ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Effect
of
vitamin
D
supplementation
on
patients
with
systemic
lupus
erythematosus:
a
systematic
review
Joyce
Ramalho
Sousa
a,
Érica
Patrícia
Cunha
Rosa
a,
Ivone
Freires
de
Oliveira
Costa
Nunes
b,∗,
Cecilia
Maria
Resende
Gonc¸alves
de
Carvalho
baFaculdadeSantoAgostinho(FSA),Teresina,PI,Brazil
bUniversidadeFederaldoPiauí(UFPI),Teresina,PI,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received22November2016
Accepted22May2017
Availableonline13October2017
Keywords:
VitaminD
Systemiclupuserythematosus
Supplementation
Systematicreview
a
b
s
t
r
a
c
t
Theobjectiveofthissystematicreviewwastoanalyzeclinicaltrialscarriedoutforthe
investigationoftheeffectofvitaminDsupplementationonsystemiclupus
erythemato-sus.TheresearchwasperformedfromAugusttoSeptember2016,withoutlimitsregarding
yearofpublication,restrictionofgender,age,andethnicity.Fortheguidingquestion,the
PICOstrategywasemployed.ToevaluatethequalityofthepublicationsthePRISMA
proto-colandJadadscalewereused.Theriskofbiasanalysisoftheclinicaltrialswasperformed
usingtheCochranecollaborationtool.Aftertheprocessofarticleselectionandremovalof
duplicates,fourarticleswereidentifiedaseligible.Theresultsofthreestudiesshoweda
positiveeffectofsupplementationondiseaseactivityreductionandsignificant
improve-mentinlevelsofinflammatorymarkers,fatigue,andendothelialfunction.Onlyonestudy
showednoimprovementindiseaseactivityaftersupplementation.Moreover,allstudies
showedanincreaseinserumvitaminDlevels.Thedatafromthisreviewprovideevidence
onthebenefitsofvitaminDsupplementationinpatientswithlupusandvitaminD
insuf-ficiency/deficiency.However,itisstillnecessarytoelucidatewhethervitaminDactsinthe
protectionagainstthismetabolicdisorder,aswellasthestandardizationofthetype,dose
andtimeofvitaminDsupplementation.
©2017PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC
BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:ivonefreirescosta@ufpi.edu.br(I.F.Nunes).
http://dx.doi.org/10.1016/j.rbre.2017.08.001
2255-5021/© 2017 Published by Elsevier Editora Ltda. This is an open access article under the CC BY-NC-ND license (http://
Efeito
da
suplementac¸ão
com
vitamina
D
em
pacientes
com
lúpus
eritematoso
sistêmico:
uma
revisão
sistemática
Palavras-chave:
VitaminaD
Lúpuseritematososistêmico
Suplementac¸ão
Revisãosistemática
r
e
s
u
m
o
Oobjetivodestarevisãosistemáticafoianalisarensaiosclínicosrealizadosnainvestigac¸ão
doefeitodasuplementac¸ãocomvitaminaDsobreolúpuseritematososistêmico.Apesquisa
foirealizadanasbasesdedadosScopus,PubMedeBibliotecaCochrane,noperíododeagosto
asetembrode2016,semlimitedeanodepublicac¸ão,restric¸ãodegênero,idadeeetnicidade.
ParaaquestãonorteadorafoiempregadaaestratégiaPICO.Paraavaliaraqualidadedas
publicac¸õesutilizou-seoprotocoloPRISMAeaescaladeJadad.Aanálisedoriscodeviés
dosensaiosclínicosocorreupelaferramentadecolaborac¸ãoCochrane.Apósoprocessode
selec¸ãoeremoc¸ãodeartigosduplicados,quatroartigosforamidentificadoscomoelegíveis.
Osresultadosdetrêsestudosmostraramefeitopositivodasuplementac¸ãonareduc¸ãoda
atividadedadoenc¸aemelhorasignificativanosníveisdemarcadoresinflamatórios,fadiga
efunc¸ãoendotelial.Emapenasumestudonãohouvemelhoranaatividadedadoenc¸aapós
asuplementac¸ão.Ademais,todososestudosapresentaramaumentodosníveisséricosde
vitaminaD.Osdadosdessarevisãofornecemevidênciasdosbenefíciosdasuplementac¸ão
comvitaminaDsobreolúpusempacientescominsuficiência/deficiência.Contudo,ainda
énecessárioelucidaraatuac¸ãodonutrientenaprotec¸ãocontraessedistúrbiometabólico,
bemcomoapadronizac¸ãodotipo,doseetempodesuplementac¸ãocomvitaminaD.
©2017PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma
licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Systemiclupuserythematosus(SLE)isachronic,autoimmune
inflammatorydiseasethataffectsseveralorgansandsystems
ofthebody.Itscauseandepidemiologyareunknown,since
acombinationoffactors,includingethnicity,gender,genetic
andenvironmentalaspectsareinvolved.Thediseaseaffects
30–50/10,000individualsworldwide,mostfrequentlyaffecting
youngwomenofreproductiveage,affecting10–12womenfor
eachman.1,2
Recentstudieshavehighlightedtherole ofvitaminDin
the developmentofautoimmune diseases.Vitamin D
defi-ciencyseems tobeassociatedwithSLEactivity,partlydue
todysregulationincytokineproductionbalance.The
photo-sensitivityandrecommendationofsunscreenuse,aswellas
othermeasuresforlesssunexposure,mayfavorthereduction
ofcutaneousvitaminDsynthesis.3
Therefore, due to the importance of the vitamin D-SLE
binomial,theaimofthisreviewwastoevaluatetheeffects
of vitamin D supplementation on systemic lupus
erythe-matosus,contributingtotheincreaseofknowledgebasedon
scientificevidence,consideringthat thesubjectisrelevant,
newand,therefore,requiresmorediscussiontoadequately
guidethedecision-makingbyhealthprofessionals.
Methods
A SystematicReview (SR) ofstudies on vitaminD
supple-mentationinpatients withSystemic LupusErythematosus
wasperformed.ThePICOstrategywasusedtoestablishthe
guidingquestionofthepresentstudy,whichrepresentsthe
acronymforproblemorpopulation(P),intervention(I),
com-parison (C) and outcome (O). These four components are
the fundamental elementsofthe researchquestion andof
the question construction for the bibliographic search for
evidence.4 Thisstrategyculminatedinthedefinitionofthe
following guiding question: Does vitamin D
supplementa-tionleadtoclinicalimprovementinSLEpatients?EachPICO
domaincorrespondedtothefollowingelements:(P)Patients
with systemic lupus erythematosus, (I) Vitamin D
supple-mentation,(C)Placebo and(O)clinicalimprovementofSLE
patients.
Thesearch wascarriedout inthe Scopus,PubMed, and
CochraneLibrarydatabasesforpublishedclinicaltrialswith
no limit regarding year of publication, and no restriction
regarding gender, age, and ethnicity, all published in the
Englishlanguage.TheBooleanconnector“and”wasusedin
thecombinationoftheMedicalSubjectHeading(MeSH)terms:
systemiclupuserythematosusandclinicaltrial,vitaminDand
supplementation and vitamin D and supplementation and
clinicaltrial.
Thetitlesandabstractsoftheselectedarticleswere
ana-lyzedtoverifywhethertheymettheinclusioncriteria:having
a controlled clinical trial design and being available as a
full-textarticle.Theevaluationoftheeligibilitycriteriawas
performedindependentlybythetwoauthors,andincaseof
divergence,athirdresearcherwasconsulted.
To ensurethe SRquality, the Preferred Reporting Items
for Systematic Reviews, and Meta-Analyses (PRISMA)
pro-tocol wasused.5 TheJadad scale6 wasused independently
bytwoblindedresearchersforthequalitativeclassification.
Scoreswereassignedtothestudies(fromzerotofive),based
onthecriteria: randomizationmethod(sequencesand
ran-domizationcriteriaofparticipants),blinding(forpatientsand
researchers)anddescriptionoffollow-uplossproportion.The
riskofbiasintheclinical trialsincluded inthisstudy was
Table1–AnalysisofthemethodologicalqualityandriskofbiasproposedbytheCochranecollaboration.
Variables Abou-Rayaetal.8
(2013)
Aranowetal.9 (2015)
Kamen;Oates10 (2015)
Limaetal.11 (2016)
Randomsequencegeneration Uncertain Uncertain Lowrisk Uncertain Allocationconcealment Lowrisk Lowrisk Lowrisk Lowrisk Blindingofparticipantsandpersonal Lowrisk Lowrisk Lowrisk Lowrisk Blindingofoutcomeevaluators Lowrisk Lowrisk Lowrisk Lowrisk Incompleteoutcomedata Lowrisk Lowrisk Lowrisk Lowrisk Selectiveoutcomereporting Lowrisk Lowrisk Lowrisk Uncertain Othersourcesofbias Lowrisk Lowrisk Lowrisk Lowrisk
Results
Thebibliographic search,carried out according to the
pre-establishedstrategy,resultedin25articles. Ofthese,seven
studieswerefromtheScopusdatabase,eightfrom PubMed
andten fromCochrane.Afterthe selectionandremovalof
articlesinduplicate,fouroriginal articlesfrom randomized
controlledtrials(RCTs)wereidentifiedaseligibleforthis
sys-tematicreview.Fig.1showstheflowchartofthesearchresult
intheinformationsources,theselectionandinclusionofthe
originalarticles intheSystematic Review,accordingtothe
PRISMAstatementprotocol.
Theclinicaltrials showedhomogeneousmethodological
quality based on biasrisk assessment using the Cochrane
tool (Table 1). Randomization was adequately reported in
25%(1/4) ofthe studies,whileallocationsequence
genera-tion,blindingofoutcomeevaluators,blindingofparticipants
andprofessionals,incomplete outcomes,and othersources
ofbiasshowed100%(4/4)oflowriskandselectiveoutcome
reportshowed75%(3/4)oflowrisk.Table2showstheresults
ofthe quality evaluation of articles analyzed according to
Jadadscale.Inrelationtotheassesseditems,allarticles
ade-quatelydescribedtheaspectsassessedintheaforementioned
scale.
Table2–EvaluationoftheclinicaltrialsusingtheJadad scale.
Author(year) Score
Abou-Rayaetal.8(2013) 5points
Aranowetal.9(2015) 5points KamenandOates10(2015) 5points Limaetal.11(2016) 5points
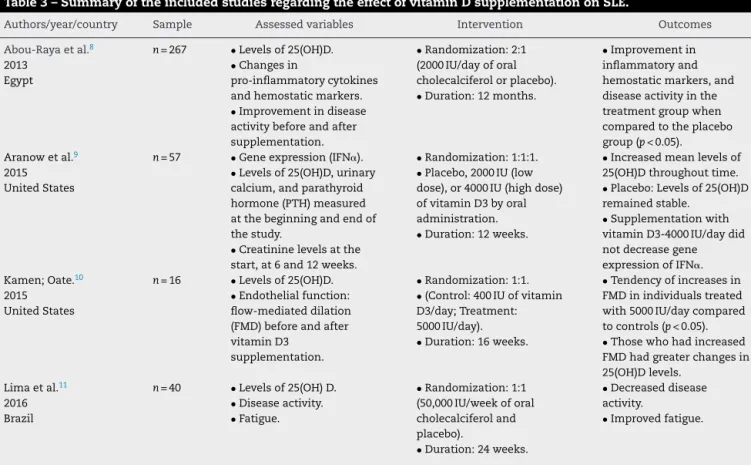
The data shown in Table 3 encompass the results of
thereviewedarticles,includingauthors,yearofpublication,
geographic location, study sample size, dose, duration of
supplementationand mainoutcomes.Itwasobservedthat
clinicaltrialswerecarriedoutindifferentcountries.Dosesof
vitaminDsupplementationrangedfrom400IUto500,000IU,
andthedurationoftheinterventionlastedfrom12weeksto
oneyear.
The main variables investigated were: changes in
lev-elsofpro-inflammatorycytokinesandhomeostaticmarkers,
IFN␣geneexpression,urinarycalciumlevels,creatinineand
parathormone(PTH)levels,endothelialfunction,fatigueand
diseaseactivity,andserumlevelsofvitaminD.
The results ofthe four clinical trials showed that
vita-minDsupplementationincreasedserumvitaminlevels.Three
Studies identified through database search: Scopus n = 07; PubMed n = 08; Cochrane n = 10
Total: n = 25 articles
IInclusion
Elegibilidade
Selected studies
Scopus n = 07; PubMed n = 04; Cochrane n = 02
Studies excluded due to Duplicity (n = 12)
Articles (full texts) of selected articles (n = 04)
Articles excluded based on the inclusion and exclusion
criteria (n = 09)
Studies included in the qualitative analysis
(n = 04)
Identification
Screening
Table3–SummaryoftheincludedstudiesregardingtheeffectofvitaminDsupplementationonSLE.
Authors/year/country Sample Assessedvariables Intervention Outcomes
Abou-Rayaetal.8 2013
Egypt
n=267 •Levelsof25(OH)D. •Changesin
pro-inflammatorycytokines andhemostaticmarkers. •Improvementindisease activitybeforeandafter supplementation.
•Randomization:2:1 (2000IU/dayoforal cholecalciferolorplacebo). •Duration:12months.
•Improvementin inflammatoryand hemostaticmarkers,and diseaseactivityinthe treatmentgroupwhen comparedtotheplacebo group(p<0.05). Aranowetal.9
2015 UnitedStates
n=57 •Geneexpression(IFN␣). •Levelsof25(OH)D,urinary calcium,andparathyroid hormone(PTH)measured atthebeginningandendof thestudy.
•Creatininelevelsatthe start,at6and12weeks.
•Randomization:1:1:1. •Placebo,2000IU(low dose),or4000IU(highdose) ofvitaminD3byoral administration. •Duration:12weeks.
•Increasedmeanlevelsof 25(OH)Dthroughouttime. •Placebo:Levelsof25(OH)D remainedstable.
•Supplementationwith vitaminD3-4000IU/daydid notdecreasegene expressionofIFN␣. Kamen;Oate.10
2015 UnitedStates
n=16 •Levelsof25(OH)D. •Endothelialfunction: flow-mediateddilation (FMD)beforeandafter vitaminD3
supplementation.
•Randomization:1:1. •(Control:400IUofvitamin D3/day;Treatment: 5000IU/day). •Duration:16weeks.
•Tendencyofincreasesin FMDinindividualstreated with5000IU/daycompared tocontrols(p<0.05). •Thosewhohadincreased FMDhadgreaterchangesin 25(OH)Dlevels.
Limaetal.11 2016 Brazil
n=40 •Levelsof25(OH)D. •Diseaseactivity. •Fatigue.
•Randomization:1:1 (50,000IU/weekoforal cholecalciferoland placebo).
•Duration:24weeks.
•Decreaseddisease activity.
•Improvedfatigue.
studiesshowedapositiveeffectofsupplementationon
dis-ease activity and a significant improvement in levels of
inflammatorymarkers,fatigue,andendothelialfunction.Only
onestudy showednoimprovementindiseaseactivity after
supplementation.
Discussion
Most of the clinical trials included in this review found
improvementinserumvitaminDlevelsandtheparameters
involvedwith SLE.8,10,11 Abou-Raya et al.8 studiedpatients
with SLE randomized 2:1 to receive 2000IU of oral
chole-calciferolper day orplacebo for12 months. Atthe end of
the treatment, 25(OH)D levels were increased. The
supple-mentedgroupshowedameanof37.8±16.3ng/mLcompared
totheplacebogroup,with19.9±16.2ng/mL(p<0.05).
More-over, therewas a significant improvement in the levels of
inflammatory and hemostatic markers, as well as in the
diseaseactivityscoremeasured bySLEDAI(SystemicLupus
ErythematosusDiseaseActivityIndex),inwhichpatientswith
vitaminDdeficiencyhadaworseSLEDAIscore.
InthestudybyKamenandOates,10theparticipantswere
randomized1:1toreceive1of2dailyoraldosesofvitamin
D3.Group1(control)received400IUofvitaminD3dailyand
Group2(treatment)received5000IU.Attheendofthe
exper-iment,therewasanimprovementinendothelialfunctionin
supplementedpatientswhencomparedtocontrols,witha
sig-nificantincreasetrendintheFMD(flow-measureddilation).
ThosewhohadanincreaseinFMDhadsignificantlygreater
changesin25(OH)Dlevels,i.e.,itwasobservedthatthehigher
thevitaminDlevel,thehighertheFMD(p<0.05).
Limaetal.11measuredserumlevelsof25(OH)D,assessed
disease activity and fatigue for a period of 24 weeks in
patientswithjuvenile-onsetSLE,whowererandomized(1:1)
toreceiveoralcholecalciferol,50,000IU/weekorplacebo.After
theintervention,itwasobservedthatsupplementationwith
cholecalciferolwaseffectiveinreducingdiseaseactivityand
improving fatigue in patients with juvenile SLE. Disease
activity was assessed by SLEDAI. Fatigue scores, including
low-intensityfatigue,fatigueduringexercise,andfatigueat
mediumeffort,were significantlylower inpatients
supple-mentedwithvitaminD(p<0.05).
However, Aranow et al.,9 when assessing 57
North-American women with SLE, randomized 1:1:1 to receive
2000IU(lowdose),4000IU(highdose)ofvitaminD3,orplacebo
orally per day for 12 weeks, found that vitamin D values
remainedstable(30ng/mL)inpatientsreceivingplaceboand
lowdose.Thehighdosegroupachievedslightlyhigherlevels
(30–35ng/mL).However,noeffectwasobtainedregardingthe
decrease inIFN␣ geneexpression,andnosignificant
corre-lationswerefoundbetweenvitaminDsupplementationand
SLEdiseaseactivity(p>0.05).Additionally,therewasno
cor-relationbetween25(OH)Dandchangesingeneexpression.
Itisemphasized that adailyconsumptionof4000IUof
vitaminD3wasconsideredsafeandwelltolerated,withno
signsoftoxicity.Casesofhypercalcemiaweremildand
infre-quent.Anunexpectedfactwasalsoobserved,characterized
byadecreaseinneutrophils,whichwasnotassociatedwith
adverseeffects.9
Moreover,itisworthnotingthestudybyAndreolietal.,12
whichshowedthatinSLEpatientsduringaone-year
supple-mentationwithcholecalciferolreceivingeitherthestandard
bolusof300,000IU,followedby50,000IUmonthly), the
lat-ter(withhigh doseofvitaminD)wassafeand effectivein
attainingsufficientvitaminlevels(>30ng/mL).However,both
supplementationregimensshowednodifferenceintheway
theyaffected diseaseactivityasassessedbySLEDAI,orthe
serologyforSLE(p>0.05).
In another study,Ruiz-Irastorza et al.13 when they
per-formedalongitudinalobservationalstudy with47patients
withSLEwhoreceived800IU/dayofvitaminD3duringamean
periodof24months(range5–24months)andanother10who
received600IU/day duringthe24-monthperiod(range7–24
months) foundatthe end ofthe experimentthat 25(OH)D
levelsincreasedin alltreated patients.Themean levels of
25(OH)Dincreasedfrom 21.7ng/mLto24.8ng/mL;however,
therewasnoimprovementinSLEactivity.Thesameauthors
recommend the use of vitamin D3 at higher doses than
800IU/dayforpatientswithSLEandvitaminDinsufficiency
ordeficiency.
InthestudybyReynoldsetal.,14oralcholecalciferol
supple-mentation(400,000IUfollowedby20,000IUweekly)inpatients
withvitaminDdeficiencypositively modulatedendothelial
functioninindividualswithstableSLE,regardlessofdisease
activity.TheseobservationssupportaroleforvitaminDin
car-diovascularhealthimprovement,byreducingtheriskofthis
diseaseinSLE.
Theresults by Petriet al.15 when assessing 763
North-Americanpatientswithameanageof49.6±13.2yearswith
SLE,observedthatindividualswithlowlevelsof25(OH)D
sup-plementedwith50,000IUofvitaminD2weeklyandwith200IU
ofCa/D3,twiceadayfor128weekshadamodest,but
signifi-cantreductionintheriskofincreaseddiseaseactivity(p<0.05)
andalsoanassociationwith25(OH)Dincreaseinthesubsetof
patientswithlowvitaminDlevels.
Terrier et al.16 foundan important increase in25(OH)D
serum levels and regulatory T-cells in 20 French patients
withhypovitaminosisD,whosemeanagewas31.0±8.0years
included in the supplementation program with 100,000IU
cholecalciferol/4 weeks, followed by 100,000IU
cholecalcif-erol/month for 6 months. There was also a decrease in
memoryBcellsandeffectorTcells(Th1andTh17),thusbeing
effectiveininducinganimmunomodulatoryeffectinlupus.
In parallel, the participation of anti-vitamin D
antibod-ies in the metabolic process of vitamin D is emphasized,
especiallybecausetheyareassociatedtothecapacityto
con-tributetotheirclearancebythebody.InthestudybyCarvalho
et al.17 171 patients with lupus were evaluated and
anti-vitaminDantibodieswerepresentin4%ofthesample.Asfor
25(OH)Dlevels,therewasnostatisticallysignificantdifference
when comparingthose with (28.4±9.6ng/mL) and without
(26.4±13.9ng/mL)(p>0.05)autoantibodies.
Moreover,this same study17 found thatthe presenceof
anti-DNAdouble-strandedantibodies (anti-dsDNA) wasthe
onlyonethatshowedastrongassociationwithanti-vitaminD
antibodies,suggestingthattheseantibodies,eventhoughthey
werefoundatlowfrequency,mayimplyinreducedserum
lev-elsofvitaminD.However,furtheranalysisisneededonthe
potentialdiagnosticandprognosticroleofthesenew
antibod-iesinSLE.
Itisnoteworthythat therewas adiscrepancy regarding
thedosesandtimeofsupplementationusedinthestudies
analyzed in this review. The studies were carried out in
different populations, which may have contributed to the
divergencesoftheidentifiedresults.
Conclusions
The results of this study showed that vitamin D
supple-mentation,inmostpublications,improvedserumvitaminD
levels,diseasesymptomsandcomplications,withsignificant
improvementinthelevelsofinflammatorymarkers,fatigue,
andendothelialfunction.
Theresultsofthisreviewprovideevidenceofthebenefitsof
vitaminDsupplementationinindividualswithSLEand
vita-minDdeficiency/insufficiency.However,furtherintervention
studiesarerequiredtoverifyamoreeffectivetherapyto
pro-tectthebodyfromthedeleteriouseffectsofthisautoimmune
disorder.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.DanchenkoN,SatiaJA,AnthonyMS.Epidemiologyof systemiclupuserythematosus:acomparisonofworldwide diseaseburden.Lupus.2006;15:308–18.
2.EdensC,RobinsonAB.Systemiclupuserythematosus,bone health,andosteoporosis.CurrOpinEndocrinolDiabObes. 2015;22:422–31.
3.LimaGL.Avaliac¸ãodasuplementac¸ãodevitaminaDem
pacientescomlúpuseritematosodeiníciojuvenil:estudo
clínicorandomizado,duplo-cego,controladoporplacebo.
LIMA,2015.79f.Tese(doutorado)-FaculdadedeMedicinada
UniversidadedeSãoPaulo,Programadeciênciasmédicas,
Áreadeconcentrac¸ão:Processosinflamatóriosealérgicos.
SãoPaulo.2015.
4.SantosCMC,PimentaCM,NobreMRC.ThePICOstrategyfor theresearchquestionconstructionandevidencesearch.Rev Latino-AmEnfermagem.2007;15:508–11.
5.MoherD,LiberatiA,TetzlaffJ,AltmanDG,PRISMAGroup. Preferredreportingitemsforsystematicreviewsand meta-analyses:thePRISMAstatement.AnnInternMed. 2009;151:264–9.
6.JadadAR,MooreRA,CarrollD,JenkinsonC,ReynoldsDJM, GavaghanDJ,etal.Assessingthequalityofreportsof randomizedclinicaltrials:isblindingnecessary.ControlClin Trials.1996;17:1–12.
7.DeCarvalhoAPV,SilvaV,GrandeAJ.Avaliac¸ãodoriscode viésdeensaiosclínicosrandomizadospelaferramentada colaborac¸ãoCochrane.RevDiagnTratamento.2013;18:38–44.
8.Abou-RayaA,Abou-RayaS,HelmiiM.TheeffectofvitaminD supplementationoninflammatoryandhemostaticmarkers anddiseaseactivityinpatientswithsystemiclupus erythematosus:arandomizedplacebo-controlledtrial.J Rheumatol.2013;40:265–72.
9.AranowC,KarmenDL,Dall’EraM,MassarottiEM,MackayMC, KoumpourasF,etal.Randomized,double-blind,
10.KamenDL,OatesJC.ApilotstudytodetermineifvitaminD repletionimprovesendothelialfunctioninlupuspatients. AmJMedSci.2015;350:302–7.
11.LimaGL,PaupitzJ,AikawaNE,TakayamaL,BonfaE,Pereira RMR.VitaminDsupplementationinadolescentsandyoung adultswithjuvenilesystemiclupuserythematosusfor improvementindiseaseactivityandfatiguescores:a randomized,double-blind,placebo-controlledtrial.Arthritis CareRes.2016;68:91–8.
12.AndreoliL,Dall’AraF,PiantoniS,ZanolaA,PivaN,CutoloM, etal.A24-monthprospectivestudyontheefficacyandsafety oftwodifferentmonthlyregimensofvitaminD
supplementationinpre-menopausalwomenwithsystemic lupuserythematosus.Lupus.2015;24:499–506.
13.Ruiz-IrastorzaG,GordoS,OlivaresN,EqurbideMV,AquirreC. ChangesinvitaminDlevelsinpatientswithsystemiclupus erythematosus:effectsonfatigue,diseaseactivity,and damage.ArthritisCareRes.2010;62:1160–5.
14.ReynoldsJA,HaqueS,WilliamsonK,RayDW,AlexanderMY, BruceIN.VitaminDimprovesendothelialdysfunctionand restoresmyeloidangiogeniccellfunctionviareduced CXCL-10expressioninsystemiclupuserythematosus.Sci Rep.2016;6:1–11.
15.PetriM,BelloKJ,FangH,MagderLS.VitaminDinsystemic lupuserythematosus:modestassociationwithdisease activityandtheurineprotein-to-creatinineratio.Arthritis Rheum.2013;65:1865–71.
16.TerrierB,DerianN,SchoindreY,ChaaraW,ZahrN,
MariampillaiK,etal.RestorationofregulatoryandeffectorT cellbalanceandBcellhomeostasisinsystemiclupus erythematosuspatientsthroughvitaminDsupplementation. ArthritisResTher.2012;14:1–10.