Received July 8, 2009 and accepted June 10, 2010. Corresponding author: tiagozootecnista@yahoo.com.br
In situ e
stimation of indigestible compounds contents in cattle feed and
feces using bags made from different textiles
1Tiago Neves Pereira Valente2, Edenio Detmann3, Sebastião de Campos Valadares Filho3, Maura da Cunha4, Augusto César de Queiroz3, Cláudia Batista Sampaio2
1Trabalho conduzido com apoio do CNPq, FAPEMIG (PPM) e INCT-Ciência Animal. 2Programa de Pós-graduação em Zootecnia – UFV. Bolsista do CNPq.
3Departamento de Zootecnia, UFV, Viçosa-MG. Pesquisador do CNPq e do INCT-Ciência Animal. 4Laboratório de Biologia Celular e Tecidual, UENF, Campos dos Goytacazes-RJ. Pesquisadora do CNPq.
ABSTRACT - The objective of this experiment was to evaluate estimates of concentration of indigestible dry matter (iDM), indigestible neutral detergent fiber (iNDF) and indigestible acid detergent fiber (iADF) in samples of cattle feed and feces processed at different particle sizes (1-, 2-, and 3-mm) using bags made from nylon (50 µm), F57 (Ankom®), and
non-woven textile (NWT – 100 g/m²) in a in situ evaluation procedure. Eight samples of concentrates, seven samples of forages and two samples of cattle feces were used. The samples were put in the bags according to a ratio of 20 mg of dry matter/cm² of surface by using three bags of each textile for each size of particle, totaling 405 bags, which were submitted to ruminal incubation for 264 hours. The contents of indigestible compounds in the samples were evaluated sequentially. Blank bags were used for microscopic evaluation of textile physical integrity and for evaluation of the physical resistance. The presence of contaminants caused high variability of results of iDM, indicating that its using as internal marker should not be recommended. By using the textiles F57 and NWT, similar estimates for contents of iNDF and iADF were obtained whereas by using nylon (50 µm), the concentration of iNDF was underestimated, possibly because of loss of particles due to its porosity. Nylon
presented loss of resistance in all analytical stages, expanding its probality of rupture, especially after extraction with acid detergent. For in situ evaluation of indigestible compounds, it is suggested grinding samples in 2-mm screen sieves.
Key Words: F57, internal markers, nylon, non-woven textile, particle size
Estimação
in situ
dos componentes indigestíveis em alimentos e fezes de
bovinos com sacos de diferentes tecidos
RESUMO -Objetivou-se avaliar as estimativas de concentração de matéria seca (MSi), fibra em detergente neutro (FDNi) e fibra em detergente ácido (FDAi) indigestíveis em amostras de alimentos e fezes bovinas processadas em diferentes tamanhos de partículas (1, 2 e 3 mm) utilizando-se sacos confeccionados com os tecidos nylon (50 µm), F57 (Ankom®) e tecido
não-tecido (TNT – 100 g/m2) em procedimento de avaliação in situ. Foram utilizadas oito amostras de alimentos concentrados, sete de alimentos volumosos e duas de fezes bovinas. O material foi acondicionado nos sacos numa relação de 20 mg de matéria seca/cm2 de superfície utilizando-se três sacos de cada tecido para cada tamanho de partícula, totalizando 405 sacos, que foram submetidos a incubação ruminal durante 264 horas. Os teores dos compostos indigestíveis nas amostras foram avaliados sequencialmente. Sacos em branco foram utilizados para avaliação microscópica da integridade física dos tecidos e para avaliação da resistência à tração. A presença de contaminantes causou alta variabilidade dos resultados de MSi, o que indica comprometimento de sua aplicação como indicador interno. Utilizando os tecidos F57 e TNT obtiveram-se estimativas similares para os teores de FDNi e FDAi, enquanto com o tecido nylon (50 µm) a concentração de FDNi foi subestimada, possivelmente em decorrência da perda de partículas devido à sua
porosidade. O nylon apresentou perda de resistência em todas as etapas analíticas, o que amplia sua probabilidade de ruptura, principalmente após extração com detergente ácido. Para procedimentos in situ de avaliação de compostos indigestíveis, sugere-se a moagem de amostras em peneiras com porosidade de 2 mm.
Palavras-chave: F57, indicadores internos, nylon, tamanho de partículas, tecido não-tecido
Introduction
Internal markers are inherent components of feeds that can be used to estimate digesta flow and fecal mass in digestion assays with ruminant animals. Currently, the most used internal markers are: indigestible dry matter (iDM), indigestible neutral detergent fiber (iNDF), and indigestible acid detergent fiber (iADF) (Detmann et al., 2004).
The capacity to be completely recovered in feces or any segment of gastrointestinal tract is one of the demanded characteristics for an ideal marker (Owens & Hanson, 1992). Under the lack of such characteristic, the estimates of fecal excretion or digesta flow could be biased. In a theoretical point of view, the recovering capacity is inherent to the marker (Detmann et al., 2007); however, indirect influences of methods applied to estimate its content in samples can apparently bias the estimates of marker recovering (Freitas et al., 2002; Detmann et al., 2007).
When internal markers contents are evaluated using
in situ procedures, different textiles can be used to make
incubation bags. Among those, it can be highlighted the nylon (50 µm), the F57 (Ankom®) and the non-woven textile
(NWT – 100 g/m2).Nylon could be assumed as standard
textile for rumen incubation procedures. However, controversial results have been reported with regard to its efficiency for obtaining contents of indigestible compounds, which seem to be associated with significant loss of particles (Casali et al., 2009).
Considering that different textiles can be used for
in situ estimation of indigestible compounds contents,
there is an imminent demand for results regarding accuracy and precision of estimates and for aspects concerning analytical costs and operational facilities.
The objective of this work was to evaluate the contents of iDM, iNDF and iADF in samples of feeds and cattle feces processed at different particle sizes and using bags made from nylon (50 µm), F57 and NWT through in situ
procedures.
Material and Methods
The experiment was carried out in the Laboratório de Nutrição Animal of the Departamento de Zootecnia at the Universidade Federal de Viçosa, Viçosa, MG. Samples of concentrates (sorghum grain, corn gluten meal, citrus pulp, cottonseed meal, soybean meal, wheat bran, corn grain and soybean hulls) and forages (signal grass hay, sugarcane, coast cross hay, corn straw, corn silage and elephant grass cut at 50 and 250 days of regrowth) were used. Feces samples taken from cattle fed 15% and 50% concentrate
diets, on dry matter (DM) basis, were also evaluated. Those feces samples were named low (LC) and high (HC) concentrate feces, respectively.
The samples of corn silage, sugarcane, elephant grass (50 and 250 days) and feces were oven-dried (60ºC) and together other samples were processed in a knife mill to pass through 1-, 2- and 3-mm screen sieves. The corn gluten meal sample was evaluated only at 1-mm because it was acquired in this form from the manufacturer. Additionally, the samples of sorghum grain, cottonseed meal, citrus pulp and soybean meal were only processed at 1- and 2-mm sieves. The sorghum grains were small enough to pass in the whole form through 3-mm sieve. On the other hand, cottonseed meal, citrus pulp and soybean meal were manufactured at 2-mm and could not be evaluated at 3-mm.
Chemical evaluations were performed by using samples processed at 1-mm. The contents of DM, organic matter (OM) and crude protein (CP) were evaluated according to Silva & Queiroz (2002). The contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were evaluated using the detergent compositions recomended by Mertens (2002) and Goering & Van Soest (1975), respectively. The micro-extraction in autoclave was used (Pell & Schofield, 1993) (Table 1). The NDF analysis was conducted using a heat-stable α-amilase (Termamyl 2X, Novozymes), but not
sodium sulfite. There were no ash or protein corrections. For the evaluation of iDM, iNDF and iADF contents, bags of 4 × 5 cm were manufactured using nylon (50 µm) and
non-woven textile (NWT - 100 g/m2). The F57 bags were
bought directly from the manufacturer (Ankom®). Three bags of each textile in each particle size were evaluated, totaling 405 bags. The samples were put in the bags following the ratio of 20 mg DM/cm2 of surface (Nocek, 1988). For all
experimental procedures, the bags were heat-sealed. The bags were incubated in the rumen of three crossbred Holstein × Zebu lactating cows. The animals were fed a mixed diet (70:30 corn silage:concentrate ratio). It was used one bag of each sample and particle size by cow. The samples were kept in the rumen for 264 hours (Casali et al., 2008). After that, the bags were cleaned with tap water, oven-dried (60ºC/72 hours and 105°C/1 hour) sequentially, put in a dissecator and then weighed, obtaining then the iDM content.
The procedures to evaluate iNDF and iADF contents were performed by using a fiber analyzer (Ankom220). The
After neutral detergent extraction, the bags were sequentially washed with hot distilled water and acetone, oven-dried (60ºC/72 hours following 105°C/1 hour), put in a dissecator and then weighed obtaining the iNDF. The iADF analysis was sequentially performed using the same procedures used for iNDF evaluation.
Before incubation procedures, all bags were cleaned with boiling neutral detergent, oven-dried and weighed as described before to obtain the blank weights.
Eight bags of each textile (blank bags) were used to evaluate the physical integrity after different analytical steps. Duplicates of bags were submitted to the following procedures: A) cleaning with neutral detergent (textile before incubation); B) all procedures described in A plus 264 hours of rumen incubation, cleaning with tap water and oven-drying (60ºC/72 hours and 105ºC/1 hour); C) all procedures described in B plus neutral detergent extraction (100ºC/1 hour), washing with hot water and acetone and oven-drying as described above; and D) all procedures described in C plus acid detergent extraction (100ºC/1 hour), washing with hot water and acetone and oven-drying as previously described. Such sequence of procedures was adopted to simulate the sequential evaluation of iDM, iNDF and iADF, respectively.
Aliquots of textiles were taken from one bag in each experimental stage and submitted to scanning microscopic evaluation (Silveira, 1998). Textile fragments were fixed on metallic holders by using a glue containing silver. After that, the textiles were covered with a gold layer and evaluated at 30 and 100× of augment using a scanning microscope (DSEM 962, Zeiss Co.). These procedures were performed in the Laboratório de Biologia Celular e Tecidual
at the Centro de Biociências e Biotecnologia of the Universidade Estadual do Norte Fluminense, Campos dos Goytacazes, RJ.
To evaluate the tensile capacity (tension of rupture), one bag of each textile and experimental stage was sent to the Laboratório de Ensaios Físicos e de Composição of SENAI/CETIQT (Rio de Janeiro, RJ). It was used a dynamometer Instron (model 4467) according to the characteristics: speed of the claw - 100 mm/min, distance between claws - 3 cm, and sizes of the claws – 2.5 x 7.5 cm. That evaluation was done according to technical norm NBR 13041/ISO 9073 (part 3).
The contents of iDM, iNDF and iADF were evaluated independently for each material (sample) by using a variance analysis according to a 3 × 3 factorial arrangement, with three textiles and three particle sizes. This last factor (particle sizes) was not constant among materials as previously reported in this section. The analysis of variance was conducted using type III sums of squares because of imbalances due to the lost of some bags. When necessary, the means were compared by using the Tukey-Kramer test (Littell et al., 1991). All statistical procedures were performed by using SAS (α = 0.05)
Results and Discussion
There was no interaction of textile and particle size (P>0.05), except for iDM and iNDF evaluated in HC feces (P<0.05).
For most materials, it was observed similar iDM contents when NWT and F57 were used (P>0.05), being those contents higher than those obtained with nylon (P<0.05). Different
Item1
Material Dry matter Organic matter Crude protein Neutral detergent Acid detergent (%) (% of dry matter) (% of dry matter) fiber (% of dry matter) fiber (% of dry matter)
Sorghum grain 88.54 99.11 8.68 11.61 3.15
Corn gluten meal 90.76 99.03 63.48 12.85 6.43
Citrus pulp 85.76 94.21 5.40 25.56 17.31
Cottonseed meal 89.28 92.53 39.93 27.92 15.36
Soybean meal 89.39 93.20 43.07 13.49 6.48
Wheat bran 83.59 93.18 14.95 47.74 14.18
Corn grain 86.28 98.65 8.25 13.61 2.64
Soybean hulls 89.77 95.96 10.83 67.34 44.62
Signal grass hay 90.54 96.59 3.71 86.29 50.29
Sugarcane 22.28 96.33 2.90 60.41 37.34
Coast cross hay 89.96 94.41 11.43 83.93 40.44
Corn straw 90.43 98.50 1.88 90.80 45.55
Corn silage 30.97 93.06 4.49 57.79 29.37
Elephant Grass (50 days) 13.55 92.17 9.07 74.35 51.88
Elephant Grass (250 days) 22.54 93.54 7.20 85.34 53.67
Feces (low concentrate) 13.37 88.83 8.88 66.03 36.44
Feces (high concentrate) 14.66 91.07 10.51 54.20 24.08
results were obtained for citrus pulp, wheat bran, corn grain and coast cross hay, where all textiles were found different from each other (P<0.05), being the lower estimates verified by using nylon. There was no difference among textiles (P>0.05) concerning iDM contents in corn gluten meal (Table 2).
On the other hand, for all materials (except corn grain), there were similar iNDF contents (P>0.05) when NWT and F57 were used; but both textiles produced higher estimates compared to nylon (Tables 3 and 5). For corn grain, all textiles were found different (P<0.05); however, iNDF
estimates obtained with F57 and NWT were closer when compared to estimates obtained with nylon (Table 3).
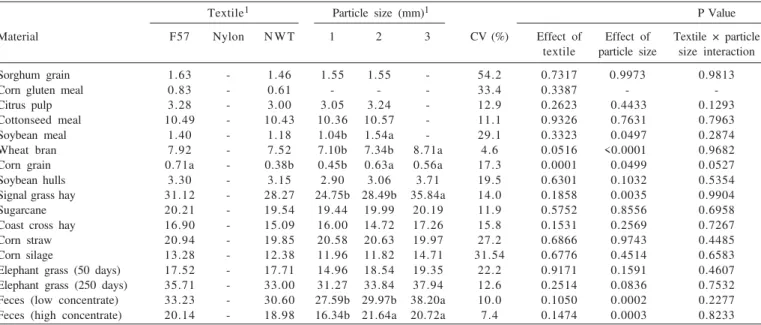
There are no estimates of iADF contents using nylon because all bags presented rupture during extraction with acid detergent. Despite this, such as observed for iNDF, similar estimates (P>0.05) of iADF contents were obtained using NWT and F57 for all materials, except for corn grain (Table 4).
Generally, it was observed higher variability among textiles for iDM contents (Table 2) when compared to iNDF and iADF (Tables 3 and 4).
Textile1 Particle size (mm)1 P Value
Material F57 Nylon N W T 1 2 3 CV (%) Effect of Effect of Textile × particle textile particle size size interaction
Sorghum grain 2.90a 0.55b 2.56a 1.88 2.13 - 45.7 0.0016 0.5731 0.9490 Corn gluten meal 1.61a 0.18b 1.36a - - - 37.5 0.0093 -Citrus pulp 4.58a 2.01b 4.08a 3.48 3.64 - 11.1 <0.0001 0.4362 0.3937 Cottonseed meal 14.02a 10.57b 14.51a 12.76 13.31 - 11.2 0.0010 0.4362 0.6737 Soybean meal 2.04a 0.16b 1.88a 1.15b 1.58a - 27.3 <0.0001 0.0296 0.1215 Wheat bran 15.11a 11.33b 14.00a 12.06b 12.88b 15.50a 7.4 <0.0001 <0.0001 0.9192 Corn grain 1.70a 0.19c 1.13b 0.97 1.11 0.93 24.5 <0.0001 0.2558 0.4319 Soybean hulls 5.40a 2.74b 5.04a 3.67 4.70 4.81 26.0 0.0004 0.1019 0.7215 Signal grass hay 48.65a 30.91b 44.07a 35.00b 39.88b 48.75a 14.2 <0.0001 0.0008 0.9173 Sugarcane 33.06a 24.56b 30.34a 27.27b 30.25ab 30.44a 7.8 <0.0001 0.0254 0.7082 Coast cross hay 35.48a 23.49b 31.99a 29.34 28.91 32.71 10.8 <0.0001 0.0693 0.1711 Corn straw 37.14a 17.04b 34.46a 28.16 30.70 29.77 26.7 0.0002 0.8120 0.8809 Corn silage 23.27a 10.34b 21.92a 17.38 17.67 20.48 29.2 0.0009 0.4801 0.8505 Elephant grass (50 days) 28.42a 17.38b 29.38a 21.06b 25.80ab 28.32a 18.7 <0.0001 0.0133 0.4742 Elephant grass (250 days) 51.85a 40.40b 47.91a 42.05b 45.95ab 52.16a 10.9 0.0016 0.0047 0.5996 Feces (low concentrate) 53.22a 36.82b 49.61a 41.25b 44.47b 53.94a 11.8 <0.0001 0.0006 0.7465 Feces (high concentrate)2 - - - - - - 5.7 <0.0001 <0.0001 0.0321
1 Means followed by different letters within textiles or particle sizes are different according to Tukey-Kramer test (P<0.05). 2 See evaluation of interaction effect in Table 5.
Table 3 - Means and coefficients of variation of indigestible neutral detergent fiber (% of dry matter) in samples processed at different particle sizes and using different textile bags
Textile1 Particle size (mm)1 P Value
Material F57 Nylon N W T 1 2 3 CV (%) Effect of Effect of Textile × particle textile particle size size interaction
Sorghum grain 5.34a 1.21b 3.83a 3.39 3.52 - 29.1 <0.0001 0.7885 0.8296 Corn gluten meal 3.58 1.58 2.91 - - - 48.2 0.2376 -Citrus pulp 6.66a 2.71c 4.91b 4.54 4.98 - 12.0 <0.0001 0.1552 0.6894 Cottonseed meal 25.09a 12.98b 20.96a 18.77 20.58 - 15.2 <0.0001 0.2228 0.2877 Soybean meal 3.93a 0.74b 2.51a 1.92 2.86 - 43.1 0.0006 0.0776 0.3252 Wheat bran 17.94a 13.32c 15.76b 13.87b 15.29b 17.86a 7.4 <0.0001 <0.0001 0.9635 Corn grain 3.63a 0.61c 1.78b 1.96 2.10 1.97 19.3 <0.0001 0.7037 0.2218 Soybean hulls 8.54a 3.55b 7.11a 5.84 6.48 6.89 29.8 <0.0001 0.5148 0.7356 Signal grass hay 54.86a 35.96b 49.10a 40.82b 45.13b 53.97a 11.4 <0.0001 0.0003 0.9244 Sugarcane 38.08a 27.40b 35.59a 32.56 34.63 33.87 9.0 <0.0001 0.3814 0.9069 Coast cross hay 41.38a 27.34c 36.86b 33.95b 33.75b 37.89a 9.5 <0.0001 0.0399 0.2565 Corn straw 42.87a 22.65b 39.73a 34.84 34.99 35.43 25.3 0.0003 0.9892 0.8956 Corn silage 29.14a 14.29b 25.81a 22.19 22.38 24.66 24.6 0.0001 0.6051 0.7871 Elephant grass (50 days) 37.70a 22.51b 34.63a 27.97b 32.45ab 34.42a 16.5 <0.0001 0.0476 0.6248 Elephant grass (250 days) 57.81a 44.63b 52.44a 47.24b 51.21ab 56.43a 9.9 0.0005 0.0101 0.5594 Feces (low concentrate) 65.19a 46.18b 58.57a 51.57b 54.23b 64.13a 10.4 <0.0001 0.0012 0.9370 Feces (high concentrate)2 - - - - - - 5.4 <0.0001 <0.0001 0.0149
1 Means followed by different letters within textiles or particle sizes are different according to Tukey-Kramer test (P<0.05). 2 See evaluation of interaction effect in Table 5.
Some authors have suggested using iDM as internal marker in ruminant nutrition research (Huhtanen et al., 1994; Detmann et al., 2001), which could be advantageous because of the low analytical cost compared to indigestible fibrous residues (Detmann et al., 2001). However, the presence of contaminants may compromise the results (Huhtanen et al., 1994; Casali et al., 2009) because detergents are not used to clean the residues after incubation and the withdrawal of microbial debris through anionic action become limited (Van Soest, 1994; Clipes et al., 2006). Additionally, the detergent action could contribute to decrease the contamination by non-microbial residues (e.g. feed or rumen content particles), that are normally observed outside of bags after removal from the rumen.
The contamination by residues in the in situ evaluation
of iDM seems to be not constant among different materials (Casali et al., 2008) and possibly is not homogeneous among replicates (Sampaio et al., 2011). Thereby, at least part of the differences among textiles concerning the iDM contents could be confounded with variations in the levels of microbial and non-microbial contaminants. This pattern may cause inconsistencies on inferences and indicates that iDM should not be used as internal marker.
Considering the results of iNDF and iADF (Tables 2 and 3), it can be assumed that estimates obtained with both F57 and NWT could be considered equivalent. The difference between these textiles for corn grain is supposed to be random or casual because it was one single difference among seventeen materials and any apparent cause could be associated with that pattern. Similar results between F57 and NWT were reported by Casali et al. (2009) and Valente et al. (2011), who worked with in situ evaluation
of iNDF and laboratorial evaluation of NDF, respectively. The lower contents of iNDF observed with nylon (Tables 2 and 5) could be associated with loss of insoluble particles through its mesh (Figure 1). Such argument is corroborated by results of other authors (Casali et al., 2009; Valente et al., 2011).
The geometrically regular arrangement of nylon mesh (Figures 1 e 2) allows the output of fibrous particles in laboratorial evaluation of NDF (Valente et al., 2011). That pattern can become more prominent when samples are submitted to long term incubations in the rumen (Casali et al., 2009), causing underestimation of the
Textile1 Particle size (mm)1 P Value
Material F57 Nylon N W T 1 2 3 CV (%) Effect of Effect of Textile × particle textile particle size size interaction
Sorghum grain 1.63 - 1.46 1.55 1.55 - 54.2 0.7317 0.9973 0.9813
Corn gluten meal 0.83 - 0.61 - - - 33.4 0.3387 -
-Citrus pulp 3.28 - 3.00 3.05 3.24 - 12.9 0.2623 0.4433 0.1293
Cottonseed meal 10.49 - 10.43 10.36 10.57 - 11.1 0.9326 0.7631 0.7963 Soybean meal 1.40 - 1.18 1.04b 1.54a - 29.1 0.3323 0.0497 0.2874 Wheat bran 7.92 - 7.52 7.10b 7.34b 8.71a 4.6 0.0516 <0.0001 0.9682 Corn grain 0.71a - 0.38b 0.45b 0.63a 0.56a 17.3 0.0001 0.0499 0.0527 Soybean hulls 3.30 - 3.15 2.90 3.06 3.71 19.5 0.6301 0.1032 0.5354 Signal grass hay 31.12 - 28.27 24.75b 28.49b 35.84a 14.0 0.1858 0.0035 0.9904 Sugarcane 20.21 - 19.54 19.44 19.99 20.19 11.9 0.5752 0.8556 0.6958 Coast cross hay 16.90 - 15.09 16.00 14.72 17.26 15.8 0.1531 0.2569 0.7267 Corn straw 20.94 - 19.85 20.58 20.63 19.97 27.2 0.6866 0.9743 0.4485 Corn silage 13.28 - 12.38 11.96 11.82 14.71 31.54 0.6776 0.4514 0.6583 Elephant grass (50 days) 17.52 - 17.71 14.96 18.54 19.35 22.2 0.9171 0.1591 0.4607 Elephant grass (250 days) 35.71 - 33.00 31.27 33.84 37.94 12.6 0.2514 0.0836 0.7532 Feces (low concentrate) 33.23 - 30.60 27.59b 29.97b 38.20a 10.0 0.1050 0.0002 0.2277 Feces (high concentrate) 20.14 - 18.98 16.34b 21.64a 20.72a 7.4 0.1474 0.0003 0.8233
1 Means followed by different letters within textiles or particle sizes are different according to Tukey-Kramer test (P<0.05). 2 See evaluation of interaction effect in Table 5.
Table 4 - Means and coefficients of variation of indigestible acid detergent fiber (% of dry matter) in samples processed at different particle sizes and using different textile bags
Particle Textile1
size (mm)1
F 5 7 Nylon N W T
Indigestible dry matter (% of dry matter)
1 43.67Ba 21.77Bc 35.67Bb
2 54.37Aa 25.95ABb 50.37Aa
3 56.02Aa 31.94Ab 49.08Aa
Indigestible neutral detergent fiber (% of dry matter)
1 30.83Ba 16.45Bb 30.38Ba
2 40.51Aa 19.82ABb 38.16Aa
3 40.17Aa 24.96Ab 36.42Aa
1 Means in the row, followed by different lower case letters, or in the column,
followed by different capital letters, are different according to Tukey-Kramer test (P<0.05).
Figure 1 - Photomicrographs of F57 (a), NWT (b) and nylon textiles (30×).
contents of indigestible compounds. Accordingly, the utilization of nylon for in situ evaluation of indigestible
compounds is not recommended.
The superficial structure of F57 (Figure 3) and NWT (Figure 4) are similar and both present irregular geometrical arrangement, being formed by deposition of synthetic fiber without weaving. From this, it could be inferred that their porosities are apparently smaller than those of nylon (Figure 2). However, the NWT does not present homogenous porosity along all surface because part of the textile is heat-sealed during textile manufacturing (Figure 1). Theoretically, this characteristic should reduce the effective porous surface of NWT. However, this fact does not seem to constraint the inflow of microorganisms as well as the outflow of degraded material, which is a desirable characteristic (Nozière & Michalet-Doreau, 2000). This observation is based on the fact that iNDF and iADF estimates using NWT were similar to those obtained with F57 (Tables 3 and 4).
On the other hand, the lower porosity of NWT and F57 could compromise the removal of fermentation gases from the bag, which could limit the microbial assessment to the sample and, as consequence, the degradation (Udén et al., 1974). So, the inner bag gas accumulation could be used as
an argument to support the higher estimates of iNDF obtained with F57 and NWT (Table 3).
However, such interferences could be considered signficant only at short incubation times (Lindberg & Knutsson, 1981, cited by Udén & Van Soest, 1984). Thereby, the influence of gas accumulation can not overestimate the contents of indigestible residues obtained with long term incubations (Casali et al., 2009), such as the ones used in this study. Once more, it is emphasized that loss of particles is the main constraint on accuracy of estimates of indigestible compounds obtained by using nylon.
There were effects of particles size (P<0.05) on eight and six materials regarding iNDF and iADF contents, respectively (Tables 3, 4 and 5). The iDM results were not examined because of the possible confounding with contaminants, as discussed before. For all materials, it was observed higher estimates for 3-mm particles compared to 1-mm particles (P<0.05).
The 2-mm particles presented varied results. Contents of iNDF obtained with 2-mm particles were similar to 1-mm particles (P>0.05), but lower (P<0.05) than 3-mm particles for wheat bran, signal grass hay and LC feces; similar to 3-mm (P>0.05) but higher (P<0.05) than 1-mm for HC feces (considering only F57 and NWT); or similar to 1-mm and
3-mm (P>0.05) presenting a intermediate position in multiple comparison procedure for sugarcane and elephant grass (Tables 3 and 5). Contents of iADF obtained with 2-mm particles were similar to 1-mm (P>0.05), but lower (P<0.05) than 3-mm for wheat bran, signal grass hay and LC feces; or similar to 3-mm (P<0.05), but higher (P<0.05) than 1-mm for corn grain and HC feces (Table 4). For soybean meal it was observed differences (P<0.05) between the two evaluated particles sizes (1- and 2-mm) for iNDF as well as iADF.
The lack of standardization constitutes one of the limitations for correct applying in situ procedures
(Vanzant et al., 1998; Broderick & Cochran, 2000). Different particles sizes are used in such procedures (Bowman & Firkins, 1993; Nocek & Kohn, 1988). Screen sieves of 2-mm and 5-mm were suggested by Nocek (1988) for concentrate and forage as an approach to compensate the lack of rumination on samples. However, big particles can compromise the microbial access to the substrate (Nozière & Michaelet-Doreau, 2000). Therefore, the utilization of 5-mm particles for forage evaluation has not been recommended in more recent approaches (Hvelplund & Weisbjerg, 2000; NRC, 2001).
On the other hand, the high variability of particles sizes effect among different materials seems to be supported on variability of true distribution of particles sizes of a sample after griding, which can be different for each material (forage
or concentrate) (Nozière & Michaelet-Doreau, 2000). When some materials are ground using a 1-mm screen sieve, very thin particles can be produced, which can escape through textile pores before degradation. This pattern could overestimate the degraded fraction and, as consequence, underestimate the non-degraded residue.
Despite of heterogeneity among materials, the utilization of particles ground to pass a 2-mm screen sieve seems to propitiate an equilibrium point between control of particles loss and an adequate specific surface for microbial degradation. This approach could be assumed as current recommendation for in situ procedures of feed evaluation
(Vanzant et al., 1998; Nozière & Michaelet-Doreau, 2000; NRC, 2001).
Although laboratorial extraction with detergents demands 1-mm particles for efficient extraction of soluble compounds, mainly with neutral detergent, such requirement does not compromise the evaluation of fibrous residues obtained with long term incubations, because it was not expected significant concentrations of cell contents in the incubation residue (Valente et al., 2011). Thereby, the extraction with neutral and acid detergents can be assuredly done on residues obtained after incubation of 2-mm particles. The evaluation of tensile capacity showed that F57 was not affected by any procedure. The values of tension of rupture were approximately constant after all experimental
stages (Table 6). Such pattern can be corroborated by visual survey of that textile (Figure 3).
On the other hand, the NWT presented decrease in tension of rupture after ruminal incubation; although the values had remained stable after detergent extractions (Table 6). When bags are submitted to long term ruminal incubations, the textile can lose resistance because the rumen continuous contractions submit the bags to tension all the time. That decrease in resistance seems to occur despite of no alterations in physical structure of textile, as observed in this study (Figure 4). Such pattern brings into evidence that NWT is not altered by chemical extractions with detergents but it can be physically affected by long term incubation in the rumen. However, that decrease in resistance during ruminal incubation does not seem to cause significant loss of particles because estimates obtained with NWT were similar to those ones obtained using F57 (Tables 3 e 4). Neverthless, it seems to indicate that reutilization of NWT for a second ruminal incubation could lead to increase in the probability of bag rupture, what possibly would not be observed for F57.
Nylon presented the lower tension of rupture when compared to the other textiles. Additionally, this textile was affected by all experimental procedures (Table 6).
Therefore, it may be affirmed that, besides lower resistance, nylon is subjected to damages caused by physical, chemical and biological events. So, there will be high probability of rupture along all analytical steps. The gradual decrease in physical resistance culminates in the rupture of fibers (Figure 2) and loss of all bags during acid detergent extraction (Table 7). Similar results were found by Senger et al. (2008), who reported that polyamides are not resistant to hot acid detergent solutions. Such pattern can compromise the accuracy of estimates and increases the experimental labor because the samples should be taken off the bags for acid detergent extraction.
There was a small loss of F57 and NWT bags during experimental procedures. Additionally, such losses should be assumed as casual because it could not be found any cause to explain that (Table 7).
According to the results of this work, it could be concluded that NWT (100 g/m²) can be a lower cost alternative to F57 for estimating indigestible compounds contents. According to Casali et al. (2009), the estimates obtained using NWT and F57 are generally similar with respect to accuracy and precision. However, the lower cost of NWT (US$ 0.02/bag) when compared to F57 (US$ 2.00/ bag) must be seen as a benefit of NWT utilization, even considering that F57 could be used twice.
Conclusions
The F57 (Ankom®) and non-woven textile (100 g/m2)
propitiate similar estimates of indigestible compounds contents in feeds and cattle feces. The use of nylon (50 µm)
underestimates the contents of such compounds because there is a significant loss of particles due to its porosity and low physical resistance. For in situ evaluation of indigestible compounds,
it is suggested grinding samples as to pass a 2-mm screen sieve.
References
BOWMAN, J.G.P.; FIRKINS, J.L. Effects of forage species and particle size on bacterial cellulolytic activity and colonization i n s i t u. J o u r n a l o f A n i m a l S c i e n c e, v.71, p.1623-1633, 1 9 9 3 .
BRODERICK, G.A.; COCHRAN, R.C. In vitro and in situ methods for estimating digestibility with reference to protein degradability. In: THEODOROU, M.K.; FRANCE, J. (Eds.).
Feeding systems and feed evaluation models. Wallingford: CAB International, 2000. p.53-85.
Ruminal incubation Extraction with neutral detergent Extraction with acid detergent
Item F 5 7 Nylon N W T F 5 7 Nylon N W T F 5 7 Nylon N W T
Lost bags 3 4 1 3 1 1 2 3 1 3 5 3
% loss 2 . 2 3 . 0 0 . 7 2 . 2 8 . 2 1 . 5 2 . 2 100.0 2 . 2
Table 7 - Cumulative loss of bags after sequential procedures for estimating indigestible compounds contents
Tecido
Experimental stage F 5 7 T N T Nylon
Before incubation 30.16 35.26 24.57
After ruminal incubation 31.47 27.38 22.36
After ruminal incubation and neutral detergent extraction 32.38 29.50 19.39 After ruminal incubation and sequential extraction with neutral detergent and acid detergent 31.72 28.02 7.89
CASALI, A.O.; DETMANN, E.; VALADARES FILHO, S.C. et al. Influência do tempo de incubação e do tamanho de partículas sobre os teores de compostos indigestíveis em alimentos e fezes bovinas obtidos por procedimentos in situ. Revista Brasileira de Zootecnia, v.37, p.335-342, 2008.
CASALI, A.O.; DETMANN, E.; VALADARES FILHO, S.C. et al. Estimação de teores de componentes fibrosos em alimentos para ruminantes em sacos de diferentes tecidos. Revista Brasileira de Zootecnia, v.38, p.130-138, 2009.
CLIPES, R.C.; DETMANN, E.; COELHO DA SILVA, J.F. et al. Evaluation of acid detergent insoluble protein as an estimator of rumen non-degradable protein in tropical grass forages.
Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v.58, p.694-697, 2006.
DETMANN, E.; PAULINO, M.F.; ZERVOUDAKIS, J.T. et al. Cromo e indicadores internos na determinação do consumo de novilhos mestiços, suplementados, a pasto. Revista Brasileira de Zootecnia, v.30, p.1600-1609, 2001.
DETMANN, E.; VALADARES FILHO, S.C.; PAULINO, M.F. et al. Avaliação da técnica dos indicadores na estimação do consumo por ruminantes em pastejo. Cadernos Técnicos de Veterinária e Zootecnia, v.46, p.40-57, 2004.
DETMANN, E.; SOUZA, A.L.; GARCIA, R. et al. Avaliação do vício de “tempo longo” de indicadores internos em ensaio de digestão com ruminantes. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v.59, p.182-188, 2007.
FREITAS, D.; BERCHIELLI, T.T.; SILVEIRA, R.N. et al. Produção fecal e fluxo duodenal de matéria seca e matéria orgânica estimados através de indicadores. Revista Brasileira de Zootecnia, v.31, p.1521-1530, 2002.
GOERING, H.K.; Van SOEST, P.J. Forage fiber analyses (apparatus, reagents, procedures and some applications). Washington, DC: USDA-ARS, 1975. 20p. (Agriculture Handbook, 379).
HUHTANEN, P.; KAUSTELL, K.; JAAKKOLA, S. The use of internal markes to predict total digestibility and duodenal flow of nutrients in cattle given six different diets. Animal Feed Science and Technology, v.48, p.211-227, 1994.
HVELPLUND, T.; WEISBJERG, M.R. In situ techniques for the estimation of protein degradability and post rumen availability In: GIVENS, D.I.; OWEN, E.; AXFORD, R.F.E. et al. (Eds.)
Forage evaluation in ruminant nutrition. London: CAB International, 2000. p.233-258.
LITTELL, R.C.; FREUND, R.J.; SPECTOR, P.C. SAS system for linear models. Cary: SAS Institute Inc., 1991. 329p. MERTENS, D.R. Gravimetric determination of amylase-treated
neutral detergent fiber in feeds with refluxing in beaker or crucibles: collaborative study. Journal of AOAC International, v.85, p.1217-1240, 2002.
NOCEK, J.E. In situ and other methods to estimate ruminal protein and energy digestibility: a review. Journal of Dairy Science, v.71, p.2051-2069, 1988.
NOCEK, J.E.; KOHN, R.A. In situ particle size reduction of alfafa and timothy hay as influence by form and particle size.
Journal of Dairy Science, v.71, p.932-945, 1988. NOZIÈRE, P.; MICHALET-DOREAU, B. In sacco methods. In:
D´MELLO, J.P.F. (Ed.) F a r m a n i m a l m e t a b o l i s m a n d nutrition. London: CAB International, 2000. p.233-253. NATIONAL RESEARCH COUNCIL - NRC. Nutrient requirements
of dairy cattle. 7.ed. Washington, DC: Academic Press, 2001. 3 8 1 p .
OWENS, F.N.; HANSON, C.F. External and internal markers for appraising site and extent of digestion in ruminants. Journal of Dairy Science, v.75, p.2605-2617, 1992.
PELL, A.N.; SCHOFIELD, P. Computerized monitoring of gas production to measure forage digestion in vitro. Journal of Dairy Science, v.76, p.1063-1073, 1993.
SAMPAIO, C.B.; DETMANN, E.; VALENTE, T.N.P. et al. Evaluation of fecal recovering and long term bias of internal and external markers in a digestion assay with cattle. Revista Brasileira de Zootecnia, v.40, p.174-182, 2011.
SENGER, C.C.D.; KOZLOSKI, G.V.; SANCHEZ, L.M. B. et al., Evaluation of autoclave procedures for fibre analysis in forage and concentrate feedstuffs. A n i m a l F e e d S c i e n c e a n d Technology, v.146, p.169-174, 2008.
SILVA, D.J.; QUEIROZ, A.C. Análises de alimentos. Métodos químicos e biológicos. 3.ed. Viçosa, MG: Editora UFV, 2002. 2 3 5 p .
SILVEIRA, M. Preparo de amostras biológicas para microscopia eletrônica de varredura. In: SOUZA, W. (Ed.) Técnicas básicas d e m i c r o s c o p i a e l e t r ô n i c a a p l i c a d a s à s c i ê n c i a s biológicas. Rio de Janeiro: Sociedade Brasileira de Microscopia Eletrônica, 1998. p.33-44.
UDÉN, P.; PARRA, R.; Van SOEST, P.J. Factors influencing reliability of the nylon bag technique. J o u r n a l o f D a i r y Science, v.57, p.622-622, 1974.
UDÉN, P.; Van SOEST, P.J. Investigation of the i n s i t u bag technique and a comparison of the fermentation in heifers, sheep, ponies and rabbits. Journal of Animal Science, v.58, p.213-221, 1984.
VALENTE, T.N.P.; DETMANN, E.; VALADARES FILHO, S.C. et al. Avaliação dos teores de fibra em detergente neutro em forragens, concentrados e fezes bovinas moídas em diferentes tamanhos e em sacos de diferentes tecidos. Revista Brasileira de Zootecnia, 2011 (in press).
Van SOEST, P.J. Nutritional ecology of the ruminant. 2.ed. Ithaca: Cornell University Press, 1994. 476p.