Introduction
Chronic kidney disease (CKD) is caused by a number of diseases that share a progressive decrease in the rate of glomerular filtration. Regardless of the initial cause, CKD progresses with glomerular sclerosis and interstitial fibrosis resulting in chronic renal failure1.
Given that people with CKD may develop renal failure, the use of substitute treatment techniques, such as hemodialysis (HD), is necessary. In HD, a semipermeable membrane is used to remove toxic substances and excess water from the body2.
Although HD prolongs the patient’s survival, it may cause complications such as nausea, vomiting, hypotension, headache, cramps, and chronic low-grade inflammation3.
Chronic low-grade inflammation affects 40% to 50% of people with CKD, it is characterized by elevated levels of inflammation markers and has been associated with malnutrition, cardiovascular disease, and mortality4-7. The causes of chronic low-grade inflammation in people with CKD is unknown but the possible causes include transmembrane passage of endotoxin during dialysis into the systemic circulation, the activation of neutrophils and monocytes during dialysis, activation of pro-inflammatory cytokines and endothelial changes8. Moreover, the
presence of inflammation in people with CKD is an independent predictor of mortality, reduced quality of life, and functional capacity9-12.
Thus, physical exercise plays a key-role as non-pharmacological treatment of CKD. Stack, Molony, Rives, Tyson, Murthy13 showed that the risk of death for CKD was lower for
active patients than for sedentary patients. Additional benefits
of exercise to patients with CKD include improved exercise tolerance, reduced cardiovascular risk, improved functional capacity, and improved glucose tolerance14, 15. Moreover, studies have reported that exercise can reduce inflammatory markers16,
17. However, remains scarce studies that have verified the effect of exercise on chronic low-grade inflammation markers in people with CKD.
In this context, intradialytic aerobic training (IAT) shows to be an important tool to CKD management. This kind of training is performed during HD session and previous studies reported improves in health outcomes18, physical capacity19, sleep quality20 and Kt/v21, an index which shows the dialysis efficiency.
However, remains scarce studies that evaluated the effect of IAT on chronic low-grade inflammation. Thus, the purpose of this study was to investigate the effect of 12 weeks of IAT on the serum levels of cytokines and functional capacity in people with CKD.
Materials and Methods
Participants and ethical aspects
Participants of this randomized controlled clinical study were treated using HD performed at the Renal Care Unit of the Federal University of Triângulo Mineiro. The inclusion criteria were: aged between 18 and 65 years, treatment with HD for more than three months with health conditions for the practice of physical
Original Article (short paper)
Intradialytic aerobic training improves inflammatory markers in patients with
chronic kidney disease: a randomized clinical trial
Lucas Gonçalves da Cruz1,3, Hugo Ribeiro Zanetti1, Alynne Christian Ribeiro Andaki1,2,
Gustavo Ribeiro da Mota1,2, Octávio Barbosa Neto1,2, Edmar Lacerda Mendes1,2
1Universidade Federal do Triângulo Mineiro, UFTM, Postgraduate Program in Physical Education, Uberaba, MG, Brazil;
2Universidade Federal do Triângulo Mineiro, UFTM, Department of Sport Sciences, Uberaba, MG, Brazil;
3Universidade Federal de Uberlândia, UFU, Postgraduate Program in Health Sciences, Uberlândia, MG, Brazil
Abstract — Aims: To investigate the effect of 12 weeks of intradialytic aerobic training on serum levels of
interleukin-1β, interleukin-6, interleukin-8, interleukin-10, tumor necrosis factor alpha and functional capacity.Methods:Thirty sedentary volunteers were randomly assigned to either to exercise or control group. Exercise group were submitted to 12 weeks of aerobic training performed on a cycle ergometer for 30 minutes at intensity rated using the Borg scale (6 to 7) three times a week while control group kept the daily habits. Results: After 12 weeks only exercise group presented a significant reduction of serum levels of interleukin-1β, interleukin-6, interleukin-8, tumor necrosis factor alpha and an increase in serum levels of interleukin-10 and functional capacity. Conclusion:Twelve weeks of intradialytic aerobic training was effective in controlling inflammation and improving the functional ability of patients with chronic kidney disease.
exercises, sedentary for at least three months prior to the study, and were not administrating anti-inflammatory drugs. Patients were excluded if they presented any acute inflammatory disease and requiring changes in access to hemodialysis, the hemodialysis time, ultrafiltrate volume and the type of filter used.
Following the selection of volunteers, 30 patients were randomly allocated to IAT (n = 15) and control (CON; n=15) groups. IAT group participated in twelve weeks of aerobic training while the CON group kept their usual daily routines. All volunteers read and signed the free and informed consent. The methods and study procedures were approved by the Ethics Committee in Research with Human Beings (Protocol number 1003882/2015).
Procedures
Participants were interviewed to obtain their demographic data partner. HD treatment time, Kt/V index, and information on the participant’s medical prescriptions were obtained from medical records. Before and after the 12 week study duration, the participants’ anthropometric measurements and blood samples, for immunological analysis were collected and participants also completed physical performance tests. The final study assessments were performed 72 hours after the last training session in order to avoid the last exercise session affecting the parameters evaluated.
Anthropometry
Body mass and height were measured using a mechanical scale and coupled stadiometer with a maximum capacity of 150 kg, a sensitivity of 100g, and an accuracy of 0.1 cm (Filizola, Campo Grande / MS, Brazil). Body mass index (BMI) was calculated using Quetelet’s index: body mass (kg) ÷ height2 (m2)].
Kt/V Index
For the calculation of Kt/V, the hemodialysis time values were used in hours (fixed in 4 hours), the value of the pre-serum urea and post-hemodialysis (measured by blood test), body mass post-dialysis patient, and ultrafiltrate volume (difference of body mass pre-and post-hemodialysis)2.
Blood sample
Participants were instructed to fast for 12-14 hours, abstain from alcohol consumption for 72 hours, and rest for 30 minutes before blood collection. The blood (20 ml) was collected using a vacuum system (BD, London, England) from the average cubital vein into tubes prepared with ethylenediamine tetra acetic acid (EDTA). Immediately after collection, the blood was taken for analysis or prepared for storage.
Inflammatory markers
The inflammatory markers evaluated were interleukin (IL) -1α, IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α). For the measure of inflammatory markers, blood was centrifuged at 3500 rpm for ten minutes and the serum was immediately separated in triplicate into Eppendorf tubes and frozen at -20°C. We used the cytometric bead array (CBA) method with commercial kits (BD Biosciences, San Jose, CA, USA) and automated equipment (Facscalibir, Becton Dickinson, USA).
Physical performance test
The six-minute walk test (6MWT) is a secure tool for assessment of the cardiorespiratory system. In cases where people with CKD is treated with HD, there is a strong correlation between the distance achieved in the 6MWT and VO2 peak; therefore, the 6MWT is a simple and inexpensive alternative to assess functional capacity22.
Patients performed the 6MWT two hours after meals and they were instructed to continue taking their usual medication. Patients rested for 10 minutes before taking the test. During this period, they were assessed for contraindications, blood pressure data, pulse oximetry, level of dyspnea (using the Borg scale) and heart rate23. The parameters evaluated before and after the test were blood pressure, heart rate, oxygen saturation, dyspnea, and respiratory rate. The reference equations were used to predict the total distance the participant walked during the 6MWT24.
Experimental Protocol
After three sessions of familiarization and adaptation with the exercise protocol, participants started IAT program performed on a mechanical cycle ergometer (Altmayer, BM 3600) controlled by the modified Borg scale23.
IAT was conducted in the first two hours of HD with an average duration of 36 minutes. IAT consisted of three stages: warm up, conditioning, and cool down. The warm up stage lasted for three minutes and the exercise intensity was low (Borg = 2-3); the conditioning stage lasted 30 minutes and the exercise intensity was severe (Borg = 6-7); the cool down stage lasted three minutes and the exercise intensity was low (Borg = 2-3). Blood pressure was monitored every 5 minutes at rest and after the cooling stage. Heart rate and oxygen saturation were constantly monitored (Polar S810i, Finland) and a portable oximeter (finger oximeter PM100C, New Tech, USA), respectively.
duration of the symptoms.
Statistical analysis
The normality of the data was verified by the Shapiro-Wilk test. The Mauchly test was used to verify the violation of sphericity (F) for repeated measures. In cases where the sphericity was violated, the Greenhouse-Geisser correction was used. To calculate main effect of time, group (IAT vs. CON), and interaction (time × group), a 2 × 2 repeated measures analysis of variance (ANOVA) was performed. The Cohen’s d was used to estimate the magnitude of the effect (η2) intervention as either small (η2 = 0.2), medium (η2 = 0.5), or large (η2 = 0.8). Statistical significance was set at α = 5% for all analyses. All analyses were performed using SPSS (version 20.0, SPSS Inc., Chicago, IL, USA).
Results
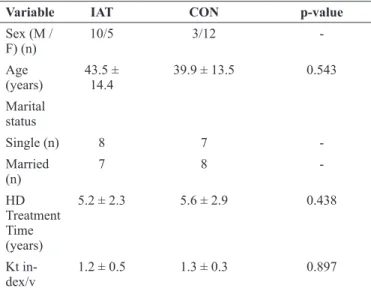
The socio-demographic data and Kt/V index of the participants are shown in Table 1. The majority of participants in the IAT group were male (67%), aged 43.5 ± 14.4, with HD treatment lengths of 5.2 ± 2.2 years. In the other hand, the majority of the participants in the CON group were female (80%), aged 39.9 ± 13.5, with HD treatment lengths of 5.6 ± 3.0 years. There were no significant differences (p>0.05) between the groups for age with HD treatment time and Kt/V index before the beginning of the study.
Table 1. Socio-demographic profile and Kt index/V of pa-tients with chronic kidney disease
Variable IAT CON p-value
Sex (M /
F) (n) 10/5 3/12
-Age (years)
43.5 ±
14.4 39.9 ± 13.5 0.543 Marital
status
Single (n) 8 7
-Married
(n)
7 8
-HD
Treatment Time
(years)
5.2 ± 2.3 5.6 ± 2.9 0.438
Kt in -dex/v
1.2 ± 0.5 1.3 ± 0.3 0.897
ITA = intradialytic aerobic training; CON = control; M = male; F = female; HD = hemodialysis; Kt/V = hemodialysis efficiency ratio
As demonstrated in Table 2, only IAT showed a significant reduction in BMI [F (1,28) = 41.317, p < 0.001; η 2 = 0.76] and increased the performance in the 6MWT [F (1,28) = 87.623, p <0.002; η 2 = 0.8].
Table 2. Body mass index and distance covered in the 6-min-ute walk test (6MWT)
Variable IAT CON p - value
Pre Post Pre Post
BMI (kg/
m2)
24.6 ±
3.7 22.9 ± 5.0* 23.6 ± 3.4 23.0 ± 4.9 0.001 6MWT
(m)
224.6
± 54.6 235.4 ± 55.7* 230.03 ± 60 224.32 ± 59.2 0.002
* Significant interaction effect between time and group (p < .05); IAT = in-tradialytic aerobic training; CON = control; BMI = body mass index; 6MWT = 6-minute walk test.
Furthermore after 12 weeks only IAT reduced the concentration of IL-1β [F (1,28) = 20.689, p = 0.027; η 2 = 0.62], IL-6 [F (1,28) = 17.646; p = 0.004; η 2 = 0.3], IL-8 [F (1,28) = 12.658; p = 0.013; η 2 = 0.6], TNF-α [F (1,28) = 53.310; p = 0.001; η 2 = 0.95], Table 3. Additionally, this group showed a significant increase in IL-10 [F (1,28) = 21.128; p = 0.042; η 2 = 0.79].
Table 3. Level of cytokines in patients with chronic kidney disease before and after the 12-week study duration
Variable
IAT group (n = 15)
CON group (n = 15)
p - value
Pre Post Pre Post
IL-1β (pg
/ L)
1.95 ±
0.99 1.18 ± 0.63 * 0.89 ± 0.47 1.17 ± 0.59 0.027 IL-6 (pg
/ L)
9.22 ±
4.48 5.45 ± 3.4 * 4.51 ± 5.05 4.8 ± 5.55 0.004 IL-8 (pg
/ L)
9.69 ±
2.48 7.97 ± 1.99 * 11.69 ± 3.18 10.39 ± 3.13 0.013 IL-10 (pg
/ L)
1.17 ±
0.69 2.72 ± 1.58 * 1.06 ± 0.68 0.93 ± 0.58 0.042 TNF-α
(pg / L)
13.12
± 3.8 11.04 ± 2.57 * ± 4.0813.61 14.87 ± 3.87 0.001
* Significant interaction effect between time and group (p < .05); IAT = intradialytic aerobic training; CON = control; IL = interleukin; TNF-α = tumor necrosis factor alpha; ANOVA: analysis of variance.
Discussion
To the best of our knowledge, this study was a pioneer to investigate the effect of IAT on CKD by assessing the participant’s inflammatory profile and the distance covered in the 6MWT. Our results show that 12 weeks of IAT is sufficient to reduce serum levels of inflammatory markers and increase the distance covered in the 6MWT.
is associated with several complications including reduced muscle mass, insulin resistance, malnutrition, atherosclerosis and, finally, cardiovascular disease28-30.
It is scientific knowledge that regular exercise has proven effective as an anti-inflammatory agent. During acute exercise there is a release of IL-6 from skeletal muscle which acts concomitantly with adrenal hormones e reduces the release and expression of TNF in monocytes and increases the IL-10 role in macrophage. Chronically, the exercise increases the number of circulating regulatory T cells and reduces the expression of toll-like receptors on monocytes and macrophages16, 31. Our results contributes to this theory, once only IAT group reduced the levels of pro-inflammatory circulating markers and increased an anti-inflammatory cytokine.
We observed a significant reduction in BMI (p<0.001). It is worth noting that reducing adiposity reduces the expression of pro-inflammatory cytokines, which is imperative for controlling inflammation in CKD patients32. Thus, regular and supervised physical activity should be encouraged during the treatment of CKD in order to counteract both the deleterious effects of the disease and the side effects of HD. The situation is worrying because the average level of physical activity is 50% to 120% lower in people with CKD than in controls33.
Moreover, our study showed an increase in the distance covered in the 6MWT. Previous studies show that individuals with CKD have a reduced exercise tolerance. Although the reason for this phenomenon is not fully understood, some research suggests the reduced exercise tolerance is related to muscle atrophy, myopathy, and malnutrition6, 28. We opted to the 6MWT due the efficient method to reproduce the VO2max and the correlation between walking distance and VO2max, as previously showed22. Volunteers of this study did not have heart failure, but the test has been applied in cases of patients with CKD due to cardiac affections generated by the condition.
The improved in 6MWT is relevant to patients with CKD once they have significantly lower values of functional capacity than the normal population, including to perform activities of daily living22, 34. Therefore, improving a patient with CKD’s physical condition may improve their quality of life35.
Regarding the limitations of our study, it is important to highlight the absence of a strict nutritional control among the study participants. Here, we demonstrate that the treatment for CKD needs to involve health professionals who can increase the quality of life and consequently the life expectancy of this population. Finally, the lack of previous studies using IAT limits the discussion of our results, mainly in relation to the impact of IAT on the inflammatory profile, mainly in patients with CKD. Thus, we suggest that this protocol of IAT can be replicated in further studies and compared with other methods of training.
Conclusion
We conclude that 12 weeks of IAT causes beneficial changes
in the body composition and functional capacity, and it reduces inflammatory markers in patients with CKD.
References
1. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16-23.
2. Breitsameter G, Figueiredo AE, Kochhann DS. Calculation of Kt/V in haemodialysis: a comparison between the formulas. J Bras Nefrol. 2012;34(1):22-6.
3. Rocco MV, Daugirdas JT, Greene T, Lockridge RS, Chan C, Pierratos A, et al. Long-term Effects of Frequent Nocturnal Hemodialysis on Mortality: The Frequent Hemodialysis Network (FHN) Nocturnal Trial. Am J Kidney Dis. 2015;66(3):459-68.
4. Astroth KS, Russell CL, Welch JL. Non-pharmaceutical fatigue interventions in adults receiving hemodialysis: a systematic review. Nephrol Nurs J. 2013;40(5):407-27; quiz 28.
5. Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009-16. 6. Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B,
Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005;67(4):1216-33. 7. Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T,
Ishiguro C, et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16(1):1-4.
8. Costa-Hong V, Bortolotto LA, Jorgetti V, Consolim-Colombo F, Krieger EM, Lima JJ. Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol. 2009;92(5):381-6, 98-403, 13-8.
9. Nascimento MM, Pecoits-Filho R, Lindholm B, Riella MC, Stenvinkel P. Inflammation, malnutrition and atherosclerosis in end-stage renal disease: a global perspective. Blood Purif. 2002;20(5):454-8.
10. Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome -- the heart of the matter. Nephrol Dial Transplant. 2002;17 Suppl 11:28-31.
11. Suliman ME, Stenvinkel P. Contribution of inflammation to vascular disease in chronic kidney disease patients. Saudi J Kidney Dis Transpl. 2008;19(3):329-45.
12. Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648-58. 13. Stack AG, Molony DA, Rives T, Tyson J, Murthy BV.
14. Daul AE, Schafers RF, Daul K, Philipp T. Exercise during hemodialysis. Clin Nephrol. 2004;61 Suppl 1:S26-30. 15. Oberley ET, Sadler JH, Alt PS. Renal rehabilitation:
obstacles, progress, and prospects for the future. Am J Kidney Dis. 2000;35(4 Suppl 1):S141-7.
16. Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis. 2004;43(4):607-16. 17. Cheema BS, O’Sullivan AJ, Chan M, Patwardhan A, Kelly
J, Gillin A, et al. Progressive resistance training during hemodialysis: rationale and method of a randomized-controlled trial. Hemodial Int. 2006;10(3):303-10. 18. Kim JS, Yi JH, Shin J, Kim YS, Han SW. Effect of acute
intradialytic aerobic and resistance exercise on one-day blood pressure in patients undergoing hemodialysis: a pilot study. J Sports Med Phys Fitness. 2018.
19. Sakkas GK, Hadjigeorgiou GM, Karatzaferi C, Maridaki MD, Giannaki CD, Mertens PR, et al. Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: a pilot study. ASAIO J. 2008;54(2):185-90. 20. Afshar R, Emany A, Saremi A, Shavandi N, Sanavi S.
Effects of intradialytic aerobic training on sleep quality in hemodialysis patients. Iran J Kidney Dis. 2011;5(2):119-23. 21. Mohseni R, Emami Zeydi A, Ilali E, Adib-Hajbaghery M,
Makhlough A. The effect of intradialytic aerobic exercise on dialysis efficacy in hemodialysis patients: a randomized controlled trial. Oman Med J. 2013;28(5):345-9.
22. Paula M. Correlation Between The Distance Covered in The Six-Minute Walk Test With Peak Oxygen Uptake in End-Stage Renal Disease Patients on Hemodialysis. Braz J Nephrol (J Bras Nefrol). 2007;29(2).
23. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-81.
24. Britto RR, Probst VS, de Andrade AF, Samora GA, Hernandes NA, Marinho PE, et al. Reference equations for the six-minute walk distance based on a Brazilian multicenter study. Braz J Phys Ther. 2013;17(6):556-63. 25. Kimoto E, Shoji T, Shinohara K, Hatsuda S, Mori K,
Fukumoto S, et al. Regional arterial stiffness in patients with type 2 diabetes and chronic kidney disease. J Am Soc Nephrol. 2006;17(8):2245-52.
26. Prinsen BH, Rabelink TJ, Beutler JJ, Kaysen GA, De Boer J, Boer WH, et al. Increased albumin and fibrinogen synthesis rate in patients with chronic renal failure. Kidney Int. 2003;64(4):1495-504.
27. Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014;29(9):1655-65.
28. Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594-601.
29. McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol. 2002;13(7):1928-36. 30. Wanner C, Zimmermann J, Schwedler S, Metzger T.
Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl. 2002(80):99-102.
31. Painter P, Moore G, Carlson L, Paul S, Myll J, Phillips W, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39(2):257-65.
32. Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25(4):352-64. 33. Johansen KL, Painter P. Exercise in individuals with CKD.
Am J Kidney Dis. 2012;59(1):126-34.
34. Cury JL, Brunetto AF, Aydos RD. Negative effects of chronic kidney failure on lung function and functional capacity. Rev Bras Fisioter. 2010;14(2):91-8.
35. Parsons TL, Toffelmire EB, King-VanVlack CE. The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol. 2004;61(4):261-74.
Corresponding author
Edmar Lacerda Mendes, Ph.D.
Postgraduate Program in Physical Education, Federal University of Triângulo Mineiro
Av. Tutunas, 490, Postcode, CEP 38025-440, Uberaba, Brazil Email: edmar.mendes@uftm.edu.br
Manuscript received on December 27, 2017 Manuscript accepted on May 28, 2018