RESEARCH ARTICLE
Minocycline protects against oxidative damage and alters energy
metabolism parameters in the brain of rats subjected to chronic
mild stress
Gislaine Z. Réus&Helena M. Abelaira&Amanda L. Maciel&Maria Augusta B. dos Santos&
Anelise S. Carlessi&Amanda V. Steckert&Gabriela K. Ferreira&Samira D. De Prá&
Emilio L. Streck&Danielle S. Macêdo&João Quevedo
Received: 4 June 2014 / Accepted: 6 August 2014 / Published online: 13 August 2014
#Springer Science+Business Media New York 2014
Abstract Studies have been suggested that minocycline can
be a potential new agent for the treatment of depression. In addition, both oxidative stress and energy metabolism present an important role in pathophysiology of depression. So, the present study was aimed to evaluate the effects of minocycline on stress oxidative parameters and energy metabolism in the brain of adult rats submitted to the chronic mild stress protocol (CMS). After CMS Wistar, both stressed animals as controls received twice ICV injection of minocycline (160 μg) or vehicle. The oxidative stress and energy metabolism parame-ters were assessed in the prefrontal cortex (PF), hippocampus (HIP), amygdala (AMY) and nucleus accumbens (Nac). Our
findings showed that stress induced an increase on protein carbonyl in the PF, AMY and NAc, and mynocicline injection reversed this alteration. The TBARS was increased by stress in the PF, HIP and NAc, however, minocycline reversed the alteration in the PF and HIP. The Complex I was incrased in AMY by stress, and minocycline reversed this effect, however reduced Complex I activity in the NAc; Complex II reduced in PF and AMY by stress or minocycline; the Complex II-III increased in the HIP in stress plus minocycline treatment and in the NAc with minocycline; in the PF and HIP there were a reduced in Complex IV with stress and minocycline. The creatine kinase was reduced in AMY and NAc with stress and minocycline. In conclusion, minocycline presented neuroprotector effects by reducing oxidative damage and reg-ulating energy metabolism in specific brain areas.
Keywords Minocycline . Oxidative stress . Energy
metabolism . Depression
Introduction
Depression is a serious disorder that has enormous conse-quences for the quality of life and can cause severe impair-ment in occupational, social, or other important areas of functioning (American Psychiatric Association, 2013). Still, depression is a clinically and biologically heterogeneous dis-order, with 10–30 % of women and 7–15 % of men suscep-tible to depression in their lifetime (Kessler and Wang,2008). For many decades, the accepted pathogenesis of major de-pression has involved the dysfunction of the monoaminergic system. In fact, the drugs which enhance monoamine function show efficacy for depression promoting serotonergic and norepinephrinergic neurotransmission (Pae et al., 2008). G. Z. Réus
:
H. M. Abelaira:
A. L. Maciel:
M. A. B. dos Santos:
A. S. Carlessi
:
A. V. Steckert:
J. QuevedoLaboratório de Neurociências, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil
G. Z. Réus
:
J. QuevedoCenter for Experimental Models in Psychiatry, Department of Psychiatry and Behavioral Sciences, Medical School, The University of Texas Health Science Center at Houston, Houston, TX, USA
G. K. Ferreira
:
S. D. De Prá:
E. L. StreckLaboratório de Bioenergética, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil
D. S. Macêdo
Neuropharmacology Laboratory, Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceara, Fortaleza, CE, Brazil
G. Z. Réus (*)
Department of Psychiatry and Behavioral Sciences, Center for Experimental Models in Psychiatry,Medical School, The University of Texas Health Science Center at Houston, Houston, TX, USA e-mail: gislainezilli@hotmail.com
However, such drugs are associated with some limitations, for example, only one-third of patients undergoing monotherapy with an antidepressant achieve complete remission of depres-sive symptoms and gain functional recovery (Skolnick,
2002).In this regard, the minocycline, a second-generation tetracycline, which presents powerful anti-inflammatory and neuroprotective effects, has been investigated as a potential new agent for the treatment of major depression (Pae et al.,
2008). Minocycline effectively crosses the bloodebrain barrier leading to the inhibition of cytochrome c release from the mitochondria, inhibition of caspase expression, and the sup-pression of microglial activation (Domercq and Matute,2004; Kim and Suh,2009). Moreover, minocycline has a regulatory effect on pro-inflammatory agents, such as nitric oxide (NO), tumor necrosis factor-alpha (TNF-a), and interleukin- 1β(Lai and Todd,2006), which are consistently reported to be in-creased in patients with major depression, and in other hand are normalized after antidepressant treatment (Maes,1995; Herken et al.,2007). Although many has been known about the neurobiology of depression in recent years, its aetiology is still largely unknown. However, recent theoretical focus has been on biological factors, including a wealth of information supporting stress as a causal factor in depression, largely concerning chronic stress-related hypothalamic–pituitary– adre-nal (HPA) axis dysregulation and toxicity from excessive glu-cocorticoid release (Lupien et al.,2009). Though other theories posit that a downregulation of hippocampal neurogenesis un-derlies the disorder (Kempermann and Kronenberg,2003), or suggest genetic or epigenetic factors for developing depression (Karg et al.,2011; Menke et al.,2012). In this way, many pre-clinical studies attempting to model aspects of depression have focused on behaviors thought to represent anhedonia, reduced locomotor activity or behavior despair by exposure to different kinds of mild stress every day (Lu et al.,2006; Rygula et al.,
2005; Willner,1997). Also, unpredictable stressors have greater negative impact in humans than predictable ones, perhaps due to temporal uncertainty and inability to anticipate the event (Anisman and Matheson,2005; Willner and Mitchell,2002).
Some evidence have reported that major depression, as well as the drugs which are used to treat this disorder can produce several adverse outcomes, among which impairment on mitochondrial function, increased production of free radi-cals and oxidative stress, leading to cellular changes and significantly affecting the patient’s quality of life (Duncan and Thompson, 2003). In this way, the present study was aimed to evaluate the effects of minocycline on stress oxida-tive parameters and energy metabolism in the brain of adult rats submitted to the chronic mild stress protocol.
Materials and methods
Animals
Male Adult Wistar rats (60 days old) were obtained from the breeding colony at UNESC (Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil). They were housed five per cage with food and water available ad libitum (except on the day that the stressor used in the stress protocol were deprived of water or food) and were maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.). All experimental procedures involving animals were performed in accordance with the NIH Guide for the Care and Usage of Laboratory Animals and under the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care, and with approval by the local Ethics Committee under protocol number 45/2012.
Experimental design and treatment
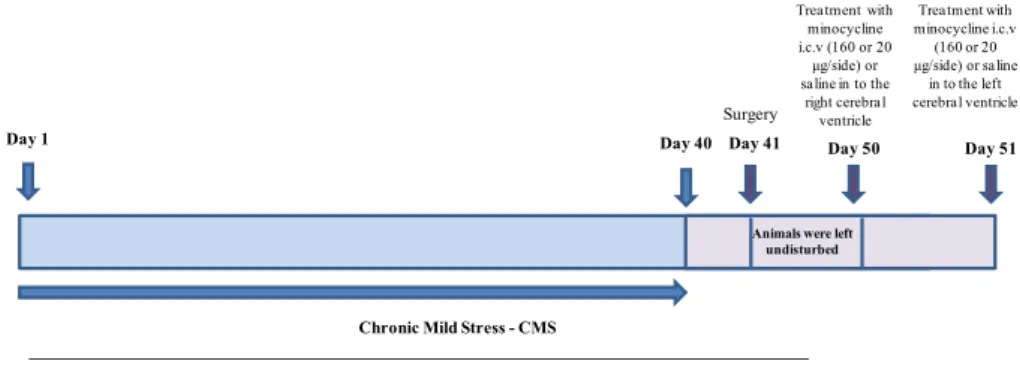
For this study, male rats used were divided into 4 experimental groups (n= 5–8): 1) Control + saline; 2) Control + minocycline; 3) Stress + saline; and 4) Stress + minociclyne. Firstly, the animals were subjected to the chronic mild stress protocol for 40 days and in the the forty-first day a surgical procedure was performed in the stereotaxic apparatus (Fig.1).
Fig. 1 Schematic drawing of the stress protocol and treatment with
minocycline. CMS procedures will be performed for 40 days. Surgery will be realized day 41st. In the fiftieth day minocycline (160 or 20μg/
After surgery, the animals were left undisturbed for nine days until the start of treatment. Minocycline hydrochloride pur-chased from (Sigma-Aldrich Corp., St Louis, USA) was dis-solved in physiological saline. In the first day of treatment, rats received bilateral microinjection of different amounts of minocycline (160 or 20μg/side) or saline (control) into the right cerebral ventricle; and in the second day of treatment, the bilateral microinjection of minocycline (160 or 20μg/side) or saline was into the left cerebral ventricle (Arakawa et al.,
2012). A total volume of 4.0μl was infused into each side over 10 min, and the injection syringe was left in place for an additional 5 min to allow for diffusion. After treatment, the animals were killed by decapitation and the prefrontal cortex, hippocampus, amygdala and nucleus accumbens were re-moved for biochemical analyzes.
Chronic mild stress protocol
The chronic mild stress (CMS) protocol was adapted from the procedure described by Gamaro et al. (2003). The animals were divided in two groups: control and stressed. The control groups were kept undisturbed in their home cages during the 40 days of treatment receiving only ordinary daily care with daily supports of food and water. The 40-days chronic mild stress paradigm was used for the animals in the stressed group. Individual stressors and length of time applied each day were as follows. The following stressors were used: (i) 24 h food deprivation was applied on days 2, 7, 15, 21 and 30; (ii) 24 h water deprivation on days 1, 10, 20 and 33; (iii) 1–3 h restraint on days 9, 23 and 31, (iv) 1.5–2 h restraint at 4 °C on days 13, 26 and 34; (v) forced swimming for a duration of 10 or 15 min on days 8, 16, 27, 35 and 40; (vi) flashing light over a duration of 120–210 min on days 6, 14, 22, 28, 32 and 39; (vii) isolationon days 3, 4, 5, 17, 18, 19, 24, 25, 36 and 37. Stressor stimuli were applied at different times every day, in order to minimize its predictability. The restraint test was carried out by placing the animal in a 25 × 7 cm plastic tube and adjusting it with plaster tape on the outside, so that the animal was unable to move. There was a 1 cm hole at the far end for breathing. The Forced swimming was carried out by placing the animal in a glass tank measuring 50 × 47 cm filled with 30 cm of water at 23±2 °C in which the animal cannot touch the bottom. The exposure to flashing light test was undertaken by placing the animal in a 60 × 60 x 25 cm plywood box divided into 16 cells of 15 × 15 × 25 cm with a frontal glass wall. A 40 w lamp, flashing at frequency of 60 flashes/min, was used.
Surgical procedure
The animals were anesthetized with ketamine 30 mg/kg and xylazine 30 mg/kg intraperitoneally. In stereotaxic apparatus the skin and scalp rat in the skull region were removed. 2 tubes
of 1 mm were placed through the following coordinates: 0.9 mm behind bregma, 1.5 mm to the right of bregma; cannula being located 2.6 mm deep to the ventricle. The fixation of the tubes was made with acrylic cement. The animals were left undisturbed for 9 days until the start of treatment.
Tissue and homogenate preparation
The rat prefrontal cortex, hippocampus, amygdala and nucleus accumbens for thiobarbituric acid reactive species (TBARS) was homogenized in 30 mM Na2PO4, 14 mM KCl, pH=7.4 and for carbonyl protein was homogenized in (12 mM KCl, 0.038 mM KH2PO4, pH=7.4.
Protein determination
All oxidative stress measures were normalized to the protein content with bovine albumin as standard (Lowry et al.,1951).
Oxidative stress parameters
MDA equivalents
To determine oxidative damage in lipid, we measured the formation of thiobarbituric acid reactive species (TBARS) during an acid-heating reaction, as previously described by Draper and Hadley (1990). The samples were mixed with 1 ml of trichloroacetic acid 10 % and 1 ml of thiobarbituric acid 0.67 %, and then heated in a boiling water bath for 30 min. Malondialdehyde equivalents were determined in tissue and in submitochondrial particles of the rat brain spectrophoto-metrically by the absorbance at 532 nm.
Carbonyls protein formation
The oxidative damage to proteins was assessed by the deter-mination of carbonyl groups content based on the reaction with dinitrophenylhidrazine (DNPH), as previously described by Levine et al. (1994). Proteins were precipitated by the addition of 20 % trichloroacetic acid and were redissolved in DNPH. The absorbance was monitored spectrophotometrical-ly at 370 nm.
Respiratory chain enzyme activities
(complex IV) was assayed according to the method described by Rustin et al. (2002), measured by following the decrease in absorbance due to the oxidation of previously reduced Cyto-chrome c at 550 nm with 580 nm as reference wavelength ( = 19.1 mM–1 × cm–1). The reaction buffer contained 10 mM potassium phosphate, pH 7.0, 0.6 mM n-dodecyl-d maltoside, 2–4 lg homogenate protein and the reaction was initiated with addition of 0.7 lg reduced cytochrome c. The activity of complex IV was measured at 25 °C for 10 min. The activities of the mitochondrial respiratory chain complexes were de-scribed as nmol. min-1. mg protein-1.
Creatine kinase activity
The creatine kinase activity was measured in the brain ho-mogenates pretreated with 0.625 mM lauryl maltoside. The reaction mixture consisted of 60 mM Tris–HCl, pH 7.5, con-taining 7 mM phosphocreatine, 9 mM MgSO4 and approxi-mately 0.4–1.2μg protein in a final volume of 100μL. After 15 min of pre-incubation at 37 °C, the reaction was started by the addition of 3.2 mmol of ADP plus 0.8 mmol of reduced glutathione. The reaction was stopped after 10 min by the addition of 1 μmol of p-hydroxymercuribenzoic acid. The creatine formed was estimated according to the colorimetric method of Hughes (1962). The color was developed by the
addition of 100μL 2%α-naphtol and 100μL 0.05 % diacetyl in a final volume of 1 mL and read spectrophotometrically after 20 min at 540 nm.
Statistical analysis
Evaluation of study variables showed that normal distribution
parametric tests would be most appropriate All data are
presented as mean ± S.E.M. Differences among experimental groups in the assessment of oxidative stress parameters and energy metabolism were determined by one ANOVA, follow-ed by Tukey post-hoc test when ANOVA was significant;P
values<0.05 were considered to be statistical significant.
Results
The Fig. 2 illustrates the oxidative stress parameters in the prefrontal cortex, hippocampus, amygdala and nucleus ac-cumbens. Figure2ashow that there was an increase on car-bonyl proteins in the control rats treated with minocycline and in the stressed rats treated with saline or minocycline, com-pared to the control rats treated with saline in the prefrontal cortex (F(3–19)=18.333;p<0.001; Fig.2a); and also that there
A
*
*
* *
# #
*/#
0 1 2 3 4 5 6 7
Prefrontal cortex Hippocampus Amygdala Nucleus accumbens
C
ar
bonyl pr
ote
in
[
n
mol/mg pr
ote
in]
Control + saline
Control + minocycline
Stress + saline
Stress + minocycline
B
* *
*
# #
0 0.001 0.002 0.003 0.004 0.005 0.006
Prefrontal cortex Hippocampus Amygdala Nucleus accumbens
MD
A
e
qui
va
le
nt
s
[nm
o
l/
m
g
pr
ot
e
in]
Control + saline Control + minocycline Stress + saline Stress + minocycline
Fig. 2 The effects of the
was an increase on carbonyl proteins in the stressed rats treated with saline in the amygdala (F (3–19)= 11.498;
p<0.001; Fig.2a) and in the nucleus accumbens (F(3–19)=
6.788;p<0.05; Fig.2a), compared to the control rats treated with saline. Interestingly, minocycline treatment reversed the increase on the carbonyl proteins in the stressed rats treated with saline in these brain regions. Figure2bshow that there was an increase on MDA equivalents in the stressed rats treated with saline, compared with the control group in the prefrontal cortex (F(3–18)=8.691;p<0.001; Fig.2b) and in the
hippocampus (F(3–18)=16.321;p<0.001; Fig.2b); however,
statistical analysis revealed that minocycline treatment re-versed the increase on the MDA equivalents in the stressed rats treated with saline. Also, there was an increase on the MDA equivalents in the stressed rats treated with saline, compared with the control rats treated with saline in the nucleus accumbens (F(3–18)=6.351; p<0.05; Fig.2b).
The Fig.3reveals the results of energy metabolism in the prefrontal cortex, hippocampus, amygdala and nucleus ac-cumbens. Figure3ashow that there was an increase on the complex I activity in the stressed rats treated with saline, compared with control rats treated with saline in the amygdala (F(3–18)=25.120;p<0.001; Fig. 3a), and minocycline
treat-ment was able to reverse this increase; and still, there was a decreased activity of complex I in the control group treated with minocycline, compared with the control group treated with saline in the nucleus accumbens (F(3–18)=6.437,p<0.05;
Fig.3a). Fig.3bdemonstrates that there was a decrease on the complex II activity in the control rats treated with minocycline and in the stressed rats treated with saline or minocycline,
compared with the control rats treated with saline, in the prefrontal cortex (F (3–19)=17.472; p<0.001; Fig. 3b); and
there was a decrease on the complex II activity in stressed rats treated with saline or minocycline, compared with the control rats treated with saline, in the amygdala (F (3–19)=8.708;
p<0.001; Fig.3b). Fig.3cillustrates that minocycline treat-ment reversed a decrease on the complex II-III in the stressed rats treated with saline in the hippocampus (F(3–19)=3.699;
p<0.05; Fig.3c); and there was an increase on the complex II-III activity in the control rats treated with minocycline, com-pared with the control rats treated with saline, in the nucleus accumbens (F(3–19)=7.071;p<0.05; Fig.3c). Fig.3dshows
that there was a decrease on the complex IV activity in the control rats treated with minocycline and in the stressed rats treated with saline, compared with the control rats treated with saline, in the prefrontal cortex (F (3–14)=18.920; p<0.001;
Fig.3d); the treatment with minocycline reversed the increase on the complex IV activity in the stressed rats treated with saline in the hippocampus; there was a decrease on the com-plex IV activity in the stressed rats treated with saline or minocycline, compared with the control rats treated with saline in the amygdala (F(3–14)=30.754;p<0.001; Fig.3d);
and still, there was an increase on the complex IV activity in the stressed rats treated with saline, compared with the control rats treated with saline in the nucleus accumbens (F (3–14)=
9.935; p<0.05; Fig.3d), however, minocycline was able to reverse the decrease on complex IV activity in the stressed group treated with saline. Also, the Fig.3ereveals that there was a decrease on creatine kinase activity in the control rats treated with minocycline, and in the stressed rats treated with
A * * */# 0 100 200 300 400 500 600 700 800 900 1000
Prefrontal cortex Hippocampus Amygdala Nucleus accumbens
Complex I activity
[nmol/min x mg protein]
Control + saline Control + minocycline Stress + saline Stress + minocycline
B * * * * * 0 2 4 6 8 10 12
Prefrontal cortex Hippocampus Amygdala Nucleus accumbens
Complex II activity
[nmol/min x mg protein]
Control + saline Control + minocycline Stress + saline Stress + minocycline
C * # 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5
Prefrontal cortex Hippocampus Amygdala Nucleus accumbens
Complex II-III activity [nmol/min x mg protein]
Control + saline Control + minocycline Stress + saline Stress + minocycline
D * * * # * */# 0 10 20 30 40 50 60 70 80
Prefrontal cortexHippocampus Amygdala Nucleus accumbens
Complex IV activity
[nmol/min x mg protein]
Control + saline Control + minocycline Stress + saline Stress + minocycline
E ** * * * 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8
Prefrontal cortex Hippocampus Amygdala Nucleus accumbens
Creatine kinase activity [nmol/min x mg protein]
Control + saline Control + minocycline Stress + saline Stress + minocycline
Fig. 3 The effects of the
saline or minocycline, compared with the control rats treated with saline, in the amygdala (F (3–19)= 12.472; p<0.001;
Fig.3e); and there was a decrease on creatine kinase activity in the stressed rats treated with saline or minocycline, com-pared with the control rats treated with saline in the nucleus accumbens (F(3–19)=10.223;p<0.001; Fig.3e).
Discussion
Chronic stress and cumulative exposure to stressful events are established risk factors for the development of many disorders in humans, including major depression (Kendler et al.,1998;
1999). In this way, chronic mild stress (CMS) is a valid model of depression in rodents (Willner,1997), modeling environ-mental factors connected to depression in humans, as chronic unexpected stressors (Willner,2005). These stressors lead to a decrease in sucrose intake, which is assumed to represent decreased responsiveness to reward that is comparable with anhedonia in humans, reduced water consumption, decrease in weight, an increase in adrenal gland, deficit in locomotor activity and cognitive dysfunction (Willner et al.,1997). In fact, stress can profoundly affect memory and produces sub-stantial changes in microglia within several brain regions critical to the regulation of mood state and cognition (Hinwood et al.,2013). Hinwood et al. (2012) showed that the antimicroglial activation by minocycline reduced the working memory disturbance induced by chronic stress, sug-gesting that a drug treatment that modifies microglial activity can improve a stress-induced cognitive impairment. Henry et al. (2008) showed that minocycline pretreatment attenuated the effect of LPS-induced weight loss, social withdrawal and anhedonia in adult mice. Furthermore, minocycline demon-strated antidepressant-like actions in the forced swimming test, and a subthreshold dose of minocycline synergized with the antidepressant actions of subthreshold doses of antidepres-sant desipramine and glutamate receptor antagonists (Molina-Hernández et al., 2008). However, minocycline did not synergize the antidepressant-like actions of fluoxetine, indi-cating that minocycline may not directly impact the seroto-nergic system (Molina-Hernández et al., 2008); and may produce antidepressant effects through modification of the noradrenergic system in the brain.
An increasing number of reports indicate that frequently observed phenomenon in the depressive episode is associated with an increase on oxidative damage to lipids (Bilici et al.,
2001; Dimopoulos et al.,2008); and still, stress-associated in experimental models of depression are accompanied by a decrease in antioxidant levels and increase on oxidative dam-age to fatty acids and proteins (Siwek et al.,2013). In this way, the present study showed an increase on carbonyl proteins in the prefrontal cortex, amygdala and hippocampus in stressed rats treated with saline and an increase on MDA equivalents in
the prefrontal cortex, hippocampus and nucleus accumbens in stressed rats treated with saline, however minocycline treat-ment was able to reversed this effect. Previous study showed an increased lipid peroxidation in the cerebellum and the striatum and protein oxidative damage in the prefrontal cortex, hippocampus and striatum in rats subjected to chronic mild stress (Lucca et al., 2009). However, Réus et al. (2010) showed that acute and chronic administration with antidepres-sants such as, imipramine and harmine (aβ-carboline alka-loid) promote antioxidant effects in the prefrontal cortex and hippocampus. In addition, consistent with our findings, Morimoto et al. (2005) showed that minocycline treatment attenuates the increase in lipid peroxidation levels in an animal model of cerebral ischemia. Moreover, Wu et al. (2002) dem-onstrated that minocycline inhibits nicotinamide adenine di-nucleotide phosphate (NADPH) oxidase, which participates in reactive oxidative stress generation. Furthermore, minocycline possesses anti-oxidant activity (Miyachi et al.,
1986), inhibits protein tyrosine nitration by scavenging peroxynitrite (Whiteman and Halliwell,1997) and blocks 6-hydroxydopamine-induced neurotoxicity and free radical pro-duction in rat cerebellar granule neurons (Lin et al.,2003). Our work is the first study to report the effects of minocycline on protein carbonyl formation. In this way, our findings suggest that minocycline exerts neuroprotective effects not just by modulating inflammatory responses, but also by virtue of its anti-oxidant action, however, such antioxidants effects were dependent on the brain area analyzed. In fact, minocycline increased the carbonyl levels in the prefrontal cortex, com-pared to control group, although this result was a surprise, we also showed previously that treatment with lamotrigine anti-depressant also had a tendency to increase the damage in proteins only in prefrontal cortex (Abelaira et al.,2013), and increased levels of protein carbonyls only in a brain region not be sufficient to impair the antioxidant properties of minocycline, as shown by the decrease in the amygdala and nucleus accumbens, as well as by decrease of TBARS levels. Evidence supports the hypothesis that metabolism impairment is involved in the pathophysiology of depression (Shao et al.,
brain area. In this way, different regions of the central nervous system can respond distinctly (Sullivan et al.,2005), and the activities of enzymes involved in energy metabolism were analyzed in different brain regions which in part represent different cell types, indicating heterogeneity in terms of phys-iological and metabolic characteristics (Sonnewald et al.,
1998). Furthermore, Rezin et al. (2009) showed that mitochon-drial respiratory chain complexes I, III and IV were inhibited after chronic mild stress in the cerebral cortex and cerebellum, and acute administration of ketamine (an antagonist of N-methyl-D-aspartate (NMDA) receptor) reversed this inhibition. Recent studies showed that antidepressants citalopram and escitalopram decreased the activity of energy metabolism (Goncalves et al.2012). Hroudova and Fisar (2010) using an in vitro study from pig brain, demonstrated that the complex I, II and IV activity decreased with antidepressants and mood stabilizers, suggesting in this study that antidepressants gener-ally act as inhibitors of electron transport chain. Our findings showed that minocycline increased the complex I activity, II-III and IV in some brain regions, without affecting the complex II activity. Still, Kumar et al. (2012) showed that minocycline at dose of 50 mg/kg significantly restored the mitochondrial en-zyme complexes (I, II and IV) and mitochondrial redox in chronic fatigue stress. We cannot explain why such discrepan-cies occur, but they may be related to the protocol used, the dose of minocycline and with the different type of administra-tion. However, the effects of minocycline on the mitochondrial respiratory chain could be positive in some complexes and brain areas, taking into account that there is impairment in energy metabolism related to depression.
Creatine kinase (CK) plays a central role in metabolism of high energy consuming tissues such as brain, being related to the pathophysiology of mood disorders (Scaini et al.,2010; Assis et al.,2009). In this context, the present study showed that creatine kinase activity was decreased in control rats treated with minocycline and in stressed rats treated with saline or minocycline in the amygdala; and in stressed rats treated with saline or minocycline in the nucleus accumbens. Contrary to these results, previous study showed that treat-ment with antidepressants imipramine and lamotrigine in-creased creatine kinase activity in rat brain after chronic mild stress (Abelaira et al.,2011). Moreover, Assis et al. (2009) reported that the acute administration of ketamine and imipramine increased creatine kinase activity in the rat brain. On the other hand, Della et al. (2013) demonstrated that CK activity was decreased in the amygdala and hippocampus of maternally deprived rats treated with saline or tianeptine. However, consistent with our findings, Aras et al. (2013)
showed that CK activity was significantly decreased follow-ing minocycline administration in cerebral ischemia-reperfusion plus treatment with minocycline. This study is the first to show the effects of minocycline in the creatine kinase in depression; however we cannot explain the
discrepancies, but could be related, at least in part, to differ-ences in the drug administration and protocol that were used. In conclusion, this is the first study to show the effects of minocycline on carbonyl proteins and creatine kinase in chronic stress. Moreover, minocycline presents neuroprotective effects on stress oxidative and energy metabolism. Further, studies are necessary to clarify the connection between depression, markers of oxidant-antioxidant status, energy metabolism and the effect of minocycline on those parameters in more detail.
Acknowledgments Laboratory of Neurosciences (Brazil) is a center
within the National Institute for Translational Medicine (INCT-TM) and is also a member of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). This research was supported by grants from CNPq (JQ, ELS, and GZR), FAPESC (JQ), Instituto Cérebro e Mente, UNESC (JQ), and L’Oréal/UNESCO/ABC Brazil Fellowship for Women in Science 2011 (GZR). JQ, and EML are CNPq Research Fellows. HMA has CAPES studentships.
Disclosures/conflicts of interest The authors have declared that no
conflicts of interest exist relevant to the current manuscript.
References
Abelaira HM, Réus GZ, Ribeiro KF, Zappellini G, Ferreira GK, Gomes LM, Carvalho-Silva M, Luciano TF, Marques SO, Streck EL, Souza CT, Quevedo J (2011) Effects of acute and chronic treatment elicited by lamotrigine on behavior, energy metabolism, neurotrophins and signaling cascades in rats. Neurochem Int 59:1163–1174
Abelaira HM, Réus GZ, Ribeiro KF, Steckert AV, Mina F, Rosa DV, Santana CV, Romano-Silva MA, Dal-Pizzol F, Quevedo J (2013) Effects of lamotrigine on behavior, oxidative parameters and signal-ing cascades in rats exposed to the chronic mild stress model. Neurosci Res 75:324–330
American Psychiatric Association (2013) Diagnostic and statistical man-ual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington
Anisman H, Matheson K (2005) Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 29: 525–546
Arakawa S, Shirayama Y, Fujita Y, Ishima T, Horio M, Muneoka K, Iyo M, Hashimoto K (2012) Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol Biochem Behav 100:601–606 Aras M, Urfal B, Serarslan Y, Ozgür T, UlutaşKT, UrfalıS, AltaşM, Yılmaz N. Protective Effects of Minocycline against Short-Term Ischemia-Reperfusion Injury in Rat Brain. Pediatr Neurosurg (Epub ahead of print)
Assis LC, Rezin GT, Comim CM, Valvassori SS, Jeremias IC, Zugno AI, Quevedo J, Streck EL (2009) Effect of acute administration of ketamine and imipramine on creatine kinase activity in the brain of rats. Rev Bras Psiquiatr 31:247–252
Bilici M, Efe H, Köroðlu MA, Uydu HA, Bekaroðlu M, Deðer O (2001) Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 64:43–51
Della FP, Abelaira HM, Réus GZ, Santos MA, Tomaz DB, Antunes AR, Scaini G, Morais MO, Streck EL, Quevedo J (2013) Treatment with tianeptine induces antidepressive-like effects and alters the neurotrophin levels, mitochondrialrespiratory chain and cycle Krebs enzymes in the brain of maternally deprived adult rats. Metab Brain Dis 28:93–105
Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A (2008) Increased plasma levels of 8-iso-PGF2a and IL-6 in an elderly population with depression. Psychiatry Res 161:59–66
Domercq M, Matute C (2004) Neuroprotection by tetracyclines. Trends Pharmacol Sci 25:609–612
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Duncan JS, Thompson PJ (2003) The cognitive consequences of epilep-sy. Ann Neurol 54:421–422
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1995) Differential in-vestigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Gamaro GD, Streck EL, Matte C, Prediger ME, Wyse ATS, Dalmaz C (2003) Reduction of hippocampal Na+, K +−ATPase activity in rats subjected to an experimental model of depression. Neurochem Res 28:1339–1344
Gardner A, Johansson A, Wibom R, Nennesmo I, von Do¨beln U, Hagenfeldt L et al (2003) Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord 76:55–68
Goncalves CL, Rezin GT, Ferreira GK, Jeremias IC, Cardoso MR, Carvalho-Silva M et al (2012) Differential effects of escitalopram administration on cortical and subcortical brain regions metabolic parameters of Wistar rats. Acta Neuropsychiatr 24:147–154 Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan
JF, Godbout JP (2008) Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedo-nia. J Neuroinflammation 13(5):15
Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M et al (2007) Adenosine deaminase, nitric oxide, superoxide dismutase, and xan-thine oxidase in patients with major depression: impact of antide-pressant treatment. Arch Med Res 38:247–252
Hinwood M, Morandini J, Day TA, Walker FR (2012) Evidence that microglia mediate the neurobiological effects of chronic psycholog-ical stress on the medialprefrontal cortex. Cereb Cortex 22:1442– 1454
Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR (2013) Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and theinhibitory effect of minocycline. Cereb Cortex 23:1784–1797
Hroudova J, Fisar Z (2010) Activities of respiratory chain complexes and citrate synthase influenced by pharmacologically different antide-pressants and mood stabilizers. Neuroendocrinol Lett 31:123–153 Hughes BP (1962) A method for estimation of serum creatine kinase and
its use in comparing creatine kinase and aldolase activity in normal and pathologic sera. Clin Chim Acta 7:597–604
Karg K, Burmeister M, Shedden K, Sen S (2011) The serotonin trans-porter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry 68:444–454
Kempermann G, Kronenberg G (2003) Depressed new neurons–adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 54:499–503
Kendler KS, Karkowski LM, Prescott CA (1998) Stressful life events and major depression: risk period, long-term contextual threat, and di-agnostic specifi- city. J Nerv Ment Dis 186:661–669
Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841
Kessler RC, Wang PS (2008) The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health 29:115–129
Kim HS, Suh YH (2009) Minocycline and neurodegenerative diseases. Behav Brain Res 196:168–179
Kumar A, Vashist A, Kumar P, Kalonia H, Mishra J (2012) Potential role of licofelone, minocycline and their combination against chronic fatigue stress induced behavioral, biochemical and mitochondrial alterations in mice. Pharmacol Rep 64:1105–1115
Lai AY, Todd KG (2006) Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia 53:809–816
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Lin S, Wei X, Xu Y, Yan C, Dodel R, Zhang Y, Liu J, Klaunig JE, Farlow M, Du Y (2003) Minocycline blocks 6-hydroxydopamineinduced neurotoxicity and free radical production in rat cerebellar granule neurons. Life Sci 72:1635–1641
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein mea-surement with the Folin phenol reagent. J Biol Chem 193:265–275 Lu X-Y, Kim CS, Frazer A, Zhang W (2006) Leptin: a potential novel
antidepressant. Proc Natl Acad Sci U S A 103:1593–1598 Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F,
Dal-Pizzol F, Gavioli EC, Quevedo J (2009) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54: 358–362
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Madrigal JL, Olivenza R, Moro M, Lizasoain I, Lorenzo P, Rodrigo J et al (2001) Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 24:420–429
Maes M (1995) Evidence for an immune response in major depression: a review and hypothesis. Prog Neuro-Psychopharmacol Biol Psychiatry 19:11–38
Menke A, Klengel T, Binder EB (2012) Epigenetics, depression and antidepressant treatment. Curr Pharm Des 18:5879–5889 Miyachi Y, Yoshioka A, Imamura S, Niwa Y (1986) Effect of antibiotics
on the generation of reactive oxygen species. J Investig Dermatol 86:449–453
Molina-Hernández M, Tellez-Alcántara NP, Pérez-García J, Olivera-Lopez JI, Jaramillo-Jaimes MT (2008) Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry 32:380–386
Morimoto N, Shimazawa M, Yamashima T, Nagai H, Hara H (2005) Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res 1044:8–15 Pae CU, Marks DM, Han C, Patkar AA (2008) Does minocycline have
antidepressant effect? Biomed Pharmacother 62:308–311 Réus GZ, Stringari RB, de Souza B, Petronilho F, Dal-Pizzol F, Hallak
JE, Zuardi AW, Crippa JA, Quevedo J (2010) Harmine and imipra-mine promote antioxidant activities in prefrontal cortex and hippo-campus. Oxidative Med Cell Longev 3:325–331
Rezin GT, Gonçalves CL, Daufenbach JF, Fraga DB, Santos PM, Ferreira GK, Hermani FV, Comim CM, Quevedo J, Streck EL (2009) Acute administration of ketamine reverses the inhibition of mitochondrial respiratory chain induced by chronic mild stress. Brain Res Bull 79: 418–421
Rustin P, Munnich A, Rotig A (2002) Succinate dehydrogenase and human diseases: newinsights into a well-known enzyme. Eur J Hum Genet 10:289–291
Scaini G, Santos PM, Benedet J, Rochi N, Gomes LM, Borges LS, Rezin GT, Pezente DP, Quevedo J, Streck EL (2010) Evaluation of Krebs cycle enzymes in the brain of rats after chronic administration of antidepressants. Brain Res Bull 82:224–227
Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM et al (2008) Mitochondrial involvement in psychiatric disorders. Ann Med 40:281–295
Siwek M, Sowa-Kućma M, Dudek D, StyczeńK, Szewczyk B, Kotarska K, Misztakk P, Pilc A, Wolak M, Nowak G (2013) Oxidative stress markers in affective disorders. Pharmacol Rep 65:1558–1571 Skolnick P (2002) Beyond monoamine-based therapies: clues to new
approaches. J Clin Psychiatry 63:19–23
Sonnewald U, Hertz L, Schousboe A (1998) Mitochondrial heterogeneity in the brain at the cellular level. J Cereb Blood Flow Metabol 18: 231–237
Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE (2005) Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res 79:231–239
Videbech P (2000) PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand 101:11–20
Whiteman M, Halliwell B (1997) Prevention of peroxynitrite-dependent tyrosine nitration and inactivation of a1-antiproteinase by antibi-otics. Free Radic Res 26:49–56
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacol (Berl) 134:319–329
Willner P (2005) Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110
Willner P, Mitchell PJ (2002) The validity of animal models of predispo-sition to depression. Behav Pharmacol 13:169–188