimicaNova13,282–284.
Bertrand,S.,Weill,F.-X.,Cloeckaert,A.,Vrints,M.,Mairiaux,E.,Praud,K., Dier-ick,K.,Wildemauve,C.,Godard,C.,Butaye,P.,Imberechts,H.,Grimont,P.A.D., Collard,J.-M.,2006.Clonalemergenceofextended-spectrumbeta-lactamase (CTX-M-2)-producingSalmonellaentericaserovarVirchowisolateswithreduced susceptibilitiestociprofloxacinamongpoultryandhumansinBelgiumand France(2000to2003).JournalofClinicalMicrobiology44,2897–2903. Braga,P.C.,Ricci,D.,1998.Atomicforcemicroscopy:applicationtoinvestigationof
Escherichiacolimorphologybeforeandafterexposuretocefodizime. Antimicro-bialAgentsChemotherapy42,18–22.
Castillo,R.A.M.,González,V.P.,1999.Plectranthusamboinicus(Lour.)Spreng.Revista CubanadePlantasMedicinales4,110–115.
CLSI,2009.PerformanceStandardsforAntimicrobialSusceptibilityTesting;18th InformationalSupplement.CLSIM07-A8.ClinicalandLaboratoryStandards Institute,Wayne,PA,pp.15–18.
Costa,J.G.,Campos,A.R.,Brito,S.A.,Pereira,C.K.B.,Souza,E.O.,Rodrigues,F.F.G.,2010. BiologicalscreeningofararipebasinmedicinalplantsusingArtemiasalinaLeach andpathogenicbacteria.PharmacognosyMagazine6(24),331–334. Derakhshan,S.,Sattari,M.,Bigdeli,M.,2008.Effectofsubinhibitoryconcentrations
ofcumin(CuminumcyminumL.)seedessentialoilandalcoholicextractonthe morphology,capsuleexpressionandureaseactivityofKlebsiellapneumoniae. InternationalJournalofAntimicrobialAgents32,432–436.
Devi,K.P.,Nisha,S.A.,Sakthivel,R.,Pandain,S.K.,2010.Eugenol(anessentialoil ofclove)actsasantibacterialagentagainstSalmonellatyphibydisruptingthe cellularmembrane.JournalofEthnopharmacology130,107–115.
Dorman,H.J.D.,Deans,S.G.,2000.Antimicrobialagentsfromplants:antibacterial activityofplantvolatileoils.JournalofAppliedMicrobiology88,308–316. Gurgel,A.,Silva,J.G.,Grangeiro,A.R.S.,Xavier,H.S.,Oliveira,R.A.G.,Pereira,M.S.V.,
Souza,I.A.,2009.AntibacterialeffectsofPlectranthusamboinicus(Lour.)Spreng (Lamiaceae)inmethicillinresistantStaphylococcusaureus(MRSA).Latin Amer-icanJournalofPharmacy28(3),460–464.
Hostacká,A.,2000.Theinvitroeffectofimipenemandofloxacinonenzymicactivity ofKlebsiellastrains.FoliaMicrobiologica45,387–390.
Koneman,E.W.,2008.DiagnósticoMicrobiológico–TextoeAtlasColorido. Guan-abaraKoogan,RiodeJaneiro,pp.209–290.
Lukhoba,C.W.,Simmonds,M.S.J.,Paton,A.J.,2006.Plectranthus:areviewof ethnob-otanicaluses.JournalofEthnopharmacology103,1–24.
Maroncle,N.,Rich,C.,Forestier,C.,2006.TheroleofKlebsiellapneumoniaeurease inintestinalcolonizationandresistancetogastrointestinalstress.Revistade Microbiologia157,184–193.
Matos,F.J.A.,2000.Plantasmedicinais:guiadeselec¸ãoeempregodeplantasusadas emfitoterapianoNordestedoBrasil,vol.2,pp.36–42.
Morais,S.M.,Dantas,J.D.P.,Silva,A.R.A.,Magalhães,E.F.,2005.Plantasmedicinais usadaspelosíndiosTapebasdoCeará.RevistaBrasileiradeFarmacognosia15, 169–177.
Murthy,P.S.,Ramalakshmi,K.,Srinivas,P.,2009.FungitoxicactivityofIndianborage (Plectranthusamboinicus)volatiles.FoodChemotherapy114,1014–1018. Nostro,A.,Blanco,A.R.,Cannatelli,M.A.,Enea,V.,Flamini,G.,Morelli,I.,Roccaro,S.,
Alonzo,V.,2004.Susceptibilityofmethicillin-resistantstaphylococcitooregano essentialoil,carvacrolandthymol.FEMSMicrobiologyLetters230,191–195. Oliveira,J.F.P.,Cipullo,J.P.,Burdmann,E.A.,2006.Nefrotoxicidadedos
aminogli-cosídeos.RevistaBrasileiradeCirurgiaCardiovascular21(4),444–452. Oyedeji,A.O.,Afolayan,A.J.,2006.Chemicalcompositionandantibacterialactivity
oftheessentialoilisolatedfromSouthAfricanMenthalongifolia(L.)L.subsp. capensis(Thunb.)Briq.TheJournalofEssentialOilResearch18,57–59. Palomino,J.C.,Martin,A.,Camacho,M.,Guerra,H.,Swing,J.,Portaels,F.,2002.
Resazurinmicrotiterassayplate:simpleandinexpensivemethodfor detec-tionofdrugresistanceinMycobacteriumtuberculosis.AntimicrobialAgentsand Chemotherapy46(8),2720–2722.
Patterson,D.L.,Bonomo,R.A.,2005.Extended-spectrumbeta-lactamases:aclinical update.ClinicalMicrobiology18,657–686.
Peres-Bota,D.,Rodriguez,H.,Dimopoulos,G.,2003.Areinfectionsduetoresistant pathogensassociatedwithaworseoutcomeincriticallyillpatients?Journalof Infection47,307–316.
968 19 (2012) 962–968
Pittet,D.,2005.Infectioncontrolandqualityhealthcareinthenewmillennium. AmericanJournalofInfectionControl33,258–267.
Rauf, A., Ahmed, F., Qureshi, A.M., Aziz-ur-Rehman, Khan, A., Qadir, M.I., Choudhary, M.I., Chohan, Z.H., Youssoufid, Z.M.H., Haddad, T.B., 2011. Synthesis and urease inhibition studies of barbituric and thiobarbituric acidderived sulphonamides. Journal ofthe Chinese Chemical Society58, 528–533.
Singh,G.,Singh,O.P.,Prasad,Y.R.,Lamposona,M.P.,Catalan,C.,2002.Studieson essentialoils.Part33.Chemicalandinsecticidalinvestigationsonleafoilof ColeusamboinicusLour.FlavourandFragranceJournal17,440–442.