RevistaBrasileiradeFarmacognosia25(2015)199–207
w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Marine
organisms
as
source
of
extracts
to
disrupt
bacterial
communication:
bioguided
isolation
and
identification
of
quorum
sensing
inhibitors
from
Ircinia
felix
Jairo
Quintana
a,
José
Brango-Vanegas
a,
Geison
M.
Costa
a,
Leonardo
Castellanos
a,∗,
Catalina
Arévalo
a,b,
Carmenza
Duque
aaGrupodeEstudioyAprovechamientodeProductosNaturalesMarinosyFrutasdeColombia,DepartamentodeQuímica,FacultaddeCiencias,UniversidadNacionaldeColombia,
Bogotá,Colombia
bGrupodeComunicaciónyComunidadesBacterianas,DepartamentodeBiología,FacultaddeCiencias,UniversidadNacionaldeColombia,Bogotá,Colombia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14November2014 Accepted26March2015 Availableonline29April2015
Keywords:
Quorumsensing Marineproducts
Irciniafelix
Furanosesterterpenes Biofilm
a
b
s
t
r
a
c
t
Inthisstudy,39extractsfrommarineorganismswereevaluatedasquorumsensinginhibitors,collected intheColombianCaribbeanSeaandtheBrazilianCoastincluding26sponges,sevensoftcorals,five algaeandonezooanthid.TheresultsshowedthatcrudeextractsfromthesoftcoralEunicealaciniata,and thespongesSvenzeatubulosa,IrciniafelixandNeopetrosiacarbonariawerethemostpromisingsource ofquorumsensinginhibitorscompoundswithoutaffectingbacterialgrowth,unliketherawextracts ofAgelascitrina,Agelastubulata,Iotrochotaarenosa,Topsentiaophiraphidites,Niphatescaycedoi,Cliona tenuis,Ptilocauliswalpersi,Petrosiapellasarca,andthealgaeLaurenciacatarinensisandLaurenciaobtusa, whichdisplayedpotentantibacterialactivityagainstthebiosensorsemployed.Thecrudeextractfrom thespongeI.felixwasfractionated,obtainingfuranosesterterpeneswhichwereidentifiedandevaluated asquorumsensinginhibitors,showingamoderateactivitywithoutaffectingthebiosensor’sgrowth.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Dueto thegreat chemical variability provided by biological diversityexistinginthemarineenvironment,researchinmarine naturalproductshasreceivedgreatinterest(Bluntetal.,2010). Manyofthesemoleculesareresponsibleforstabilityand adapt-abilityofmarineorganismstoachangingenvironment;besides theyareinvolvedinthesymbioticrelationshipbetweenmarine macro-organisms and associatedmicroorganisms thathasbeen thoroughlydocumented.Thisentirechemicalsignalingmakesthis multiorganismalconsortiumbehaveasabiochemicaland physio-logicalunitfundamentaltotheequilibriumofthewholeecosystem (Harvelletal.,2007).
Structure and relative density of microbial populations are associated with macro-organisms’ change in time and loca-tion. This alteration directly influencesthe organism health as wellasthe productionof bioactive substances.Therefore, mul-tiorganism consortium couldregulate settlement of exogenous bacteriausingcompoundstoavoidabiofilmwithanundesirable
∗ Correspondingauthor.
E-mail:lcastellanosh@unal.edu.co(L.Castellanos).
composition(TeplitskiandRitchie,2009).Coordinationbetween macro-organisms and associated bacteria is done, as well as betweenbacteria,bymeansofcommunicationsystemsregulated by chemicalmolecules. In bacteria,this phenomenon is known asquorumsensing(QS),andsignals[N-acylhomoserinelactones (AHL)forGramnegativebacteria]involvedinthismechanismare alsoresponsibleforinter-kingdomcommunication(Loweryetal., 2008).
DuetoQSsystems,bacteriabehaveinaverydifferentwaywhen theyareorganizedas acomplex community becauseinsucha way,theycoordinatedifferentphenotypesthatprovidethe com-munitywithmoreresistanceandadaptabilitytotheenvironment, likeantibioticproduction,sporulation,DNAexchange,expression andsecretionofvirulencefactors,bioluminescence,pigment pro-duction,andremarkablebiofilmmaturation(NgandBassler,2009; Greenberg,2003;BoyerandWisniewski-Dyé,2009).
Bacterial biofilms are sessile communities of differentiated cells associated with a surface, submerged in an extracellular matrixofexcretedpolysaccharides(alsotermed exopolysaccha-rides,EPS),proteinsandDNA.Specificstructureandcomposition ofbiofilmsvarywiththeresidentspeciesandenvironmental con-ditions(Dickschat,2010).Biofilmmaturationisalsocontrolledby QSthatregulatesmanyphenotypesasEPSproduction,adhesines
http://dx.doi.org/10.1016/j.bjp.2015.03.013
200 J.Quintanaetal./RevistaBrasileiradeFarmacognosia25(2015)199–207
and otherfundamental requirementsfor theconsolidation of a strongmatrix(Dickschat,2010).Sincebiofilmsareknowntobe importantcuesforsettlementoflarvaeofmanymarine inverte-brates(Dobretsovetal.,2009),thecontrolofbiofilmformationon thesurfacebecomesatargetforbiofoulingcontrol.Furthermore, biofilmhasbeendisruptedoverunanimatedsurfacesusingquorum sensinginhibitors(QSI),leadingtothereductionofmacrofouling (Dobretsovetal.,2009).
Oneof themostpromisingalternativesforbiofilmcontrolis the use of marine natural products, which are extracted from someinvertebrateorganismswithamacro-foulingfreesurfacethat couldbeanindicatoroftheproductionofcompoundswith set-tlementdeterrenceproperties(Qianetal.,2007;Mesegueretal., 2004).Followingthenecessityofantifoulingcompoundswithout toxiceffectsoncolonizingspecies,quorumsensingdisruptionhas becomeapromisingalternative.QSImayactinfourdifferentways: first,inhibitingthesignalmoleculesbiosynthesis(i.e.AHL); sec-ond,inducingdegradationofthesignalmolecules;third,blocking specificbindsitesofAHLtoLuxRtypeproteins;and finally,by inhibitionof DNAtranscription (Dobretsovet al.,2009).A quo-rumsensing inhibitor suppressesspecific geneticexpression of bacteriawithoutcausingdeath. Sincesurvivablemechanismsof bacteriaarenotinducedbyQSIcompound,bacterialresistanceis notdeveloped;thusithasalsobeenconsideredforyearsbythe pharmaceuticalindustryasapromisingstrategyforthedesignand developmentofantipathogeniccompounds,usefulincontrolling microbialchronicinfections,duetotheobservedmicrobial devel-opmentofresistancetoantibiotics(RasmussenandGivskov,2006). Theseincreasingeffortsfocusedonnewalternativesintendedto preventandtreatsevereinfectionsinordertominimizethetotal antimicrobialexposure,andincludesseveralQS-modulating ther-apiessuchasmacrolideantibiotics,QSvaccines,andcompetitive QSinhibitorsthathavebeenprovedtobehelpfulindiminishing thetranslationofQS-directedtoxinsorbyprematurelyactivating theQSresponsetoalerttheimmunesystemtobacteriahidingina lowcelldensity(Martinetal.,2008).
ThisstudyisaimedtoextendknowledgeabouttheColombian andBrazilianmarinebiodiversityandtoevaluatetheQSIactivity ofmarineorganisms’extracts,whichhavenotbeenwidelystudied aroundtheworldand,asfarasweareaware,neverdonebefore attheSouth-EasternCaribbeanSeaandSouthBrazilianCoast.We presentevaluationsof theQSI activityof 39 marineorganisms’ extractsfromtheColombianCaribbeanSeaandtheBrazilianCoast (Santa Catarina State), using Chromobacterium violaceum ATCC 31532andEscherichiacolipSB401asbiosensorscoupledwith Pseu-domonasputidaIsoFbydiscdiffusionassays.Besides,weevaluated a furanosesterterpeneenriched-fraction fromtheactive sponge Irciniafelix,asanapproachtoidentifysomeofthepossibleactive compounds.
Materialsandmethods
Organismsandextractpreparation
OrganismswerecollectedbyscubadivinginSantaMartabay andIslasdelRosarioattheColombianCaribbeanSea,andinSanta Catarina State onthe Brazilian Atlantic Coast (Table 1; autho-rizationcode: DTC-C-33 11/7ofUAESPNN; permissionNo.4 of 10/02/2010).Organismsweredriedandkeptfrozenuntil extrac-tion.Driedorganismswerethencutintosmallpiecesandextracted three timeswith dichloromethane:methanol(1:1); solventwas thenevaporateduntilthecrudeextractswereobtained,andstored at4◦Cbeforeusing.TheextractsofSvenzeatubulosaandNeopetrosia carbonaria were partitioned using Kupchan methodology (Anta
etal.,2002)andthefractionssolubleindichloromethane(DCM), butanol(WB)andwater(WW)weretested.
ExtractionandisolationoffuranosesterterpenesfromI.felix
Thecrudeextract(35.3g)ofI.felix,obtainedasdescribedabove from562.2goffreshmaterial,waspartitionedwithamixtureof DCM:H2O(1:1,v/v),yieldingthedichloromethanefractionthatwas
concentratedunderreducedpressure.Thisfraction(4.5g)was sub-mittedtovacuumcolumnchromatographyoversilicagel(Merck 60) using a gradient increasing in polarity, starting with 100% benzenetoethylacetate:methanol(1:1,v/v).Sevenfractions (IFF1-IFF7)wereyieldedandevaluatedintheQSIassay.FractionIFF3 (1.7g),elutedwithbenzene:ethylacetate(7:3,v/v),showedQSI activityandwasfurtherchromatographedagaininvacuum col-umnchromatographyoversilicagel(Merck60);andelutedwith hexane:ethylacetate(7:3,v/v),hexane:ethylacetate(1:1,v/v)and 100%ethylacetate,ledtoninefractions(FractionsIFF3-1to IFF3-9).FractionIFF3-3(600mg),elutedwithhexane:ethylacetate(7:3, v/v),containingamixtureofsesterterpenescompounds,showing QSIactivity,wasacetylated.Briefly,analiquot(300mg)offraction IFF3-3wassubmittedtoacetylationwith6mlofamixtureofacetic anhydride:pyridine(1:2,v/v)underagitationfor24h.The reac-tions’productsweremonitoredbythin-layerchromatography.The solventmixturewaspouredover10mlofhydrochloricacid(5%) coldsolutionandextractedwithethylacetate.Theorganicphase wasdriedwithanhydroussodiumsulfate,filtratedanddriedunder reducedpressure,yielding332.7mgofanacetylated furanosestert-erpenefraction.Thisfractionwassubmittedtohigh-performance liquid chromatography(HPLC) (forchromatographic conditions, see‘PreparativeHPLCseparations’section)toyieldcompounds1a and1b(2.0mg),2aand2b(2.5mg)and3(6.0mg),identifiedby1H
and13CNMR,andbycomparisonwiththeliteraturedata(Martínez etal.,1995,1997).
FractionI(mixtureofthetwocompounds)
Colorlessoil.[␣]25D 23.0(c0.193,CH3OH).1a.(7Z,13Z,18R,20Z
)-felixininacetate.1HNMR(400MHz,CDCl
3)ı(ppm):7.35(1H,m,
H-1),7.21(1H,s,H-4),6.27(1H,s,H-2),5.15(1H,t,J=6.7Hz,H-7), 5.08(1H,t,J=6.8Hz,H-15),5.04(1H,d,J=10.2Hz,H-20),2.82(1H, m,H-18),2.43(2H,t,H-5aandH-5b),2.35(3H,s,H-27),2.22(2H,c, J=7.5Hz,H-6aandH-6b),1.96–2.06(m,H-16,H-12andH-10),1.82 (3H,s,H-25),1.65(3H,s,H-14andH-9),1.43–1.33(m,H-17and H-11),1.05(3H,d,J=6.8Hz,H-19).1b.(8Z,13Z,18R,20Z)-strobilinin acetate.1HNMR(400MHz,CDCl
3)ı(ppm):7.34(1H,m,H-1),7.21
(1H,s,H-4),6.27(1H,s,H-2),5.12(1H,t,J=6.7Hz,H-10),5.08(1H, t,J=6.8Hz,H-15),5.04(1H,d,J=10.2Hz,H-20),2.82(1H,m,H-18), 2.39(2H,t,H-5aandH-5b),2.34(3H,s,H-27),2.03(2H,m,H-7), 1.96–2.06(m,H-16andH-12),1.82(3H,s,H-25),1.67(3H,s,H-14 andH-9),1.62(m,H-6),1.43–1.33(m,H-17andH-11),1.05(3H,d, J=6.8Hz,H-19).
FractionII(mixtureofthetwocompounds)
Colorlessoil.[␣]25D 23.5(c0.20,CH3OH).2a.(7E,13Z,18R,20Z
)-felixinin acetate. 1H NMR (400MHz, CDCl
3) ı (ppm): 7.34
(1H, m, H-1), 7.20 (1H, s, H-4), 6.27 (1H, s, H-2), 5.16 (1H, m, H-7), 5.08 (m, H-15), 5.05 (1H, d, J=10.3Hz, H-20), 2.82 (1H, m, H-18), 2.44 (2H, t, J=6.5Hz, H-5a and H-5b), 2.35 (3H, s, H-27), 2.25 (2H, t, J=6.5Hz, H-6a and H-6b), 1.95–2.09(m, H-11and H-10),1.88–1.95 (m,H-16,H-12), 1.81 (3H,s,H-25),1.65(3H,s,H-14),1.57(3H,s,H-9),1.30–1.50(m, H-17),1.06(3H,d,J=6.7Hz,H-19).2b.(8E,13Z,18R,20Z)-strobilinin acetate.1HNMR(400MHz,CDCl
3)ı(ppm):7.34(1H,m,H-1),7.20
J.
Quintana
et
al.
/
Revista
Brasileira
de
Farmacognosia
25
(2015)
199–207
201
Table1
QSIandantibacterialresultsofmarineextractsfromColombianCaribbeanSeaandBrazilianSouthCoast.
Typeof collected sample
Marineorganism extractsource
Collection number
Collectiondate Geographic origin
EscherichiacolipSB401 ChromobacteriumviolaceumATCC31532
Growinginhibitiona QSinhibitiona Growinginhibitiona QSinhibitiona
200g 400g 200g 400g 100g 200g 400g 100g 200g 400g
Sponges Agelascitrina INV-POR969 05/04/2008 IslasdelRosario(Col) 7–9 7–9 5–7 >12 – – – – – 7–9
Agelastubulata ICN-MHN(Po) 154
07/12/2005 SantaMarta(Col) 7–9 7–9 – >12 – – NT – – NT
Agelasmarron ICN-MHN(Po) 0149
07/12/2005 IslasdelRosario(Col) – – – – – – – – – –
Akacachacrouense INV-POR-412 07/12/2005 SantaMarta(Col) – 5–7 – – – – NT 9–12 9–12 NT Biemnacribaria INV-POR-890 07/12/2005 SantaMarta(Col) 7–9 5–7 – 7–9 NT – – NT – –
Cinachyrella kuekenthalli
INV-POR-878 18/11/2009 SantaMarta(Col) – – 5–7 5–7 NT NT NT NT NT NT
Clionadelitrix INV-MHN(Po)-189
18/09/2005 SanAndrés(Col) – – – 7–9 NT – – NT – –
Clionatenuis INV-POR-669 06/04/2008 IslasdelRosario(Col) – 7–9 – – 9–12 9–12 NT – – NT Clionavarians INV-POR-339 07/09/2005 SantaMarta(Col) – – – – NT – – NT – –
Iotrochotaarenosa
(Iotrochotainminuta)b
INV-POR-883 15/11/2006 SantaMarta(Col) >12 – – 7–9 – – – – – –
Irciniafelix INV-POR-014 18/11/2009 SantaMarta(Col) NT NT NT NT – – – 7–9 7–9 7–9
Lissodendoryx colombiensis
ICN-MHN-PO 0246
06/04/2008 IslasdelRosario(Col) – – – – NT – – NT – –
Muriceopsissp. ICN-MHN-PO 0254
13/02/2006 SantaMarta(Col) – 5–7 – – – – NT - – NT
Neopetrosiacarbonaria
(DCM)(Xestospongia carbonaria)b
ICN-MHN-PO 0244
05/04/2008 IslasdelRosario(Col) – – – 7–9 – – – >12 – 9–12
Neopetrosiacarbonaria
(WB)
ICN-MHN-PO 0244
05/04/2008 IslasdelRosario(Col) – – 7–9 7–9 – – – – – >12
Neopetrosiacarbonaria
(WW)
ICN-MHN-PO 0244
05/04/2008 IslasdelRosario(Col) – – – – NT – – NT – –
Neopetrosiamuta
(Xestospongiamuta)b
ICN-MHN-PO 0247
06/04/2008 IslasdelRosario(Col) – – – – NT – – NT – –
Neopetrosiaproxima
(Xestospongia proxima)b
ICN-MHN-PO 0239
04/04/2008 IslasdelRosario(Col) – – – – NT – – NT – –
Neopetrosiarosariensis
(Xestospongia rosariensis)b
ICN-MHN-PO 0245
05/04/2008 IslasdelRosario(Col) – – – - NT – – NT – –
Neopetrosia subtriangularis
(Xestospongia subtriangularis)b
ICN-MHN-PO 0242
05/04/2008 IslasdelRosario(Col) – – – – – – – >12 7–9 7–9
Ptilocauliswalpersi ICN-MHN-PO 0248
06/04/2008 IslasdelRosario(Col) – – – 5–7 NT 9–12 9–12 NT – –
Svenzeatubulosa(crude extract)
ICN-MHN-PO 0249
06/04/2008 IslasdelRosario(Col) – – 9–12 >12 – – – – >12 9–12
Svenzeatubulosa(DCM) ICN-MHN-PO 0249
06/04/2008 IslasdelRosario(Col) NT NT NT NT – – – 7–9 9–12 9–12
Topsentiaophiraphidites ICN-MHN-PO 0243
202
J.
Quintana
et
al.
/
Revista
Brasileira
de
Farmacognosia
25
(2015)
199–207
Table1(Continued)
Typeof collected sample
Marineorganism extractsource
Collection number
Collectiondate Geographic origin
EscherichiacolipSB401 ChromobacteriumviolaceumATCC31532
Growinginhibitiona QSinhibitiona Growinginhibitiona QSinhibitiona
200g 400g 200g 400g 100g 200g 400g 100g 200g 400g Softcorals Eunicealaciniata
ICN-MHN-CR-106
13/02/2006 SantaMarta(Col) – – 7–9 7–9 NT NT NT NT NT NT
Euniceasuccinea ICN-MHN-PO 0251
13/02/2006 SantaMarta(Col) – – – 5–7 NT – – NT – –
Euniceafusca ICN-MHN-PO 0252
13/02/2006 SantaMarta(Col) – – – 5–7 NT – – NT – –
Euniceasp1 – 13/02/2006 SantaMarta(Col) 5–7 5–7 – – NT – – NT – –
Erythropodium caribaeorum
INV-CNI-1193 07/09/2005 SantaMarta(Col) – – – – – – – – – 9–12
Pseudopterogorgia elisabethae
(Providencia)
INV-CNI-1612–1614
25/05/2008 Providenciaand SanAndrés(Col)
5–7 – – – – – NT – – NT
Pseudopterogorgia elisabethae(San Andrés)
INV-CNI-1615-1616
18/09/2005 Providenciaand SanAndrés(Col)
– – – 9–12 NT – – NT – –
Zoanthid Palythoacaribaeorum ICN-MHN-PO 0255
07/09/2005 SantaMarta(Col) – – – – – – – – 9–12 –
Algae Laurenciacatarinensis FLOR14516 02/2008 IlhadoArvoredo, Florianópolis(Bra)
– – – – 7–9 9–12 9–12 – – –
Laurenciaflagellifera FLOR14521 03/2006 PraiadaSepultura, Bombinhas(Bra)
– – – – – – – – – –
Laurenciamajuscule FLOR14524 11/2006 IlhadoFrancês, Florianópolis(Bra)
– – – – – 7–9 – – – –
Laurenciamicrocladia FLOR14520 03/2008 PraiadaSepultura, Bombinhas(Bra)
– – – – – – – – – –
Laurenciaobtusa FLOR14512 08/2009 PraiadeCanasvieiras, Florianópolis(Bra)
– – – – 7–9 9–12 >12 – – –
ahalo,inmm.
J.Quintanaetal./RevistaBrasileiradeFarmacognosia25(2015)199–207 203
H-10),5.04(1H,d,J=10.3Hz,H-20),2.82(1H,m,H-18),2.37(2H,t, H-5aandH-5b),2.34(3H,s,H-27),1.95–2.09(m,H-16,H-12,H-7), 1.81(3H,s,H-25),1.67(3H,s,H-14),1.58(3H,s,H-9),1.64(m, H-6),1.30–1.50(m,H-17andH-11),1.05(3H,d,J=6.7Hz,H-19).
FractionIII(3)
(7E,12E,18R,20Z)-variabilinacetate,colorlessoil. [˛]25D 26.1(c
0.167,CH3OH).1HNMR(400MHz,CDCl3)ı(ppm):7.33(1H,s,
H-1),7.20(1H,s,H-4),6.28(1H,s,H-2),5.16(1H,t,J=6.9Hz,H-7), 5.07(1H,t,J=6.9Hz,H-12),5.04(1H,d,J=10.2Hz,H-20),2.81(1H, m,H-18),2.44(2H,t,J=7.4Hz,H-5aandH-5b),2.35(3H,s,H-27), 2.23(2H,c,J=14.7and7.4Hz,H-6aandH-6b),2.05(2H,m,J=8.0 and7.6Hz,H-11aandH-11b),1.98(2H,t,J=7.8Hz,H-10aand H-10b),1.94(2H,t,J=7.0Hz,H-15aandH-15b),1.82(3H,s,H-25), 1.58(3H,s,H-9),1.55(3H,s,H-14),1.34(4H,m,H-16),1.34(4H, m,H-17),1.05(3H,d,J=6.7Hz,H-19).RMN13C(100MHz,CDCl
3) ı(ppm):142.5(CH,C1),138.8(CH,C4),124.4(CH,C12),123.7(CH, C7),111.1(CH,C2),39.7(CH2,C10),39.5(CH2,C15),36.6(CH2,C17),
31.1(CH,C18),28.4(CH2,C6),26.6(CH2,C11),25.7(CH2,C16),25.0
(CH2,C5),20.6(CH3,C27),20.5(CH3,C19),16.1(CH3,C9),15.8(CH3,
C14),116.7(CH,C20),8.4(CH3,C26).
PreparativeHPLCseparations
HPLCseparationswere carriedout ona Merck-Hitachi6000 systemequippedwithaL-6000Abinarypump,Rheodyneinjector andaL-4250UV–Visdetector.Thestationaryphaseemployedwas ScharlauScienceNucleosil120C-18column,(300mm×8mm;i.d.;
10m),usingisocraticMeOH:H2O(95:5)asmobilephase,witha
flowrateat2ml/min,anddetectionat310nm.
Bacterialstrainsandcultureconditions
TwodifferentbiosensorswereusedtoevaluateQSIactivity:C. violaceumATCC31532andE.colipSB401coupledwithP.putidaisoF WTastheexogenoussourceofAHLtoinducebioluminescence pro-duction.E.coliandP.putidaisoFWTwereculturedinLuria–Bertani brothat37◦C,andthosestrainswerekindlyprovidedbyProf.Dr. KathrinRiedel(DepartmentofMicrobiology,InstituteofPlant Biol-ogy,UniversityofZürich).C.violaceumwasculturedinCASOAgar® (Merck,Germany)atroomtemperature.
QSIassays
C.violaceumATCC31532wasinoculatedovernightatroom tem-perature in CASO brothand 100lof bacteria werespread on agarplates.Incontrast,E.colipSB401andP.putidaisoFWTwere inoculatedseparatelyat37◦Covernighttogetacellulardensity equalto0.5MacFarlandstandard.AnequalamountofLBmedium withagarat1.6%waspreparedandmixedwithbacteriacultures withtheintentionof preparing0.8%agarplates,already inocu-latedwithboth,thebiosensorandtheAHLproducerstrain.Sterile paperdiscs(5mmdiameter)wereloadedwiththeappropriate vol-umeofcrudeextractssolutions,dissolvedinmethanol,inorder toobtain100, 200and 400gperdisc.Thesolventwasleftto evaporate.Methanolwasusedas blankcontroland methanolic fractionfromCecropiapachystachyawasusedaspositivecontrol. Discswereplacedonplatespreviouslyinoculatedwiththe appro-priatebiosensorandwereincubatedfor24hat37◦Cfortheassay withE.colipSB401andP.putidaisoFWT;andat28◦CforC. vio-laceumATCC31532assay.AQSIpositiveresultwastheinhibitionof phenotypeexpression(bioluminescenceorviolacein,respectively) withoutaffectingthegrowingprocessofbacteria.Thepresenceofa growthinhibitionzonearoundthediscswastakenasantibacterial activity.Thisprocedurewasmadebyatriplicate.Theinhibitionof
quorumsensinginC.violaceumwasmeasuredbyvisualanalysisof violaceinproduction,andforthebioassaywithE.colipSB401and P.putidaisoFWTthebioluminescenceinhibitionwasregisteredby autoradiography.
Resultsanddiscussion
Manydifferentstudieshavedemonstratedtheabilityofnatural compoundstoblockQSsystems.Ornamental,medicinaland edi-bleplantshavebeenthemoststudiedsourcesofQSIcompounds (Dickschat,2010; Dobretsovet al., 2009);however, only a few examplesofsystematicscreeningstudieshavebeendocumented usingrawextractsfrommarinesources.Aninvestigationbased on78extractsof25speciesofmarineorganismscollectedfrom FloridawatersshowedthatcyanobacteriahavehighQSinhibitory potential(Dobretsovetal.,2011).Anotherstudyusedmarinecrude extractsoforganismsfromtheGreatBarrierReefinAustralia.The authorsidentifiedinthespongeLuffariellavariabilisthemanoalide asresponsiblefortheQSIactivityoftherawextract.Thiscompound isknownasananti-inflammatoryagent,anditsstructure hasa lactonicringwhichresemblesthestructureofAHL,andhasbeen foundtobeanactiveQSIcompoundinthefusionsystemlasB::gfp (ASV)ofPseudomonasaeruginosa(Skindersoeetal.,2008).Some othercompoundshavebeenidentifiedasQSIfrommarinesources, andthemostrepresentativeQSIcompoundsarethehalogenated furanones,isolatedfromtheredalgaDeliseapulchra,whichhave beendemonstratedtobeoneofthemostpromisingQSIcompounds andpotentantifoulingagents(BoyerandWisniewski-Dyé,2009). Ontheotherhand,threecompounds(floridoside,betonicineand isethionicacid)isolatedfromtheredalgaAhnfeltiopsisflabelliformis showedpromisingQSIactivitywiththeAgrobacteriumtumefaciens NTL4QSsystem(Kimetal.,2007).Anotherexampleisthe bromi-natedtriptamine-likealkaloidsthatwerefoundinbryozoanFlustra foliaceaandweretestedusingtheP.putida(pKR-C12),P.putida (pAS-C8)andE.coli(pSB403)QSsystemsasbiosensors(Petersetal., 2003).
Extractstestedinthepresentstudywereobtainedfrommarine organismsselectedspeciallybecauseoftheircleansurface;such organismshavemicroorganismcommunitiesinthesurfaceswhich mayhaveanecologicalcontrolthatavoidsettlementof macro-organisms. This control can be done by chemical compounds produced by the marine organism (the host) or by associated microorganismsasbacteria,orbyboth,theholobiont.As previ-ouslymentioned,inthisecologicalcontrol,QSplaysamajorrole duetoregulationofbiofilmmaturation,causingaselective settle-mentofbacteria,larvaeorothereukaryoticmicroorganisms(Boyer andWisniewski-Dyé,2009).
Discdiffusionassays wereusedtotestQSI andantibacterial activityof39marineorganismsextracts.Table1showstheresults usingdifferentconcentrationsofextractswithbothbiosensors.A promisingextractassourceofQSIcompoundsmustshowaQS inhi-bitionwithoutaffectingthebacterialgrowingprocesstoavoidlater developmentofbacterialresistance.
204 J.Quintanaetal./RevistaBrasileiradeFarmacognosia25(2015)199–207
Concentration
200 µg
400 µg
Petri dish pictures
A
1
1
2
3
1
1
2
3
2
3
2
3
C
D
B
X-ray plate
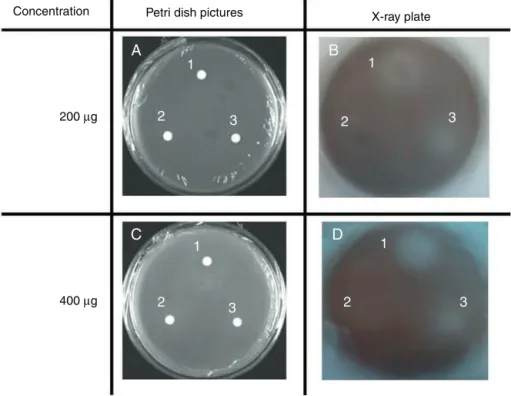
Fig.1.DiscdiffusionassayshowinginhibitionofbioluminescenceofEscherichiacolipSB401coupledwithP.putidaIsoFbymarineextracts.AandCarepicturesofPetri dishesandsensi-discs.BandDaretheX-rayplaquesofthesepetridishes.1:Svenzeatubulosa(crudeextract),2:Petrosiapellasarca(crudeextract)and3:Eunicealaciniata
(crudeextract).Theclearzonesinautoradiography(BandD)andtheabsenceofgrowthinhibitionzones(AandC)showQSinhibitoryactivityoftheextracts.
ofspongeN.carbonaria.AmildQSIactivitywasshownbycrude extractsfromthespongesClionadelitrix,dichloromethanefraction ofN.carbonariaandalowactivitywasshownbycrudeextracts fromtheoctocoralsEuniceasuccinea,Euniceafuscaandthesponge Ptilocauliswalpersi.
WhenC.violaceumATCC31532wasusedasabiosensor,nine (23%) of the39 extracts resultedactive. Of these,seven corre-sponded to sponges, one to an octocoral, and the other to a zoanthid. These results are in contrast withthe onesobtained by using the E. coli biosensor because with C. violaceum the most active extract belonged to sponges, 27% of the extracts were active (7 of 26) and so were just 14.3% of the octoco-ralextracts(1of7).Likewise,algaeextractsshowednoactivity withthisbiosensor.Fig.2showstheresultsfortherawextracts of the sponges S. tubulosa and I. felix in the assay with C. violaceum which, in conjunction with the crude extracts from sponges Neopetrosia subtriangularisand Aka cachacrouense, and as well as extracts from octocoral Erythropodium caribaeorum, dichloromethane extracts of S. tubulosa and N. carbonaria, and butanol fraction of N. carbonaria, showed high bacterial com-municationdisruption(QSI)activity.ExtractsfromthespongeE. caribaeorumandthezoanthidPalythoacaribaeorumshowed mod-erateactivity.
Withthesetestswecouldalsoseesomeextractswith antibacte-rialactivitiesagainstthreeGramnegativebacteria:E.coli,P.putida andC. violaceum.Extracts fromthesponges Agelas citrina, Age-lastubulata,IotrochotaarenosaandTopsentiaophiraphiditeswere themost potentantibacterial against E.coli and P.putidaIsoF, whileextractsfromthespongesNiphatescaycedoi,Clionatenuis, P.walpersi,PetrosiapellasarcaandthealgaeLaurenciacatarinensis andLaurenciaobtusashowedhighantibacterialactivityagainstC. violaceumATCC31532.Theseresultscouldbeexplainedduetothe factthatthespongesbelongingtothegenusAgelasarerecognized asasourceofbromopyrrolidinicalkaloids(Braekmanetal.,1992) withanimportantecologicalkeyroleasfishfeedingdeterrents (Assmannetal.,2000).Ithasalsobeenfoundthatsuchcompounds possessinteresting antiviral (Keifer et al.,1991), antihistaminic
(Cafierietal.,1997)andantimicrobialactivities(Shenetal.,1998). The sponges of the genus Iotrochota are known for producing brominatedalkaloids(Carlettietal.,2000)withmildantibacterial activity,andsulfatedpyrrolealkaloidswithpromising anti-HIV-1activity(Fan etal.,2010).Thesponges ofthegenusTopsentia arerecognizedassourceofsulfatedsteroidswithawidespread range of bioactivities, for example, ophirapstanol trisulfate – a sulfate steroidisolated fromT. ophiraphidites which hasshown activityasaninhibitorintheguanosinediphosphate/G-proteinRAS exchangeassay(GDPX),whichisdirectlyrelatedwithanticancer activity(Gunasekeraetal.,1994).Thespongesofthegenus Ptilo-caulishavebeenreportedbyproducingguanidinetypealkaloids withantimicrobialactivity,amongothers.In2003,acyclic guani-dine,withantibacterialactivityagainstStaphylococcusaureus,was identifiedfromthespongePtilocaulisspiculifer(Yangetal.,2003). AlgaeofthegenusLaurenciahaveshown sesquiterpenes, diter-penes,triterpenesandacetogeninsthatusuallyhaveoneormore halogenatoms.Thesetypesofcompoundswererecognizedfor var-iousactivities,suchascytotoxicandantibacterial(Lhullieetal., 2010).
Thoseresultsareinagreementwithapreviousworkdonein ourlaboratory, where many of theextracts used in this study weretestedagainstacollectionoftwelvemarinebacteriastrains, isolatedfrom colonizedsurfaces(Mora-Cristancho etal., 2011). Additionally,formanyofthesemarinestrains,presenceofQS sys-tems byidentificationof itsAHLproduction wasdemonstrated (Cuadrado,2010).Therefore, itcouldbeachallengetoevaluate thepropertiesoftheseQSIactiveextractsinthesemarinebacteria involvedinthebiofoulingprocessofQSsystems.
J.Quintanaetal./RevistaBrasileiradeFarmacognosia25(2015)199–207 205
A, C1 A, C2 B, C2 B, NC
PC A, C4 B, C4 A, NC
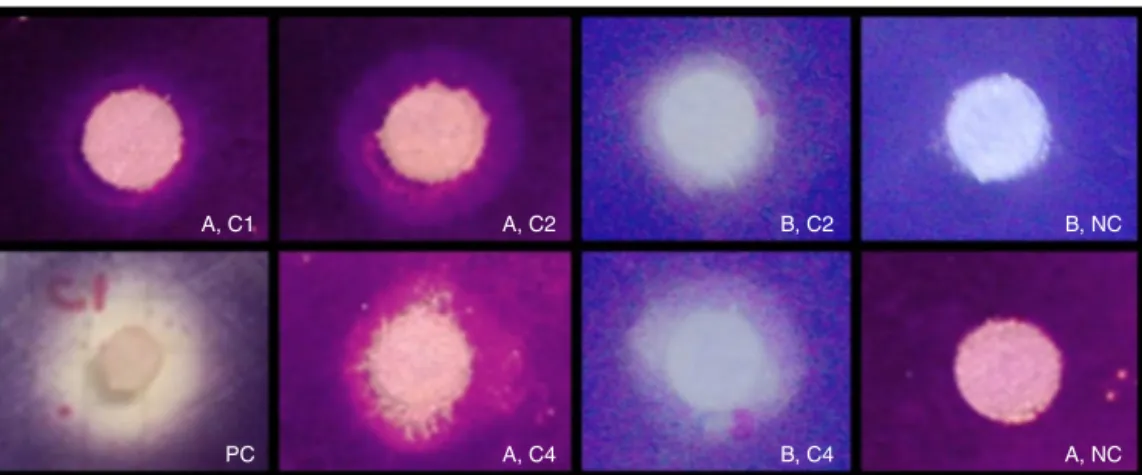
Fig.2. DiscdiffusionassayshowinginhibitionofviolaceinaproductioninC.violaceumATCC31532.Thepicture(A)isdichloromethanefractionfromSvenzeatubulosa
and(B)iscrudeextractfromIrciniafelix.C1=100g,C2=200g,C3=300g,andC4=400gperdisco.Positivecontrol,PC=100gofmethanolfractionfromCecropia pachystachya;negativecontrol,NC=20lofmethanolwithpriorevaporationbeforetoplacing.TheclearzonesurroundingthepaperdiscshowsQSinhibitoryactivityof theextracts.
abletocombatagainstantibioticresistantbacterialstrainsthat representaseriousthreattohumanhealth.
Inthissense,bioguidedisolationwasconductedwiththecrude extractofI.felix.Throughoutdifferentchromatographicsteps,we obtaineda furanosesterterpenes-enriched fraction (IFF3-3) that presentedamoderateQSIactivity.Duetotheknowninstability ofthesecompounds,thisfractionwasacetylatedandsubmitted toapreparativeHPLCtoyieldthreesub-fractions(FractionI–III). FractionIshowedamixtureof(7Z,13Z,18R,20Z)-felixininacetate
(1a)and(8Z,13Z,18R,20Z)-strobilininacetate(1b).Verysimilarly, fractionIIalsopresentedamixtureofthesefuranosesterterpenes, butwithadistinctconfiguration:(7E,13Z,18R,20Z)-felixininacetate (2a)and(8E,13Z,18R,20Z)-strobilininacetate(2b).InfractionIII, thecompound(7E,12E,18R,20Z)-variabilinacetate(3)wasisolated. Allthesefivecompounds,whichpresentsimilarstructuretoAHL (Dobretsovet al., 2009), have already been reportedfor I. felix (Martínezetal.,1995,1997).Besides,propertiessuchasanalgesic, antimicrobial, cytotoxicand anti-tumor of this furanosesterter-penesarewelldocumented(Martínezetal.,1997).
1
5
4
9 8
9 8
14 13
14 13
18 22 23
24 25
19
18
19 18
19
18
19 13
14 O
1
5
4 9
8 14
13
O
1
5
4 9
8 14
13
1
5
4 O
1
5
4 O
O O
22 23
24 25
O O
22 23
24 25
O O O O O
OAc 18 22 23
24 25 19 O
O OAc
22 23
24 25 O
O OAc OAc
1a
2a
1b
2b
206 J.Quintanaetal./RevistaBrasileiradeFarmacognosia25(2015)199–207
The other two promising QSI sponges, S. tubulosa, formerly knownasPseudoaxinellatubulosa,havenoreportsaboutits chem-istry,butthespongesofthisgenusareknownassourceoflong chainfattyacids,steroidal,andpeptidecompounds,someofthem withantibacterial activities(Kong et al.,1992; Sjöstrand et al., 1981).InourpreliminaryQSIbioassay-guidedfractionationofthis sponge,wefoundthat organicfractionsareresponsible forthe activity,andthesefractionsarerichinepidioxysterolsandlipids, which couldbe theQS inhibitors in thecrude extract (unpub-lishedresults)consideringthat,forsomefattyacids,QSIactivity isreported(Osornoetal.,2012).ThegenusNeopetrosiaisknownas asourceofquinolinictypealkaloidsandothernitrogencompounds thatexhibitcytotoxic,antimicrobialactivity,amongothers(Nakao etal.,2004;DeAlmeidaetal.,2008).ParticularlyfromN.carbonaria, previouslyknownasXestospongiacarbonaria,itwaspossibleto isolateacridinetypepolaralkaloids(Thaleetal.,2002)with recog-nizedanticanceractivity.Inapreviousstudy,thesealkaloidswere presentinthebutanolicfractionwhich,inthepresentstudy,isalso activedisruptingQSbiosensorsystems.Consequently,itcouldbe assumedthatthesealkaloidsareresponsiblefortheQSIactivityin ourextract,butithasnotbeendemonstratedyet.
In conclusion, we were able to identify the extracts of the spongesN.carbonaria,S.tubulosaandI.felixasthemost promis-ingQuorumsensinginhibitorsdisruptingtheexpressionofboth biosensors’phenotypeswithoutaffectingtheirgrowingprocess. Differentfromtheseextracts,theextractsofthespongesA.citrina, A.tubulata,I.arenosa,andT.ophiraphiditesshowedantibacterial activityagainstE.coliandP.putida,andtheextractsofthesponges N.caycedoi, C. tenuis, Ptilocaulis wallpersi, P.pellasarca and the algaeL.catarinensisandLaurenciaobtusewerehighlyantibacterial againstC.violaceum.FromthespongeI.felix,afuranosesterterpenes enriched-fractionshowedamoderateQSIactivitywithoutaffecting thebiosensor’sgrowth.Ingeneral,thesefuranosesterterpenesare structurallyrelatedwithAHLandotherQSI,whichcouldexplain theobservedactivityagainstC.violaceum,additionallypossessing asimilarmechanismofaction.Notwithstanding,otherstudiesare requiredtoimprovetherealpotentialofthesecompoundsasQSI exploitablebyman.
Authors’contributions
JQandJBV(PhDstudents)contributedbyrunningthe labora-torywork,biologicalstudies,analysisofthedataanddraftingthe paper.GMC(Postdoctoral researcher) contributedto chromato-graphicproceduresandtocriticalreadingofthemanuscript.CA contributed bycollecting samples,identification,and biological studies.LCandCDdesignedthestudy,supervisedthelaboratory workandcontributedtocriticalreadingofthemanuscript.Allthe authorshavereadthefinalmanuscriptandapprovedits submis-sion.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Thisworkwasconductedaspartofajointprojectfinancially supportedbyCNPq/MCT/Brazil(grantnumber490151/2007-8)and COLCIENCIAS/Colombia(grantnumber358-2007).Theworkwas alsofinanced by other grants fromColciencias and “Fundación paralaPromocióndelaInvestigaciónylaTecnologíadelBanco delaRepública”.TheColombianauthorsgreatlyacknowledgeProf. Dr.KathrinRiedel(DepartmentofMicrobiology,InstituteofPlant Biology,University of Zürich) and Prof. Dr. Sven Zea from the
UniversidadNacionaldeColombiafortheirinterestandassistance inthiswork,andProf.Dr.PauloHorta,Dr.CintiaLlhulierandProf. Dr.EloirP.Schenkelforthecollectionandidentificationofthealgae.
References
Anta,C.,Gonzáles,N.,Santafé,G.,Rodríguez,J.,Jiménez,C.,2002.NewXenia diter-penoidsfromtheIndonesiansoftcoralXeniasp.J.Nat.Prod.65,766–768. Assmann,M.,Lichte,E.,Pawlik,J.R.,Köck,M.,2000.Chemical defensesofthe
CaribbeanspongesAgelaswiedenmayeriandAgelasconifera.Mar.Ecol.Prog.Ser. 22,255–262.
Blunt,J.W.,Copp,B.R.,Munro,M.H.G.,Northcote,P.T.,Prinsep,M.R.,2010.Marine naturalproducts.Nat.Prod.Rep.27,165–237.
Boyer,M.,Wisniewski-Dyé,F.,2009.Cell–cellsignallinginbacteria:notsimplya matterofquorum.FEMSMicrobiol.Ecol.21,1–19.
Braekman,J.C.,Daloze,D.,Stoller,C.,VanSoest,R.W.M.,1992.Chemotaxonomyof Agelas(Porifera:Demospongiae).Biochem.Syst.Ecol.20,417–431.
Cafieri,F.,Carnuccio,R.,Fattorusso,E.,Taglialatela-Scafati,O.,Vallefuoco,T.,1997. Anti-histaminicactivityofbromopyrrolealkaloidsisolatedfromCaribbean Age-lassponges.Bioorg.Med.Chem.Lett.9,2283–2288.
Carletti,I.,Banaigs, B.,Amade, P.,2000. Matemonea newbioactive bromine-containingoxindolealkaloidfromtheIndianOceanspongeIotrochotapurpurea. J.Nat.Prod.63,981–983.
Cuadrado,C.T.,(Master’sthesis)2010.AislamientodeN-acilhomoserinlactonasde algunasbacteriasprocedentesdelMarCaribeColombiano,comoevidenciadela existenciadecircuitosdequorumsensing.UniversidadNacionaldeColombia, Bogotá,270pp.
DeAlmeida,L.P.,Carroll,A.R.,Towerzey,L.,King,G.,McArdle,B.M.,Kern,G.,2008. Exiguaquinol:anovelpentacyclichydroquinonefromNeopetrosiaexiguathat inhibitsHelicobacterpyloriMurI.Org.Lett.19,2585–2588.
Dickschat,J.S.,2010.Quorumsensingandbacterialbiofilms.Nat.Prod.Rep.27, 343–369.
Dobretsov,S.,Teplitski,M.,Paul,V.,2009.Mini-review:quorumsensinginthe marineenvironmentanditsrelationshiptobiofouling.Biofouling25,413–427. Dobretsov,S.,Teplitski,M.,Bayer,M.,Gunasekera,S.,Proksch,P.,Paul,V.,2011. Inhi-bitionofmarinebiofoulingbybacterialquorumsensinginhibitors.Biofouling 27,893–905.
Fan,G.,Li,Z.,Shen,S.,Zeng,Y.,Yang,Y.,Xu,M.,2010.BaculiferinsA-O,O-sulfated pyrrolealkaloidswithanti-HIV-1activity,fromtheChinesemarinesponge Iotro-chotabaculifera.Bioorg.Med.Chem.18,5466–5474.
Greenberg,E.P.,2003.Bacterialcommunicationandgroupbehavior.J.Clin.Invest. 112,1288–1290.
Gunasekera,P.S.,Sennett,S.H.,Kelly-Borgues,M.,Bryant,R.W.,1994.Ophirapstanol trisulfate,anewbiologicalactivesteroidsulfatefromthedeepwatermarine spongeTopsentiaophiraphidites.J.Nat.Prod.57,1751–1754.
Harvell,D.,Jordán-Dahlgren,E.,Merkel,S.,Rosenberg,E.,Raymundo,L.,Smith,G., Weil,E.,2007.Coraldiseaseenvironmentaldrivers,andthebalancebetween coralandmicrobialassociates.Oceanography20,172–195.
Keifer,P.A.,Schwartz,R.E.,Koker,M.E.S.,HughesJr.,R.G.,Rittschof,D.,Rinehart,K.L., 1991.BioactivebromopyrrolemetabolitesfromtheCaribbeanspongeAgelas conifera.J.Org.Chem.56,2965–2975.
Kim,J.S.,Kim,Y.H.,Seo,Y.W.,Park,S.,2007.Quorumsensinginhibitorsfromthered algaAhnfeltiopsisflabelliformis.Biotechnol.Bioproc.Eng.30,308–311. Kong,F.,Burgoyne,D.L.,Andersen,J.,Allen,T.M.,1992.Pseudoaxinellin,acyclic
heptapeptideisolatedfromthePapuaNewGuineaspongePseudoaxinellamassa. TetrahedronLett.33,3269–3272.
Lhullie,C.,Falkenberg,M.,Ioannou,E.,Quesada,A.,Papazafiri,P.,Antunes-Horta,P., Schenkel,E.P.,Vagias,C.,Roussis,V.,2010.Cytotoxichalogenatedmetabolites fromtheBrazilianredalgaLaurenciacatarinensis.J.Nat.Prod.73,27–32. Lowery, C.A.,Dickerson,T.J.,Janda,K.D.,2008. Interspecies andinterkingdom
communicationmediatedbybacterialquorumsensing.Chem.Soc.Rev.37, 1337–1346.
Martin,C.A.,Hoven,A.D.,Cook,A.M.,2008.Therapeuticfrontiers:preventingand treatinginfectiousdiseasesbyinhibitingbacterialquorumsensing.Eur.J.Clin. Microbiol.27,635–642.
Martinez, A., Duque, C., Hara, N., Fujimoto, Y., 1995. Variabilin 11-metiloctadecanoate a branched-chain fatty acidester offuranosesterpene tetronicacid,fromspongeIrciniafelix.Nat.Prod.Lett.6,281–284.
Martínez,A.,Duque,C.,Sato,N.,Fujimoto,Y.,1997.(8Z,13Z,20Z)-strobilininand (7Z,13Z,20Z)-felixinin:newfuranosesterterpenetetronicacidsfrommarine spongesofthegenusIrcinia.Chem.Pharm.Bull.45,181–184.
Meseguer,D.,Kiil,S.,Dam-Johansen,K.,2004.Antifoulingtechnology–past,present andfuturestepstowardsefficientandenvironmentallyfriendlyantifouling coatings.Progr.Org.Coat.50,75–104.
Mora-Cristancho,J.A.,Arevalo-Ferro,C.,Ramos,F.A.,Tello,E.,Duque,C.,Lhullier, C.,Falkenberg,M.,Schenkel,E.P.,2011.Antifoulingactivitiesagainstcolonizer marinebacteriaofextractsfrommarineinvertebratescollectedinthe Colom-bianCaribbeanSeaandontheBrazilianCoast(SantaCatarina).Z.Naturforsch. C66,515–526.
Nakao,Y.,Shiroiwa,T.,Murayama,S.,Matsunaga,S.,Goto,Y.,Matsumoto,Y.,2004. IdentificationofRenieramycinAasanantileishmanialsubstanceinamarine spongeNeopetrosiasp.Mar.Drugs25,55–62.
J.Quintanaetal./RevistaBrasileiradeFarmacognosia25(2015)199–207 207
Osorno,O.,Castellanos,L.,Ramos,F.A.,Arévalo,C.,2012.Gaschromathographyas atoolinquorumsensingstudies.In:Salih,B.,C¸elikbıc¸ak,Ö.(Org.).Gas Chro-matography–Biochemicals,NarcoticsandEssentialOils.Intech,Croatia,pp. 67–96.
Peters,L.,König,G.M.,Wright,A.D.,Pukall,R.,Stackebrandt,E.,Eberl,L.,2003. Sec-ondarymetabolitesofFlustrafoliaceaandtheirinfluenceonbacteria.Appl. Environ.Microbiol.69,3469–3475.
Qian,P.Y.,Lau,S.C.K.,Dahms,H.U.,Dobretsov,S.,Harder,T.,2007.Marinebiofilmsas mediatorsofcolonizationbymarinemacroorganisms:implicationsfor antifoul-ingandaquaculture.Mar.Biotechnol.12,399–410.
Rasmussen,T.B.,Givskov,M.,2006.Quorumsensinginhibitorsasanti-pathogenic drugs.Int.J.Med.Microbiol.296,149–161.
Shen,X.,Perry,T.L.,Dunbar,C.D.,Kelly-Borges,M.,Hamann,M.T.,1998. Debro-mosceptrin,analkaloidfromtheCaribbeanspongeAgelasconifera.J.Nat.Prod. 61,1302–1303.
Sjöstrand,U.,Kornprobst,J.M.,Djerassi,C.,1981.Minorandtracesterolsfrom marine invertebrates 29 (22E)-ergosta-5,22,25-trien-3-ol and (22E,24R)-24,26-dimethylcholesta-5,22,25(27)-trien-3-ol.Twonewmarinesterolsfrom thespongePseudopxinellalunachata.Steroids38,355–364.
Skindersoe,M.,Ettinger-Epstein,P.,Rasmussen,T.,Bjarnsholt,T.,DeNys,R.,Givskov, M.,2008.Quorumsensingantagonismfrommarineorganisms.Mar.Biotechnol. 20,56–63.
Teplitski,M.,Ritchie,K.,2009.Howfeasibleisthebiologicalcontrolofcoraldiseases? TrendsEcol.Evol.24,378–385.
Thale,Z.,Johnson,T.,Tenney,K.,Wenzel,P.J.,Lobkovsky,E.,Clardy,J.,2002. Struc-turesand24cytotoxicpropertiesofsponge-derivedbisannulatedacridines.J. Org.Chem.67,9384–9391.