Review
Oral
films as breakthrough tools for oral delivery of proteins/peptides
Pedro M. Castro
a,b, Pedro Fonte
b,c, Flávia Sousa
b, Ana Raquel Madureira
a,
Bruno Sarmento
b,d,e, Manuela E. Pintado
a,⁎
a
CBQF— Centro de Biotecnologia e Química Fina — Laboratório Associado, Escola Superior de Biotecnologia, Universidade Católica Portuguesa/Porto, Rua Arquiteto Lobão Vital Apartado 2511, 4202-401 Porto, Portugal
b
CESPU, Instituto de Investigação e Formação Avançada em Ciências e Tecnologias da Saúde, Rua Central de Gandra 1317, 4585-116 Gandra-PRD, Portugal
c
REQUIMTE, Department of Chemical Sciences— Applied Chemistry Lab, Faculty of Pharmacy, University of Porto, Rua de Jorge Viterbo Ferreira 228, 4050-313 Porto, Portugal
d
Instituto de Investigação e Inovação em Saúde, Universidade do Porto, Portugal
eINEB— Instituto de Engenharia Biomédica, University of Porto, Rua do Campo Alegre 823, 4150-180 Porto, Portugal
a b s t r a c t
a r t i c l e i n f o
Article history: Received 14 April 2015
Received in revised form 7 May 2015 Accepted 8 May 2015
Available online 13 May 2015 Keywords: Bioactive peptides Bioactive proteins Bioavailability enhancement Oral delivery Oralfilms Stability
Therapeutic proteins and peptides demonstrate unique, peerless, pharmacological characteristics such as high specificity to receptors and superior biological mimicking of physiological mechanisms, resulting in a better therapeutic index compared to conventional chemical-derived drugs. However, proteins also present inherent bioavailability limitations. Thus, this paper proposes several effective tools to improve protein/peptide drugs stability, permeability and pharmacokinetics with special emphasis on oral polymericfilms as oral delivery platforms. Indeed, oralfilms present inherent characteristics that can greatly enhance biological performance of proteins and peptides and patient compliance along with other advantages that are critically discussed in this review. A rational choice of excipients addressed in and manufacture processes are also focused. In addition, possible toxicity issues to be overtaken and critical analysis regarding current market tendencies respecting oral films and protein/peptides along with future prospects are disclosed.
© 2015 Elsevier B.V. All rights reserved.
Contents
1. Introduction . . . 64
2. Advantages and disadvantages of oralfilms as delivery systems for bioactive proteins and peptides . . . 64
3. Polymer-based oralfilms for delivery of proteins and peptides . . . 65
3.1. Relevant oralfilms excipients for protein delivery . . . 66
3.1.1. Polymers . . . 66
3.1.2. Amino acids and proteins . . . 66
3.1.3. Ions . . . 66
3.1.4. Sugars and polyols . . . 66
3.1.5. Surfactants . . . 67
3.1.6. Other approaches to achieve protein/peptides bioavailability enhancement . . . 67
4. Toxicity concerns . . . 68
5. Walkthrough on pipeline products . . . 69
6. Future perspectives . . . 70
7. Conclusion . . . 70
References . . . 71
Abbreviations: CMC, carboxymethylcellulose; CPP, cell penetrating peptide; FPT,flexographic printing technology; HSPC, hydrogenated phosphatidylcholine; PEG-b-PLA, poly(ethylene glycol)methyl ether-block-polylactide; PLGA, poly(lactic-co-glycolic acid); PVP, polyvinylpyrrolidone; MW, molecular weight; SDS, sodium dodecyl sulfate; SLN, solid lipid nanoparticle.
⁎ Corresponding author.
E-mail address:mpintado@porto.ucp.pt(M.E. Pintado).
http://dx.doi.org/10.1016/j.jconrel.2015.05.258
0168-3659/© 2015 Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
Journal of Controlled Release
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / j c o n r e l1. Introduction
Pharmacological active proteins and peptides have been extensively investigated regarding hormonal, immune, hypertensive, anti-nociceptive, among other biological properties, with the advantages of great target-specificity and safety comparatively to the majority of conventional drugs. Bioactive proteins and derived peptides may be ob-tained from a broad array of natural resources (e.g. concentrated pep-tide extract obtained by hydrolysis of whey proteins demonstrated antinociceptive effect, peptides obtained from enzymatic degradation ofβ-lactoglobulin and α-lactalbumin demonstrated antihypertensive activity by inhibition of angiotensin-converting enzyme) or by chemical synthesis[1].
Nevertheless, some protein and peptides delivered by oral route present inherent drawbacks, namely low intestinal absorption profile through transcellular and paracellular routes due to their hydrophilic nature. Other problems include potential loss of bioactivity due to the harsh pH conditions and enzymatic activity prevailing throughout gas-trointestinal tract. Consequently, protein formulations are usually ad-ministered recurring to invasive route in order to assure the expected efficacy[2]. However, oral administration is still the preferred one among all administration routes, mainly because it is noninvasive, which implies a better compliance to treatment[3,4].
Oralfilms are delivery systems used to administrate active pharma-ceutical ingredients per os, and can be tailored attending to promote local absorption of proteins/peptides and to offer stability and improve-ment of the permeability profile along the gastrointestinal tract, for those molecules that are eventually swallowed due to mouth mechani-cal forces and saliva turnover. Additionally, like other oral delivery sys-tems, oralfilms may also be used for molecules that act locally in the gastrointestinal tract.
Hence, this review proposes several approaches to optimize the pharmacokinetic profile of proteins and peptides, mainly regarding lib-eration and absorption. Stability and overall bioavailability improve-ments will also be discussed, suggesting oralfilms as relevant protein/ peptide oral delivery systems. Additionally, commonly used excipients for oralfilm production along with different manufacturing process-es are analytically reviewed to help tailor an oralfilm that better suits each protein/peptide and ensures biopotency enhancement along with maximized compliance. Complementary strategies to im-prove protein/peptide bioavailability, namely nano- and microencapsu-lation, use of permeability enhancers and chemical modification of biomacromolecules, along with toxicity issues will be discussed. Fur-thermore, oralfilm-based pipeline products will be overviewed along with a hypothetical prediction of the potential benefits brought by the scale-up production of protein/peptides incorporated into oralfilms. 2. Advantages and disadvantages of oralfilms as delivery systems for bioactive proteins and peptides
Oralfilms present pleasant flavor and texture, are small-sized (fitting comfortably in the oral cavity) and are easily dissolved in the oral cavity, immediately releasing the active pharmaceutical ingredient (disintegration time is ca. 30 s). Additionally, oralfilm characteristic mucoadhesion is ideal for uncooperative patients since, once in the oral cavity, oralfilms are very difficult to remove. Moreover, oral films do not require water to be administered and, thus, are much more prac-tical than tablets, capsules or syrups, being also very interesting tools re-garding protein/peptide oral delivery[5].Fig. 1reveals the common appearance of oralfilms, wherein very small thickness is evidenced by translucency. Dimensions indicate that oralfilms comfortably fit into the oral cavity.
Hence, a delivery system such as oralfilms can be specifically tailored to represent a good solution regarding the improvement of pharmacokinetic properties of proteins and peptides to be delivered, along with a great enhancement of compliance to treatment. Indeed,
buccal administration and absorption of peptides/proteins is favorable, avoiding inherent harsh gastrointestinal conditions. Also, keratinization profile of buccal cavity, low enzymatic activity and rich vascularization are positive factors for administration of bioactive proteins/peptides
[6]. However, oral films have a limited incorporation capacity of active ingredients (ca. 5%, w/w, to 30%, w/w) implying that bioactive molecules must be potent enough at low doses, being considered an im-portant drawback of oralfilms. The fact that buccal epithelium presents a molecular weight cut-off of ca. 500–1000 Da, above which protein/ peptide permeation is hindered must be considered, requiring an opti-mization of oralfilm formulation to overcome buccal permeability is-sues[7]. Subsequently, if proteins/peptides carried by oralfilms are not totally absorbed in the oral cavity, protection from gastrointestinal tract conditions (e.g. extreme pH and enzymatic degradation) must be granted and permeability-enhancing techniques need to be effective. Oralfilm formulation must be designed so that proteins and peptides are able to pass throughout the gastrointestinal tract without losing ac-tivity and must offer capacity to permeate intestinal epithelium to car-ried biomolecules, assuring that systemic circulation is reached[8]. A list of proteins and peptides with therapeutic interest that are potential-ly good candidates to be delivered by oralfilms (with an expected biopotency increment) is depicted inTable 1.
Furthermore, since oralfilms are very thin layers usually composed of polymers, plasticizers, saliva stimulating agents, disintegrants (or super-disintegrants), sweetening agents,flavoring agents, coloring agents and stabilizers, special care must be taken in order to avoid peptide- or protein–excipient interactions. Excipients may yield posi-tive effects, increasing overall bioavailability of carried proteins/pep-tides, but may also lead to problems such as precipitation, structure loss and bioavailability modification[4,22,23]. Also, some excipients may induce alterations in gastrointestinal tract motility and/orfluid se-cretion rate, therefore interfering with biomolecules absorption[24].
Thus, despite the inherent advantages of protein and peptide deliv-ery systems, due to the intimate contact between all excipients and the active ingredients, oralfilm formulation must be thoroughly ideal-ized regarding functional structure maintenance of carried molecules along with preservation of biological function.
Moreover, classic oralfilm production methods may also represent some struggles regarding the stability of bioactive proteins and pep-tides. Among the different methodologies to preparefilms, the solvent casting is the most feasible, cost-effective technique and, therefore, the most used one. The solvent-casting method is performed by
homogenizing excipients and active pharmaceutical ingredients using an aqueous or alcoholic solvent[5]. The mixture is then casted into a surface or mold and is heated for the solvent to be eliminated, possibly compromising stability of thermo labile bioactive molecules as proteins or peptides. Finally, once dried,films are cut, stripped and packaged. Solvent-casting procedure sometimes implies energetic stirring during the homogenization or de-aeration steps, factors that can also lead to protein denaturation[25]. Moreover, in order to decrease solvent cast-ing time, organic solvents are frequently used, which can represent a se-rious problem regarding protein structure maintenance. Finally, since active ingredients are added to the oralfilm formulation before the solvent is casted, the possibility of phase separation and precipitation of incorporated proteins or peptides must be taken into account, which could not only alter biomacromolecule activity but also induce a modification in texture properties and flavor of the oral film formulation.
Hot-melt extrusion is an alternative method to solvent-casting for oralfilm production that allows avoiding solvents[26]. Briefly, instead of using a solvent, both excipients and active pharmaceutical ingredi-ents are homogenized in a dry state and subsequently molten and ex-truded. Finally, oralfilms are cut from the mold in appropriate shapes and dimensions. Hot-melt extrusion method is impracticable for pro-teins, especially thermo labile propro-teins, not only due to heating but also to the shear stress applied during the process.
Semisolid casting technique is performed by adding a previously prepared solution of water-soluble polymer to a solution of acid-insoluble polymer (prepared in ammonium or sodium hydroxide) in a 4:1 ratio. Remaining excipients and active ingredients are then added and resulting gel is cast by heating, using heat control drums. Again, cast process occurs by heating which may compromise bioactivity of carried proteins and peptides. Moreover, recurring to alkali solutions may denature proteins with consequent lost of activity.
Solid dispersion extrusion consists of the dispersion of active phar-maceutical ingredients (pure or in solution) into melted polyethylene glycol (PEG) or another polyol. It is required that dispersed drug, either pure or in solution, is insoluble in the polyol. Immiscible components are then extruded, to obtain solid dispersions. Finally, solid dispersions acquirefilm shape by means of dies.
Flexographic printing technology (FPT) is a gentle process that deposits an active pharmaceutical substance onto oralfilms by con-tact printing[26]. FPT can be particularly suitable for heat-sensitive molecules such as proteins and peptides thus representing a very promising tool to effectively incorporate proteins/peptides into oralfilms[27]. Since mixing and drying of the oralfilm formulation
is performed before drug incorporation (drug is posteriorly added by rotating rollers), mechanical stress, dehydration and active ingredi-ent losses are obviated. Additionally, FPT does not alter mechanical properties of oralfilms. Moreover, since both scale-up and scale-down are easy to perform and considering that great production speed (530 oralfilms per minute were reported) can be achieved, FTP represents a good industrial solution to elaborate oralfilms with incorporated peptides or proteins.
Inkjet-printing is another suitable oralfilm preparation method that can be applicable for incorporation of proteins or peptides. Like FPT, inkjet-printing adds solution drops containing the active sub-stances onto (and not into) the oralfilm but with a very high precision (5–15 pL/droplet)[28]. Inkjet-printing mechanism of adding active pharmaceutical ingredients does not affect the mechanical properties of oralfilms which is an important advantage compared to solvent casting[26]. Nevertheless, since proteins and peptides are very prone to suffering hydrolytic degradation, incorporation into droplets must be performed with care especially regarding solvent choice.
Both FPT and inkjet-printing allow two or more proteins or peptides to be added to the same oralfilm in individualized, pre-designed pat-terns[29]. Thus, different proteins/peptides can be printed on different spots of the oralfilm matrix, avoiding interaction between bioactive molecules during storage time.
3. Polymer-based oralfilms for delivery of proteins and peptides Structural stability of proteins or peptides is a major factor regarding biologic activity and, therefore, physical and chemical stability must be guaranteed, in which excipients may play a central role[30]. Also, pro-teins/peptides administered by oral route are extremely prone to suffer-ing pre-systemic degradation[24]. However, stabilization procedures and stabilizing excipients are not the same for all kinds of proteins or peptides and, therefore, oralfilm formulation must be tailored accord-ing to the protein/peptide to be delivered.
Oral film production methods imply that proteins or peptides contact directly with excipients either in dry or wet states, a factor that must be considered since excipients play a central role in protein/ peptide stability[31]. In order to achieve stable and functional formula-tions, excipients may be used in order to assure proteins/peptides cor-rect delivery and expected bioactivity[30,32]. Type and concentration of stabilizing excipients depend on the type of proteins/peptides to be delivered and must be carefully considered. Oralfilm formulations may also, in some cases, benefit from absorption enhancers to obviate inherent protein/peptide absorption problems, allowing disruption of
Table 1
Potential protein/peptide candidates to be delivered by oralfilms.
Native protein/peptide Active form MW (Da) Uses Absorption Ref.
Linaclotide Linaclotide (CCEYCCNPACTGCY) ~1526.7 – Treatment of chronic constipation. Gastrointestinal local action
[9]
Plecanatide Plecanatide (NDECELCVNVACTGCL) ~1681.8 – Treatment of chronic constipation. Gastrointestinal local action
[10,11]
α-Lactalbumin (α-LA) Peptide segment (YGLF) ~499 – Antinociceptive activity; – Antihypertensive activity; – Intestinal motility stimulator.
Potential buccal absorptionb
[12]
α-Lactalbumin (α-LA) Peptide segment (EQLTK) ~618.3 – Antibacterial activity Potential buccal absorptionb
[1,13]
β-LB Peptide segment (VAGTWY) ~695,3 – Antihypertensive activity; – Antibacterial activity
Potential buccal absorption
[1,14]
Octreotide Octreotide (FCFWKTCT) ~1019,2 – Acromegaly and gigantism treatment Buccala/intestinal [15,16] Salmon calcitonin Salmon calcitonin ~3431.8 – Osteoporosis treatment and/or management Buccala
/intestinal [17]
Insulin Insulin ~5808 – Diabetes mellitus management. Buccala
/intestinala
[18]
Stichodactyla helianthus neurotoxin
Stichodactyla helianthus neurotoxin
(RSCIDTIPKSRCTAFQ----CKHSMKYRLSFCRKTCGTC)
~4060.8 – Treatment of autoimmune diseases. Buccala
[19,20]
Buserelin Buserelin peptide ~1299.4 – Fertility pre-treatment; – Endometriosis treatment.
Buccala
[21]
a
Formulation must contain permeability enhancers.
b
epithelial barriers, interfering with tight junctions closing mechanism, increasing thefluidity of membranes or by decreasing mucus viscosity
[33]. Thus, different approaches to enhance pharmacokinetic profile (especially regarding liberation and absorption) of proteins and pep-tides delivered by oralfilms are addressed.
3.1. Relevant oralfilms excipients for protein delivery 3.1.1. Polymers
Polymers are the backbone of oralfilm formulations and may also be used as absorption enhancers[34]. Mucoadhesive polymers are able to reduce the clearance of drugs from the local site of absorption, attenuat-ing saliva turnover effect. Consequently, active biomacromolecule con-tact with the oral mucosa is maximized, increasing absorption through the mucosa. Some polymers may also work as protein stabilizers and as-semblers by specific mechanisms, as occurs for charged polymers, and by non-specific mechanisms, as occurs for hydrophilic polymers[22]. Polymer molecular size demonstrated to be positively related to protein protection, due to a non-specific protection mechanism called crowding effect[35]. Also, some polymers (e.g. dextran) are able to avoid protein aggregation during lyophilization and to prevent the difficulty of lyophilizate reconstitution[36].
Gelatin and starch, two polymers commonly used in oralfilms pro-duction, provide stabilization to proteins/peptides. Indeed, charged polymers seem to better protect proteins and peptides due to their metal chelating ability[37]. Also, most polymers used in oralfilms for-mulations are mucoadhesive, allowing concentration of the formulation in the absorption area. Chitosan is a good example of a mucoadhesive molecule that can promote both buccal and intestinal permeation by prolonged adhesion to the mucosa, disrupting lipid organization of the buccal epithelium thereby facilitating paracellular permeation[38]. Effectively, even though in the format of bilaminated buccalfilm, chitosan was used as a polymer to deliver insulin through buccal muco-sa[39,40]. A relevant and sustained decrease of blood glucose was verified 5 h after administration. It was also reported that thiolated polymers (e.g. thiolated chitosan and polyacrylate) are particularly good permeation enhancers, promoting reversible and efficient para-cellular permeation (by inhibition of tyrosine phosphatase involved in tight junction closing process) of hydrophilic molecules such as a wide array of proteins and peptides[41–43]. Additionally, thiolated polymers increase mucoadhesivity of the formulation, contributing to an effective drug liberation and absorption[4]. Polymers such as chito-san may also present antibacterial activity, contributing to the elimina-tion of bacteria that are prone to degrade proteins/peptides. Some other polymers such as polycarbophil were found to protect proteins from proteolytic enzymes.
Effectively, polymers may work as stabilizers for proteins by the above-mentioned mechanisms. Polymers also enhance permeability of proteins and peptides through mucosal tissues, which can be translated into an improved absorption performance.
Advantages and disadvantages offilmogenic polymers to be included in formulations of oral strips, aiming transport and delivery of proteins and peptides, are shown inTable 2.
3.1.2. Amino acids and proteins
Some amino acids are good examples of physical stabilizing excipients by favoring the proteins to be surrounded by water molecules and, there-fore, becoming more stable and reducing the probability of aggregation
[54,55]. Indeed, aggregation of proteins and peptides represents a struc-tural problem that leads to immediate disapproval of the formulation. Ar-ginine, although not a stabilizing agent, binds to some proteins (and is excluded from the surface of others) preventing aggregation with other molecules, increasing the shelf life of the formulation. Moreover, arginine promotes refolding of some proteins, being an important additive regard-ing protein bioactivity maintenance. The mechanisms of stabilizregard-ing/ destabilizing effects of arginine seem to be protein-specific which can
explain why, at the same concentration, arginine causes stabilization of some proteins and destabilization of others. Nevertheless, it has been claimed that multiple direct interactions with the charged portions of pro-teins may be the cause of stabilizing effects[56]. Moreover, arginine stabi-lizing effects are preferentially performed when proteins are in their dissociated form, apparently due to the existence of more free arginine binding sites[31]. Arginine may also increase the solubility of proteins, seemingly due to protein suppressing effects[57].
Oligo-arginine and penetratin are able to promote the endocytic path-way and adsorption to glycosaminoglycans present on the surface of mammalian cells and were thus reported as permeability enhancers for hydrophilic molecules as some proteins and peptides[58–60]. Indeed, cell-penetrating peptides (CPP) have been successfully used as absorption enhancers, improving absorption and bioavailability profiles of proteins/ peptides with intracellular activity, that were only liable to be adminis-trated by invasive routes[61]. Moreover, protein enzyme inhibitors (chy-motrypsin, trypsin, carboxy peptidases and aminopeptidase inhibitors) are effective permeability enhancers and reported as interesting tools re-garding protein/peptide bioavailability enhancement[62]. Nevertheless, toxicity issues, mainly digestion problems of diet proteins, are prone to happen.
It is therefore observed that amino acids, if used properly, may aid to stabilize, increase solubility and enhance absorption profile of protein and peptide drugs across physiological barriers, meaning an overall phar-macokinetic improvement.
3.1.3. Ions
Some ions promote aggregation of proteins in specific binding sites, working as stabilizers, zinc being an important example by inducing the formation of insulin hexamers. Actually, metal ions may contribute to stabilization or destabilization of proteins[30]. Thus, metallic composi-tion must be thoroughly controlled, since structure alteracomposi-tion caused by a specific metallic element is dependent on the protein or peptide to be carried and delivered. When preparing an oralfilm formulation with the purpose of protein or peptide delivery, ions from the Hofmeister series may also be chosen (CO32−N SO42−N S2O32−N H2PO4−N F−N Cl−N Br−
~ NO3−N I−N ClO4− N SCN−)[63]. Anions on the left side of the
Hofmeister series, or Kosmotropic ions, are prone to precipitate proteins and to prevent from unfolding. On the other hand, anions from the right side of the Hofmeister series, or Chaotropic ions, tend to increase protein solubility but also to promote denaturation of proteins. Anions are reported to influence protein stability by se-lective accumulation on the protein surface[23]. Chloride is one of the most used anions from the series, being located in the dividing line between Kosmotropic and Chaotropic ions and has demonstrat-ed, when in the form of guanidinium chloride, to decrease the rate of thermal destabilization and unfolding of proteins by binding to one or more cation binding sites, as verified for ribonuclease T1[64]. Once again, it is warned that choice of ions must be performed taking into account that each protein or peptide might behave differently when in contact with different anions. In addition, some excipients are used in the salt form (e.g. saliva stimulating agents such as citric acid, malic acid, tartaric acid, lactic acid) and released ions should be accounted.
Some metallic ions are also prone to promote the formation of re-active oxygen substances that may oxidize proteins and disrupt three-dimensional structure, compromising bioactivity. Citric acid, a saliva-stimulating agent broadly used in oralfilms formulations, may also act as a chelating agent, binding to the proteins and pep-tides or by capturing destabilizing metal ions. Moreover, citric acid captures calcium ions leading to disruption of intracellular junctions, increasing permeability of proteins and peptides[65].
3.1.4. Sugars and polyols
Other important stabilizers, currently used in oralfilms formula-tions, are polyols and sugars such as glycerol, sorbitol (commonly
used as plasticizers in oralfilms formulations) and sucrose, lactose, mannitol and dextrose (used as sweeteners/saliva stimulating agents in oralfilms formulations)[66,67]. It was verified for some proteins and peptides that, during purification processes, sugars present at high concentrations are able to prevent loss of bioactivity[31,68,69]. This protein activity maintenance offered by sugars seems to be due both to water replacement and vitrification phenomena[70]. Water re-placement occurs when proteins are freeze-dried and hydrogen bonds that were previously established between proteins and water are now settled between proteins and sugars (preferentially in the amorphous state, in order tofit the surface of the protein), favoring protein struc-ture maintenance. On the other hand, vitrification is explained by reten-tion of the protein or peptide inside the sugar matrix, restricting aggregation.
Potential of polyols to hinder aggregation of proteins correlates to two main factors: polyol size and chemical composition, especially regarding the number of–OH groups. Indeed, polyol size seems to be positively correlated with protein protection ability but, above the mo-lecular weight of sorbitol (182.2 g/mol), this relation does not exist any-more. Moreover, the number of–OH groups of polyols also seems to be positively related to protein protection efficacy, presumably due to an increased possibility of hydrogen bonds establishment between polyols and proteins or peptides. Sorbitol, for instance, is commonly used both as a plasticizer and sweetening agent in the elaboration of oralfilms and was proven to inhibit heat-induced aggregation of some proteins, even though the effect decreased for high concentra-tions[71]. Glycerol also induces stabilization of certain peptides but may potentially destabilize others and, therefore, must be used care-fully[72]. Nevertheless, reducing sugars may be harmful due to the possible occurrence of Maillard reactions that may generate toxic compounds[32,73].
Briefly, sugars and polyols may offer stability, protection and aggre-gation inhibiting of proteins and peptides, assuring that carried proteins
and peptides successfully reach the local of action, performing expected bioactivity.
3.1.5. Surfactants
Surfactants are known to disrupt phospholipid bilayer and intercel-lular lipids when administered at concentrations above critical micellar concentration, thus being considered rather aggressive permeability en-hancers[74]. Nevertheless, the potential epithelial damage caused by surfactants such as sodium dodecyl sulfate (SDS), nonylphenoxy-polyoxyethylene-10.5 or taurodeoxycholate is reported to be reversible within few hours after disruption. Still, chronic toxicity effects need to be assessed. Yet, non-ionic surfactants such as polysorbate (also used in oralfilms formulations as stabilizing agents) also offer protection to proteins, inhibiting chemical degradation and/or working as chaper-ones to proteins and peptides, offering stability[30,75]. Actually, it was reported that Tween 40 might stabilize human growth hormone, inhibiting peptide aggregation. On the other hand, tween 40 seems to exert a null effect on immunoglobulin G or even present a destabilizing effect on interferon-γ.
Overall, if used properly, surfactants can lead to important pharma-cokinetic improvements of proteins and peptides carried by oralfilms, by enhancing permeability and possibility to successfully reach system-ic circulation.
3.1.6. Other approaches to achieve protein/peptides bioavailability enhancement
Besides benefits that can be obtained from commonly used excipi-ents in oralfilms formulations to enhance bioavailability of carried pro-teins and peptides, other tools may be used to reach similar goals. Next approaches can be used to enhance liberation profile and increase stability and permeability across buccal and/or intestinal epithelium.
The encapsulation of proteins/peptides into nanoparticles, for in-stance, has been used to increase stability and intestinal permeability
Table 2
Advantages and disadvantages of polymers used in the production of oralfilms containing proteins and peptides.
Polymer Advantages Disadvantages Ref.
PVP – Nonionic polymer; – Good filmogenic properties;
– Promote stabilization of proteins/peptides from heat-induced aggregation;
– Bulking agent for peptide formulations.
– Possible formation of amide bonds between proteins/peptides and PVP during oralfilms production process, leading to an alteration of delivery profile.
[44–46]
Gelatin – Stabilizer of proteins by preventing aggregation – Mitigates protein buccal absorption;
– Disruption of the three-dimensional functional structure of proteins; – Formation of complexes with anionic proteins, altering delivery profile.
[47,48]
PLGA – Mucoadhesive, biodegradable, facilitating oral delivery of molecules;
– pH variation which may compromise proteins/peptides bioactivity; – Sensible to degradation from heat treatment in solvent casting process.
[49,50]
Chitosan – Mucoadhesive, biodegradable, facilitating oral delivery of molecules;
– Maintains drugs in the oral activity, compensating the saliva turnover; – Protein permeability enhancer;
– Thiolated chitosan is a reversible paracellular permeation enhancer;
– Transient opening of tight-junctions, allowing polar drugs to penetrate intestinal barriers (GIT absorption only);
– Antibacterial effect.
– Presents astringency and may be unpleasant when in high concentration; – Formation of complexes with anionic proteins, altering delivery profile; – Soluble in water at low pH, being unsuitable to carry some proteins.
[41–43]
Guar gum – Nonionic polymer, reducing the possibility of negative interactions with proteins; – Guar gum conjugated with alginate and cross-linked with glutaraldehyde allows controlled release of proteins.
– Oral films production implies heating to avoid gelation; – High viscosity at low temperatures.
[51]
Carrageenan gum – Potential to act as protein/peptide stabilizer by steric stabilization;
– Suitable for oral delivery systems, promoting sustained release
– Leads to water absorption when in contact with proteins/peptides.
– Negative charge may destabilize or alter delivery profile of proteins; – Protein/peptide stabilizing effects are concentration-dependent
of biomacromolecules. Hence it was verified that PLGA nanoparticles with a hydrogenated phosphatidylcholine (HSPC) surface coating im-proved ovalbumin intestinal delivery and absorption[76]. PLGA nano-particles not only offered protection from gastrointestinal extreme conditions but also granted controlled release to the proteins along with enhanced affinity to intestinal M-cells, which can be especially in-teresting regarding intestinal delivery of vaccines. In another approach, poly(ethylene glycol)methyl ether-block-polylactide (PEG-b-PLA) was used as copolymer in a nanoparticle formulation with the purpose of encapsulating insulin and impregnating nanoparticles into chitosan oralfilms, obtaining a suitable buccal delivery system for insulin[77, 78]. It was concluded that chitosan oralfilms impregnated with insulin entrapped into PEG-b-PLA nanoparticles represent a buccal delivery system that offers insulin biphasic and sustained release. Indeed PEG-b-PLA nanoparticles erosion was shown tofirst depend on the swelling of polymeric matrix and then from the nanoparticles. Similarly, another oralfilm formulation was impregnated with nanoparticles coated with lysozyme attaining sustained release (4 h) observable when using a Franz diffusion cell to mimic buccal cavity[79].
Solid lipid nanoparticles (SLN) are also suitable carriers for proteins as stated for insulin intestinal delivery and absorption, demonstrating a significant hypoglycemic effect that lasted for 24 h, after oral adminis-tration in mice[18,80]. Also, SLN combination with mucoadhesive and absorption enhancing polymers (e.g. chitosan) showed to be very effec-tive calcitonin delivery systems, offering improved bioavailability and continuous release[81]. Moreover, controlled release of proteins was demonstrated to be possible by manipulating relative amounts of chito-san, tripolyphosphate (TPP) and protein as evidenced using bovine serum albumin (BSA) as model[82]. Indeed, an initial burst release (30–70% release after 3–6 h) was obtained and a posterior protein re-lease was obtained, having proceeded for some days after oral adminis-tration. Furthermore, it was demonstrated that SLN are prone to be tailored in order to obtain controlled release of peptides (as shown for insulin) also offering protection from hostile gastrointestinal environ-ment and promoting absorption, inducing significant hypoglycemia in diabetic rats[80,83].
As verified for nanoparticles, good results were also obtained using PLGA microparticles as cholera toxin B carriers administered per os, in-ducing an effective immune response observable in vivo[84].
Moreover, the encapsulation of proteins/peptides may avoid di-rect contact with oralfilms matrix. This approach has been explored for oralfilms formulations improvement regarding the delivery of several molecules with pharmaceutical or nutraceutical interest
[85,86].
Micro- and nanoencapsulation demonstrated to be suitable carriers for proteins and peptides, offering protection and controlled release but also increased permeability either by specific or unspecific path-ways. Thus, microparticles and nanoparticles are prone to be tailored in order to favorably enhance pharmacokinetic profile of proteins and peptides and can easily be carried into or onto oralfilms.
Another interesting tool may be adapting colon-specific delivery approaches, creating microparticles or nanoparticles coated with methacrylic acid copolymers or Eudragit, allowing preferential disin-tegration and preferential release of proteins at alkaline pH values
[87]. Moreover, nanoparticles and microparticles coating polymers (e.g. chitosan, pectin, chondroitin sulfate or inulin) that are prefer-entially degraded by the colonic microflora are also good candidates to achieve colon-specific delivery of proteins.
Chemical modification of proteins and peptides is another bio-availability enhancement tool. Indeed, desmopressin is achieved from deamination of thefirst amino-acid and inversion of the stereo-chemical configuration – from levo to dextro – of the arginine at the eighth position of vasopressin, thus offering an exponentially higher permeability across intestinal epithelia and enzymatic stability[88]. Hence, desmopressin presents much higher bioavailability after oral administration in comparison with vasopressin.
An effective way of protein chemical modification is lipidization, resulting from conjugation of fatty acids and proteins, leading to an in-creased lipophilicity and consequently pharmacokinetic improvement
[89]. Lipidization is a successful resource regarding in vivo oral absorp-tion of calcitonin conjugated with N-palmitoyl cysteinyl 2-pyridyl disulfide, comparatively to unmodified calcitonin, leading to overall bio-availability improvement. Lipidization was also a breakthrough mean to obtain detemir (Levemir®) and degludec insulin modifications. Detemir was obtained by myristoylation of the penultimate lysine residue in position B29 and removal of the terminal threonine resulting in an in-creased affinity to albumin and consequent half-life and stability in-crease[90]. Degludec results from an addition of a palmitoyl group to the B29ε-lysyl amino group using a γ-glutamic acid spacer with an ad-ditional removal of terminal threonine, resulting in a more stable insulin analogue, with a longer lasting glycemic control. Recently, acyclovir was conjugated with a lipophilic raft that overcomes low permeability of the drug across cell membranes by increasing docking affinity to membrane transporter (sodium multivitamin transporter for biotin)[91]. Conse-quently, acyclovir is more rapidly translocated across cell membrane, representing an effective bioavailability improvement that can be extrapolated for peptides and proteins.
Creating prodrugs that are activated from enzymatic or non-enzymatic processes, from proteins/peptides is also a suitable tool regard-ing the optimization of bioavailability[92]. Prodrugs are engineered to obtain improved stability and permeability across gastrointestinal bar-riers. Even though this tool was unsuccessfully used attempting to modify some proteins due to structural complexity and chemical processes with overall low yield, a successful case was obtained for opioid peptides ([Leu5]-enkephalin), achieving a significantly higher permeability profile
using Caco-2 cell models, by cyclization technique[93,94].
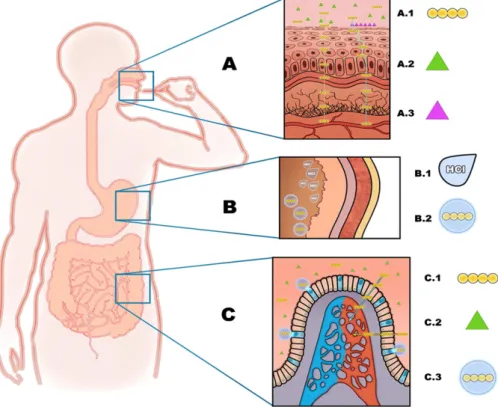
Fig. 2schematizes the behavior of proteins/peptides in the presence of referred excipients that offer enhancement of permeability and/or structural stability along gastrointestinal harsh conditions, allowing proteins to be absorbed to systemic circulation without losing desired bioactivity.
4. Toxicity concerns
When protein or peptide delivery is intended, parameters such as immunogenicity and other adverse effects are very important aspects to control. Additionally, since oralfilms formulations are complex, ex-cipients may induce collateral, unexpected and harmful effects. Also, permeability enhancement occurs differently in the intestine and in the mouth tissues and thus, toxicity concerns might also be different
[95]. For instance, sodium dodecyl sulfate (SDS) seems to enhance per-meability almost exclusively for non-polar molecules (such as a wide array of proteins/peptides) by extracting lipid molecules that block the passage of hydrophilic molecules, but may also allow crossing of toxins, pathogens or other harmful xenobiotics[96]. Moreover, for high concentrations of bile salts or other surfactants, both transcellular and paracellular permeation are increased due to polar lipids and re-moval of cholesterol from cell membranes [97]. Thus, with non-specific removal of transcellular and paracellular components, wider arrays of harmful molecules or organisms are able to permeate oral tis-sues. It also must accounted that many permeability and toxicity studies reported in the literature were performed in rat or rabbit tissues, not being as comparable to human tissues as, for instance, porcine oral mucosa samples due to distinct keratinization profiles and/or lipid chemical composition[98].
Permeability enhancement obtained when using excipients that de-stabilize epithelium physico-chemical structure may also induce cell damage, mainly in buccal and intestinal cells which may result in acute and, possibly, chronic toxic effects (e.g. permeability enhance-ment of toxic molecules and potential carcinogenic effect caused by constant aggressions, respectively). As a matter of fact, besides being a film forming polymer, chitosan is also a permeability enhancer due to
bioadhesion but also to the capability to open tight junctions, allowing permeation by a wide array of molecules and delaying intestinal mucus turnover[99]. Thus, even though chitosan is considered biode-gradable and biocompatible, is also a tight-junction disrupting agent, which may facilitate invasion by harmful xenobiotics. Chitosan nano-particles have demonstrated significant hepatotoxicity after 4 h of expo-sure so that overconfidence on chitosan safety shall be considered[100]. Also, hydroxypropylmethylcellulose (HPMC), an extensively used polymer in oralfilms formulations along with glycerol (plasticiz-er), is known to induce laxative or constipation effects[101]. Dietary products containing ethanol, an extensively studied permeability enhancer, must also be avoided when oralfilms containing proteins or peptides are being taken. Actually, ethanol greatly enhances perme-ation of molecules by transcellular route, which may unexpectedly increase protein/peptide permeation to systemic circulation or, oppo-sitely, denature proteins, decreasing or even eliminating proteins/ peptides bioactivity[102,103]. Ethanol is also known to be intimately correlated with oral cancer occurrence and should be avoided as solvent or permeability enhancer[104].
Protease inhibitors are also commonly used in formulations to deliv-er proteins/peptides in orddeliv-er to avoid enzymatic degradation, which may yield digestion problems if chronic administration is intended
[99]. Many toxicity studies were performed regarding permeability enhancers, polymeric matrices or nanoparticle formulations especially regarding acute toxicity, but there is a lack of toxicity studies regarding oralfilms as protein/peptide oral delivery systems. Therefore, further investigation needs to be performed in thisfield along with more chron-ic toxchron-icity assays to obtain acceptably safe products.
5. Walkthrough on pipeline products
Oralfilms were developed around 1970, so they are not actually new. Nevertheless, many new pharmaceutical products based on
oralfilms as delivery systems are being developed, with a predict-able growth in the market in the next few years. All inherent advan-tages of oral films as delivery systems, allows pharmaceutical companies to manage a new life cycle to reference pharmaceutical products that lost or are about to lose patenting protection. Addi-tionally, as oralfilms are different delivery systems from tablets or capsules, when prescribed, they cannot be substituted in the phar-macy for the conventional oral dosage forms, hence representing a smart alternative strategy to achieve a higher market quote. More-over, practicality is arguably one of the most important characteris-tics that any product may present nowadays, which is a key point not only for pharmaceutical but also food industries, to be develop-ing new oralfilms formulations as drugs and nutraceuticals delivery systems. However, oralfilms as protein or peptide oral delivery sys-tems are not available yet and this gap might represent a marketing opportunity for the pharmaceutical industry. Moreover a significant amount of oralfilm-based products are already in pipeline or have already been marketed.Table 3enlists some examples of different products based on oralfilms and similar delivery systems, in differ-ent stages of developmdiffer-ent, indicating the wide array of utilization of oralfilms along with industry emerging interest.
Additionally, different oralfilms systems are being developed. VersaFilm™ and IntelGenX were designed to obtain immediate release of incorporated bioactive molecules. Bio-FX™ (NAL Pharma) promote absorption of bioactive molecules directly in the oral cavity. Also, BEMA® (BioDelivery Sciences) are highly mucoadhesive oralfilms ap-plied directly in the cheek to obtain maximum buccal absorption.
On the other hand, protein/peptide oral delivery has been pursued for quite some time, ambitioning to avoid invasive drug administration and to maximize compliance to treatment. Insulin is a classic case that would deeply benefit from oral delivery, obtaining painless administra-tion without any stigma and, perhaps more importantly, obviate co-morbidities that result from noncompliance to subcutaneous treatment.
Fig. 2. When oral strips are introduced in the oral cavity (A), carried proteins (A.1) cross epithelial barriers by transcellular and paracellular routes, aided by the action of permeability enhancers that facilitate internalization (A.2) and/or adhesion to the mucosa (A.3). Nanoparticles (B.2) may be used to protect proteins from gastrointestinal extreme conditions as low pH (B.1) in the stomach (B). If proteins reach the intestine (C), permeability enhancers (C.2) facilitate transcellular and paracellular protein (C.1) permeation. Nanoparticles with ma-trices such as PLGA, besides the protection offered, also favor internalization of proteins by interacting with intestinal M-cells (represented in blue) and promote proteins controlled release.
Effectively, an effective insulin oral delivery system would seriously im-prove lifestyle of the patients and avoid substantialfinancial burdens in-herent to untreated (or poorly managed) diabetes. Indeed, several companies are currently investing in research and development that regards oral delivery of proteins and peptides.Table 4indicates exam-ples of products that delineate the paradigm of oral delivery of proteins and peptides, along with the techniques used to obviate bioavailability problems.
Nevertheless, there are still very few studies on oralfilm formula-tions that contemplate protein/peptide delivery, which can represent an opportune market gap regarding patenting and/or marketing or new pharmaceutical/nutraceutical products with great relevance in therapy enhancement.
6. Future perspectives
User-friendly vaccines, hormonal treatments or simply life-enhancing products may be achieved, just by adding new excipients (e.g. absorption enhancers, enzyme inhibitors), incorporating proteins or peptides into micro- or nanocarriers or simply by using common ex-cipients in oralfilms formulations. Such a wide array of products, when optimized, can be very interesting from the patient/consumer point of view.
Research and development of new oralfilm formulations can also be expensive if protein/peptide delivery is intended since, as mentioned, each protein/peptide may interact differently with excipients, which may lead to a significant increase of costs. In addition, oral films are only able to carry a maximum of about 30%, w/w, of active ingredient, thus being restricted either to potent drugs or to molecules that play both excipient and active pharmaceutical/nutritional ingredient roles as observed for Gas-X® (in which simethicone plays both polymer
and active ingredient roles and, thus, the bioactive molecule incorpora-tion rate is ca. 50%, w/w)[4]. Moreover, for new proteins/peptides deliv-ered in the oral cavity using oralfilms as carriers and penetration enhancers as excipients, regulating entities will certainly require exten-sive toxicity studies. Hence, oralfilms formulations must be tailored considering not only stability but also, in most cases, an overall incre-ment of proteins/peptides biopotency.
Moreover, production techniques such as inkjet printing or FPT that offer greater dosage accuracy, stability for thermally labile drugs (e.g. peptides), enables individualized dosage, avoids potential contamina-tions and are liable to be automated are very important factors to con-sider if production scale-up is pursued[112]. Added to all above-mentioned advantages of oralfilms, either for the patient or industries, oralfilms have the potential to offer a breakthrough in protein/peptide delivery and overall acceptance, improving every-day of patients. Effec-tively, oralfilm production costs are not as high as for tablets or capsules and offer the possibility of a broader therapeutic customization simply by cutting larger or smallerfilms.
7. Conclusion
It is expected that purposed strategies to achieve delivery or proteins and peptides using oralfilms as carriers, culminate into safer and more effective products that overcome inherent problems of invasive routes or even conventional delivery systems. Indeed, as mentioned, there is a wide array of excipients and techniques that demonstrated to poten-tially contribute to enhance pharmacokinetic characteristics of proteins, especially regarding liberation and absorption of proteins and peptides. Even though the development of proper oralfilms, with mandatory tailored manufacturing process and excipients, may represent a laborious and time-consuming task, the convenience, compliance to treatment and
Table 3
Examples of products in different stages of development that serve diverse purposes.
Product Owner company Uses Development stage Ref.
Listerine® Pocketpacks® Johnson & Johnson Breath fresheners Marketed [105]
Ondansetron Rapidfilms® Labtec GmbH Post-operatory- and chemotherapy-induced nausea and vomiting Marketed [4]
Aripiprazole Labtec Schizophrenia management Recently completed clinical studies [105]
Apomorphinea Labtec Treatment of Parkinson's disease and depressive disorders Currently running phase II experiments [106] Breakyl®b(fentanyl) BioDelivery Sciences Chronic pain management Marketed [107]
a
Sublingualfilm.
b
Buccalfilm.
Table 4
Examples of different approaches to achieve protein/peptide oral delivery that are under development or already marketed. Protein/peptide
drug
Uses Dosage form Owner Company Bioavailability enhancement techniques
Development stage
Ref.
Insulin (POD™) – Diabetes mellitus management
Capsules Oramed Pharmaceuticals, Inc. – Enzyme inhibitors; – Permeability enhancers; – Emulsifiers.
Phase II [105]
Desmopressin – Diabetes insipidus management
– Primary nocturnal enuresis
Tablets Sanofi Aventis – Chemical modification of vasopressin.
Marketed [105]
Cyclosporine (Sandimmune®)
– Immunomodulator Capsules Novartis None. Market [105,108]
Linaclotide (Linzess)
– Tratment of irritable bowel syndrome
– Treatment of chronic idiopathic constipation
Capsules Ironwood Pharma – Permeability enhancers; – Protease inhibitors.
Market [105,109]
Calcitonin (Ostora®)
– Treatment of osteoporosis Tablets Tarsa therapeutics – Colon-specific permeability enhancer (tetradecyl maltoside); – Citric acid as peptide stabilizing excipient; – Calcitonin incorporated into vesicles.
Phase III completed [110]
Octreotide (Octreolin®)
– Acromegaly treatment Transient Permeability Enhancer (TPE™)
Chiasma, Inc. – Protectors and permeability enhancers in gastrointestinal tract (TPE™)
overall acceptability of the product by the consumer/patient are factors that are not going unnoticed by food or pharmaceutical companies. Furthermore, due to the fact that oralfilms may represent a breath of fresh air namely for drugs with falling patents and that, being distinct delivery forms, oralfilm products cannot be switched in a pharmacy for other oral delivery forms when prescribed, research and development efforts will certainly be profitable both for patients/consumers and man-ufacturers/retailers. Also, oralfilms can represent an innovative, non-invasive, way of delivering bioactive proteins or peptides with maximum compliance from the patients, especially geriatric, pediatric or those with swallowing complications.
References
[1] A.R. Madureira, T. Tavares, A.M. Gomes, M.E. Pintado, F.X. Malcata, Invited review: physiological properties of bioactive peptides obtained from whey proteins, J. Dairy Sci. 93 (2010) 437–455,http://dx.doi.org/10.3168/jds.2009-2566. [2] J. Shaji, V. Patole, Protein and peptide drug delivery: oral approaches, Indian
J. Pharm. Sci. 70 (2008) 269–277,http://dx.doi.org/10.4103/0250-474X.42967. [3] D.J. Brayden, D.J. O'mahony, Novel oral drug delivery gateways for biotechnology
products: polypeptides and vaccines, Pharm. Sci. Technol. Today 1 (1998) 291–299,http://dx.doi.org/10.1016/S1461-5347(98)00075-3.
[4] R.P. Dixit, S.P. Puthli, Oral strip technology: overview and future potential, J. Con-trol. Release 139 (2009) 94–107,http://dx.doi.org/10.1016/j.jconrel.2009.06.014. [5] T. Nagaraju, R. Gowthami, M. Rajashekar, S. Sandeep, M. Mallesham, D. Sathish, Y.S.
Kumar, Comprehensive review on oral disintegratingfilms, Curr. Drug Deliv. 10 (2013) 96–108.
[6] J.O. Morales, S. Huang, R.O. Williams III, J.T. McConville, Films loaded with insulin-coated nanoparticles (ICNP) as potential platforms for peptide buccal delivery, Colloids Surf., B 122 (2014) 38–45,http://dx.doi.org/10.1016/j.colsurfb.2014.05. 025.
[7] H.P. Merkle, G. Wolany, Buccal delivery for peptide drugs, J. Control. Release 21 (1992) 155–164,http://dx.doi.org/10.1016/0168-3659(92)90017-L.
[8] B.F. Choonara, Y.E. Choonara, P. Kumar, D. Bijukumar, L.C. du Toit, V. Pillay, A re-view of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules, Biotechnol. Adv. 32 (2014) 1269–1282,http://dx.doi.org/10.1016/j.biotechadv.2014.07.006.
[9] R.W. Busby, A.P. Bryant, W.P. Bartolini, E.A. Cordero, G. Hannig, M.M. Kessler, S. Mahajan-Miklos, C.M. Pierce, R.M. Solinga, L.J. Sun, J.V. Tobin, C.B. Kurtz, M.G. Currie, Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit, Eur. J. Pharmacol. 649 (2010) 328–335,http://dx.doi.org/10.1016/j.ejphar.2010.09.019. [10] K.A. Jadallah, S.M. Kullab, D.S. Sanders, Constipation-predominant irritable
bowel syndrome: a review of current and emerging drug therapies, World J. Gastroenterol. 20 (2014) 8898–8909,http://dx.doi.org/10.3748/wjg.v20.i27.8898. [11] K. Shailubhai, S. Comiskey, J.A. Foss, R. Feng, L. Barrow, G.M. Comer, G.S. Jacob, Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointesti-nal tract, is safe and well-tolerated in single doses, Dig. Dis. Sci. 58 (2013) 2580–2586,http://dx.doi.org/10.1007/s10620-013-2684-z.
[12] J. Otte, S.M.A. Shalaby, M. Zakora, M.S. Nielsen, Fractionation and identification of ACE-inhibitory peptides from α-lactalbumin and β-casein produced by thermolysin-catalysed hydrolysis, Int. Dairy J. 17 (2007) 1460–1472,http://dx. doi.org/10.1016/j.idairyj.2007.04.008.
[13] M. Gulzar, S. Bouhallab, J. Jardin, V. Briard-Bion, T. Croguennec, Structural conse-quences of dry heating on alpha-lactalbumin and beta-lactoglobulin at pH 6.5, Food Res. Int. 51 (2013) 899–906,http://dx.doi.org/10.1016/j.foodres.2013.02.025. [14] S. Català-Clariana, F. Benavente, E. Giménez, J. Barbosa, V. Sanz-Nebot, Identifica-tion of bioactive peptides in hypoallergenic infant milk formulas by capillary elec-trophoresis–mass spectrometry, Anal. Chim. Acta 683 (2010) 119–125,http://dx. doi.org/10.1016/j.aca.2010.10.002.
[15] E.A. Popova, S.K. Nikolskaia, I.A. Gluzdikov, R.E. Trifonov, An efficient synthesis of a novel analog of octreotide with an unnaturalL-lysine-like tetrazolyl amino acid, Tetrahedron Lett. 55 (2014) 5041–5046,http://dx.doi.org/10.1016/j.tetlet.2014. 07.067.
[16] F. Veuillez, Y.N. Kalia, Y. Jacques, J. Deshusses, P. Buri, Factors and strategies for im-proving buccal absorption of peptides, Eur. J. Pharm. Biopharm. 51 (2001) 93–109,
http://dx.doi.org/10.1016/S0939-6411(00)00144-2.
[17] Y.-H. Lee, P.J. Sinko, Oral delivery of salmon calcitonin, Adv. Drug Deliv. Rev. 42 (2000) 225–238,http://dx.doi.org/10.1016/S0169-409X(00)00063-6.
[18] F. Sousa, P. Castro, P. Fonte, B. Sarmento, How to overcome the limitations of cur-rent insulin administration with new non-invasive delivery systems, Ther. Deliv. 6 (2015) 83–94,http://dx.doi.org/10.4155/tde.14.82.
[19] V. Chi, M.W. Pennington, R.S. Norton, E.J. Tarcha, L.M. Londono, B. Sims-Fahey, S.K. Upadhyay, J.T. Lakey, S. Iadonato, H. Wulff, C. Beeton, K.G. Chandy, Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases, Toxicon 59 (2012) 529–546,http://dx.doi.org/10.1016/j.toxicon.2011.07.016. [20] L. Jin, B.J. Boyd, P.J. White, M.W. Pennington, R.S. Norton, J.A. Nicolazzo, Buccal
mu-cosal delivery of a potent peptide leads to therapeutically-relevant plasma concen-trations for the treatment of autoimmune diseases, J. Control. Release 199 (2015) 37–44,http://dx.doi.org/10.1016/j.jconrel.2014.12.001.
[21] A.J. Hoogstraate, J.C. Verhoef, B. Tuk, A. Pijpers, L.A.M.G. van Leengoed, J.H.M. Verheijden, H.E. Junginger, H.E. Boddé, Buccal delivery of fluorescein
isothiocyanate-dextran 4400 and the peptide drug buserelin with glycodeoxycholate as an absorption enhancer in pigs, J. Control. Release 41 (1996) 77–84,http://dx.doi.org/10.1016/0168-3659(96)01358-2. [22] A. Garcia-Arieta, Interactions between active pharmaceutical ingredients and
ex-cipients affecting bioavailability: impact on bioequivalence, Eur. J. Pharm. Sci. 65 (2014) 89–97,http://dx.doi.org/10.1016/j.ejps.2014.09.004.
[23] T.J. Kamerzell, R. Esfandiary, S.B. Joshi, C.R. Middaugh, D.B. Volkin, Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development, Adv. Drug Deliv. Rev. 63 (2011) 1118–1159,
http://dx.doi.org/10.1016/j.addr.2011.07.006.
[24] S. Maher, D.J. Brayden, Overcoming poor permeability: translating permeation en-hancers for oral peptide delivery, Drug Discov. Today Technol. 9 (2012) e71–e174,
http://dx.doi.org/10.1016/j.ddtec.2011.11.006.
[25] E.M. Hoffmann, A. Breitenbach, J. Breitkreutz, Advances in orodispersiblefilms for drug delivery, Expert Opin. Drug Deliv. 8 (2011) 299–316,http://dx.doi.org/10. 1517/17425247.2011.553217.
[26] A.B. Buanz, C.C. Belaunde, N. Soutari, C. Tuleu, M.O. Gul, S. Gaisford, Ink-jet printing versus solvent casting to prepare oralfilms: effect on mechanical properties and physical stability, Int. J. Pharm. (2014)http://dx.doi.org/10.1016/j.ijpharm.2014. 12.032.
[27] E.M. Janssen, R. Schliephacke, A. Breitenbach, J. Breitkreutz, Drug-printing by flexo-graphic printing technology— a new manufacturing process for orodispersible films, Int. J. Pharm. 441 (2013) 818–825,http://dx.doi.org/10.1016/j.ijpharm. 2012.12.023.
[28] N. Genina, D. Fors, H. Vakili, P. Ihalainen, L. Pohjala, H. Ehlers, I. Kassamakov, E. Haeggström, P. Vuorela, J. Peltonen, N. Sandler, Tailoring controlled-release oral dosage forms by combining inkjet andflexographic printing techniques, Eur. J. Pharm. Sci. 47 (2012) 615–623,http://dx.doi.org/10.1016/j.ejps.2012.07.020. [29] M. Preis, J. Breitkreutz, N. Sandler, Perspective: concepts of printing technologies
for oral film formulations, Int. J. Pharm. (2015)http://dx.doi.org/10.1016/j. ijpharm.2015.02.032.
[30] W. Wang, Instability, stabilization, and formulation of liquid protein pharmaceuti-cals, Int. J. Pharm. 185 (1999) 129–188,http://dx.doi.org/10.1208/pt040342. [31] S. Ohtake, Y. Kita, T. Arakawa, Interactions of formulation excipients with proteins
in solution and in the dried state, Adv. Drug Deliv. Rev. 63 (2011) 1053–1073,
http://dx.doi.org/10.1016/j.addr.2011.06.011.
[32] S. Li, C. Schoneich, R.T. Borchardt, Chemical instability of protein pharmaceuticals: mechanisms of oxidation and strategies for stabilization, Biotechnol. Bioeng. 48 (1995) 490–500,http://dx.doi.org/10.1002/bit.260480511.
[33] D.Z. Liu, E.L. LeCluyse, D.R. Thakker, Dodecylphosphocholine-mediated enhance-ment of paracellular permeability and cytotoxicity in Caco-2 cell monolayers, J. Pharm. Sci. 88 (1999) 1161–1168,http://dx.doi.org/10.1021/js990094e. [34] J. Renukuntla, A.D. Vadlapudi, A. Patel, S.H. Boddu, A.K. Mitra, Approaches for
en-hancing oral bioavailability of peptides and proteins, Int. J. Pharm. 447 (2013) 75–93,http://dx.doi.org/10.1016/j.ijpharm.2013.02.030.
[35] A.P. Minton, Models for excluded volume interaction between an unfolded protein and rigid macromolecular cosolutes: macromolecular crowding and protein stabil-ity revisited, Biophys. J. 88 (2005) 971–985,http://dx.doi.org/10.1529/biophysj. 104.050351.
[36] H.R. Costantino, S.P. Schwendeman, R. Langer, A.M. Klibanov, Deterioration of ly-ophilized pharmaceutical proteins, Biochemistry (Mosc) 63 (1998) 357–363.
[37] J.H. Suh, Y.H. Cho, K.J. Lee, Macrocyclic metal-complexes built on polyethylenimine, J. Am. Chem. Soc. 113 (1991) 4198–4202,http://dx.doi.org/10.1021/Ja00011a022. [38] S.Şenel, M.J. Kremer, S. Kaş, P.W. Wertz, A.A. Hıncal, C.A. Squier, Enhancing effect of chitosan on peptide drug delivery across buccal mucosa, Biomaterials 21 (2000) 2067–2071,http://dx.doi.org/10.1016/S0142-9612(00)00134-4.
[39] F. Cui, C. He, M. He, C. Tang, L. Yin, F. Qian, C. Yin, Preparation and evaluation of chitosan-ethylenediaminetetraacetic acid hydrogelfilms for the mucoadhesive transbuccal delivery of insulin, J. Biomed. Mater. Res. A 89 (2009) 1063–1071,
http://dx.doi.org/10.1002/jbm.a.32071.
[40] J.S. Boateng, O. Okeke, Chitosan-basedfilms for the sustained release of peptides: a new era in buccal delivery? Ther. Deliv. 5 (2014) 497–500,http://dx.doi.org/10. 4155/tde.14.21.
[41] A. Bernkop-Schnurch, C.E. Kast, D. Guggi, Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems, J. Control. Release 93 (2003) 95–103,http://dx.doi.org/10.1016/j.jconrel.2003.05.001.
[42] G. Borchard, H.L. Lueβen, A.G. de Boer, J.C. Verhoef, C.-M. Lehr, H.E. Junginger, The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorp-tion. III: effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro, J. Control. Release 39 (1996) 131–138, http://dx.doi.org/10.1016/0168-3659(95)00146-8.
[43]C.M. Lehr, From sticky stuff to sweet receptors— achievements, limits and novel approaches to bioadhesion, Eur. J. Drug Metab. Pharmacokinet. 21 (1996) 139–148.
[44] A.J. D'Souza, R.L. Schowen, R.T. Borchardt, J.S. Salsbury, E.J. Munson, E.M. Topp, Re-action of a peptide with polyvinylpyrrolidone in the solid state, J. Pharm. Sci. 92 (2003) 585–593,http://dx.doi.org/10.1002/jps.10316.
[45] W.R. Gombotz, S.C. Pankey, D. Phan, R. Drager, K. Donaldson, K.P. Antonsen, A.S. Hoffman, H.V. Raff, The stabilization of a human IgM monoclonal antibody with poly(vinylpyrrolidone), Pharm. Res. 11 (1994) 624–632,http://dx.doi.org/10. 1023/A:1018903624373.
[46] K. Izutsu, S. Kojima, Freeze-concentration separates proteins and polymer excipients into different amorphous phases, Pharm. Res. 17 (2000) 1316–1322,
http://dx.doi.org/10.1023/A:1026412107574.
[47] E. Cao, Y. Chen, Z. Cui, P.R. Foster, Effect of freezing and thawing rates on denatur-ation of proteins in aqueous solutions, Biotechnol. Bioeng. 82 (2003) 684–690,