Caste Asymmetries in the Neotropical
Swarm-Founding Wasp
Polybia (Trychothorax) ignobilis
(Hymenoptera, Vespidae, Epiponini)
by
Ivan Cesar Desuó1; Sulene Noriko Shima1; Alexandre Capelete Lima Oliveira2; Bruno Gomes2 & Carlos Tadeu Santos Dias3
ABSTrACT
Epiponini wasps are dominant in Neotropical regions and represent, in
several ways, exceptions for general theories regarding social insects. They are
highly eusocial and most species build large and complex nests. These wasps
form large populations, and colony reproductive fission occurs by swarming.
On the other hand, differently from other highly social insects, in several species
morphological differences among castes are slight or absent, suggesting that
caste determination in the Epiponini differs from better known systems. In
fact, the mechanisms that lead to caste differentiation in this tribe still remain
obscure. The aim of this study is to investigate the pattern of caste
differentia-tion during the colonial cycle of the Neotropical Epiponini wasp
Polybia (T.)
ignobilis
. Measurements were taken from 13 morphometric variables regarding
the head, metassoma, messosoma, and wing. Ovarian development, relative
age, and fat bodies were also examined. Four patterns of ovarian development
were found and varied from filamentous ovarioles with no visible oocytes to
well-developeded ovaries with mature oocytes. ANOVA showed that queens
were significantly larger than workers in all colony phases, and also showed
some significant differences between workers and intermediates in some
body parts. However, multivariate statistics revealed that intermediates are
in fact morphologically similar to workers, whereas queens were identified as
a different group composed of larger individuals. Caste differentiation varied
according to the colony cycle: the morphological differences between queens
and workers tend to increase with colony maturity.
1Departamento de Zoologia, Instituto de Biociências, UNESP, Campus rio Claro, SP, Brazil, ivan. desuo@yahoo.com.br, CEP:13506-900, (5519) 35264298.
2Departamento de Zoologia e Botânica, IBILCE, UNESP, Campus São José do rio Preto, SP, Brazil, CEP:15054-000, (5519)32212373.
52 Sociobiology Vol. 57, No. 1, 2011
Key-Words:
Social Insects, caste differences, kin selection, cyclical
oli-gogyny, Vespidae, Epiponini.
INTrODUCTION
The division into castes is a keystone in studies of social evolution in
social insects, and a higher degree of caste differentiation usually indicates a
well-defined division of labor and, consequently, a higher level of sociality
(Bourke 1999; Noll
et al.
2004). In this context, the Vespidae attracts special
attention of sociobiologists, because a remarkable sequence of sociality
pat-terns is found in this group, and every step of social organization is observed
(Wilson 1971).
The Neotropical Epiponini comprises 19 genera and at least 229
spe-cies representing the Neotropical swarm-founding wasps, distributed from
Argentina to the southwest of the USA (Carpenter 2004). In Brazil the
Epiponini are ecologically dominant, showing the highest level of diversity
and endemism ( Jeanne 1975 1980). The Epiponini present polygyny and a
complex caste system (Carpenter 1993, 1997), and represents an important
group in caste evolutionary studies in social wasps (richards & richards
1971; richards 1971; richards 1978). Unlike other highly eusocial insects
(such as honeybees and vespines), societies of the Epiponini are better
char-acterized as a “conspiracy of workers” and, apparently, queens do not play
an important role in the control of sociality (Noll & Wenzel 2008). The
Epiponini are also polygynous and queen demography is regulated by worker
policing, which usually selects only the queens with the highest
reproduc-tive status (West-Eberhard 1978). This flexibility in the number of queens
results in few queens in the advanced phases of the colony cycle and allows
the maintenance of a reasonable level of genetic relatedness among nestmates,
representing a remarkable manifestation of kin selection theory (Hamilton
1964a,b; Hamilton 1972). Furthermore, the presence of intermediate females
brings more complexity to caste determination as these females may show
totipotency (e.g.,
Parachartergus colobopterus
, Strassmann
et al.
2002), may
lay eggs, and also seem to play an important role in the process of cyclical
oligogyny, which regulates the number of queens in the colony (Strassmann
richards (1978) documented that castes in Polistinae (mainly in the
Epiponini) present three main types of differentiation: (1) queens larger than
workers [e.g.
A. areata
(Jeanne & Fagen 1974);
A. vicina
(Sakagami
et al.
1996);
Protonectarina sylveirae
(Shima
et al.
1996b);
Epipona guerini
(Hunt
et al.
1996);
Polybia scutellaris
(Noll
et al.
1997a)]; (2) conspicuous dimorphism,
in which queens present different body proportions compared to workers,
resulting in shape differences. This could be a consequence of differential
ontogenetic reprogramming of growth parameters ( Jeanne
et al.
1995), and
in this case intermediate females are absent [e.g.
Apoica flavissima
(Shima
et
al.
1994);
Polybia dimidiata
(Shima
et al.
1996a)]; (3) slight or undetectable
differences with the presence of intermediate females [e.g.
Pseudopolybia
vespiceps
(Shima
et al.
1998);
Protopolybia exigua
(Noll
et al.
1996)].
Studies on castes of Epiponini wasps through the colony cycle have
in-creased considerably during the last decade. According to Noll and Zucchi
(2002), five different types of social regulation are now recognized within
this tribe: (1) absence of morphological differentiation between queens and
workers during the whole nest cycle, in which the intermediate females may
achieve reproductive status [e.g.,
Chartergellus communis
(Mateus 1999);
Parachartergus smithii
(Mateus 1997);
Pseudopolybia vespiceps
(Shima
et al.
1998
); Brachygastra lecheguana
(Shima
et al.
2000)]; (2) absence of caste
differences, in which young females may develop ovaries only during some
phases of the colony cycle [e.g.,
Metapolybia aztecoides
and
Synoeca.
suri-namensis
(West-Eberhard 1981),
Synoeca cyanea
(Noda
et al.
2003)]; (3)
morphological discontinuities among castes varying according to the nest
cycle and the constant presence of intermediate females [e.g.
Protopolybia
exígua
and
P. sedula
(Noll & Zucchi 2002)]; (4) presence of uninseminated
egg-laying females only during some phases; the differences between castes
increase throughout the colony cycle [e.g.
Chartergus globiventris
(Noll &
Zucchi 2002);
Polybia scutellaris, P. paulista
,
and
P. occidentalis
(Noll &
Zuc-chi 2002)]; (5) morphological differences between queens and workers are
conspicuous during the whole colony cycle and there are no uninseminated
egg-laying females [e.g.
Apoica flavissima
(Shima
et al.
1994)
; A. pallens
54 Sociobiology Vol. 57, No. 1, 2011
al.
(2008) these syndromes are easily interpreted phylogenetically and suggest
that castes in Epiponini evolved several times in different lineages, however
from an ancient condition of casteless societies.
The Neotropical Epiponini wasp
Polybia (Trychothorax) ignobilis
(Haliday,
1836) is widely distributed throughout South America, occurring from Panama
to Paraguay and Argentina. This species is extremely aggressive and builds
enveloped nests inside cavities (richards 1978). The aim of this study was to
investigate the patterns of caste differentiation in
P. (T.) ignobilis
during the
colony cycle and address questions regarding the role of cyclical oligogyny
in the caste system of this species.
Material and Methods
For this study, six colonies of
Polybia (Trichothorax) ignobilis
in different
phases of development were analyzed. All colonies were collected in the
state of São Paulo, Brazil, in the following cities: colonies I (30/04/95), II
(20/11/95), III (31/07/96), and V (28/09/1995) were collected in rio Claro,
(22º24’36’’S; 47º33’36’’W); colonies IV (30/03/2007) and VI (22/10/93)
in ribeirão Preto (21º12’’42’S; 47º48’24’’W). All adult wasps from the
colonies were immediately killed and fixed in Dietrich’s solution and then
preserved in 70% ethanol.
In order to assess the reproductive status of each female picked for the
morphological study, they were dissected under a stereomicroscope and their
ovaries were drawn, permitting the establishment of an ovarian development
pattern. Inseminated females with well-developeded ovaries were regarded as
queens. Unmated females with no or a low degree of ovary development were
regarded as workers. When the females were not inseminated but presented
substantial ovarian development, they were regarded as intermediates. The
relative age was estimated by observing the progressive pigmentation of the
transverse apodeme across the hidden base of the 4
thsternum. According to
56 Sociobiology Vol. 57, No. 1, 2011
richard & richards (1951), richards (1971), and West-Eberhard (1973), the
progressive pigmentation of this structure is related to the age of individuals.
Females were classified as: (1) without pigmentation, (2) light brown, (3)
dark brown, and (4) black.
We used SAS 9.2 for Windows to perform statistical analysis. All
measure-ments herein given in millimeters were log converted to facilitate arranging
them in a normal distribution. From the measurements, the means and
standard deviations were calculated. Aiming to verify the differences among
queens, workers and intermediates, a One-Way ANOVA was performed
using PrOC ANOVA procedure using the option BON to test the means
differences using the Bonferroni-correction
t
-test.
Discriminant analysis was conduct using PrOC DISCrIM procedure
in SAS to allocate the intermediates females into worker or queen classes.
We used the option POOL=TEST to test homogeneity of covariance
matrices within groups, and if the null hypothesis was rejected, then the
software proceeded the quadratic form of discriminant function. We used
the CrOSSVALIDATE option to provide rigorous classification accuracy
and the misclassified observation was listed using CrOSSLISTEr option
in SAS software.
Canonical variate analysis (CVA) was implemented to describe differences
among queens, workers and intermediates. This procedure finds a new set
of axes or canonical variates that are linear combinations of the original set
of variables, which provide the best discrimination between groups by
maxi-mizing the between-group variance relative to the within-group variance .
To perform this analysis we used PrOC CANDISC in SAS software. The
Canonical Plots were obtained using JMP 7.0 linked to SAS database.
Finally, we used PrOC STEPDISC to perform a stepwise discriminant
analysis in order to choose the most important variables which discriminate
castes in
P. (T.) sericea
. We use the option SLENTrY=0.15, which specifies
the significance level for adding variables, in this case, variables with a P-value
greater than 0.15 were removed from the model. Graphs were made using
JMP 7.0 Statistical Software linked to SAS 9.2 database.
rESULTS
relative age of individuals, nest architecture,
and presence of males (Table 1). Based on this
analysis and according to Noll & Zucchi (2000,
2002), the colonies were classified as following:
(Colony I) - Swarm; (Colonies II and III) - Pre
emergence, (Colonies IV, V and VII) - Male
Production.
The results regarding the ovarian
develop-ment of
Polybia (T.) ignobilis
are shown in Fig.
2. Four general patterns of ovarian development
were found, although some variation was
ob-served: pattern A - filamentous ovarioles with
no visible oocytes; pattern B: B1 - ovaries with
slight oocyte development; pattern B2 -
ovari-oles with small well defined oocytes; pattern C:
C1 - characterized by only one nearly mature
oocyte located in the base of the ovary; pattern
C2 – more than one nearly mature oocyte in
the basal region of the ovary; pattern D: D1 –
well-developed ovaries with mature oocytes;
pattern D2 – extremely well-developed (and
usually twisted) ovaries with many nearly
ma-ture oocytes in each ovariole.
Females that were not inseminated and
pre-sented patterns A, B1, and B2 were regarded
as workers; those which presented patterns C1
and C2 were regarded as intermediates. Since
insemination was exclusively detected in females
presenting type D1 and D2 ovaries, these were
considered queens.
The data on relative age (Fig. 3) showed that
workers of colony I were represented mostly
by young individuals (Patterns 1 and 2), in the
other five colonies workers were characterized
by different-aged broods. Queens were always
T ab le 1 . Co lo ny a nd N es t c om posit io n (
W – W
or
ke
rs, I – I
nt
er
me
dia
tes, Q – Q
58 Sociobiology Vol. 57, No. 1, 2011
the oldest individuals (Patterns 3 and 4), and young queens were found only
in colonies II, II and IV in small percentages. Intermediate females were
represented mostly by young individuals.
Univariate analysis of variance (ANOVA) showed that queens were
sig-nificantly larger than workers and intermediates for most characters; this
pattern occurred in all colonies (Table 2). Some differences between workers
and intermediates were found in colonies II, III, IV and V. Except for colony
IV; intermediates were smaller than workers in some variables (Table 2). The
results of MANOVA confirmed that queens were larger than workers and
intermediates, as multivariate contrasts were strongly significant (Table 3).
Considering all variables together intermediates differed from workers only
in colonies IV and V (Table 4).
Discriminant function analysis allocated the intermediates mostly within
the worker group and the percentage of correctly classified intermediates
was noticeablely low. Otherwise, queens presented a higher percentage of
correct classification, which reinforces the results cited above (Table 3). The
canonical Variate plot (Fig. 4) clearly shows that queens form a well
sepa-rated group from workers and intermediates in every phase of the colonial
cycle. However, such a distinction was not observed between workers and
intermediates. Queens are better characterized for higher values of CAN1,
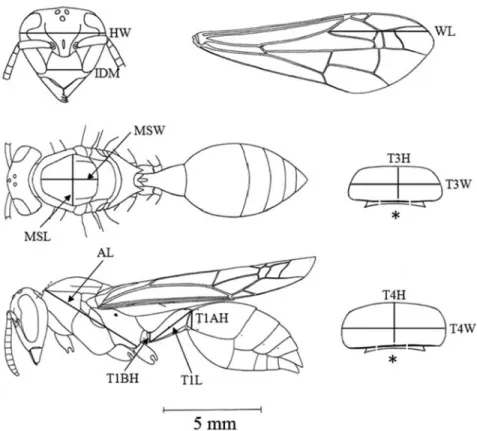
which is mostly represented by abdominal variables, such as T4H and T4W
(see the values of CAN1 in Table 2). Thus, queens are represented by larger
individuals, especially in the abdominal characters. However, a contrast among
abdominal variables, head length and wing length may also be important in
discriminating castes in this species (Table 2)
60
So
cio
bio
lo
gy V
ol. 57, N
o. 1, 2011
Table 2. Mean and standard deviationof most important variables, One-Way ANOVA significance, Stepwise Discriminant Analysis results and Canonical Scores of Polybia (T.) ignobilis. Q-queens, W-Workers, I-Intermediates.
Colony Characters Q WMean±SD I ANOVA F λ F to Enter p>F Can1 Can2
Colony I
T4H 1.78±0.11 1.49±0.05 - 239.89d 0.32 239.89 <0.001 1.12
-T4W 4.90±0.15 4.37±0.30 - 10.62d 0.28 14.81 <0.001 0.64
-MSW 2.29±0.07 2.13±0.22 - 9.63d 0.27 4.48 0.0366 0.47
-HW 3.03±0.03 3.00±0.06 - 2.07ns 0.25 9.19 0.0030 -0.35
-Colony II
T4H 1.83±0.07 1.53±0.08 1.49±0.05 380.44a,b 0.19 380.44 <0.001 1.98 -0.46
MSL 2.49±0.10 2.45±0.14 2.34±0.09 8.99a,c 0.14 34.62 <0.001 0.79 0.81
MSW 2.47±0.08 2.27±0.05 2.24±0.04 173.41a,b 0.12 11.13 <0.001 0.83 -0.11
WL 4.54±0.12 4.46±0.15 4.37±0.09 11.64a,b 0.11 7.56 <0.001 -0.38 0.52
T4W 5.25±0.37 4.69±0.19 4.63±0.12 260.14a,b 0.11 5.30 0.006 0.75 -0.21
HW 3.16±0.06 3.09±0.08 3.09±0.08 18.61a,b 0.10 2.62 0.075 -0.19 -0.43
Colony III
T4H 1.81±0.07 1.53±0.09 1.50±0.05 525.96a,b 0.15 525.96 <0.001 2.10 1.09
HW 3.02±0.04 3.03±0.05 2.98±0.04 3.68c 0.11 33.32 <0.001 -0.78 -0.55
T4W 5.06±0.12 4.57±0.14 4.47±0.10 328.26a,b 0.09 20.16 <0.001 0.82 -0.54
T1AH 0.99±0.06 0.87±0.04 0.86±0.05 124.89a,b 0.08 4.01 0.02 0.31 0.07
AL 4.79±0.09 4.56±0.14 4.50±0.08 88.35a,b 0.08 2.85 0.06 0.29 0.33
Colony IV
T4H 1.77±0.04 1.52±0.06 1.57±0.04 142.71a,c 0.30 142.71 <0.001 1.45 -1.41
T1AH 1.06±0.06 0.92±0.05 0.94±0.05 59.83a,b 0.26 8.96 <0.001 0.44 -0.55
T4W 4.95±0.13 4.52±0.14 4.75±0.08 79.81a,c 0.23 7.37 0.001 0.24 1.70
HW 3.07±0.04 3.05±0.06 3.06±0.03 0.63ns 0.20 8.84 <0.001 -0.42 -0.38
MSW 2.40±0.08 2.19±0.06 2.26±0.07 94.16a,c 0.19 3.48 0.03 0.62 0.15
AL 4.94±0.16 4.71±0.26 4.85±0.09 16.58d 0.18 2.88 0.06 0.29 0.33
a – Queens larger than workers and intermediates (Bonferroni p<0.05); b – No differences between workers and intermediates (Bonferroni p<0.05); c – Workers and Intermediates differ significantly (Bonferroni P<0.05); d – Queens larger than workers (Bonferroni p<0.05);
61
D
es
uo, I.
C.
et a
l.
— C
ast
e As
ym
met
ries i
n
P
oly
bia ig
no
bi
lis
Table 2. Mean and standard deviationof most important variables, One-Way ANOVA significance, Stepwise Discriminant Analysis results and Canonical Scores of Polybia (T.) ignobilis. (Continued). Q-queens, W-Workers, I-Intermediates.
Colony Characters Mean±SDQ W I ANOVA F λ F to Enter p>F Can1 Can2
Colony V
T4H 1.78±0.04 1.50±0.06 1.52±0.32 128.07a,b 0.45 128.07 <0.001 1.29 -0.64
HW 3.11±0.04 3.09±0.07 3.03±0.08 14.60c,e 0.36 26.49 <0.001 -0.73 0.40
MSW 2.51±0.06 2.25±0.08 2.18±0.09 82.52a,c 0.29 20.88 <0.001 0.72 0.62
WL 4.51±0.09 4.43±0.15 4.27±0.30 17.30a,c 0.27 9.13 <0.001 -0.44 0.70
T1AH 1.03±0.04 0.88±0.05 0.87±0.05 51.50a,b 0.25 5.88 0.003 0.29 -0.39
T4W 5.07±0.12 4.54±0.15 4.43±0.29 99.35a,c 0.24 4.21 0.02 0.42 0.15
Colony VI
T4W 4.87±0.23 4.50±0.16 4.50±0.11 55.94a,b 0.57 55.94 <0.001 0.98 0.31
HW 3.01±0.08 3.02±0.06 3.00±0.07 0.03ns 0.45 18.74 <0.001 -0.71 -0.17
T4H 1.61±0.10 1.48±0.06 1.44±0.06 40.02a,b 0.40 10.28 <0.001 0.78 -1.11
MSL 2.33±0.11 2.31±0.10 2.31±0.08 0.51ns 0.38 3.13 0.05 0.41 -0.48
MSW 2.30±0.10 2.16±0.07 2.16±0.08 32.97a,b 0.35 6.38 0.002 0.63 -0.002
T1AH 0.94±0.07 0.88±0.06 0.90±0.05 11.54a,b 0.34 1.99 0.14 0.10 0.94
a – Queens larger than workers and intermediates (Bonferroni p<0.05); b – No differences between workers and intermediates (Bonferroni p<0.05); c – Workers and Intermediates differ significantly (Bonferroni P<0.05); d – Queens larger than workers (Bonferroni p<0.05);
62 Sociobiology Vol. 57, No. 1, 2011
The values of Wilks’ lambda (λ) are shown in Table 2. The mean values of λ
ranged from 0.1 to 0.5, indicating that the characters are good discriminators
and castes in this species are well defined.
Table 3. Classification matrix for group comparison after discriminant function analysis, using the option CrOSSVALISATE in SAS software.
Correctly Classified Females (%)
Predicted Classification Workers Intermediates Queens
Colony I WorkersQueens 100.00100.00 1000 -- 09
Total 100 100 0 9
Colony II
Workers 72.00 72 27 1
Intermediates 1.37 12 1 0
Queens 98.66 0 1 72
Total 59.44 84 29 73
Colony III WorkersIntermediates 89.0010.00 899 111 00
Queens 100.00 0 0 76
Total 66.33 98 12 76
Colony IV
Workers 96.94 95 1 2
Intermediates 0.00 8 0 1
Queens 94,74 1 0 18
Total 63.89 104 1 21
Colony V WorkersIntermediates 66,0068,00 6632 3468 00
Queens 100,00 0 0 12
Total 78.00 98 102 12
Colony VI WorkersIntermediates 66,0041.67 6613 2810 61
Queens 77.78 4 2 21
Total 28.00 28 40 20
Table 4. results of MANOVA. Multivariate contrasts considering all variables together (Q-queens, W-Workers, I-Intermediates).
Colony F W vs. Q p Multivariate ContrastF W vs. Ip F I vs. Q p Colony I
Wilk´s Lambda 40.02 <0.0001 - - 20.52 <0.0001 Colony II
Wilk´s Lambda 164.89 <0.0001 1.81 0.0774 44.72 <0.0001 Colony III
Wilk´s Lambda 225.35 <0.0001 1.74 0.0918 49.77 <0.0001 Colony IV
Wilk´s Lambda 55.23 <0.0001 3.39 <0.001 14.43 <0.0001 Colony V
Wilk´s Lambda 59.80 <0.0001 4.09 <0.001 59.97 <0.0001 Colony VI
DISCUSSION
In
Polybia (T.) ignobilis
, queens were physiologically distinct from workers
and intermediates. They presented more developed ovaries, a larger fat body,
they were the oldest individuals and were the only inseminated females, a
pattern also observed in several species of Epiponini (Noll
et al.
2004; Noll
& Zucchi 2000; Noll
et al.
1997a,b; Shima
et al.
1996a,b; Shima
et al.
1994)
(Fig. 2 and 3). However, some flexibility in the ovarian development of queens
was observed: in the early stages of colonial cycle queens presented smaller
ovaries with fewer eggs and oocytes in the final stage of development than
in advanced stages (see Fig. 2). This is evidence of the actuation of worker
policing and confirms cyclical oligogyny and queen selection for this species.
This mechanism selects only the most productive queens in the colony (those
with larger ovaries and fat bodies, and longer longevity), and the maintenance
of queen dominance may inhibit ovarian development in workers. In fact,
Strassmann
et al.
(2002) verified that, in manipulated colonies of
Parachartergus
colobopterus
from which queens were removed, 86.8% of workers developed
their ovaries. As some species may present totipotency (see Strassmann
et al.
2002 and West-Eberhard 1978 and 1981) the loss of queen dominance may
represent a chance for workers to remain longer in the nest, feeding more,
accumulating a greater fat body and becoming reproductive.
The role of intermediate females in the Epiponini has been gradually
de-fined (Desuó
et al. 2
009). Some authors suggested that intermediates could
represent young or noninseminated queens (Forsyth 1978; West-Eberhard
1978; and Strassmann
et al.
2002). Naumann (1970) proposed that these
fe-males are important for the energetic flow within the colony, since their main
function would be laying trophy eggs. Intermediates could also be responsible
for producing males, as stated by Simões (1997). However, ratnieks (1988)
demonstrated that when queen mating frequency exceeds two, worker
polic-ing acts against the production of males by other workers or intermediates.
Moreover, the authors (1998) showed that males of
Brachygastra mellifica
64 Sociobiology Vol. 57, No. 1, 2011
2004). This process is likely to be related to queen senescence, which probably
leads to a decrease in the efficiency of pheromones and loss of reproductive
dominance by queens, although the mechanisms related to this process are not
clear yet. Nascimento
et al.
(2004) showed that cyclical oligogyny in
Asteloeca
ujhelyii is behaviorally mediated, and the authors observed the occurrence
of conflicts among queens and workers/queens; however, the first seems to
be more related to the initial phases of colony development. Nevertheless,
few studies addressed the role of intermediates in queen elimination in
Epi-ponini. Mateus (2005) analyzed the behavioral repertoire of intermediates
of
Parachartergus fraternus
and showed that these females also acted directly
in queen elimination through aggressive acts. In this study, intermediates
competed against queens for reproductive dominance through differential
oophagy. Morphological and physiological data showed that intermediates
of
Polybia (T.) ignobilis
are young workers, with more fat body and more
developed ovaries.
Several syndromes of caste differentiation are found in
Polybia
genera
(Noll
et al.
2004; Noll and Wenzel 2008). This study showed that castes in
Polybia (T.) ignobilis
are characterized mainly by differences in overall size
of females (Table 2). Queens were larger than workers and intermediates,
especially in the abdominal variables, which presented the higher values of
CAN1 (Table 2). However, multivariate analysis also revealed that a contrast
among variables may discriminate castes in
P. (T.) ignobilis
, meaning that a
shape component may be important in caste differentiation in this species.
Table 2 shows this contrast and indicates that individuals which present
larger abdomens also present smaller heads and wing length. Such a pattern
were reported for other species of
Polybia
and it is a widespread pattern of
caste differentiation in the Epiponini (Desuó
et al.
2009; Noll
et al.
2004).
According to Jeanne
et al.
(1995) such differences are related to a differential
growth of imaginal discs during immature period, however, developmental
studies are need to evaluate this theory.
66 Sociobiology Vol. 57, No. 1, 2011
1978) and other
Polybia
species (Noll and Zucchi 2000), and in the present
study for
Polybia (T.) ignobilis
(Fig. 3). The canonical Scatterplot (Fig. 4)
indicates that caste differences increased as the colony developed and castes
are not well defined during initial phases of colonial cycle (Colony I - swarm)
and final stages (Colony VI - late male production).
Such differences through the colony cycle may indicate that body size may
be important in competition among queens, since larger queens tend to be
more reproductively dominant and a hierarchical system based on
repro-ductive dominance should select larger individuals during variations in the
number of queens through the colonial cycle (Queller & Strassamann 1989;
reeve 1991; Noll & Zucchi 2000). Another important implication of queen
selection regards the maintenance of reasonable levels of genetic relatedness
in the advanced phases of colonial cycle, making Hamiltons’ kin selection
applicable (Nascimento
et al.
2004). Because of the importance of workers
in the control of queen demography, Noll and Wenzel (2008) defined the
Epiponini society as “a conspiracy of workers”.
According to Bourke (1999) and Strassmann
et al.
(2002), colony size
may also influence caste differentiation. In social wasps which present small
colonies (i.e.,
Polistes
,
Mischocyttarus
) no morphological caste differences are
observed, and then small societies are better characterized by direct conflicts
for reproduction. On the other hand, in insects which build large colonies
(i.e., ants, termites, honeybees, stingless bees, and vespines) and also present
monogyny, castes are remarkable and the conflicts for reproduction tend to
be indirect, reflecting directly in kin composition. Once again the Epiponini
represent an important exception for this generalization, since in several
spe-cies which build large colonies, morphological castes are absent or slightly
defined (richards 1978; Jeanne 1980; Jeanne
et al. 1
995; Hunt
et al.
1996;
O’Donnell 1998; Strassmann
et al.
2002; Noll & Wenzel 2008). According to
Strassmann
et al.
(2002), the low level of caste differentiation in the Epiponini
is the result of a post-imaginal (no differential feed during larval period) caste
determination. For these authors, females of some species are totipotent at
emergence as adults, but they are quickly suppressed by other workers when
more than one queen is present. This view matches that of West-Eberhard
(1978 and 1981) in
Metapolybia aztecoides
and
Synoeca surinama
. Mateus
post-imaginally determined. However, based on physiological and morphological
data presented in this study caste determination in
Polybia (T.) ignobilis
has
a strong pre-imaginal component, a result of differential feeding during the
larval stage or developmental switches responsible for the shape differences
detected ( Jeanne
et al.
1995) .
ACkNOWLEDGMENTS
The authors gratefully acknowledge the financial support of CNPq. We
also give special thanks to Prof. Dr. Sidnei Mateus and Prof. Dr. ronaldo
Zucchi for their suggestions and for providing colony VIII, and to Mr. Jaime
r. Somera, the illustration expert, for the artwork.
rEFErENCES
Baio, M.V., F.B. Noll, r. Zucchi, & D. Simões 1998. Non allometric differences in Agelaia vicina (Hymenoptera, Vespidae, Epiponini). Sociobiology32(33):465-476.
Bourke, A.F.G. 1999. Colony size, social complexity and reproductive conflict in social insects. Journal of Evolutionary Biology 12(2):245-25.
Carpenter, J.M. 1993. Biogeograph patterns in the Vespidae (Hymenoptera): two views of Africa and South America. In: Goldblatt, P. (ed), Biological relationships between Africa and South America. New Haven (CT): Yale University Press. p. 139-155. Carpenter, J.M. 1997. A note on the names of paper wasps tribes (insecta:Hymenoptera:Vespidae).
Natural History Bulletin of Ibaraki University 1:5-16.
Carpenter, J.M. 2004. Synonymy of the Genus Marimbonda richards 1978, with Leipomeles Mobius, 1856 (Hymenoptera:Vespidae:Polistinae), and a New key to the Genera of Paper Wasps of the New World. American Museum of Novitates 16,16p.
De Oliveira, O.A.L., F.B. Noll, S. Mateus & B. Gomes 2007. Castes and Asynchronous colony cycle in Polybia bistriata (Fabricius) (Hymenoptera: Vespidae). Neotropical Entomology 36(6):817-827
Desuó, I.C., A.S.N. Murakami, V.C. Oliveira & S.N. Shima 2009. Overview of Caste Differentiation in the Polistinae, Emphasizing the Neotropical Swarm-Founding Polistinae (Hymenoptera, espidae, Epiponini). Sociobiology 53:851-889.
Forsyth, A. 1978. Studies on behavioral ecology of polygynous wasps. [Thesis]. [Cambridge(MA)]: Harvard University. p. 226.
Hamilton, W.D. 1964a. The genetical evolution of social behavior I.Journal of Theorical Biology7:1-16.
Hamilton, W.D. 1964b. The genetical evolution of social behavior II. Journal of Theorical Biology7:17-52.
68 Sociobiology Vol. 57, No. 1, 2011
Hastings, M.D., D.C. Queller, F. Eischen, & J.E. Strassmann 1998. kin selection, relatedness and worker control of reproduction in a large-colony epiponine wasp, Brachygastra mellifica. Behavior Ecology9:573–581.
Herman, r.A., D.C. Queller, & J.E Strassmann 2000. The role of queens in colonies of the swarm-founding wasp Parachartergus colobopterus. Animal Behavior 59:841-848 Hunt, J.H., D.k. Schmidt, S.S. Mulley & M.A. Williams 1996. Caste dimorphism in Epipona
guerini (Hymenoptera, Vespidae): Further evidence for larval determination.Journal of kansas Entomological Society69(4):362-369.
Jeanne, r.L. 1975. The adaptativeness of social wasp nest architecture. Quarterly reviews of Biology 50: 67-287.
Jeanne, r.L. 1980. Evolution of social behavior in Vespidae. Annual reviews of Entomology 25:371-395.
Jeanne, r.L. & r. Fagen 1974. Polymorphism in Stelopolybia areata (Hymenoptera, Vespidae). Psyche 81:155-166.
Jeanne, r.L., C.A. Graf & B.S. Yandell 1995. Non-size-based morphological castes in a social insect. Naturwissenschaften82:296-298.
Mateus, S. 2005. Análise dos comportamentos envolvidos na organização social e no processo de enxameio de Parachartergus fraternus (Hymenoptera, Polistinae, Epiponini) [Thesis]. [ribeirão Preto (SP)]:Faculdade de Filosofia Ciências e Letras, Universidade de São Paulo. p. 143.
Mateus, S. & F.B. Noll 1997. Nectarinella xavantinensis, a new neotropical social wasp (Hymenoptera, Vespidae, Polistinae). Journal of New York Entomological Society 105(1): 45-49.
Mateus, S., F.B. Noll & r. Zucchi 1999. Castes differences and related bionomic aspects of Chartergellus communis, a Neotropical swarm-founding polistinae wasp (Hymenoptera, Vespidae, Epiponini). Journal of New York Entomological Society 107(4):391-406. Mateus, S., F.B. Noll, & r. Zucchi 2004. Caste flexibility and variation according to the colony
cycle in the swarm-founding wasp, Parachartergus fraternus (Gribodo) (Hymenoptera: Vespidae, Polistinae). Journal of kansas Entomological Society 77(4):470-483. Nascimento, F.S., I.C. Tannure-Nascimento & r. Zucchi 2004. Behavior mediators of
cyclical oligogyny in the Amazonian swarm-founding wasp Asteloeca ujhelyii (Vespidae, Polistinae, Epiponini).Insectes Sociaux 51:17-23.
Naumann, M.G. 1970. The nesting behavior of Protopolybia pumila (Saussure, 1863) (Hymenoptera, Vespidae) in Panama [Thesis]. [kansas(kS)]: University of kansas. p. 182.
Noda, S.C.M., S.N. Shima, & F.B. Noll 2003. Morphological and physiolocal caste differences in Synoeca cyanea (Hymenoptera, Vespidae, Epiponini) according to the ontogenetic development of the colonies. Sociobiology41:547-570.
Noll, F.B., S. Mateus, & r. Zucchi 1997a. Morphological caste differences in neotropical swarm-founding and polygynous Polistinae wasps, Polybia scutellaris (Hymenoptera, Vespidae).Studies on Neotropical Fauna and Environment 32:76-80.
Noll, F.B., D. Simões, & r. Zucchi 1997b. Morphological caste differences in Neotropical swarm-founding polistinae wasp. Agelaia multipicta and A. pallipes (Hymenoptera:Vespidae). Ecology, Ethology and Evolution 9:361-372.
Noll, F.B. & r. Zucchi 2000. Increasing caste differences related to life cycle progression in some neotropical swarm-fouding polygynic wasps (Hymenoptera, Vespidae, Epiponini). Ecology, Ethology and Evolution 12(1):43-65.
Noll, F.B. & r. Zucchi 2002. Caste and influence of colony cycle in swarm-founding Polistinae wasps (Hymenoptera, Vespidae, Epiponini) Insectes Sociaux48:01-13.
Noll, F.B., J.W. Wenzel & r. Zucchi 2004. Evolution of castes in neotropical swarm-founding wasps (Hymenoptera, Vespidae, Epiponini).American Museum of Novitates 3467:1-12.
Noll, F.B. & J.W. Wenzel 2008. Castes in the swarming wasps: “queenless” societies in highly social insects. Biological Journal of LinneanSociety93:509-522.
O’Donnel, S. 1998. reproductive caste determination in social wasps (Hymenoptera, Vespidae). Annual reviews of Entomology43:323-346.
Platt, T.G., D.C. Queller, & J.E. Strassmann 2004. Aggression and worker control of caste fate in a multiple-queen wasp, Parachartergus colobopterus. Animal Behavior 67:1-10. Queller, D.C. & J.E. Strassmann 1989. Measuring inclusive fitness in social wasps. In: M.D.
Breed & r.E Page (eds.). The genetics of social evolution. Boulder (CO): WestView Press. p. 103-112.
ratneiks, F.L.W. 1988. reproductive harmony via mutual policing by workers in eusocial hymenoptera. The American Naturalist 132:217-236.
reeve, H.k. 1991. Polistes. In: ross k.G. & r.W. Matthews (eds.). The Social Biology of Wasps., Ithaca(NY): Cornell University Press. p. 99-149.
richards, O.W. & M.J. richards 1951. Observations on the social wasps of South America (Hymenoptera, Vespidae). Transaction of royal Entomological Society of London 102:1-170.
richards, O.W. 1971. The biology of the social wasps (Hymenoptera:Vespidae). Biological reviews46:483-528.
richards, O.W. 1978. The social wasps of the Americas excluding the vespinae. London British Museum (Natural History). p. 580.
Sakagami, S.F., r. Zucchi, S. Yamane, F.B. Noll & J.M.F Camargo 1996. Morphological caste differences in Agelaia vicina, the neotropical swarm-founding polistinae wasp with the largest colony size among social wasps (Hymenoptera, Vespidae). Sociobiology 28(2):207-223.
70 Sociobiology Vol. 57, No. 1, 2011
Shima, S.N., S. Yamane, & r. Zucchi 1996a. Morphological caste differences in some neotropical swarm-founding Polistinae wasps II. Polybia dimidiata (Hymenoptera, Vespidae). Japanese Journal of Entomology64(1):131-134.
Shima, S.N., S. Yamane, & r. Zucchi 1996b. Morphological caste differences in some neotropical swarm-founding Polistinae wasps III. Protonectarinae Sylveirae (Hymenoptera, Vespidae). Bulletin of the Faculty of Education of Ibaraki University 45:57-67.
Shima, S.N., F.B. Noll, r. Zucchi & S. Yamane 1998. Morphological caste differences in some neotropical swarm-founding Polistinae wasps VI. Pseudopolybia vespiceps, with preliminary considerations on the role of the intermediate females in social organization of the Epiponini (Hymenoptera, Vespidae). Journal of Hymenoptera research 7:280-295.
Shima, S.N., F.B. Noll & r. Zucchi 2000. Morphological caste differences in some neotropical swarm-founding Polistinae wasps. Brachygastra lecheguana (Hymenoptera: Vespidae, Polistinae, Epiponini). Sociobiology 35(3):131-134.
Shima, S.N., F.B. Noll & r. Zucchi 2003. Influence of colony cycle on physiological and morphological caste variation in the perennial Neotropical swarm-founding social wasp, Protonectarina sylveirae (Hymenoptera:Vespidae:Epiponini). Sociobiology 42(2):449-446.
Simões, D. 1977. Etologia e diferenciação de castas em algumas espécies de vespas sociais (Hymenoptera, Vespidae) [Thesis]. [ribeirão Preto(SP)]: Faculdade de Filosofia Ciências e Letras, Universidade de São Paulo.
Strassmann, J.E., k.F. Goodnight, C.J. klinger & C.r. Queller 1998. The genetic structure of swarms and the timing their production in the queens cycles of Neotropical wasps. Molecular Ecology 7:709-718.
Srassmann, J.E., B.W. Sullender & D.C. Queller 2002. Caste totipotency and conflict in a large-colony social insect. Proceedings of the royal Society of London B 269:263-270. West-Eberhard, M.J. 1973. Monogyny in “polygynous” social wasps. Proceedings of the VII
CongressIUSSI, London. p. 396-403.
West-Eberhard, M.J. 1978. Temporary queens in Metapolybia wasps: non-reproductive helpers without altruism? Science200:441-443.
West-Eberhard, M.J. 1981. Intragroup selection and the evolution of insects societies. In: Alexander, r.D. & D.W. Tinkle (eds), Natural selection and social behavior. New York(NY): Ed Chiron Press. p. 3-17.