w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
In
vitro
anti-
Leishmania
infantum
activity
of
essential
oil
from
Piper
angustifolium
Lauriane
S.S.
Bosquiroli
a,
Daniel
P.
Demarque
b,
Yasmin
S.
Rizk
a,
Marillin
C.
Cunha
c,
Maria
Carolina
S.
Marques
d,
Maria
de
Fátima
C.
Matos
c,
Mônica
C.T.
Kadri
e,
Carlos
A.
Carollo
b,
Carla
C.P.
Arruda
a,∗aLaboratóriodeParasitologiaHumana,CentrodeCiênciasBiológicasedaSaúde,UniversidadeFederaldeMatoGrossodoSul,CampoGrande,Brazil
bLaboratóriodeFarmacognosia,CentrodeCiênciasBiológicasedaSaúde,UniversidadeFederaldeMatoGrossodoSul,CampoGrande,Brazil
cLaboratóriodeBiologiaMoleculareCulturasCelulares,CentrodeCiênciasBiológicasedaSaúde,UniversidadeFederaldeMatoGrossodoSul,CampoGrande,Brazil
dLaboratóriodeMicrobiologia,CentrodeCiênciasBiológicasedaSaúde,UniversidadeFederaldeMatoGrossodoSul,CampoGrande,Brazil
eLaboratóriodeBiofisiofarmacologia,CentrodeCiênciasBiológicasedaSaúde,UniversidadeFederaldeMatoGrossodoSul,CampoGrande,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27August2014 Accepted15March2015 Availableonline31March2015
Keywords:
Visceralleishmaniasis Chemotherapy Naturalproducts Sesquiterpenes
a
b
s
t
r
a
c
t
PiperangustifoliumLam.,Piperaceae,popularlyknownas“matito”,“pimenta-de-macaco”,
“pimenta-longa” or “jagurandi” in Brazil, has been commonly used in the treatment of cutaneous
leishmaniasis-associatedlesions,buttherearefewstudiesontheactivityagainstvisceral leishmaniasis-associatedspecies.ThisstudydemonstratesthefirstinvitroantileishmanialactivityoftheP.angustifolium essentialoil,ofwhichthephytochemicalprofileshowedthepresenceofsesquiterpenesand monoter-penes.Themaincompoundswerespathulenol(23.8%)andcaryophylleneoxide(13.1%).P.angustifolium essentialoilwashighlyactive[thehalfmaximuminhibitoryconcentration=1.43g/ml]against intra-cellularamastigotesofLeishmaniainfantum,theetiologicalagentofvisceralleishmaniasisintheNewand OldWorld.Activitywasobtained24hafteradditionoftheoil(6.25–50g/ml),withareductionof100% intheinfectionindexatconcentrationsof25and50g/ml.P.angustifoliumessentialoilshowedlow cytotoxicityformammalianfibroblastsandmacrophages(thehalfmaximuminhibitoryconcentration valuesof31.67and48.22g/ml,respectively),anditwas33and22timesmoretoxictoamastigotesthan tomammaliancells,asindicatedbyselectivityindexes.TheresultsdemonstratedthatP.angustifolium essentialoilisapromisingalternativeforthestudyofpotentialdrugsforvisceralleishmaniasis.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Visceral leishmaniasis(VL)is a severe systemic chronic dis-easecaused bythe Leishmaniadonovani complex inEastAfrica andtheIndiansubcontinent,andLeishmaniainfantuminEurope, northernAfricaandLatinAmerica(Lukesetal.,2007).Itisoneof themostimportantneglecteddiseasestoday,givenitshigh inci-denceandmortality,especiallyamonguntreatedindividualsand malnourishedchildren. It isalsoconsidereda diseaseemerging inimmunocompromisedindividuals(JarvisandLockwood,2013). Evenduringtreatment,acase-fatalityratioof10–20%isestimated (Collinetal.,2004).
Thefirst choice drugs in thetreatment of leishmaniasisare thepentavalentantimonials(WHO,2010).Thesedrugs,however,
∗ Correspondingauthor.
E-mail:carla.arruda@ufms.br(C.C.P.Arruda).
presentsignificantlimitationsconcerningtheirtherapeuticsafety astheyhave ahighleveloftoxicity. Highfrequency ofadverse effectsandteratogenicriskhavebeendescribed(Mirandaetal., 2006).LipidformulationsofamphotericinB,miltefosineand paro-momycinhavebeenapproved,butthecorrectdoseandefficacyof thesedrugshavenotbeenproveninallendemicareasofthe dis-ease(WHO,2010).Combinationsofthesedrugshavebeenrequired inthecasesofinfectionsresistanttoantimony(SeifertandCroft, 2006).Consideringthescenarioofresistancetothemaindrugsin use,researchershave beenstudyingnew,lesstoxicdrugs, with greateravailabilityandwithinreachoftheunderprivileged popula-tionaffectedbythedisease(Freitas-Junioretal.,2012).Inthesearch fornewandbettercompounds,productsofplantoriginhavebeen testedsincetheyareeasilyobtainedatlowcost(LiandVederas, 2009).
Piper is one of thegenera of great ecological and economic importancewithinthePiperaceaefamily(Monzoteetal.,2010). SeveralclassesofcompoundshavebeenisolatedfromPiperspecies,
http://dx.doi.org/10.1016/j.bjp.2015.03.008
includingthosewithantileishmanialactivitysuchasbenzoicacids (Flores et al., 2007, 2009), dillapiole (Parise-Filho et al., 2012), and adunchalcone(Dal Picoloetal., 2014).Moreover,in recent years itsessential oilshave becomean importanttarget inthe searchfornewtherapeuticoptionsagainstparasites(Antonyetal., 2005).
Piper angustifolium Lam. (sin.=Piper aduncum L.), popularly known as “matito”, “pimenta-de-macaco”, “pimenta-longa” or “jagurandi”inBrazil,isaplantnativetotropicalregionssuchas theSouthandCentralAmericas,Asia,andthePacificOceanislands (Martínez etal., 2003).Itsleavesare usedin folkmedicine for thetreatmentofstomatitis,vaginitis,erysipelasand liver disor-ders(LorenziandMatos,2002)orasanantiseptic,antidiarrheal, tonic,astringent,andantirheumaticmedicine,andalsoasaninsect repellent (Schaus and Desmarchelier, 2000).In addition,it has beencommonlyusedinthetreatmentofcutaneous leishmaniasis-associated lesions(Martínezet al., 2003), which motivatedthe developmentofsomeworksonLeishmaniaspeciesassociatedwith thisclinicalformofthedisease(Bragaetal.,2007).Incontrast,few studieshavebeenconductedontheactivityagainstLV-associated speciesanduptonow,therehasbeennoresearchontheessential oilextractedfromthisplant.Thus,theaimofthisstudywasto char-acterizethechemicalcompositionoftheP.angustifoliumessential oil(PAEO)andevaluateitsactivityagainstL.infantum.
Materialsandmethods
Plantmaterial
PiperangustifoliumLam.,Piperaceae,wascollectedinJanuary 2014fromtheAbobralSubregionofthePantanalofMatoGrosso doSul.AftertheidentificationperformedbyDr.GeraldoAlves Dam-ascenoJunior,abotanicalvoucherwasdepositedintheherbarium ofCampoGrande/MS,Brazil,undernumber20182.
Extractionandanalysisoftheessentialoil
TheP.angustifoliumessentialoil(PAEO)wasextractedfromthe plantleavesbydistillationinaClevengerapparatus(Vidrolex).The oilwasdriedwithanhydroussodiumsulfate(Vetec,RiodeJaneiro, Brazil)withyieldof0.41%(w/w),andstoredat−5◦Cinasealed
containeruntilthetimeofanalysis.Thechemicalcompositionwas determinedbygaschromatography–massspectrometry(GC–MS) usingaShimadzuQP2010PlussystemwithaRTX5MScapillary col-umn(30m×0.25mm×0.25m).Nitrogenwasappliedascarrier gasusingaflowrateof1.13ml/min.Theconstituentswere con-firmedbycomparisonwithlibrariesandcalculationoftheKovats index.
Parasites
Thestandard strainMHOM/BR/1972/BH46 ofL. (Leishmania)
infantumwasusedforinvitroassaysofantileishmanialactivity. Theamastigotes wereroutinely isolatedfrom Goldenhamsters (Mesocricetusauratus)andmaintainedaspromastigotesin Schnei-der’sInsectMedium(Sigma)supplementedwith20%fetalbovine serum(FBS,Sigma)and140g/mlgentamicin(Sigma)at26◦C.On
the7thdayofcultivation,promastigotesfromuptothreeserial passagesafterisolationwereusedintheexperiments.
Animals
BALB/cmiceagedsixweekswereusedtoobtaintheperitoneal cellsusedintheantileishmanialactivityassays.Theanimalswere obtainedfromthecentralanimalfacilityoftheCenterfor Biolog-icalandHealthSciences(CCBS)oftheFederalUniversityofMato
GrossodoSul(UFMS,Brazil)ingoodhealthandfreeofinfections orparasitescommontorodents,maintainedinindividually venti-latedcagesequippedwithmini-isolators,andfedabalancedfeed (NuvilabCR-1,Nuvital®)withfreeaccesstowater.Thestudywas
approvedbytheEthicsCommitteeonAnimalUse–CEUA/UFMS, underprotocol432/2012.
ActivityagainstintracellularamastigotesofL.infantum
PeritonealmacrophagesfromBALB/cmicewereisolatedafter rinsingwithRPMI1640medium(Sigma)andplacedina24-well plate(1×105cells/well)inRPMI 1640medium(Sigma)
supple-mentedwith10%FCS(Cultilab)and140g/mlgentamicin(Sigma). Afterincubationat37◦Cfor1h,cellswereinfectedwithL.infantum
promastigotes(1×106cells/well)andincubatedat35◦C for4h.
PAEOwasaddedatconcentrationsof6.25–50g/mlinsetsof sex-tuplicateexperiments.Untreatedinfectedcellsandamphotericin B(Sigma)wereusedasnegativeandpositivecontrol,respectively. Thecellswereincubatedat37◦Cin5%CO2,fixedandstainedwith
Giemsaafter24h.Thepercentageofinfectedmacrophagesandthe totalnumberofamastigotesweredeterminedbycounting200cells sixfold.Theinfectionindexwasdeterminedbymultiplyingthe per-centageofmacrophagesthathadatleastoneintracellularparasite bythemeannumberofamastigotespermacrophage,asdescribed byPaladietal.(2012).Anonlineardose–responseregressioncurve wasusedtocalculatethehalfmaximuminhibitoryconcentration (IC50).Theresultswereexpressedasthemean±standard
devia-tion(SD)andthedatawereanalyzedusingtheStudent’st-test. Differenceswereconsideredsignificantatp<0.05(representedby anasterisk).
Nitricoxide(NO)evaluation
To evaluate the production of NO by the infected periton-eal cells, supernatants of the aforementioned cultures (100l) were collected after 24h of treatment and incubated with an equal volume of Griess Reagent (1% sulfanilamide/0.1% (naph-thyl)ethylenediaminein5%phosphoricacid)atroomtemperature for 10min. The accumulation of nitrite was quantified accord-ingtoDingetal.(1988)andtheabsorbancewasdeterminedat 540nm.AbsorbancewasconvertedtoMofNO2−bycomparing
the samples witha standard curve obtainedwith known con-centrations(1–10M)ofsodiumnitritedilutedinRPMImedium. Theresultswereexpressedasthemean±standarddeviation(SD). The data were analyzed using the Student’s t-test and differ-enceswereconsideredsignificantatp<0.05(representedbyan asterisk).
Cytotoxicityassay
Fibroblast(NIH/3T3) and murinemacrophage(J774.A1) cells purchasedfromtheRiodeJaneiroCellBank(Brazil)weretreated withPAEO at concentrations of 0.25–250g/ml in triplicate to estimateIC50.PAEOwasdissolvedinDMSO(dimethylsulfoxide
sodium)anddilutedincompletemedium(thehighest concentra-tionofDMSOusedinthetestwas0.25%,anddidnotaffectcell viability).Cellsinculturemediumwereusedasnegativecontrol andamphotericinB(0.025–25g/ml)aspositivecontrol.Cell via-bilitywasdeterminedusingthesulforhodamineBassay(Skehan etal.,1990).Thepercentageofgrowthofeachtest samplewas calculatedasdescribedbyMonksetal.(1991).TheIC50was
deter-minedbynonlinearregression(MicrocalOriginVersion6.0and MicrosoftOfficeExcel2007).Selectivityindex(SI)wascalculated bythefollowingformula:IC50onmammaliancells/IC50on
Table1
ChemicalconstitutionoftheessentialoilfromPiperangustifolium.
Compound % Retentiontime TheoreticalKI CalculatedKI
1 ␣-pinene 5.87 4.64 939 932
2 camphene 0.53 5.05 946 947
3 cymene 2.77 7.10 1025 1032
4 limonene 4.27 7.20 1032 1037
5 limoneneoxide 0.64 8.50 1137 1103
6 cis-verbenol 0.32 8.62 1135 1113
7 cryptone 1.75 9.67 1186 1193
8 cuminaldehyde 1.29 10.24 1242 1249
9 p-cymen-7-ol 0.70 10.71 1291 1297
10 ␦-elemene 1.11 11.14 1339 1339
11 ␣-copaene 0.44 11.55 1393 1388
12 -elemene 4.01 11.68 1389 1402
13 trans-caryophyllene 2.24 12.04 1444 1437
14 aromadendrene 1.80 12.25 1469 1455
15 longifolene 4.5 12.59 1487 1405
16 ␣-muurolene 3.56 12.91 1512 1500
17 ␥-cadinene 3.74 13.13 1534 1526
18 cis-calamenene 0.50 13.24 1543 1535
19 nerolidol 5.80 13.63 1562 1561
20 isospathulenol 1.67 14.04 1589 1630
21 spathulenol 23.78 14.15 1578 1598
22 caryophylleneoxide 13.06 14.28 1613 1605
23 viridiflorol 0.51 14.41 1620 1610
24 torreyol 3.81 15.24 1643 1651
25 ␣-cadinol 4.37 15.52 1656 1665
KI,Kovatsindex.
Resultsanddiscussion
Twenty-five constituentswere identified in theessential oil from P. angustifolium, representing 93.04% of the total com-pounds.Themajorityoftheseweresesquiterpenesandthetwo majorcompoundsidentified,spathulenolandcaryophylleneoxide, correspondedto36.8%ofthetotalcompounds(Table1).The phyto-chemicalprofileofPAEOdifferedfromthatfoundbyAlmeidaetal. (2009),whoobservedthepredominanceofaphenylpropanoid (dil-lapiole–86.9%)intheessentialoilfromtheaerialpartsoftheplant collectedinPará,Brazil.Parise-Filhoetal.(2012)alsofound dil-lapioleasthemajorcompoundofessentialoilfromtheleavesof thesameplantcollectedinSãoPaulo,Brazil.Tirillinietal.(1996), whocollectedthesameplantinPeru,nearCuzco,observedthe monoterpenescamphor(25.3%)andcamphene(22.4%)tobethe mainconstituents.
Theantileishmanialactivityofseveralsesquiterpeneshasbeen describedintheliterature(Mikusetal.,2000;Santosetal.,2008; Arrudaetal.,2005).Valadeauetal.(2009)demonstratedthe activ-ity of sesquiterpene-rich ethanolic extract from Piper dennisii;
Monzoteetal.(2010)foundanIC50of22.3g/mlfortheessential
oilfromP.auritumagainstL.donovaniamastigotes.Themethanolic eugenol-richextractfromPiperbetlewasactiveagainstL.donovani
amastigotesandpromastigotes(Misraetal.,2009).Other sesquiter-peneswithleishmanicidalactivityhavepreviouslybeenreported, asshownbyMarquesetal.(2011),whoidentifiedE-nerolidolinthe essentialoilfromPiperclaussenianum(83%).Whentestedagainst
Leishmaniaamazonensisarginase,itshowedanenzymeinhibition of62.2%.Oliveiraetal.(2014)testedtheessentialoilfrom Boca-geopsismultiflorawith16.2%ofspathulenolagainstL.amazonensis
promastigotes.
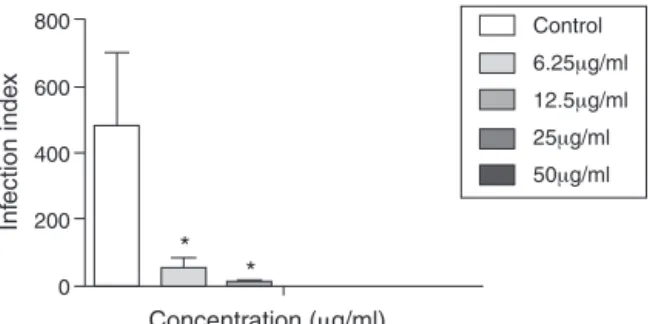
Thisstudypresentsthefirstdescriptionofanti-L.infantum activ-ityassociatedwiththeessentialoilobtainedfromP.angustifolium. Ourresultsdemonstratedadose-dependentincreaseinthe inhi-bitionoftheintracellularamastigoteproliferation24hafterthe oilwasaddedtoinfectedcells.Theinfectionindexdecreased,in arangefrom88.1to100%fromthelowesttothehighest concen-tration,incomparisonwithuntreatedinfectedcells(Fig.1).TheIC thatreduced50%oftheintracellularformsof L.infantum(IC50)
Control 800
Concentration (μg/ml)
*
*
Inf
ection inde
x 600
400
200
0
6.25μg/ml
12.5μg/ml
25μg/ml
50μg/ml
Fig.1.AntileishmanialactivityofPAEOonL.infantumintracellularamastigotes. Barsrepresentthemean±SDofsextuplicates.*p<0.01,forthedifferent concentra-tionscomparedtountreatedcells(control)(Student’st-test).
was1.43g/mlwithlowcytotoxicitytomammaliancells com-paredwithamphotericinB(Table2).WithregardtoSI,PAEOwas 33and22timesmorecytotoxictointracellularamastigotesthanto NIH/3T3andJ774.A1cells,respectively(Table2).Although ampho-tericinBwasaboutfourtimesmoreactivethanPAEO,thisreference drugwasnotasselectiveastheoil,sinceitwasmorecytotoxicto mammaliancells(Table2).
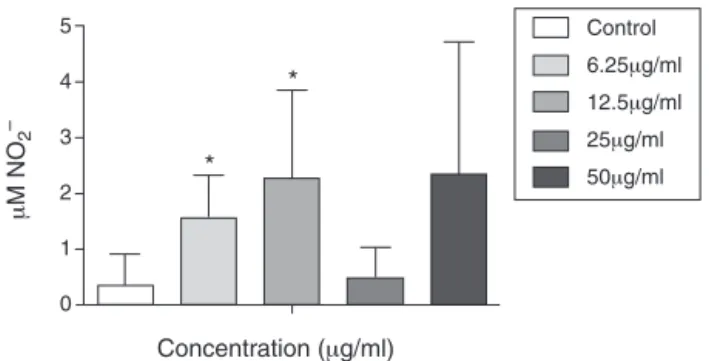
Activation of macrophages was investigated by NO release (Fig.2).Asignificantincrease(p<0.05)inNOreleasewasfound aftertreatmentwithPAEOatconcentrationsof6.25and12.5g/ml, suggestingthatitsactivitymaybeassociatedwiththis antileishma-nialmechanism(Stengeretal.,1994).Atconcentrationsof25and 50g/ml,however,PAEOdidnotinduceasignificantincreasein NOrelease,showinganatypicalresultthatmaybeduetothe pres-enceofcertaincompoundsintheoil.Theliteraturehasreported severalcompoundsinducingthisbiphasicresponse(Nothnickand Soloway,1998;O’Flahertyetal.,1989,1990;Adedapoetal.,2009; Linneretal.,1993;Calabrese,2005).
Table2
EffectofPAEOonintracellularamastigotesofLeishmaniainfantum,cytotoxicitytomammaliancellsandcorrespondingselectivityindex(SI).
Testsamples Intracellularamastigotes NIH/3T3 J774.A1
IC50(g/ml)a IC50(g/ml)a SIb IC50(g/ml)a SIb
PAEO 1.43 48.22 33.72 31.67 22.15
AmphotericinB 0.33 2.19 6.63 4.32 13.09
aIC
50,halfmaximuminhibitoryconcentration. bSI,selectivityindex:IC
50onmammaliancells/IC50onintracellularamastigotes.
Control 5
4
3
2
1
0
Concentration (μg/ml)
μ
M NO
2
–
*
*
6.25μg/ml12.5μg/ml
25μg/ml
50μg/ml
Fig.2.EffectofadditionofdifferentconcentrationsofPAEOontheproduction ofnitricoxidebyperitonealcellsinfectedwithL.infantum.Infectedcells with-outtreatmentwereusedascontrols.Thedatarepresentmean±standarddeviation ofquadruplicates.*p<0.05forthedifferentconcentrationsofPAEOversuscontrol (Student’st-test).
Authors’contributions
LSSB,YSRand MCC(MScstudents)contributed tobiological studies.DPD(PhDstudent)contributedbycollectingplant sam-ples,andperformingchromatographicanalysis.MCTKcontributed totheNOevaluationtest.MFCMcontributedtothecytotoxicity test.MCSMcontributedtocriticalreadingofthemanuscript.CCPA andCACdesignedthestudy,supervisedthelaboratoryworkand contributedtocriticalreadingofthemanuscript.Alltheauthors havereadthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Theauthorsthankthe(CNPq)forthefinancialsupportreceived, andDr.GeraldoAlvesJuniorDamasceno,foridentificationofthe species.
References
Adedapo,A.A.,Jimoh,F.O.,Koduru,S.,Masika,P.J.,Afolayan,A.J.,2009.Assessment ofthemedicinalpotentialsofthemethanolextractsoftheleavesandstemsof Buddlejasaligna.BMCComplement.Altern.Med.9,1–8.
Almeida,R.R.P.,Soutob,R.N.P.,Bastosc,C.N.,Milton,H.L.,DaSilvad,M.H.L.,Maia, J.G.S.,2009.ChemicalvariationinPiperaduncumandbiologicalpropertiesofits dillapiole-richessentialoil.Chem.Biodivers.6,1427–1434.
Antony,J.F.,Fyfe,L.,Smith,H.,2005.Plantactivecomponents–aresourcefor antipar-asiticagents?TrendsParasitol.21,462-458.
Arruda,D.C.,D’Alexandri,F.L.,Katzin,A.M.,Uliana,S.R.B.,2005.Antileishmanial activityofterpenenerolidol.Antimicrob.AgentsChemother.49,1679–1687. Braga,F.,Bouzada,M.L.,Fabri,R.L.,Matos,M.O.,Moreira,F.O.,Scio,E.,Coimbra,
E.S.,2007.Antileishmanialandantifungalactivityofplantsusedintraditional medicineinBrazil.J.Ethnopharmacol.111,396–402.
Calabrese,E.J.,2005.Hormeticdose–responserelationshipsinimmunology: occur-rence,quantitativefeaturesofthedoseresponse,mechanisticfoundations,and clinicalimplications.Crit.Rev.Toxicol.35,89–295.
Collin,S.,Davidson,R.,Ritmeijer,K.,Keus,K.,Melaku,Y.,Kipngetich,S.,Davies,C., 2004.Conflictandkala-azar:determinantsofadverseoutcomesofkala-azar amongpatientsinSouthernSudan.Clin.Infect.Dis.38,612–619.
DalPicolo,S.R.,Bezerra,M.P.,Gomes,K.S.,Passero,L.F.,Laurenti,M.D.,Martins,E.G., Sartorelli,P.,Lago,J.H.,2014.Antileishmanialactivityevaluationof adunchal-cone,anewprenylateddihydrochalconefromPiperaduncumL.Fitoterapia97, 28–33.
Ding,A.H.,Nathan,C.F.,Stuer,D.J.,1988.Releaseofreactivenitrogen intermedi-atesandreactiveoxygenintermediatesfrommouseperitonealmacrophages: comparisonofactivatingcytokinesandevidenceforindependentproduction.J. Immunol.141,2407–2412.
Flores,N.,Cabrera,G.,Jimenez,J.A.,Pinero,J.,Jimenez,A.,Bourdy,G.,Cortes-Selva,F., Bazzochi,I.L.,2007.LeishmanicidalconstituentsfromtheleavesofPiperrusbyi. PlantaMed.73,206–211.
Flores,N.,Jiménez,I.A.,Giménez,A.,Ruiz,G.,Gutiérrez,D.,Bourdy,G.,Bazzocchi, I.L.,2009.AntiparasiticactivityofprenylatedbenzoicacidderivativesfromPiper species.Phytochemistry70,621–627.
Freitas-Junior,L.H.,Chatelain,E.,Kim,H.A.,Siqueira-Neto,J.L.,2012.Visceral leish-maniasistreatment:whatdowehave,whatdoweneedandhowtodeliverit? Int.J.Parasitol.:DrugsDrugResist.2,11–19.
Jarvis,J.N.,Lockwood,D.N.,2013.ClinicalaspectsofvisceralleishmaniasisinHIV infection.Curr.Opin.Infect.Dis.26,1–9.
Li,J.W.H.,Vederas,J.C.,2009.Drugdiscoveryandnaturalproducts:endofaneraor anendlessfrontier?Science325,161–165.
Linner,K.M.,Nicol,S.E.,Sharp,B.M.,1993.IL-1betamodulatesthe concanavalin-A-induced expressionof proenkephalinAmRNA inmurinethymocytes.J. Pharmacol.Exp.Ther.267,1566–1572.
Lorenzi,H.,Matos,F.J.A.,2002.PlantasMedicinaisdoBrasil.Plantarum,NovaOdessa. Lukes,J.,Mauricio,I.L.,Schönian,G.,Dujardin,J.C.,Soteriadou,K.,Dedet,J.P.,Kuhls, K.,Tintaya,K.W.,Jirk ˚u,M.,Chocholová,E.,Haralambous,C.,Pratlong,F.,Oborník, M.,Horák,A.,Ayala,F.J.,Miles,M.A.,2007.Evolutionaryandgeographicalhistory oftheLeishmaniadonovanicomplexwitharevisionofcurrenttaxonomy.Proc. Natl.Acad.Sci.U.S.A.104,9375–9380.
Marques, A.M.,Barreto, A.L.S.,Curvelo,J.A.R., Romanos,M.T.V., Soares,R.M.A., Kaplan,M.A.C.,2011.Antileishmanialactivityofnerolidol-richessentialoilfrom Piperclaussenianum.Rev.Bras.Farmacogn.21,908–914.
Martínez,J.,Rosa,P.T.V.,Ming,L.C.,Marques,M.O.M.,Meireles,A.A.,2003.Extraction ofvolatileoilfromPiperaduncumL.leaveswithsupercriticalcarbondioxide.In: Proceedingsofthe6thInternationalSymposiumonSupercriticalFluids, Ver-sailles.
Mikus,J.,Harkenthal,M.,Steverding,D.,Reichling,J.,2000.Invitroeffectofessential oilsandisolatedmono-andsesquiterpenesonLeishmaniamajorand Try-panosomabrucei.PlantaMed.66,366–368.
Miranda,E.S.,Miekeley, N.,De-Carvalho,R.R.,Paumgartten,F.J.R.,2006. Devel-opmentaltoxicityofmeglumineantimoniateandtransplacentaltransferof antimonyintherat.Reprod.Toxicol.21,292–300.
Misra,P.,Kumar,A.,Khare,P.,Gupta,S.,Kumar,N.,Dube,A.,2009.Pro-apoptotic effectofthelandraceBanglaMahobaofPiperbetleonLeishmaniadonovanimay beduetothehighcontentofeugenol.J.Med.Microbiol.58,1058–1066. Monks,A.,Scudiero,D.,Skehan,P.,Shoemaker,R.,Pau,K.,Vistica,D.,Hose,C.,
Cro-nise,P.,Vaigro-Wolff,A.,1991.Feasibilityofahigh-fluxanticancerdrugscreen usingadiversepanelofculturedhumantumorcelllines.J.Natl.CancerInst.83, 757–766.
Monzote,L.,García,M.,Montalvo,A.M.,Scull,R.,Miranda,M.,2010.Chemistry, cyto-toxicityandantileishmanialactivityoftheessentialoilfromPiperauritum.Mem. Inst.OswaldoCruz105,168–173.
Nothnick,W.B.,Soloway,P.D.,1998.Novelimplicationsinthedevelopmentof endometriosis:biphasiceffectofmacrophageactivationonperitonealtissue expressionoftissueinhibitorofmetalloproteinase-1.Am.J.Reprod.Immunol. 40,364–369.
O’Flaherty,J.T.,Jacobson,D.P.,Redman,J.F.,1989.Bidirectionaleffectsofprotein kinaseCactivators.Studieswithhumanneutrophilsandplatelet-activating factor.J.Biol.Chem.264,6836–6843.
O’Flaherty,J.T.,Redman,J.F.,Jacobson,D.P.,1990.Mechanismsinvolvedinthe bidirectionaleffectsofproteinkinaseCactivatorsonneutrophilresponsesto leukotrieneB4.J.Immunol.144,1909–1913.
Oliveira,E.S.C.,Amaral,A.C.F.,Lima,E.J.,Silva,J.R.A.,2014.Chemicalcompositionand biologicalactivitiesofBocageopsismultifloraessentialoil.J.Essent.OilRes.26, 161–165.
Paladi,C.S.,Pimentel,I.A.S.,Katz,S.,Cunha,R.L.O.R.,Judice,W.A.S.,Caires,A.C.F., Barbieri,C.L.,2012.Invitroandinvivoactivityofapalladacyclecomplexon Leishmania(Leishmania)amazonensis.PLoSNegl.Trop.Dis.6,e1626. Parise-Filho,R.,Pasqualoto, K.F.M.,Magri,F.M.M.,Ferreira,A.K.,Silva, B.A.V.G.,
preliminarySARstudiesofdillapioleanalogues.Arch.Pharm.Chem.LifeSci.345, 934–944.
Santos,A.O.,Ueda-Nakamura,T.,Dias,B.P.,Veiga,V.F.,Pinto,A.C.,Nakamura,C.V., 2008.EffectofBraziliancopaibaoilsonLeishmaniaamazonensis.J. Ethnophar-macol.120,204–208.
Schaus,F.W.,Desmarchelier,C.,2000.SixtyMedicinalPlantsfromthePeruvian Amazon:Ecology,EthnomedicineandBioactivity.Bio2000,Lima.
Seifert,K.,Croft,S.L.,2006.Invitroandinvivointeractionsbetweenmiltefosineand otherantileishmanialdrugs.Antimicrob.AgentsChemother.50,73–79. Skehan,P.,Storeng,R.,Scudiero,D.,Monks,A.,Mcmahon,J.,Vistica,D.,Warren,J.T.,
Bokesch,H.,Kenney,S.,Boyd,M.R.,1990.Newcolorimetriccytotoxicityassay foranticancer-drugscreening.J.Natl.CancerInst.82,1107–1112.
Stenger,S.,Thüring, H.,Röllinghoff,M.,Bogdan,C.,1994.Tissueexpressionof induciblenitricoxidesynthaseiscloselyassociatedwithresistanceto Leish-maniamajor.J.Exp.Med.180,783–793.
Tirillini, B., Velasquez, E.R., Pellegrino, R., 1996. Chemical composition and antimicrobialactivityofessentialoilofPiperangustifolium.PlantaMed.62, 372–373.
Tiuman,T.S.,Ueda-Nakamura,T.,Cortez,D.A.G.,DiasFilho,B.P.,Morgado-Díaz,J.A., deSouza,W.,Nakamura,C.V.,2005.Antileishmanialactivityofparthenolide,a sesquiterpenelactoneisolatedfromTanacetumparthenium.Antimicrob.Agents Chemother.49,176–182.
Valadeau,C.,Pabon,A.,Deharo,E.,Albán-Castillo,J.,Estevez,Y.,Lores,F.A.,Rojas,R., Gamboa,D.,Sauvain,M.,Castillo,D.,Bourdy,G.,2009.Leishmanicidalmedicinal plantsfromtheYanesha(Peru):evaluationoftheandantimalarialactivityof selectedextracts.J.Ethnopharmacol.123,413–422.