ii
DECLARAÇÃO
Nome: CARLA ANDREIA MACHADO DE SOUSA
Endereço electrónico: PG28832@alunos.uminho.pt Telefone: 918572190 Número do Bilhete de Identidade: 13028180
Título dissertação:
Screening for antioxidant activity in extracts obtained from extremophile microalgae Orientador(es): Prof. Doutor José António Teixeira e Prof. Doutor Rui Tavares Ano de conclusão: 2018
Designação do Mestrado:
Mestrado em Biologia, Biotecnologia e Bioempreendedorismo em Plantas
DE ACORDO COM A LEGISLAÇÃO EM VIGOR, NÃO É PERMITIDA A REPRODUÇÃO DE QUALQUER PARTE DESTA TESE/TRABALHO
Universidade do Minho, 31/ 10 / 2018
iv
ACKNOWLEDGMENTS
I would like to recognize all the effort of my supervisors Luis Loureiro, Dr. Bruno Fernandes, Prof. José Teixeira and Prof. António Vicente, that affectionately welcome me. Without their providential contribute, important reflections and wise opinions I wouldn’t be able to conceive the work necessary to this demanding project, specially being a working-student.
I would like to address a special thanks to all of my colleagues and friends from LIP (Indrusty and Processes Laboratory, Centre of Biological Engineering, University of Minho) for their care and constant support.
For last, I would like to dedicate this thesis to the family and friends that supported and believed or even to those who doubt of me, they were the reason for the strength that stood me up in every step of this tough journey.
Screening for antioxidant activity of bioactive compounds extracted from extremophile microalgae
ABSTRACT
Both in the health sector or food industry there is a persistent focus on the search for antimicrobial, antioxidant, inflammatory, obesity, diabetic, neuroprotective and anti-carcinogenic substances. In the attempt to prevent or control some pathology conditions related to oxidative stress damage industry has been exploring the use of natural antioxidant substances capable of protect cells and organisms from the damage caused by pro-oxidant factors. Aiming to delay the natural aging process, to achieve a superior resilience and cellular survival skills, industry initiated a constant demand for antioxidant substances. Natural products often unveil new bioactive molecules with a promising health and industrial potential application. One possible natural source of bioactive antioxidant metabolites is extremophile lichens and their microalgae, that have been gaining investigators’ and industry’s attention. Microalgae’s bioactivities explored so far are relevant to different target markets, such as health, cosmetics and functional foods. Innovative biotechnological approaches are nowadays being studied to optimize the production, extraction and gathering of those bioactive molecules. For this work and in the attempt of studying antioxidant potential activity, several lichens specimens were identified and collected from different environments. This study focused on six lichen species, collected from different extremophile environments. An improved isolation method (differential centrifugation) was implemented and a cell disruption technique, bead milling, was used in order the cells rupture. A basic screening of microalgae extracts was assayed for antioxidant activity to determine the specimens of major interest for these properties. Fruticose lichens were identified as the best sources for bioactive compounds with antioxidant activity. Comparing the results of FRAP, ORAC and ABTS antioxidant assays, it seems reasonable to infer that fruticose lichens LFR2, LFR3 and foliose LFO2 and LFO1 are potential good sources of bioactive molecules with antioxidant relevant potential. Our results demonstrated that algae extracts has antioxidant in vitro activity, suggesting that extremophile microalgae may represent a promising source of bioactive molecules for antioxidant therapeutic applications.
ii
Deteção de atividade antioxidante em extratos de microalgas provenientes de líquenes extremófilos
RESUMO
Existe, na atualidade, uma demanda por compostos com potencial antioxidante, antimicrobiano, anti-inflamatório, anti obesidade, antidiabéticos, neuro protetores ou anticancerígenos. Produtos de origem natural são frequentemente fontes de compostos bioativos com potencial aplicação ao nível industrial ou mesmo na área da cosmética ou saúde. Na tentativa de prevenir ou mesmo controlar o desenvolvimento de patologias relacionadas com stress oxidativo, a indústria tem vindo a dar especial relevância ao potencial uso de substâncias de origem natural. Na tentativa de interferir e retardar o processo de envelhecimento celular, com intuito de alcançar maior resiliência e até sobrevivência, têm sido exploradas substâncias de origem natural com capacidade antioxidante. Uma das possíveis fontes de metabolitos com propriedades antioxidantes apontadas pela investigação são os líquenes e as respetivas microalgas, nomeadamente os que se encontram sujeitos a condições ambientais extremas. De acordo com a bibliografia e investigação existentes, as principais bioatividades associadas aos extratos de microalgas recolhidos de líquenes em condições extremas são relevantes para o sector da saúde, cosmética e indústria alimentar. Em virtude desta constatação, novas abordagens biotecnológicas têm sido estudadas de forma a otimizar o processo de produção, extração e recolha desses compostos bioativos. De modo a inferir o potencial antioxidante de diferentes extratos de microalgas provenientes de líquenes, foram recolhidas seis espécies com características distintas e provenientes de diferentes ambientes. Foi aplicado um método de isolamento otimizado, a centrifugação por gradiente, bem como uma técnica de rutura celular, o moinho de bolas, por forma a alcançar os melhores resultados ao nível da extração de compostos provenientes das microalgas. Foi realizada uma verificação preliminar da capacidade antioxidante dos extratos provenientes das microalgas isoladas dos líquenes através de ensaios antioxidantes como o ORAC, FRAP e ABTS. Os extratos provenientes das microalgas dos líquenes fruticosos, LFR2 e LFR3, bem como dos foliosos, LOF1 foram os que apresentaram melhor desempenho nos ensaios, sugerindo ser boas fontes de compostos bioativos com capacidade antioxidante. Os resultados obtidos sobre a actividade antioxidante in vitro evidenciam um potencial antioxidante para os extratos das microalgas testadas. Esta constatação poderá representar a indicação de que os compostos bioativos com atividade antioxidante existentes extraídos das microalgas possuem potencial terapêutico.
Index
ABSTRACT I
RESUMO III
LIST OF GENERAL NOMENCLATURE VII
LIST OF FIGURES IX
LIST OF TABLES XI
1. INTRODUCTION 1
1.1. THE INTEREST OF NATURAL BIOACTIVE COMPOUNDS 1
1.2. LICHEN - THE SYMBIOTIC ORGANISM 2
1.3. LICHENS ENVIRONMENT CHALLENGE ADAPTATION PROCESS 4
1.3.1. MORPHOLOGICAL ADAPTATIONS 4
1.3.2. BIOCHEMICAL ADAPTATIONS 4
1.4. LICHENS – A POTENTIAL SOURCE FOR BIOACTIVE SUBSTANCES 5
1.4.1. MICROALGAE BIOACTIVE MOLECULES 7
1.5. LICHENS KNOWN BIOACTIVITIES 9
1.6. THESIS PURPOSE AND GOALS 12
2. BIOTECHNOLOGICAL APPROACH FOR LICHENS EXPLOITATION 12
2.1. ISOLATION METHODS 12
2.2. CELL DISRUPTION METHODS 13
3. MATERIAL AND METHODS 16
3.1. COLLECTION AND IDENTIFICATION OF LICHENS SAMPLES 16
3.2. ISOLATION PROCESS 19
3.3. CELL DISRUPTION METHOD – USING BEAD MILLING 20
3.3.1. CELL DISRUPTION EFFICIENCY EVALUATION 21
3.4. ANTIOXIDANT ACTIVITY ASSESSMENT 21
3.4.1. ABTS RADICAL SCAVENGING ACTIVITY 21
3.4.2. FRAP ANTIOXIDANT ACTIVITY ASSAY 22
vi
4. RESULTS AND DISCUSSION 23
4.1. ISOLATION METHOD 23
4.2. CELL DISRUPTION METHOD EFFICIENCY EVALUATION 25
4.3. ANALYSIS OF THE MICROALGAE CONTENT FROM THE LICHENS SAMPLES 26
4.4. ANTIOXIDANT POTENTIAL 30
4.4.1. ABTS RADICAL SCAVENGING ACTIVITY 30
4.4.2. FRAP ANTIOXIDANT ACTIVITY ASSAY 31
4.4.3. ORAC ASSAY - OXYGEN RADICAL ABSORBANCE CAPACITY 32
5. CONCLUSIONS 33
6. FUTURE PERSPECTIVES 35
LIST OF GENERAL NOMENCLATURE
CHEMICAL SYMBOL
CO2 – Carbon Dioxide CsCl2 -Caesium chloride
FeCl3 - Iron chloride
Fe(SO4)3 - Iron Sulphate
KI - Potassium Iodate
ABBREVIATIONS AA – Arachidonic Acid
Abs– absorbance
ABTS – (2,2-azinobis (3-ethylbenzothiaazoline-6-sulphonic acid)
Alt – Altitude
AUC - Area Under Curve
DHA – Docosahexaenoic acid
DNA – Deoxyribonucleic acid
EPA – Eicosapentaenoic acid
FRAP - Ferric Reducing Antioxidant Power
IOMR - Intracellular Organic Matter Release
ORAC - Oxygen Radical Absorbance Capacity
PI - Percentage of inhibition
PTP1B - Protein Tyrosine Phosphatase
PUFAs – Polyunsaturated Fatty Acids ROS - Reactive Oxygen Species
LIST OF FIGURES
FIGURE 1. LICHENS MAIN BIOACTIVE METABOLITES CLASSIFICATION ACCORDING TO DAYAN AND ROMAGNI (2001). ... 6
FIGURE 2. LICHENS BIOACTIVE COMPOUNDS CLASSIFICATION ON A METABOLIC PATHWAYS
PERSPECTIVE (ADAPTED FROM NASH III, 2008). ... 7
FIGURE 3. SCHEMATIC REPRESENTATION OF LICHENS POTENTIAL FOR COMMERCIAL AND INDUSTRIAL PURPOSES AND POSSIBLE APPLICATIONS. ... 8
FIGURE 4. SCHEMATIC REPRESENTATION OF MAIN CELL DISRUPTION METHODS. ... 14
FIGURE 5. SCHEMATIC DIAGRAM OF THE GRADIENT CENTRIFUGATION PERFORMED FOR THE ISOLATION OF PHOTOBIONT CELLS EXTRACTED FROM THE SAMPLES OF LICHENS COLLECTED. ... 20
FIGURE 6. CELL DISRUPTION ASSESSMENT (BASED ON VIABLE CELLS COUNTING AND ON IOMR
VARIATION) EVALUATED BY FLOW CYTOMETRY ANALYSIS AND IOMR TECHNIQUE. ... 25
FIGURE 7. CELL DISRUPTION TECHNIQUE - TREATMENT TIME EFFECT IN METHOD EFFICIENCY
EVALUATED BY FLOW CYTOMETRY ANALYSIS. ... 26
FIGURE 8. ABTS RADICAL SCAVENGING ACTIVITY ASSAY FOR THE EXTRACTS OF MICROALGAE
ISOLATED FROM LICHENS ... 30 FIGURE 9. FRAP ANTIOXIDANT ACTIVITY ASSAY FOR THE EXTRACTS OF MICROALGAE ISOLATED FROM
LICHENS ... 31 FIGURE 10. RESULTS OF ORAC ASSAY FOR THE EXTRACTS OF MICROALGAE ISOLATED FROM LICHENS
LIST OF TABLES
TABLE 1 - LICHENS’ MAJOR MORPHOLOGY GROUPS CLASSIFICATION WITH FOCUS ON STRUCTURAL AND ENVIRONMENTAL DISTINCT CHARACTERISTICS ADAPTED FROM (NASH III, 2008). ... 3
TABLE 2. LICHENS USED IN THIS WORK, WITH RESPECTIVE PHOTOGRAPHIC RECORD, IDENTIFICATION AND DATA REGARDING COLLECTION SITE ... 17 TABLE 3. FLOW CYTOMETRY ANALYSES OF LICHEN MICROALGAE HOMOGENATE AFTER APPLYING
ISOLATION TECHNIQUE DESCRIBED. ... 24
TABLE 4. FLOW CYTOMETRY ANALYSIS ON THE MICROALGAL EXTRACTED FROM EACH LICHEN ... 27
1. Introduction
1.1. The interest of natural bioactive compounds
Historically, natural products or plants are the genesis for primordial medicines and also for some poisons. Firsts chemical, pharmacological, clinical studies or even traditional medicines accrued from plants and were the basis to some currently ubiquitous drugs like aspirin, digitoxin, morphine, quinine and pilocarpine (Butler, 2004). The screening for bioactive molecules from natural sources still provide a high percentage of the new compounds tested in clinical trials. Nowadays, natural products derived drugs are still a significant source for new medicines or treatments, especially for anticancer therapies, gathering about 62% of the existing drugs, for infectious diseases (75%) and antihypertensive therapies (65%) (Newman et al., 2003; Pan et al., 2010; Rout et al. 2009). Emerging antibiotic resistance and also an urge for more efficient anticancer therapies are becoming true public health concerns. Severe pathology conditions like cancer, microbial infections, obesity or even hypertension could benefit through the use of natural substances. For example, cancer natural chemoprevention, a pharmacological approach to arrest or to reverse the process of carcinogenesis recurring to phytochemicals substances as gaining scientific evidence (Russo et al., 2010; Singh et al, 2013b).
Antioxidant activity of compounds have been linked to prevention of important and emergent diseases that include cancer, heart diseases, neurological and inflammatory disorders and also the aging process. Reactive oxygen species (ROS) formed under photooxidation stress can react with macromolecules like lipids and proteins leading to cellular damage. Antioxidants are substances that have the ability to reduce ROS and prevent macromolecules from oxidation. An important example are the polyphenolic compounds, isolated from algae sources, closely related to their antioxidant proprieties and pharmacological or nutraceutical potential. Also, carotenoids - a natural pigment synthesized by plants, fungi or algae, possesses relevant antioxidant activity. Fucoxanthin or Astaxanthin, are carotenoid pigments found in algae that contribute to prevention of several diseases associated with oxidation processes (Ibañez et al., 2012; Xia et al., 2013). Polyketides, like usnic acid, are another example of natural molecules that are being used and studied for their pharmacological proprieties: cytotoxicity, antifungal or antiviral agents (Ziehl, et al. 2005). The potential use of microalgae for industrial and commercial applications is one of the clearest examples of the interest on natural bioactive compounds (Suganya et al., 2016). Nowadays, innovative biotechnological approaches are being developed and could be applied in order to optimize the production, extraction and gathering of those natural bioactive compounds. The growing interest for natural bioactive compounds research impels the need to improve the speed of screening, isolation, structural and functional comprehension but also entail the need for large-scale production strategy
2
for these nutraceuticals. One possible natural source of bioactive metabolites is extremophile lichens, more specifically, their microalgae that have been gaining investigators’ and industry’s attention.
1.2. Lichen - the symbiotic organism
Lichens are a symbiotic association between two very different species: a fungus (mycobiont) and an autotrophic partner (photobiont). The photobiont can either be a microalga (Protista and Plantae kingdom), a cyanobacterium (Monera kingdom), or both organisms, in the case of tripartite lichens (Rafat, 2014; Zambare and Christopher, 2012). In the symbiotic association, mycobiont is responsible for providing access to water and nutrients captured from the substrate and surrounding environment in order to fulfil the needs of the photobiont. Also, the thallus formed by fungus hyphae, provides protection to photobiont from the environmental factors (Sanders, 2001). As for the photobiont, which is autotrophic, meaning that can use carbon dioxide from the atmosphere, as well as the light and the water available to produce sugar alcohols and glucose, essential to the lichen energy production and survival (Friedl and Budel, 2008; Nash III, 2008). The combination of all these singular characteristics result in a symbiotic association, where lichens became a more autonomous organism. Despite of all possible environmental constraints lichens are perfectly capable of colonizing different terrestrial environments and types of surfaces, some of them with almost no food resources (inorganic or indigestible substrates) and to survive in inhospitable environments (Boustie et al., 2011; Nash III, 2008).
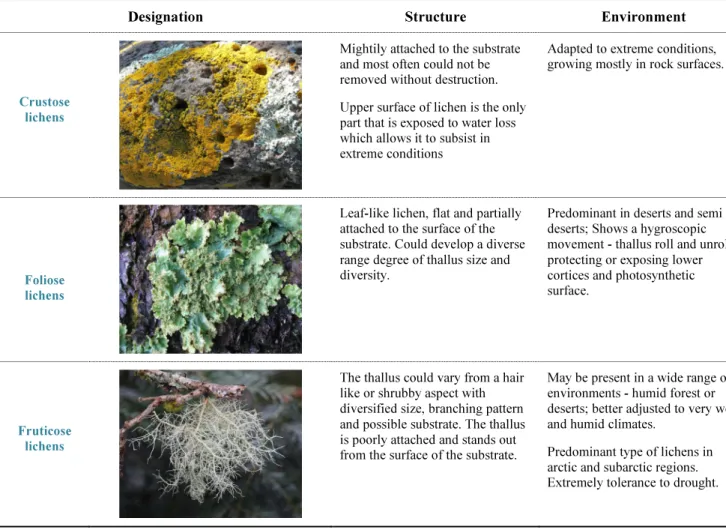
The fungus development is responsible for building and determining the structure of the lichen, as well as to bind and fix the symbiont into a surface (Nash III, 2008). Lichens can be classified using different criteria, but the most common classification is based on the morphologic characteristics, such as the colour of the basal thallus, frequency of segmentation, secondary branches patterns, the presence and conformation of the reproductive structures. Lichens morphology also depends on the photobiont/mycobiont interaction (Zambare and Christopher, 2012). According to their morphological characteristics, lichens can be divided into different main groups: crustose, fruticose and foliose.
Encrustation lichens could be characterized by the placement of the photobiont in a distinct layer that remains below the mycobiont cortical layer. These organisms are known for being firmly attached to substrate, that is crucial and favours their survival in extreme habitats like tree branches and specially in rock surfaces. The fruticose lichens have a thallus structure resembling to a bush, that naturally stands out from the surface of the substrate, in which the phycobiont is located in the inside layer of the outer cortex. The size of the branch in this kind of lichens is extremely variable,
ranging from one millimetre to several meters in some Usnea species. The highly branched structure of the fruticose lichens grants a high surface ratio when compared to other lichens resulting in a more efficient water exchange structure. For this reason, this morphology is more adjusted to tropical wet and also humid environments than any other lichen structures. As for the foliose lichens, they typically present a flat leafy aspect where the phycobiont remains in a layer below the upper cortex. Moreover, a discrete cortex below is observed in this type of morphology, well separated from the substratum, which means that kind of lichens are only partially attached to the surface. The thallus of foliose lichens is composed of lobes that might display several degrees of branching (Nash III, 2008; Zambare and Christopher, 2012).
Table 1 summarizes the most important morphological characteristics of crustose, fruticose and foliose lichens previously referred.
Table 1 - Lichens’ major morphology groups classification with focus on structural and environmental distinct characteristics adapted from (Nash III, 2008).
Designation Structure Environment
Crustose lichens
Mightily attached to the substrate and most often could not be removed without destruction. Upper surface of lichen is the only part that is exposed to water loss which allows it to subsist in extreme conditions
Adapted to extreme conditions, growing mostly in rock surfaces.
Foliose lichens
Leaf-like lichen, flat and partially attached to the surface of the substrate. Could develop a diverse range degree of thallus size and diversity.
Predominant in deserts and semi deserts; Shows a hygroscopic movement - thallus roll and unroll protecting or exposing lower cortices and photosynthetic surface.
Fruticose lichens
The thallus could vary from a hair like or shrubby aspect with diversified size, branching pattern and possible substrate. The thallus is poorly attached and stands out from the surface of the substrate.
May be present in a wide range of environments - humid forest or deserts; better adjusted to very wet and humid climates.
Predominant type of lichens in arctic and subarctic regions. Extremely tolerance to drought.
4
The growth rate of lichens is normally very slow, and it is measured in millimetres per year. Hale (1973) established the following pattern of growth in lichens: foliose species grow about 0.5-4 mm/year, fruticose species 1.5–5 mm/year and crustose species 0.5–2 mm/year.
1.3. Lichens environment challenge adaptation process
Environment conditions often exert a significant impact on the life forms that colonize and inhabit a certain region or environment. For that, biotic and abiotic factors are responsible for perpetuating a natural selection and thus, extremophile conditions limit the development of more fragile species.
Some of the most determining factors that influence adaptation and survival are: high salinity levels, CO2 prevailing atmosphere, presence of heavy metals, UV (ultraviolet) radiation exposure, extreme pH ranges, scarcity of water and organic matter, extreme humidity and/or temperature and also the potential contact with xenobiotic substances. Few species can cope with harsh environmental conditions, as described. For the adaptation process, lichens’ unique symbiotic association is critical and is responsible for the resilience and survival skills of these life forms in some of the most inhospitable places on Earth (Boustie et al., 2011; Nash III, 2008). For many reasons that will be soon approached lichen’s symbiotic organism are for fact prepared to face extreme environmental conditions.
1.3.1. Morphological adaptations
Lichens morphology is quite variable and well-adjusted to the limitations imposed by the environment (e.g. associated with the uptake of light or water availability). Those limitations not only influence the thallus structure but also their capability to grow and size but also could define the degree of development of thallus size and form. Lichen’s thallus structure is well adjusted to several specific environmental conditions and adaptability is the reason why these organisms belong to a restrict group that survive under extreme environmental conditions, being classified as stress tolerant organisms (Nash III, 2008).
1.3.2. Biochemical adaptations
The frequent exposure to extremophile environments resulted in several biochemical adaptations, influencing the production of different molecules to confer them high resistance and survival capacity in extreme environmental conditions (Boustie and Grube, 2005; Boustie et al., 2011; Gauslaa and Solhaug, 2001; Nash III, 2008; Rancan et al., 2002; Torres et al., 2004).
Main lichens cellular structures can only maintain their integrity because of the presence of some stabilizing-molecules such as polyols, polysaccharides, nucleotides, proteins, and some membrane lipids (Boustie et al., 2011). Also, the need to withstand extreme dissection and radiation, and consequent formation of ROS, only allowed the survival of the lichens that produced more complex and efficient scavenging molecules (Kranner, et al., 2005). Significant increases in temperature influence the synthesis and content of secondary metabolites, like salazinic acid which is responsible to attenuate the effects of higher temperatures due to its hydrophobic properties. Also, lichens with high content in usnic acid are less prone to survive in acidic environments (Deduke et al., 2012). Lichens bioactive metabolites that act as UV radiation absorbents or improves their resistance to drought or bacteria (Al-Qasmi et al., 2012; Boustie et al., 2011) contributed to their resistance to stressful environmental conditions (Suzuki et al., 2016).
1.4. Lichens – a potential source for bioactive substances
Lichens, as several other natural product sources, revealed to be a potential source for new potent and innovative bioactive molecules with several industrial and health applications. Contrary to primary metabolites, that are crucial to survival, the secondary metabolites produced by the lichen are not essential for the organism. Those metabolites are produced from intermediates generated from the primary metabolism, are influenced for biotic and abiotic factors and their prevalence could be extremely variable among endemic species. Lichens metabolites molecules could be divide as aliphatic substances, aromatic substances, diphenyleneoxide derivatives, and polysaccharides (Boustie et al., 2005; Stocker-Wörgötter et al., 2013) and are represented in Figure 1. As for the main aromatic compounds, terpenes for example, are isoprenoids that are the main building blocks of steroids (e.g. cholesterol) and also for essential oils synthesis. Aliphatic acids, contain a hydrocarbon fragment derived from a nonaromatic substance, like methane, propane or hexane. These natural substances are responsible for flammable characteristics, allowing their use in fuel production (e.g. biofuel or biogas). Polysaccharides, like β-glucans or galactomannan, are polymeric carbohydrates molecules already used in food products as binders or stabilizers. In pharmaceutical or cosmetic industries, polysaccharides are being used as excipients or even active ingredients, like Fenugreek plant example (Williams et al., 2004).
6
Figure 1. Lichens main bioactive metabolites classification according to Dayan and Romagni (2001).
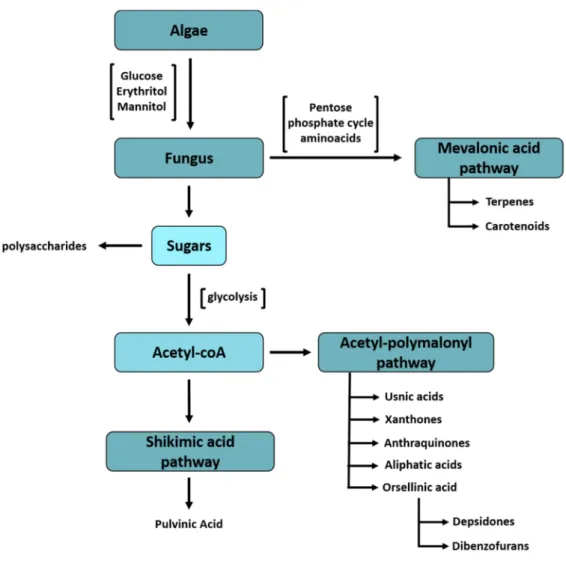
All of lichens secondary metabolites result from three major biosynthetic metabolite secondary pathways: mevalonic acid, shikimic acid and acetyl-polymalony, represented in Figure 2.
Figure 2. Lichens bioactive compounds classification on a metabolic pathways perspective (adapted from Nash III, 2008).
Besides the natural production of several bioactive compounds, lichens can also harbour different bioactive microorganisms (e.g. Actinobacteria – Microcystis, Nostoc). Those organisms are involved in biosynthesis or intervein in the conversion of metabolites found in lichens, also producing substances with high medicinal and therapeutic value (e.g. antimicrobial or antiproliferative activity), becoming another major focus of interest in the use of lichens (Boustie et al., 2011; Costa et al. 2013; González et al., 2005; Parrot et al., 2015; Sarmiento-Vizcaíno et al., 2016; Singh et al., 2005; Suzuki et al., 2016).
1.4.1. Microalgae bioactive molecules
The research and exploration for new, renewable and sustainable environment friendly bioactive molecule sources has gaining attention. In most of the microalgae species, starch or starch-like compounds, lipids - mainly in the form of polyunsaturated fatty acids (PUFAs), including
8
arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) are the typical contents. Fatty acids and sterols are also found in cellular membranes, being more difficult to extract than the storage intracellular lipids. The algae lipid-rich composition ideal for biofuel production and also because of it’s a biodegradable, non-toxic, low impact CO2 emission that contribute to pollution control and that has a positive environmental impact (Chisti et al., 2007; Meher et al., 2006). Microalgae symbiont species also produce unique compounds like carotenoids, fatty acids, enzymes, polymers, peptides, toxins and sterols. Those bioactive molecules have numerous commercial applications like their use in human or animal food industry, for drug development or supplementation purposes, for cosmetic industry, the fluorescent pigments use in textile industry or their use as a biofertilizer (Suganya et al., 2016). Some of the mentioned applications are represented in Figure 3.
Figure 3. Schematic representation of lichens potential for commercial and industrial purposes and possible applications.
As for the human nutrition, several supplements or fortified foods recur to microalgae source for quality protein (e.g. snacks, gums, beverages). Most know species used are Chlorella or Spirulina. Spirulina has a notable high protein level and also a great source for essential fatty acids (linolenic acid), beta-carotene, B-vitamins and phycocyanin. Chlorella has been related to cholesterol
control, having a positive effect on immune system in virtue of its antiviral, anti-inflammatory and anticancer properties (Singh et al., 2005; Suganya et al., 2016). Some algae species (e.g. Arthrospira) are used to aquaculture feed because of their content in vitamins, minerals, fatty acids improving health and fertility of the animals. In fact, 30% of the current world algal production is sold for animal feed applications (Suganya et al., 2016). Red microalgae, for example, are rich in chlorophyll, carotenoids, phycocyanin and phycoerythrin. All these algae pigments are used in food, cosmetic and pharmaceutical industry, replacing the use of synthetic dyes (Singh et al., 2005). Blue-green microalgae, like Spirulina, produces phycocyanin and also contains omega 3 – fatty acids, an essential fat which contribute to inhibition of inflammatory prostaglandins syntheses, contributing for an improved immune system and preventing heart and other inflammatory diseases, like rheumatoid arthritis. Omega 3-fatty acids, eicosapentanoic acid (EPA) or docosahexaenoic acid (DHA), have a wide range of therapeutic application and for that the annual worldwide demand of EPA rises to about 300 tons. The algae source for omega 3-fatty acids are a more sustainable one and superior in the sense that doesn’t carry any off flavors unlike fish sources, has a low cholesterol content and a more affordable cost (Belarbi et al., 2000; Singh et al., 2005). Green microalgae, like Dunaliella, are an excellent source of beta-carotene, lycopene and lutein, bioactive substances that have relevant antioxidant and anti-inflammatory properties and are relevant in the treatment of degenerative diseases. The industry and commercial interest on β-carotene extracted from Dunaliella, as growing since 1980 (Olaizola, 2003). β-carotene is a precursor of vitamin A that is absorbed from the diet and is required for normal growth and tissue repair in animals and for that is currently used to fortification of cattle feed, resulting in improved health and fertility of animals. For humans, it is used as food addictive for improving the color of flesh, fish and egg yolk (Singh et al., 2005).
1.5. Lichens known bioactivities
Lichens unique secondary metabolites have already been investigated and tested for several pharmaceutical properties: antioxidant, antimicrobial, anti-inflammatory, neuroprotective or even anticancer. Lichens metabolites have been often associated with antimicrobial potential (Padhi and Tayung, 2015) and one of the most recognized compounds is usnic acid (Francolini et al., 2004; Ivanova et al., 2010; Kukla et al., 2014; Rankovic et al., 2014; Sultana and Afolayan, 2011). For that characteristics lichens extracts could be applied as potential therapeutic alternatives to antibiotics or, at least, as a preventive barrier. Theoretically, usnic acid has an uncoupling action of oxidative phosphorylation cycle in mitochondria organelles. Several studies determined the antimicrobial effect of usnic acid on gram positive anaerobic bacteria (Ristic et al., 2016). Maciag-Dorszynska et al. (2013) reveal that usnic acid’s antibacterial effect is caused by inhibition of RNA and DNA
10
synthesis, which affects the bacterial cell membrane. Still, usnic acid is not the only metabolite of interest regarding to the antimicrobial activity since atranorin has also shown a notorious bioactivity (Kosanic et al., 2014; Rankovic et al., 2014; Toledo-Marante et al., 2003; Turk et al., 2006;). Many lichens have been linked to antiproliferative properties through scientific evidences, which have a great interest mainly due to the increasing incidence of cancer and death rates among adults, children and adolescents (Siegel et al., 2016). Atranorin and protocetraric acid arise as promising compounds found in lichens that demonstrated antiproliferative effect on tumour cells (Backorová et al., 2012; Brandão et al., 2012; Fernandez-Moriano et al., 2016; Kosanic et al., 2014; Rankovic et al., 2014; Ristic et al., 2016; Toledo-Marante et al., 2003). Usnic acid was also pointed as a promising compound as an antiproliferative agent (Bazin et al., 2008; Bézivin et al., 2004; Fernandez-Moriano et al., 2016; Koparal et al., 2006; Mayer et al., 2005; Nguyen et al., 2014; Schinkovitz et al., 2014; Singh et al., 2013; Singh et al., 2013b). Some lichens’ metabolites demonstrated a great potential to interact with enzymes that interfere with insulin sensitive tissues (Choudhary et al., 2011; Seo et al., 2009; Seo et al., 2009b). This finding is of significant importance because of the growing number of diabetes- and obesity-related problems, two emerging and concerning global health problems. The most common tests used to evaluate antidiabetic bioactivity are the antiglycation assays and the urease inhibition assay, that allow the investigators to realize the extent of enzyme activity and to infer about the potential of the compound to treat or prevent diabetes disease. Usimines, an usnic acid derivative, appear to present a potent inhibitory activity against PTP1B (protein tyrosine phosphatase), which is implied in the control of insulin activity in tissues and in leptin regulation (Seo et al., 2008). Some lichens’ secondary metabolites might act as a protective mechanism against other fungi, helping in the growth control of pathogenic fungi (Basile et al., 2015; Kowalski et al., 2011). As example, parietin and emodin are thought to be promising agents on pathogen fungal control (Basile et al., 2015; Wei et al., 2008; Manojlovic et al., 2005; Manojlovic et al., 2005b). Other bioactivities such as herbicidal, insecticidal or antiviral, have been reported lichens as well(Cetin et al., 2008; De Carvalho et al., 2004; Fazio et al., 2007; Goel et al., 2014; Nimis and Skert, 2004). However, many other bioactivities still need more investigation and could, ultimately, lead to the discovery or development of unique substances to improve our quality of life.
Importance of antioxidant activity Environmental or biological stress factors many times lead to damage in DNA (deoxyribonucleic acid) and often result in damage for the organisms or cells. Aging, cancer, some inflammatory disorders are examples of consequences from DNA oxidant aggressions. Antioxidants are substances that protect cells and organisms from prooxidant aggression factors. As so, antioxidant commercial
demand as gaining importance and it’s a relevant scientific field. Interference with enzyme production or activity, adjustment in molecules synthesis that are responsible that confer stability to molecules or cell structures may be some of the mechanisms that result in a better resistance to stress factors (Collins and Horváthová, 2000).
In the hope to achieve superior resilience and survival skills for cells and organisms, industry initiated a constant demand for antioxidant substances, responsible for protecting cells and organisms from the damage caused by pro-oxidant factors. Comprehensibly and also due to cost-effectiveness and developing investigation reasons natural antioxidant compounds have become a growing focus of commercial interest manly for cosmetic, food and medicinal purposes. As many other potential applications for these bioactive molecules, antioxidant purposes metabolites have been gaining investigators and industry attention. Innovative biotechnological approaches are nowadays being studied to optimize the production, extraction and gathering of those compounds. Atranorin and usnic acid are two of the main lichens antioxidant metabolites that have linked to antioxidant properties (Fernandez-Moriano et al., 2016; Kosanic et al., 2014; Kumar et al., 2010; Mitrovic et al., 2011; Rankovic et al., 2012; Sisodia et al., 2013).
Lichens’ phenolic compounds have demonstrated pronounced capability for protecting cells from the damage caused by oxidative stress residues. Phenolic compounds and other antioxidant substances capable of scavenging ROS often protect plants and lichens from sun and UV light exposure. Therefore, lichens have shown to be an interesting source of natural UV filters, a characteristic of particularly interest for the cosmetic industry (Gauslaa and Solhaug, 2001; Rancan et al., 2002; Solhaug et al., 2003; Torres et al., 2004). Antioxidant characteristics are also crucial to protect neural cells from oxidative stress-related diseases like neurodegenerative disorders (Fernandez-Moriano et al., 2016). Perlatolic acid, a secondary metabolite found in Cladonia portentosa, is a good example of a compound with proven neuroprotective capabilities (Sumana et al., 2016). Some compounds of lichens are naturally capable of reducing or down-regulate inflammatory molecular agents formed in response to stress conditions that result in an inflammation. Longissiminone A present in Usnea lichens and stereocalpin A from Ramalina terebarata are examples of secondary metabolites that revealed a relevant anti-inflammatory effect (Beyon et al., 2011; Choudhary et al., 2005).
12
1.6. Thesis purpose and goals
Lichens’ bioactive natural molecules are relevant to specific markets, such as health, cosmetics and functional foods. Also, there are a wide range of classified microalgae species, as well as potential bioactive compounds that accrue from them. Microalgae found in extremophile lichens are also considered stress tolerant and have singular antioxidant characteristics because of their ability to adjust the metabolite synthesis in result of environmental conditions.
Regarding all these facts, the purpose of this thesis is to gather information about antioxidant potential of lichens’ microalgae secondary metabolites. Therefore, our goal was to optimize and implement an efficient isolation and cell disruption technique, in some lichens subjected to extremophile conditions that we collected from their natural habitats. After collecting the samples, a basic screening will be executed to determine antioxidant properties potential in the microalgae isolated extract from the several lichens collected in Portugal. The main objective for this master’s degree work was to evaluate lichens microalgae potential interest for isolation and/or synthesis of antioxidant substances with commercial and possible industrial potential.
2. Biotechnological approach for lichens exploitation
Although bioactive compounds extracted from lichens evidenced a potential commercial value, there are still no lichens that are cultivated at large scale. Some constraints affect the process of lichens microalgae mass cultivation representing a limiting factor to their commercial use. Because of known difficulties to use lichens, one of the natural sources for bioactive metabolites that already collected commercial and industrial attention, is microalgae. Regarding the photobiont, the average biomass productivities reach up to 20 kg.m2.year-1 with a potential for further improvement by means of strain selection and process engineering (Duong et al., 2012; Varshney et al., 2015). To perform a successful, effective and mild cell disruption procedure to fully extract secondary metabolites without destroying them or affecting their bioactivity is a considerable need (Boustie and Grube, 2005). Because of that, most of the laboratory work on lichens developed so far encompasses the use of methods focused on the isolation of the photobiont.
2.1. Isolation methods
Photobiont isolation from lichen structure is the first step in the process of obtaining the bioactive metabolites. The purpose of isolation technique is to successfully extract photobiont cells from the mycobiont structure. It is important that the isolated cells are free of fungus contamination
and fully functional if the aim is to culture them in lab environment. Another major factor is that isolation method should be easy, rapid to perform and have a reasonable cost.
To successfully perform the isolation process and separate the photobionts from the homogenates there are three studied possible methods: the filtration method, the micropipette method and the centrifugation method. Filtration, also called the Yamamoto method – is a procedure based on a sequence of filtrations trough different mesh sizes (Calatayud et al., 2001). The micropipette method uses a micropipette to transfer drops of photobiont isolated from the lichen suspension cells to microscope slides (Ahmadjian, 1993). The limitation of the above-mentioned methods is that requires times for further culture of the photobiont under axenic conditions (Calatayud et al., 2001). Centrifugation methods perform a photobiont separation based on the particle size and density differences. There are many protocols using differential and gradient centrifugation, serves for microalgae and cyanobacterial, and on the centrifugation method different photobionts are separated from the homogenate by their size using different speed for the centrifugation. Lower-speed centrifugation are more prone to separate larger fragments, like thallus. The gradient centrifugation is useful for isolation and photobiont purification. Gradients can be generated throughout Caesium chloride (CsCl2), Potassium Iodate (KI), Percoll® and/or sucrose solution (Backor et al., 1998). Percoll® gradient centrifugation is the preferred method when it’s important to maintain intact the algae extracted to further cultivation or inoculation, because this method doesn’t affect the function of PSII, having no photoinhibition impact on photobiont, which is determining when batch culture of the photobiont is being considered. Photoinhibition is one of the first signs of stress in photosynthetic cells. Gradient centrifugation methods are still time consuming and demands for further steps to concentrate photobiont cells but are the most efficient methodology (Calatayud et al., 2001; Gasulla et al., 2010).
2.2. Cell disruption methods
Perform a successful, effective and mild cell disruption procedure to fully extract secondary metabolites without destroying them or affecting their bioactivity is a considerable need. Several extraction processes and cell disruption methods have been already performed, tested and optimized in order to obtain higher extraction capacity. Cell disruption techniques resort to mechanical and non-mechanical methods (described in Figure 4.), essential to obtain the maximum bioactive molecules often accumulated in lichen cortex and microalgae cells (Boustie and Grube, 2005; Gunerken et al., 2015).
14
Figure 4. Schematic representation of main cell disruption methods.
Mechanical cell disruption methods are more common and consists in techniques that use mechanical forces to destroy cell walls and collect the intracellular biochemicals liberated throughout the process. Some of the mechanical methods currently used are solid-shear forces (ball milling), liquid-shear (high pressure homogenization), high speed homogenization, energy transfer (ultra-sonication, microwave treatment), ionic liquids-based extraction and pulsed electric field treatment (Bonny et al., 2009; Bonny et al., 2011; Boustie and Grube, 2005; Komaty et al., 2016; Günerken et al., 2015; Parrot et al., 2015).
Among mechanical cell disruption techniques, ball milling has a significant high disruption efficiency in single-pass operation and it’s also easy to scale up for industrial implementation. Ball milling is a mechanical compaction and shear stress cell disruption mechanism. The most important factors that influence this process are: speed, time, amount of dry weight, microalgae type and size, material and agitation disk design. Microwave extraction has gaining attention as an alternative to solid liquid solvent extraction, because needs less time to extraction and also have a more efficient extraction ratio. Microwave allows the transference of electromagnetic radiation evenly into the sample without physical contact, altering cell wall thickness and pore diameter. Microwave allows the significant reduction of organic solvents and results in a drastic reduction of extraction time (Bonny et al., 2009; Bonny et al., 2011; Günerken et al., 2015; Kim et al., 2015). Ultrasound method generate microbubbles in liquid medium producing a cavitation effect that raises temperature and affects cell wall integrity (Kim et al., 2015). Ionic liquids, like 1-butyl-3-methylimidazolium
tetrachloroferrate, for example, are being used for their potential less harmful environmental impact than conventional solvents. The use of ionic liquid-based extraction has a significant influence on the efficiency of the extraction (Bonny et al., 2011).
The performance of mechanical methods can vary significantly with some conditions like: solvent, processing time or the power used. The difficulty of use mechanical methods is the substantial energy consumption, moderate extraction efficiency and also the difficulty of performing the methodology in larger-scale manner (Kim et al., 2015).
Non-mechanical methods such as enzymatic cell lysis and chemical cell disruption can also be used. Chemical based extraction are considered low energy consuming, require modest investments and are easy to scale-up. However, some practical questions arise, like chemical cost, bio-toxicity and lipid degradation. The efficiency of using organic solvents is also a problem (Günerken et al., 2015; Kim et al., 2015). Ferric salts, such as FeCl3 or Fe(SO4)3, due to their positive charges are used to aggregate negative charged microalgae, acting as chemical coagulants in the harvesting of microalgae. For enzymatic extraction the problem seems to be the prolonged reaction time and high enzyme costs (Kim et al., 2015). Non-mechanical methods are more expensive but are capable of sparing molecules’ bioactivity being thus more suitable to preserve their normal structure and functionality (Boustie and Grube, 2005; Günerken et al., 2015). Some procedure by themselves are not enough but cell wall disruption can be optimized, and the efficiency can be improved by mixing different rupture strategies (Kim et al., 2015).
16
3. Material and Methods
3.1. Collection and Identification of lichens samples
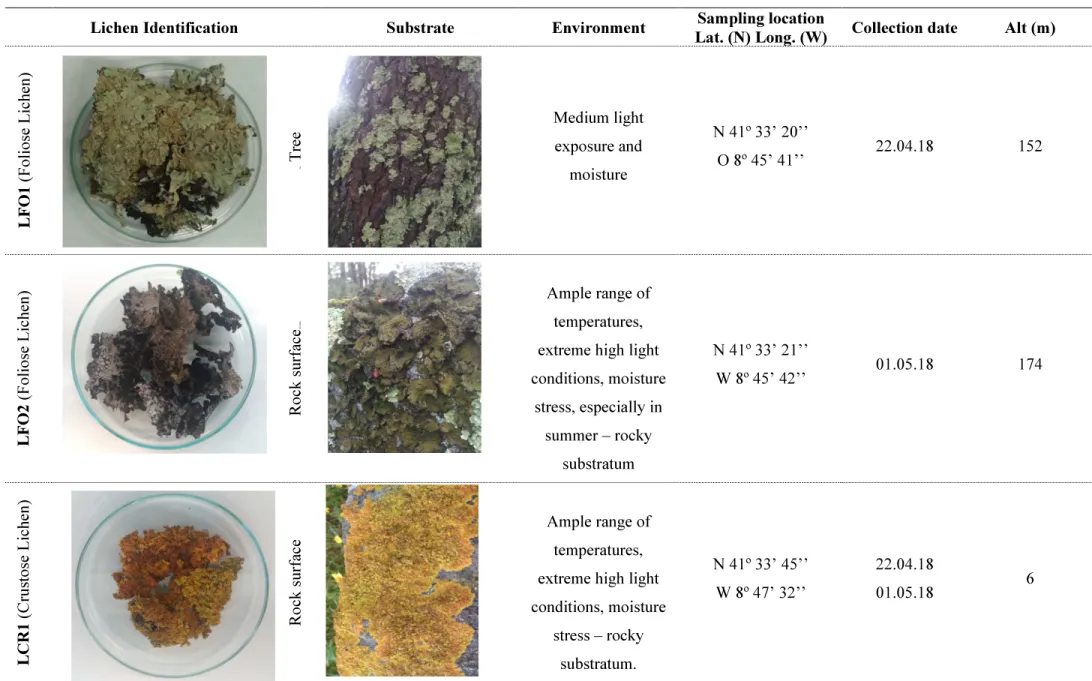
The selection of microalgae was performed accounting exposure to harsh conditions, like sunlight conditions, high salinity and low humidity, ruff and poor substrate. Six species of fruticose, foliose and crustose lichens from natural growing sites in Esposende – Portugal, were collected. Lichens identification, sampling and environment conditions are described in Table 2.
To collect the lichen specimens’ specific tools and material were necessary: sample bags, notebook/register collecting sheet, GPS location system, spray bottle filled with clean water for some crustose lichens. Some lichens specimens are more attached to the substrate and for those is necessary a knife, a stone chisel/hammer to pull it off in some cases. In the collecting process it was vital to register location, environment specific conditions, ecosystem surrounding. In laboratory, lichen material was washed, air dried and stored at room temperature (25º Celsius) in a desiccator.
Table 2. Lichens used in this work, with respective photographic record, identification and data regarding collection site
Lichen Identification Substrate Environment Sampling location
Lat. (N) Long. (W) Collection date Alt (m)
L FO 1 ( F o li o se L ic h en ) Tree Tr ee Medium light exposure and moisture N 41º 33’ 20’’ O 8º 45’ 41’’ 22.04.18 152 L FO 2 ( F o li o se L ic h en ) R o ck su rf ac e Rock surface Ample range of temperatures, extreme high light conditions, moisture stress, especially in summer – rocky substratum N 41º 33’ 21’’ W 8º 45’ 42’’ 01.05.18 174 L C R 1 ( C ru st o se L ic h en ) R o ck su rf ac e Ample range of temperatures, extreme high light conditions, moisture stress – rocky substratum. N 41º 33’ 45’’ W 8º 47’ 32’’ 22.04.18 01.05.18 6
18 L FR 1 ( F ru ti co se L ic h en ) Tree R o ck su rf ac e Low temperature,
high light conditions, moisture stress – rocky substratum. N 41º 33’ 21’’ W 8º 45’ 42’’ 22.04.18 174 L FR 2 ( F ru ti co se L ic h en ) Tree R o ck su rf ac e Medium light exposure and moisture N 41º 33’ 42’’ W 8º 45’ 52’’ 01.05.18 182 L FR 3 ( F ru ti co se L ic h en ) Tree R o ck su rf ac e Medium light exposure and moisture N 41º 33’ 42’’ W 8º 45’ 52’’ 01.05.18 182
3.2. Isolation Process
For the isolation process, first the air-dried thalli of several species of lichens were washed in a slow agitation warm (30º Celsius) bath with distilled water, for 15 min. After a careful wash and removal of the dirt, rocks and any wood chips the thallus were cut into small pieces. Then lichens fragmented thallus were macerated in a mortar with a pestle in sterile isotonic buffer (0.3M sorbitol in 50mM HEPES pH 7.5) (Ahmadjian, 1967; Gasulla et al., 2010). After adequate homogenisation in the mortar and adding 5 mL of isotonic buffer, the homogenate was filtrate with sterile muslin. The filtration process of the homogenate was repeated through a height-layer of sterile muslin (Backor et al., 1998). After filtration, the homogenate included a mixture of different cell types from photobiont and mycobiont. For obtaining photobiont cell material gradient centrifugation were performed using a fix-angle rotor (Beckman Coulter Allegra®, USA) based on density gradient segregation (Ahmadjian, 1967). The isolation process performed on lichens collected is described in the scheme of Figure 5.
Lichens homogenate first centrifugation was at 2300 rpm for 5 min, aiming to collect the pellet. After diluting the pellet in isotonic buffer, the centrifugation was repeated. The supernatant is discarded, and the resulting pellet is resuspended 0,25M sucrose solution. A gradient is then created by adding the sucrose-algae solution carefully layered onto KI solution (80% W/V). Tubes with the suspension is centrifugate for 10 min at 4500 rpm to extract the interphase (Backor et al., 1998). After this centrifugation, different layers were visible in the tube: a) upper layer pellet; b) diffuse light green layer above KI layer - interphase; c) bottom pellet; d) bottom layer cells debris. The interphase recovered goes under more two centrifugations at 6000 rpm for 10 min. The final pellet containing the isolated algal cells is then resuspended into distilled water.
20
Figure 5. Schematic diagram of the gradient centrifugation performed for the isolation of photobiont cells extracted from the samples of lichens collected.
After centrifugation procedure, the homogenates must be filtered through Teflon filters (22 µm mesh sizes). The samples of isolated microalgae filtered suspension were preserved at -20º Celsius into the freezer until needed.
3.3. Cell disruption method – using bead milling
The samples of microalgae isolated from lichens were subjected to the bead milling technique, the mechanical method selected to perform cell disruption. Bead milling assays occurred using glass beads of different diameters and a vortex mixer Clifton Cyclone CM-1 (Nickel Electro Ltd., UK). The assay was performed using the microalgae extracted from the foliose lichen - LFO1. This disruption method was carried out using several beads ratios (between 0 and 52% of the culture volume) and treatment time intervals (1-10 min).
3.3.1. Cell disruption efficiency evaluation
After disruption treatment, samples were centrifuged at 12000 rpm for 10 min. The absorbance of the supernatant was measured at 254 nm in SynergyTM HT Multi-Detection Microplate Reader (BioTek Instruments. Inc., USA). Before starting disruption process, extracellular organic matter of the original sample was also determined. IOMR (intracellular organic matter release) factor of each sample was calculated by the following equation:
IOMR factor = Abst - Abs0 Abs0
where Abst refers to the absorbance measured at 254 nm after disruption treatment during a certain period of time (t, min) and Abs0 is the absorbance measured at 254 nm of the original sample (before treatment).
To evaluate efficiency of cell disruption method flow cytometry analyses of the samples was performed by using an EC800TM flow cytometer analyser (Sony Biotechnology Inc., USA). Total chlorophyll content was determined by red fluorescent signals collected by a 665 nm long-pass filter FL3. Red fluorescence is linked to total chlorophyll content, and chlorophyll is present in all photoautotrophic microalgae species. The number of photobiont events and fluorescence signals were evaluated through EC800 analyses flowing software (Sony Biotech).
3.4. Antioxidant activity assessment
In the attempt of studying bioactivity antioxidant potential of natural lichens extracts, several lichens specimens were used to perform in vitro antioxidant activity assays. In laboratory, stoichiometric methods are used to infer antioxidant activity of extracts or substances through the discolouration or coloration obtained in result of the chemical reaction when compared to know substances results. Common antioxidant assays used within food industry like ABTS (2,2-azinobis (3-ethylbenzothiaazoline-6-sulphonic acid radical scavenging activity), FRAP (ferric reducing antioxidant power) and ORAC (oxygen radical absorbance capacity) assays were performed (Huang et al., 2005).
3.4.1. ABTS radical scavenging activity
Determination of the antioxidant capacity can be performed by the ABTS assay, as described elsewhere (Gião et al., 2007). ABTS methodology relays on the conversion of ABTS (2,2-azinobis (3-ethylbenzothiaazoline-6-sulphonic acid)) in its radical cation in the presence of sodium persulfate.
22
The radical cation has a blue colour and absorbs light at 734 nm and reacts with most common antioxidants. During the chemical reaction, the blue ABTS radical cation is reduced and converted to its neutral colourless form. The reactivity of the antioxidants tested are then compared to ascorbic acid capacity.
For the ABTS assay was prepared an ABTS stock solution prepared by adding, at 1:1 (v/v), of 7 mM ABTS (2,2-azinobis (3-ethylbenzothiaazoline-6-sulphonic acid)) (Sigma-Aldrich, USA) to a solution of 2.45 mM of Potassium Persulphate (Merck, Germany) diluted in water. This solution had an incubation time between 12 to 16 hours in the dark. Then a 10 mL aliquot of the sample was assayed for percentage of inhibition (PI). Each experiment was run in triplicates and included controls. The absorbance of the samples against a blank was determined at the spectrophotometer SynergyTM HT Multi-Detection Microplate Reader (BioTek Instruments. Inc., USA).
The calibration curve was performed using Trolox. Percentage of inhibition (PI), was determined according to the equation:
PI % = ((Abs ABTS – Abs sample) / Abs ABTS) x 100
Abs ABTS denotes the initial absorbance of diluted ABTS, and Abs sample denotes the absorbance of the sample by 6 min of reaction.
3.4.2. FRAP antioxidant activity assay
This method is used to determine the antioxidant activity by measuring the ferric reducing antioxidant power (FRAP). The reducing capacity of samples is expressed as millimoles of ferrous equivalent (mM Fe (II).g-1 of dry weight material (Benzie and Strain, 1996).
Frap assay uses several reagents solutions that must be always freshly prepared: solution A, the acetate buffer (300 mM, pH 3.6); solution B - HCl (40 mM); solution C, TPTZ - 2,4,6-tri-(2-pyridyl)-s-triazine (10 mM) and solution D - FeSO4.7H2O stock solution, for calibration curve. The FRAP solution is prepared by adding the following proportion of solutions - 10(A):1(C):1(D). After preparation, FRAP reagent must be putted into a water bath for 15-20 min at 37 °C. For the assay 10 μL of sample was mixed with 290 μL of FRAP reagent into well microplate. The plate was incubated at 37 °Celsius for 15 min and then determine the absorbance at 593 nm against a blank sample in the spectrophotometer SynergyTM HT Multi-Detection Microplate Reader (BioTek Instruments. Inc., USA).
The calibration curve was prepared by plotting the absorbance value obtained for the FeSO4.7H2O standard solutions (200, 400, 600, 800 and 1000 μM) versus the ferrous solutions' concentration (μmol.L-1). From the calibration curve, a linear regression can be obtained:
in which AbsS is the absorbance of the sample; AbsB is the absorbance of the blank; [Fe] is the concentration of Fe (II), in μmol.L-1; I is the slope of the linear regression; and b is the point at which the line crosses the y-axis.
3.4.3. ORAC assay - oxygen radical absorbance capacity
The ORAC assay uses the AAPH [2,2'-azobis(2-amidinopropane) dihydrochloride] radical/oxidant to generate peroxyl radicals when heated in the presence of oxygen. The reaction is monitored by the rate of fluorescence light detected from the fluorescein in the presence of the antioxidant. The higher the efficacy of the antioxidant compound for scavenging free radicals, the lower the decrease of the fluorescein intensity, that is, the area under the curve (intensity emission/time) would be higher (Ou et al., 2001).
For the ORAC assay, 20 μL of antioxidant (samples or standards) was dispensed in each well microplate. Then was added 120 μL of Fluorescein (F6377, Sigma-Aldrich) solution 117mM, prepared freshly with Phosphate buffer 75 mM at pH 7.4 to each well using an automatic dispenser (Eppendorf Multipette plus). A blank (Fluorescein and AAPH solution) with 20 μL of phosphate buffer instead of the antioxidant solution is also carried out. The mixture putted in the microplate was then incubated with automatic for 15 min at 37º Celsius. After that 60 μL of AAPH (440914, Sigma-Aldrich) solution 40 mM diluted in Phosphate buffer was rapidly added to microplate wells and fluorescence of the misture was determined every 5 minutes for 120 min, using 485-P excitation and 520-P emission filters in the spectrophotometer SynergyTM HT Multi-Detection Microplate Reader (BioTek Instruments. Inc., USA). The calibration was made from a solution of 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, 238813, Sigma-Aldrich) 100 μM in phosphate buffer 75 mM at pH 7.4, which must be prepared daily by diluting from a fresh 1 mM Trolox solution in the same buffer. A suitable concentration range is 5-80 μM (0.5-8 μM, final concentration) with six calibration points. To determine the antioxidant activity, first is necessary to calculate the area under curve (AUC) as:
AUC = 1 + f1/f0 + f2/f0 + f3/f0 + … + f24/f0
where f0 is the initial fluorescence reading at 0 min after addition of AAPH and fi is the fluorescence reading at time i. Then, calculate the net AUC according to the following equation:
Net AUC = AUCSample - AUCBlank
4. Results and Discussion 4.1. Isolation method
24
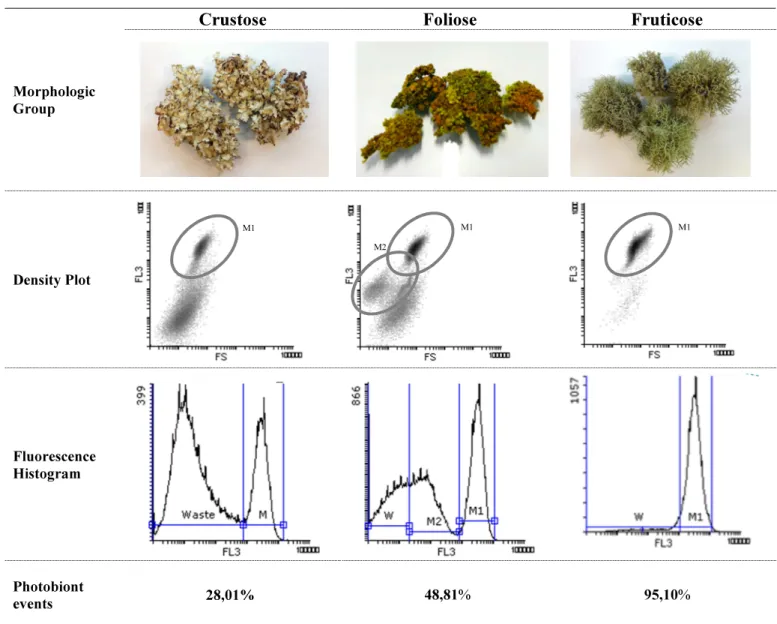
In order to optimize the isolation method and maximize the harvest of photobiont from the lichens, first it was used a generic sample of the three lichen morphological groups. This analysis allowed to infer methodology efficiency. In Table 3 it is possible to compare the results obtained by application of the same isolation method to crustose, foliose and fruticose lichens. Using flow cytometry to evaluate the fluorescence results of the cells in the isolated samples it was possible to perspective the effect of the isolation process over different morphology lichens.
Table 3. Flow Cytometry analyses of lichen microalgae homogenate after applying isolation technique described.
Morphologic Group
Crustose Foliose Fruticose
Density Plot Fluorescence Histogram Photobiont events 28,01% 48,81% 95,10% Photobiont
concentration 2012 Nr Cells/µL 4371 Nr Cells/µL 12600 Nr Cells/µL
Density plot of microalgae isolated from crustose lichens show a distinct population (M1) with 28,01% events and a counting of 2012 Nr cells/ µL. The microalgae isolated from foliose lichens has showed two different populations, M1 and M2, constituting 48,81% of cell events and a
M1 M1
M1
photobiont concentration of 4371 Nr cells/µL. M2 cells could be a different microalgae population or even cyanobacteria because of the fluorescence detected. Fruticose exhibited the best isolation response to treatment, having M1 population, constituting 95,10% of cell events and a photobiont concentration of 12600 Nr cells/µL. For these results the isolation process had a better effect on fruticose lichens. According to the previous results and by analysing microalgae cell isolates at the microscope it was possible to confirm that hyphae fragments were reduced, and cell aggregates became minimal after isolation. The isolation method by KI gradient centrifugation was found to be effective after appropriate optimization. Also, KI gradient centrifugation has shown to be have a good control over mycobiont possible contamination (Fontaniella et al., 2000), and that was the reason why the protocol was chosen over other possible gradient centrifugation methods, like Percoll® gradient centrifugation.
4.2. Cell disruption method efficiency evaluation
Bead milling process was carried out in order to compare the effect of using different glass beads relative percentage and time of procedure. Cell disruption assessment for 0,149-0,25 mm and 0,5 mm using different relative percentage of beads were analysed based on percentage of viable cells remaining in the solution and also intracellular organic matter release (IOMR). The cell disruption process was carried out for 5 min, and the results are represented in Figure 2, where the error bars represent the standard deviation for the three experiments.
Beads Relative Percentage
Figure 6. Cell disruption assessment (based on viable cells counting and on IOMR variation) evaluated by flow cytometry analysis and IOMR technique.
26
When comparing beads size and its efficiency by viable cells and IOMR factor, the experiment results show that 0,5 mm beads have a more efficient cell disruption effect than the smaller beads (0,149-0,25 mm). As for the relative percentage of beads used in cell disruption evaluation, the 42% concentration showed to be the optimum relative percentage for these kind of samples (Figure 6). Cell disruption effect was also tested for the optimal time of treatment.
Time of Procedure
Figure 7. Cell disruption technique - treatment time effect in method efficiency evaluated by flow cytometry analysis.
The results based on cell viability and IOMR factor showed better results at 5 minutes of procedure (Figure 7). In perspective and analyzing figure 6 and 7, it was possible to conclude that the rate of organic matter liberated by cells was considerably promoted by the use of 42% beads with 5 min treatment, using the bead milling technique. For these facts, cell disruption technique was adjusted to the use of 42% beads and for 5 min of treatment.
4.3. Analysis of the microalgae content from the lichens samples
Flow cytometry analyses and microscopic evaluation were performed on the microalgae content from each lichen sample. The resume of data collected from microalgae content isolated from lichen samples is compiled into Table 4.
FS S S 100 101 102 103 104 105 100 101 102 103 104 SS C o u n t 100 101 102 103 104 105 FS 100 101 102 103 104 0 684 1369 2053 2737 FL3 C o u n t 100 101 102 103 104 105 0 116 231 347 462 M1 13,46% SS C o u nt 100 101 102 103 104 105 FS 100 101 102 103 104 0 2640 5281 7921 10561
Table 4. Flow cytometry analysis on the microalgal extracted from each lichen
Lichens Density Plot Surface Plot Fluorescence Histogram Dot Plot
L F O 1 L FO 2 L C R 1 FS S S 100 101 102 103 104 105 100 101 102 103 104 FL3 C o u n t 100 101 102 103 104 105 0 455 910 1364 1819 M1 2,52% FS S S 100 101 102 103 104 105 100 101 102 103 104 FS S S 10 0 10 1 10 2 10 3 10 4 10 5 100 10 1 10 2 10 3 10 4 SS C o u n t 100 101 102 103 104 105 FS 100 101 102 103 104 0 2895 5791 8686 11581 FS SS 100 101 102 103 104 105 100 101 102 103 104 FL3 C o u n t 100 101 102 103 104 105 0 527 1054 1580 2107 M 1 4,78% FS S S 100 101 102 103 104 105 100 101 102 103 104
28 FL3 C o u n t 100 101 102 103 104 105 0 97 194 290 387 M1 21,93% SS C o u n t 100 101 102 103 104 105 FS 100 101 102 103 104 0 876 1751 2627 3502 FS S S 100 101 102 103 104 105 100 101 102 103 104 L F R 1 L F R 2 L F R 3 FL3 C o u n t 100 101 102 103 104 105 0 76 151 227 302 M1 50,01% FS S S 100 101 102 103 104 105 10 0 10 1 10 2 10 3 10 4 SS C o u n t 100 101 102 103 104 105 FS 100 101 102 103 104 0 1204 2407 3611 4814 FS S S 100 101 102 103 104 105 100 101 102 103 104 SS C o un t 100 101 102 103 104 105 FS 100 101 102 103 104 0 1118 2236 3354 4472 FL3 C o u n t 100 101 102 103 104 105 0 96 192 287 383 M1 41,88% FS S S 100 101 102 103 104 105 100 101 102 103 10 4 FS S S 100 101 102 103 104 105 100 101 102 103 104 FS S S 100 101 102 103 104 105 100 101 102 103 104
Comparing flow cytometry analysis on the algal isolates extracted from each lichen it was possible to determine that LOF1 had 2,52%, LFO2 had 13,46%, LCR1 had 4,78%, LFR1 21,93% of events and LFR2 had 50,01%, LFR3 had 41,88% of florescence events detected corresponding to microalgae extract successfully extracted. Analysing collected data (green fluorescence population) from microalgae extracts isolated, lichens cell disruption process had exerted a better effect in fruticose lichens, LFR1, LFR2 and LFR3. In fact, the florescence histogram of LFR2 showed an isolation process with highest percentage of events in the region corresponding to microalgae population (50,01%). LFR3 also exhibit 41,88% of events detected with same florescence intensity.
In order to check efficiency of cell disruption and evaluate the purity of the culture obtained, isolated microalga extracts were observed with transmission-light microscopes and recorded by photomicrography - Table 5.
Table 5. Photobiont extract isolated from lichens microscopy evaluation
It was possible to confirm that for all the tested samples, after bead milling technique execution, the number of cell debris, associated with the photobiont cells decreased, when compared to initial results before the optimization of isolation method, as predicted. Also, looking at the collected samples it is possible to observe that foliose and crustose microalgae extracts had less microalgae concentration treated with the same isolation method. These results are congruent with the percentage photobiont concentration collected from each lichen sample and evaluated in previous results analysed through flow cytometry.
Microalgae Microscopy Evaluation
LFO1 LFO2 LCR1 LFR1 LFR2 LFR3
30
4.4. Antioxidant potential
4.4.1. ABTS radical scavenging activity
In ABTS assay antioxidant activities of microalgae extracts were compared to Trolox, which is a standard antioxidant. In this test an inferior absorbance demonstrates a higher reducing and antioxidant power. The results for the ABTS antioxidant assay of tested samples, are summarized in Figure 8.
Figure 8. ABTS radical scavenging activity assay for the extracts of microalgae isolated from lichens
When compared to Trolox, a standard antioxidant, extracted microalgae suspension results varied between 10 mg/mL Trolox and 20 mg/mL, the strongest activity measured in the assay. The inhibition of oxidant activity of ABTS varied from 77% (in LFR1) to 91,2% (LFR3). The antioxidant results indicated that the reducing power of the samples decreased in the following order: LFR3, LFR2, LFO2, LCR1, LFO1 and LFR1. Extracts of the studied lichens showed moderate ABTS antioxidant activity and fruticose lichen LFR3 had demonstrated the strongest antioxidant value 18,3 mg.mL-1, similar to Trolox 20 mg/mL result with similar inhibition power (91,2%).
55,0 91,1 91,2 77,0 80,8 84,0 78,8 91,2 0 10 20 30 40 50 60 70 80 90 100 10 mg/mL Trolox LFR2 LFR3 LFR1 LCR1 LFO2 LFO1 20 mg/mL Trolox % PI
ABTS
4.4.2. FRAP antioxidant activity assay
FRAP assay results for tested samples are represented in Figure 9.
Figure 9. FRAP antioxidant activity assay for the extracts of microalgae isolated from lichens
It is possible to infer that, like the other antioxidant assays, Fruticose lichens had the most active results. LFR2 and LFR3 had shown the most antioxidant potential. All the other samples had a similar result (about 500 µm/L) with LCR1 having a slightly detach in terms of antioxidant result.
872 1860 719 531 535 503 506 0 400 800 1200 1600 2000 400 mg/mL Trolox LFR2 LFR3 LFR1 LCR1 LFO2 LFO1 F E ( II ) C O N C E N T RA T IO N ( µ m o l/ L -1 ) FRAP