4 7 0 4 7 04 7 0 4 7 0
4 7 0 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors: determinants
associated with seropositivity in a low risk
population
HTLV-I/II e doadores de sangue: determinantes
associados à soropositividade em população
de baixo risco
Bernadette Catalan Soaresa, Anna Bárbara de F Carneiro Proiettia, Fernando Augusto
Proiettib and GIPH (Interdisciplinary HTLV-I/II Research Group)*
aFundação Hemominas. Belo Horizonte, MG, Brasil. bDepartamento de Medicina Preventiva e
Social da Faculdade de Medicina da Universidade Federal de Minas Gerais. Belo Horizonte, Minas Gerais, Brasil
Correspondence to:
Fernando Augusto Proietti Faculdade de Medicina - UFMG
Avenida Prof Alfredo Balena, 190 10º Andar 30130-100 Belo Horizonte, MG, Brasil E-mail: proietti@medicina.ufmg.br
*The name of the investigators of the GIPH Group is listed in the end of the article.
Partially supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig - Process n. 408/01). Received on 11/7/2002. Approved on 9/4/2003.
Keywords
HTLV-I infections, blood. HTLV-II infections, blood. Blood donors. Risk factors. Prevalence. Serologic tests. Enzyme-linked immunosorbent assay. Blot. Western.
Descritores
Infecções por HTLV-I, sangue. Infecções por HTLV-II, sangue. Doadores de sangue. Fatores de risco. Prevalência. Testes sorológicos. ELISA.
Abstract
Objective
Blood donors in Brazil have been routinely screened for HTLV-I/II since 1993. A study was performedto estimate the prevalence of HTLV-I/II infection in a low risk population and to better understand determinants associated with seropositivity.
Methods
HTLV-I/II seropositive (n=135), indeterminate (n=167) and seronegative blood donors (n=116) were enrolled in an open prevalence prospective cohort study. A cross-sectional epidemiological study of positive, indeterminate and seronegative HTLV-I/II subjects was conducted to assess behavioral and environmental risk factors for seropositivity. HTLV-I/II serological status was confirmed using enzyme-linked immunosorbent assay (EIA) and Western blot (WB).
Results
The three groups were not homogeneous. HTLV-I/II seropositivity was associated to past blood transfusion and years of schooling, a marker of socioeconomic status, and use of non-intravenous illegal drugs.
Conclusions
The study results reinforce the importance of continuous monitoring and improvement of blood donor selection process.
Resumo
Objetivo
Doadores de sangue no Brasil têm sido avaliados sorologicamente para o HTLV-I/ II desde 1993. Assim, realizou-se estudo para estimar a prevalência dessa infecção em população de baixo risco e para melhor compreender os determinantes associados à soropositividade.
Métodos
4 7 1 4 7 1 4 7 1 4 7 1 4 7 1 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors Catalan-Soares B et al
INTRODUCTION
Human lymphotropic viruses types I and II (HTLV I/II) were the first reported human retroviruses, hav-ing been isolated in the beginnhav-ing of the 80’s (Poiez et al 13). In regions considered endemic (Caribbean,
Africa and Southwest Japan), HTLV-I antibody preva-lence range from 3% to 15%, it is higher in older individuals and in females (Blattner et al;1 Chavance
et al;3 Tokudome et al15). Although it shares 65% of
its genome with type I, HTLV-II is much less preva-lent, predominating in native populations of the American continent and among injecting drug users in the United States and Europe (Hall et al5). The
vi-rus is transmitted vertically, especially through breastfeeding (Hirata et al6), sexually (Kajiyama et
al8), and with the exposure to infected blood, mostly
through blood transfusions or syringes and needles sharing among intravenous drug users (Sullivan et al,14 Okochi et al12).
HTLV-I/II serological screening has become man-datory in Brazilian blood banks since November 1993. Among the 405,437 volunteers donating blood at Hemominas Blood Center, between January 1994 to January 1999, 3,203 (0.79%) were EIA positive. For those 405,437 blood donors, 244 (0.06%) were confirmed as positive in the Western Blot test and 1,454 (0.36%) were considered indeterminate.
An open prevalence prospective cohort study (The Interdisciplinary HTLV-I/II Research Group [GIPH] Cohort Study) is being conducted at the Hemominas Blood Center since March 1997. In an attempt to better understand the epidemiological aspects of the virus seroprevalence, and to define the infection’s determinants in this population, as well as to subsi-dize the screening criteria in local blood banks, a-case-control study with cross-sectional features of the social-economic, demographic and behavior vari-ables at baseline of the GIPH Cohort Study partici-pants was conducted.
de risco comportamentais e ambientais para soropositividade. O status sorológico foi definido usando a reação de EIA (enzyme linked immunosorbent assay) e o teste Western blot (WB).
Resultados
Os três grupos apresentaram heterogeneidade entre si. A soropositividade mostrou-se associada à história pregressa de transfusão de sangue, em nível educacional, como um marcador de condição socioeconômica e ao uso de drogas ilegais não endovenosas.
Conclusões
Os resultados confirmam a importância de um monitoramento e refinamento do processo de seleção dos doadores de sangue.
METHODS
Hemominas Institute is located in the metropoli-tan area of Belo Horizonte , state of Minas Gerais, Southeast Brazil. Belo Horizonte has about 4,000,000 inhabitants and it is the third largest city in Brazil. Hemominas Institute, the blood bank of the state of Minas Gerais, is the largest blood bank in the city. In 2000 an average of 7,000 individuals/ month donated blood at Hemominas and 15,000 blood transfusions/month were made. After a rou-tine pre-donation screening questionnaire and clini-cal examination, individuals who are considered eligible for blood donation - aged between 18 and 60 years, good general health, not being exposed to retrovirus risk factors (for example, use of illegal injecting drugs, unsafe sexual behavior, tattoos), and not having received blood or blood products trans-fusions in the last 10 years - are tested for blood transmitted infections, namely HIV-1/2, HBV, HCV, T. cruzi, T. pallidum and HTLV-I/II. Blood donors in Brazil are all volunteers because any reimbursement for blood donation is prohibited by law.
For HTLV-I/II, an eligible donor is considered posi-tive when their enzymatic serological testing (ELISA) is reactive, and the confirmatory testing, Western Blot (WB), also shows positive results ac-cording to the manufacturer’s instructions: there is a band at p24 and gp46 or p21env (1998); reactivity to GAG (p19 and p24) and ENV (GD21) - (1998 to 2000). A reactive EIA and the presence of any band pattern different from manufacturer’s criteria for positivity is classified as indeterminate. Individu-als whose EIA test was not reactive are considered seronegative; also, those whose EIA test was not reactive and there were no bands in the Western Blot are considered seronegative.
4 7 2 4 7 24 7 2 4 7 2
4 7 2 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors Catalan-Soares B et al
start of the GIPH Cohort Study were eligible to the study. A sample of seronegative donors (n=348) selected using a systematic random sampling, who donated blood during the same period of time in the same network, was included in the study. Subjects with abnormal serology, as well as the seronegative group, were contacted by mail and invited to an initial interview. After informed and counseled about their serological status, the study objective and proce-dures, an informed consent was obtained from those willing to participate. Data collected during the baseline visit of study participants’ were analyzed and results are presented here.
All subjects answered the same questionnaire to the same interviewer in a private environment. The interviewer, who is one of the authors (BCS), was aware of participants’ serological results. The inter-views were conducted from March 1997 to June 1999.
The questionnaire was designed to elicit data on (a) demographics such as education level, personal monthly income, and occupation; (b) HTLV-I/II trans-mission: breastfeeding, blood transfusion at any time in the past, intravenous injection of illegal drugs, syringe and needle sharing, accidents involving an-other person’s blood, tattoos, and acupuncture treat-ment. Questions about use of any recreational illegal drug and; (c) sexual history: number of partners, paid sex and male homosexual contacts were also asked. For women, information about reproductive history was also obtained.
To guide the interpretation, crude and stratified
prevalence rates were calculated along with preva-lence odds ratio and 95% confidence interval. Since all the variables were categorical, the answers were compared within the groups (positive, indetermi-nate and negative) using cross tabulation. The dif-ferences were tested for significance using the X² test or, when indicated, Fisher ’s exact test. Multinomial logistical regression models were also fitted to the data to better compare the three groups simultaneously (Hosmer & Lemeshow7). The
vari-ables shown to be statistically significant in the bivariate analysis were added to the logistical model (forward selection), and maintained in the final model if p<0.05 or if presenting biological plausibility to justify their remaining in the model, despite the p-value. Data analysis was conducted using Stata 6.0.
The Hemominas Institutional Board approved the study procedures.
RESULTS
During the study period, of all seropositive sub-jects (n=180) invited to participate in the GIPH Co-hort Study, 75.0% (n=135) signed a consent as com-pared to about 33.0% of the indeterminate (n=506) and negative (n=348) groups. No significant differ-ence in behavior and demographic variables distri-bution was found when comparing responding and non-responding subjects as a group or according to serologic results (data not shown).
The average subject age was 33 years, and 66.8%
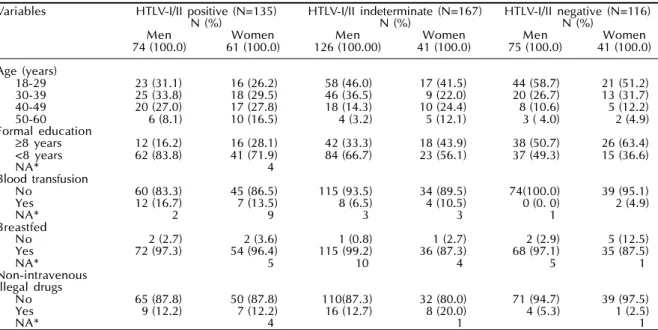
Table 1 - Frequency distribution of HTLV-I/II serostatus according to demographic and behavior characteristics at baseline. The GIPH Cohort Study. Belo Horizonte, Brazil, 1997-1999.
Variables HTLV-I/II positive (N=135) HTLV-I/II indeterminate (N=167) HTLV-I/II negative (N=116)
N (%) N (%) N (%)
Men Women Men Women Men Women
74 (100.0) 61 (100.0) 126 (100.00) 41 (100.0) 75 (100.0) 41 (100.0)
Age (years)
18-29 23 (31.1) 16 (26.2) 58 (46.0) 17 (41.5) 44 (58.7) 21 (51.2)
30-39 25 (33.8) 18 (29.5) 46 (36.5) 9 (22.0) 20 (26.7) 13 (31.7)
40-49 20 (27.0) 17 (27.8) 18 (14.3) 10 (24.4) 8 (10.6) 5 (12.2)
50-60 6 (8.1) 10 (16.5) 4 (3.2) 5 (12.1) 3 ( 4.0) 2 (4.9)
Formal education
≥8 years 12 (16.2) 16 (28.1) 42 (33.3) 18 (43.9) 38 (50.7) 26 (63.4)
<8 years 62 (83.8) 41 (71.9) 84 (66.7) 23 (56.1) 37 (49.3) 15 (36.6)
NA* 4
Blood transfusion
No 60 (83.3) 45 (86.5) 115 (93.5) 34 (89.5) 74(100.0) 39 (95.1)
Yes 12 (16.7) 7 (13.5) 8 (6.5) 4 (10.5) 0 (0. 0) 2 (4.9)
NA* 2 9 3 3 1
Breastfed
No 2 (2.7) 2 (3.6) 1 (0.8) 1 (2.7) 2 (2.9) 5 (12.5)
Yes 72 (97.3) 54 (96.4) 115 (99.2) 36 (87.3) 68 (97.1) 35 (87.5)
NA* 5 10 4 5 1
Non-intravenous Illegal drugs
No 65 (87.8) 50 (87.8) 110(87.3) 32 (80.0) 71 (94.7) 39 (97.5)
Yes 9 (12.2) 7 (12.2) 16 (12.7) 8 (20.0) 4 (5.3) 1 (2.5)
NA* 4 1 1
4 7 3 4 7 3 4 7 3 4 7 3 4 7 3 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors Catalan-Soares B et al
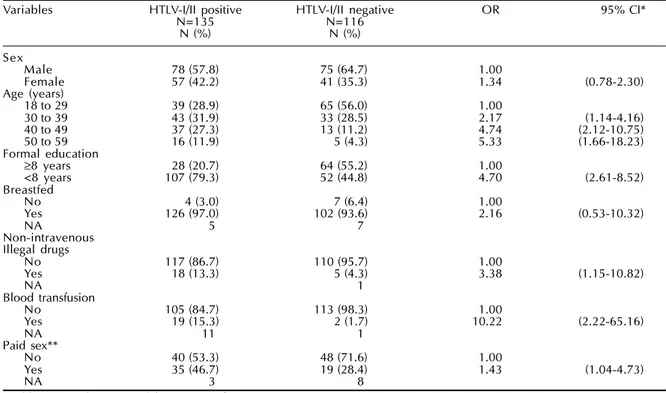
Table 2 - Bivariate analysis for HTLV-I/II serologic status according to selected determinants at baseline: seropositive versus seronegative groups. The GIPH Cohort Study. Belo Horizonte, Brazil, 1997-1999.
Variables HTLV-I/II positive HTLV-I/II negative OR 95% CI*
N=135 N=116
N (%) N (%)
Sex
Male 78 (57.8) 75 (64.7) 1.00
Female 57 (42.2) 41 (35.3) 1.34 (0.78-2.30)
Age (years)
18 to 29 39 (28.9) 65 (56.0) 1.00
30 to 39 43 (31.9) 33 (28.5) 2.17 (1.14-4.16)
40 to 49 37 (27.3) 13 (11.2) 4.74 (2.12-10.75)
50 to 59 16 (11.9) 5 (4.3) 5.33 (1.66-18.23)
Formal education
≥8 years 28 (20.7) 64 (55.2) 1.00
<8 years 107 (79.3) 52 (44.8) 4.70 (2.61-8.52)
Breastfed
No 4 (3.0) 7 (6.4) 1.00
Yes 126 (97.0) 102 (93.6) 2.16 (0.53-10.32)
NA 5 7
Non-intravenous Illegal drugs
No 117 (86.7) 110 (95.7) 1.00
Yes 18 (13.3) 5 (4.3) 3.38 (1.15-10.82)
NA 1
Blood transfusion
No 105 (84.7) 113 (98.3) 1.00
Yes 19 (15.3) 2 (1.7) 10.22 (2.22-65.16)
NA 11 1
Paid sex**
No 40 (53.3) 48 (71.6) 1.00
Yes 35 (46.7) 19 (28.4) 1.43 (1.04-4.73)
NA 3 8
*Odds ratio and 95% Confidence Interval. **For males only.
were males. HTLV-I/II prevalence was higher in women (41.0%) than men (28.0%) (Table 1).
In the bivariate analysis (Table 2), when compared to seronegative donors, HTLV-I/II seropositive sub-jects were more often older, had fewer years of school-ing, used non-intravenous recreational illegal drugs (marijuana as almost the single drug used), had blood transfusions in the past and paid for sex (males only). The indeterminate subjects when compared to seron-egative donors (Table 3) were more likely to have fewer years of schooling, breastfeeding, use of non-intravenous recreational illegal drugs, blood transfu-sions in the past and paid sex (males only). No sig-nificant difference between the groups was found for the frequency distribution of tattoos, acupuncture and intravenous drugs use (data no shown).
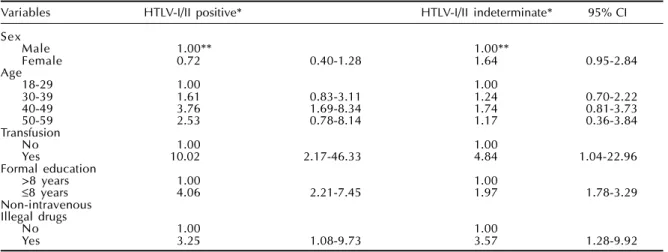
The multinomial logistical regression model was applied to the group as a whole (Table 4). The final model comparing positive and indeterminate subjects to the negative group identified three main variables after adjusting for age and sex: history of previous transfusions, less than eight years of schooling, and use of non-intravenous recreational illegal drugs in the past. In Brazil schooling years is a well-recog-nized and accurate surrogate of socio-economic sta-tus. As education and monthly income are strongly correlated, only the variable education was included in the study models. For this study, education was
assumed to be a measurement with higher accuracy and reliability than income.
DISCUSSION
The study results revealed remarkable lack of ho-mogeneity between the three studied groups. This is very important when considering that all study sub-jects had self-assessed themselves as healthy enough to volunteer for blood donation. Also, all subjects were clinically asymptomatic when submitted to the routine pre-donation screening questionnaire and clinical examination conducted at Hemominas Insti-tute. For the seropositive group it was observed an growing trend in the OR with increasing in the study population age (Table 4). The greater 95% confidence intervals may have resulted from small numbers es-pecially for the 50-59 age group. Several different hypothesis can be formulated: the higher antibody titers in subjects infected for longer periods of time, the continuous addition of new infected individuals and the cohort effect, with older groups reflecting the highest infection prevalence rate in the past (Yamaguchi,16 Murphy et al11).
4 7 4 4 7 44 7 4 4 7 4
4 7 4 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors Catalan-Soares B et al
Table 3 - Bivariate analysis for HTLV-I/II serologic status according to selected determinants at baseline: indeterminate group versus negative group. The GIPH Cohort Study. Belo Horizonte, Brazil, 1997-1999.
Variables HTLV-I/II indeterminate HTLV-I/II negative OR 95% CI*
N=166 N=116
N (%) N (%)
Sex
Male 126 (75.9) 75 (64.7) 1.00
Female 40 (24.1) 41 (35.3) 0.57 (0.33-0.98)
Age (years)
18 to 29 74 (44.6) 65 (56.0) 1.00
30 to 39 55 (33.1) 33 (28.5) 1.44 (0.81-2.58)
40 to 49 28 (16.8) 13 (11.2) 1.87 (0.84-4.17)
50 to 59 9 (5.4) 5 (4.3) 1.56 (0.45-5.68)
Formal education
≥8 years 60 (36.1) 64 (55.2) 1.00
<8 years 106 (63.9) 52 (44.8) 2.19 (1.32-3.67)
Breastfed
No 2 (1.3) 7 (6.4) 1.00
Yes 152 (98.7) 102 (93.6) 5.22 (0.97-37.1)
NA 12 7
Non-intravenous Illegal drugs
No 143 (86.1) 110 (95.7) 1.00
Yes 23 (13.9) 5 (4.3) 3.54 (1.22-10.99)
NA 1
Blood transfusion
No 149 (92.5) 113 (98.3) 1.00
Yes 12 (7.5) 2 (1.7) 4.55 (0.94-30,06)
NA 5 1
Paid sex**
No 58 (48.3) 48 (71.6) 1.00
Yes 62 (51.7) 19 (28.4) 2.70 (1.36-5.40)
NA 6 8
*Odds ratio and 95% Confidence Interval. **For males only.
Table 4 - Multinomial logistic regression of HTLV-I/II serological status according to selected variables, at baseline. GIPH Cohort Study. Belo Horizonte, 1997-1999.
Variables HTLV-I/II positive* HTLV-I/II indeterminate* 95% CI
Sex
Male 1.00** 1.00**
Female 0.72 0.40-1.28 1.64 0.95-2.84
Age
18-29 1.00 1.00
30-39 1.61 0.83-3.11 1.24 0.70-2.22
40-49 3.76 1.69-8.34 1.74 0.81-3.73
50-59 2.53 0.78-8.14 1.17 0.36-3.84
Transfusion
No 1.00 1.00
Yes 10.02 2.17-46.33 4.84 1.04-22.96
Formal education
>8 years 1.00 1.00
≤8 years 4.06 2.21-7.45 1.97 1.78-3.29
Non-intravenous Illegal drugs
No 1.00 1.00
Yes 3.25 1.08-9.73 3.57 1.28-9.92
*HTLV-I/II negative is the comparison group. **Odds ratio and 95% Confidence Interval.
from men to women, and/or to hormonal factors and possibly other unknown determinants and exposures. All these findings corroborate previous reports (Manns & Blattner,10 Clark4) where the interplay of two or more
determinants was suggested (Kajiyama et al,8 Clark et
al,4 Brodine et al2). However, in the final logistic
re-gression model, after adjusting for age, previous his-tory of transfusion, years of schooling and use of ille-gal non-intravenous drugs, it was found that HTLV-I/II serological status was independent of participants’ sex. It is possible that in the study population, sex may be
a marker of social inequality and/or a confounder of the association of HTLV-I/II serology and education - a marker for socio-economic status.
The results of the indeterminate group should be considered bearing in mind that this group has sub-jects with false positive results (cross-reaction? ma-laria? dengue? other retroviruses?) who could be infected with different virus strains (Chavance et al,3 Kaplan et al9) as well as the individuals
4 7 5 4 7 5 4 7 5 4 7 5 4 7 5 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors Catalan-Soares B et al
serological response detectable in the Western blot.
The present results clearly show that the indetermi-nate group is different from the seropositive and se-ronegative groups, presenting demographics and behavior characteristics (e. g., sex, age, breastfeeding) similar to the negative group, but matching the posi-tive group in terms of past history of blood transfu-sions, less than eight years of schooling and use of recreational non-intravenous drugs (Tables 2, 3 and 4). Eleven percent of the indeterminate subjects had positive polymerase chain reaction (PCR). Of these, some have shown overt seroconversion at the time of the biannual reevaluation (manuscript in preparation). To one’s knowledge, not much has been investigated about the epidemiological characteristics of the in-determinate group, which makes it difficult to com-pare the study data with the literature.
The present study has allowed the evaluation of various demographics, socio-economic and behavior characteristics associated to HTLV-I/II in-fection, and contrasts between positive and indeter-minate serology groups. Some new determinants associated with HTLV-I/II seropositivity were iden-tified, such as non-intravenous use of recreational illegal drug use, especially marijuana, and low socio-economic conditions, measured by years of school-ing, both of them relatively valid markers for in-come and social status. These data point out toward significant inequalities as determinants of exposure to HTLV-I/II infection. The study population, where well-known determinants of HTLV-I/II infection were by definition exclusion criteria of study participa-tion, make possible to show inequalities of the study population as a determinant of HTLV-I/II seroprevalence. Although lower education has been associated with higher risk of other blood-borne virus infections, such as HIV and Hepatitis B and C (Goubau*), it has not been conclusively associated with HTLV infection.
Some study limitations should be also considered. For example, blood donors are not representative of the base population in age and sex distribution and may be also healthier, a possible “healthy blood do-nor effect”. Concerning HTLV-I/II distribution in the base population, seropositive blood donors are more likely to include younger asymptomatic males in-dividuals who were more recently infected. Al-though most of HTLV-I/II seropositive individuals are apparently asymptomatic through out their lives, the study cross-sectional data analysis may have resulted in an undetected survival bias. Also, as for
all individuals donating blood in a public health blood center, participants were informed of their serologic results before being invited to join the study. As a consequence, neither participants nor the interviewer were blind as for participants’ sero-logical status. Although only one experienced and well-trained interviewer conducted all interviews, interviewer bias may not be ruled out. The advan-tages of this population are that blood donors’ sta-tus is more easily and less expensively ascertained as compared to a random sample of the base popula-tion, and these individuals are also a healthier non-institutionalized population.
The strong association between blood transfusions in the past year and uncertainties concerning chronic infection, as well as the actual significance of inde-terminate results call for a careful approach regard-ing counselregard-ing and follow-up protocols.
In this manner, it will be possible to elaborate pub-lic health measures to minimize the risk of asympto-matic HTLV infected individuals developing HTLV-I/II-related diseases, and to control virus transmis-sion. It is believed that the study’s findings may be valuable in the reevaluation of criteria for blood do-nor selection, further understanding of virus infec-tion spread and preveninfec-tion, and reinforcing the need of a more in depth assessment of the indeterminate serology group.
GIPH INVESTIGATORS GROUP
GIPH (Interdisciplinary HTLV-I/II Research Group) investigators are: Anna Bárbara F. C. Proietti, Hemominas Blood Center; Antônio Carlos Guedes, Universidade Federal de Minas Gerais (UFMG); Bernadette Catalan Soares, Hemominas Blood Center; Fernando A Proietti, UFMG; José Roberto Lambertucci, UFMG; Sônia Regina Pinheiro, UFMG; Denise Utsch Gonçalves, UFMG; Marina Lobato Martins, Hemominas Blood Center; Gerhard Werner Thorun, Social Pioneers Association, Sarah’s Hospi-tal of the Locomotor System Belo Horizonte (BH); João Gabriel Ramos Ribas, Social Pioneers Associa-tion, Sarah’s Hospital of the Locomotor System, BH; Erna Geesien Kroon, UFMG; Olga Maria Carvalho Pfeilstiker, UFMG; Claúdio Bonjardim, UFMG; Olindo Assis Martins, Centro de Pesquisa René Rachou; Hilda Maria de Oliveira, Centro de Pesquisa René Rachou; Edel Figueiredo Barbosa, UFMG; Gustavo Eustáquio B. Alvim de Melo, Centro de Pesquisa René Rachou; Eric Bassetti Soares, Hemominas Blood Center; Vandack Nobre, UFMG.
4 7 6 4 7 64 7 6 4 7 6
4 7 6 Rev Saúde Pública 2003;37(4):470-6
www.fsp.usp.br/rsp
HTLV-I/II and blood donors Catalan-Soares B et al
REFERENCES
1. Blattner WA, Nomura A, Clark JW, Ho GY, Nakao Y, Gallo R et al. Modes of transmission and evidence for viral latency from studies of Human T-cell
Lymphotropic Virus type I in Japanese migrant populations in Hawaii. Proc Natl Acad Sci USA 1986;83:4895-8.
2. Brodine SK, Oldfield EC 3rd, Corwin Al, Thomas RJ, Ryan AB, Holmberg J et al. Human T-cell Leukemia/ lymphoma virus among U.S. marines stationed in a hyperendemic area - evidence for female-to-male sexual transmission. J Acquir Immune Defic Syndr 1992;21:75-9.
3. Chavance M, Frery N, Valette I, Schaffar-Deshayes L, Monplaisir N. Sex ratio of Human T lymphotropic virus type I infection and blood transfusion. Am J Epidemiol 1990;131:395-9.
4. Clark J, Saxinger C, Gibbs N, Lofters W, Lagranade L, Blattner WA et al. Seroepidemiologic studies of Human T-cell Leukemia/lymphoma Virus type I in Jamaica. Int J Cancer 1985;36:37-41.
5. Hall WW, Kubo T, Lijichi S, Takahashi H, Zhu SW. Human T cell leukemia/lymphoma virus, type II (HTLV-II): emergence of an important newly recognized pathogen. Semin Virol 1994;5:165-78.
6. Hirata M, Hayashi J, Noguchi A, Nakashima K, Kajiyama W, Sawada T et al. The effects of breastfeeding and presence of antibody to p40tax protein of Human T-cell Lymphotropic Virus type-I on Mother to Child Transmission. Int J Epidemiol 1992;21:989-94.
7. Hosmer D, Lemeshow S. Applied logistic regression. 2nd ed. New York: John Wiley & Sons; 2000.
8. Kajiyama W, Kashiwagi S, Ikematsu H, Hayashi J, Nomura H, Okochi K et al. Interfamilial transmission of adult T-cell leukemia virus. J Infect Dis
1986;154:851-7.
9. Kaplan JE, Khabbaz RF, Murphy EL, Hermansen S, Roberts C, Lal R et al. Male-to- female transmission of human T-cell lymphotropic virus types I and II: association with viral load. J Acquir Immune Defic Syndr Hum Retrovirol 1996;12:193-201.
10. Manns A, Blattner WA. The epidemiology of the Human T-cell lymphotropic virus type I and type II: etiologic role in human disease. Transfusion 1991;31:67-75.
11. Murphy EL, Figueroa JP, Gibbbs WN, Alexander SS, Blattner WA, Cranston B et al. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I Demographic determinants. Am J Epidemiol 1991;133:1114-24.
12. Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang 1984;46:245-53.
13. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn P, Minna J, Gallo RC et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 1980;77:7415-9.
14. Sullivan MT, Willians AE, Fang CT, Grandinetti T, Poiesz BJ, Ehrlich GD Transmission of human T-lymphotropic virus types I and II by blood transfusion: a retrospective study of recipients of blood
components. Arch Int Med 1991;151:2043-8.
15. Tokudome S, Tokunaga O, Shimamoto Y, Miyamoto Y, Sumida I, Wishizumi M et al. Incidence of adult T-cell leukemia/lymphoma among human T-lymphotropic virus type I carriers in Saga, Japan. Cancer Res 1989;49:226-9.