w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Co-extracted
bioactive
compounds
in
Capsicum
fruit
extracts
prevent
the
cytotoxic
effects
of
capsaicin
on
B104
neuroblastoma
cells
Viktorija
Maksimova
a,∗,
Liljana
K.
Gudeva
b,
Rubin
Gulaboski
a,
Karen
Nieber
c aFacultyofMedicalSciences,GoceDelcevUniversity,Stip,MacedoniabFacultyofAgriculture,GoceDelcevUniversity,Stip,Macedonia
cFacultyofPharmacy,BiologyandPsychology,InstituteofPharmacyUniversityofLeipzig,Leipzig,Germany
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received21April2016
Accepted13June2016
Availableonline11August2016
Keywords:
Antioxidants Capsaicin Capsicum Cytotoxicity
Neuroblastomacells
Voltammetry
a
b
s
t
r
a
c
t
TheaimofthisstudywastoinvestigatetheeffectofcapsaicinandethanolicCapsicumextractsonB104 neuroblastomacellsasapotentialanticanceragent.Additionally,thisstudyalsoaimstoexaminethe influenceofco-extractedbioactivecompounds(vitaminE,vitaminCandquercetin)inCapsicumfruit extractsonthecytotoxiceffectsofcapsaicininneuroblastomacells.MTTandLDHassayswereusedto determineviabilityandcelldeathinB104neuroblastomacells.Antioxidativepropertiesofcapsaicin, vitaminE,vitaminCandquercetinwereestimatedbymeansofcyclicandsquarewavevoltammetry. Therewasasignificantcytotoxicityofcapsaicin(100mol/l)after24hincubationandforcapsaicin (250mol/l),evenwhencellsaretreatedfor1h.Ontheotherhand,ethanolicCapsicumextractswhich containedcapsaicin(0.5–2.1mmol/l)didnotshowanycytotoxiceffect.Wesuggesttherefore,thatother co-extractedcompoundswithintheethanolicextractsinteractantagonisticwiththecytotoxiceffectof capsaicinandtheirinteractionsshouldbefurtherinvestigated.Ourresultsindicatethatcapsaicininhigh concentrationinducescytotoxiceffectsinadosedependentmanner,butotherbioactivecompounds presentinCapsicumfruitspreventthecytotoxiceffectsoftheextractsonneuroblastomacells.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Capsaicin(N-vanillyl-8-methyl-6-nonenamide,1)isthemajor componentofcapsaicinoids.Thisalkaloidisasecondary metabo-liteindifferentspeciesofthegenusCapsicum(Buczkowskaetal., 2013).Itgivesthepungencyofhotpeppersandisresponsiblefor manyphysiologicalandpharmacologicalpropertiesofthisplant. Althoughtopicalcreamswithcapsaicinareusedtotreat periph-eralneuropathicpainconflictingepidemiologicdata,manybasic researchstudiesresultssuggestthatcapsaicincanactasacytotoxic orasacytoprotectiveagent(BodeandDong,2011).Themajority ofresearchstudiessuggestthatcapsaicininducescell-cyclearrest orapoptosisorinhibitsproliferationindifferentmalignantcells includinglungcancer,adenocarcinoma,pancreaticcancer,breast cancerDíaz-LaviadaandRodríguez-Henche(2014)hepatocellular carcinomaBaeketal.(2008),osteosarcomaandmanyothersWon etal.(2013).Variousmechanismsforcapsaicin-induced apopto-sishave beenproposedfor differentcellsystems. Physiological
∗ Correspondingauthor.
E-mail:viktorija.maksimova@ugd.edu.mk(V.Maksimova).
processeslinkedtotheintracellularcalciumincrease,reactive oxy-gen species generation, disruptionof mitochondrial membrane transitionpotentialandactivation ofsometranscriptionfactors areinvolved ClarkandHo-Lee(2016)are closelyrelated tothe capsaicinactivity.
AccordingtoSanchezetal.(2006),capsaicincanactascytotoxic agentthroughevokingapoptosisinprostatecancercellsthrough mechanismwhichincludesincreasedproductionofreactive oxy-genspecies(ROS),disruptionofinnermitochondrial membrane potentialandactivationofcaspase-3.Pramaniketal.(2011)and Zhangetal.(2008)showedthatapoptosisprovokedbycapsaicin in pancreatic cells is accompanied by 4–6fold increase of the concentrationoffreeradicalsandconsequentlydisruptionofthe mitochondrialmembranepotential.Thereforecapsaicinhas pro-vokedaninhibitionofcellproliferationandinducedapoptosisina dosedependentmanner.
Incontrast,hotpeppersfruitsarewidelyusedineveryday nutri-tionandhaveshownmanybenefitsforhumanhealth.Asknown fromtheliterature,beside capsaicin,theseextractsrepresent a complexmixtureofmanyotherbioactivecompoundsasvitamin C(2),vitamin E(3), carotens,quercetinandluteolin (Asninand Park,2015).Thesemoleculesarereportedascompoundswhich
http://dx.doi.org/10.1016/j.bjp.2016.06.009
0102-695X/©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
haveshownhighantioxidativepotentialandprotectiverolein car-cinogenesis(MaterskaandPerucka,2005).Actingasantioxidants, thesemoleculesarecapabletoneutralizeorscavengethefree radi-calswhichareresponsibleformanydegenerativediseasesaswell asprogressionofcancer(Uttaraetal.,2009).Therefore,thetotal anitoxidativecapacityofpepperextractscanmodulatethe cyto-toxicityofcapsaicinpresentintheextracts.
1 2 3
Theaimofthepresentworkwastostudythecytotoxic proper-tiesofcapsaicinonB104neuroblastomacells,andalsotoexamine thetoxicityoftheCapsicumfruitextractsobtainedfromaseveral differentvarietiesofhotpeppers.Thecurrentstudyalsoaimsto addressthepossibleinteractionsandthesynergisticantioxidant effectsoftheco-extractedcompoundswithcapsaicin.Tothebest ofourknowledge,theinfluenceoftheotherco-extractedbioactive compoundsinCapsicumfruitextractsonthecytotoxiceffectsof capsaicinonB104neuroblastomacells,havenotbeenevaluatedso far.
Materialsandmethods
Plantmaterials
CapsicumfruitsfromfourdifferentgenotypesofC.annuumL. Solanaceae,(hotpeppers)weretakenforthisexperiment.Different plantseedswerestoredinthegenbankatGoceDelcev Univer-sity,FacultyofAgriculture,atthecampusofStrumica,Macedonia. Plantnamehasbeencheckedontheweb:www.theplantlist.orgon February10,2015.Theseseedshavebeentakenforcultivationand theirfruitswerecollectedfromthefieldinthephaseofbotanical maturity.Thefruitsfromfourgenotypesofhotpepperswithlocal names:Bombona,Feferona,Vezena,andSivrija,weredriedonroom temperatureforabouttwoweeks.Afterward,theyweregrounded andthepowderwasusedforextraction.
Cellline
Cells of the rat neuroblastoma line B104 (ATCC, Manassas, VA,Schubertetal.1974)weremaintainedinDMEM/Ham’swith l-glutamine (Dulbecco’smodifiedEagle’smedium)(PAAGmbH)
supplementedwith15%fetalbovineserum(FBS),andantibiotics1% penicillin/streptomycinsolution.Themediumwaschangedevery 2–3days.Cellswereincubatedat37◦Cinanatmospherecontaining 5%CO2andsaturatinghumidity.Cellswereallowedtobeadherent for24hbeforetreatmentwithcapsaicinorCapsicumextracts.
Reagents
Stock solutions of capsaicin (1) (>95%, natural capsaicin), vitamin E (2), quercetin and ascorbic acid were freshly pre-paredbyusingstandardsubstancesobtainedfromSigma-Aldrich and 96% ethanol (reagent grade) (3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazoliumbromide)(MTT),sodiumdodecylsulfate (SDS),dimethylformamide(DMF),lactate,NAD,diaphorase,HCl, SDSlysisbuffer,DMEM,phosphatebuffersolution)PBS,phosphate buffer(pH7.4),fetalbovineserum,penicillin,streptomycin(PAA GmbH),tritonX,ethanol96%(Sigma–Aldrich).Allsolutionswere storedat4◦C.Inallsolutionsusedforelectrochemical measure-ments,KClwasaddedasanadditionalelectrolyteatconcentration of0.010M.
Methods
Extractionmethod
Extraction wasperformed by maceration using ethanol 96% (v/v) as a solvent (Rafajlovska et al., 2007). Maceration was
performedfor5h,on60◦C,andafterwardtheextractswere fil-teredbygouchfilter,usingavacuumpump.0.2gofthepulverized plantmaterialwasmaceratedwith25mlsolvent.Stocksolution waspreparedbyusingcapsaicin,with96%ethanolandthendiluted toappropriateconcentrations.
Spectrophotometricmethod
UV/VISspectrophotometrywasusedforquantificationof cap-saicininethanolicextractsandstandardssolutions(Peruckaand Oleszek,2000).Theconcentrationofcapsaicinwasmeasuredby usingaCary100spectrometer,instrumentversionno.9.00,ona specificmaximumwavelengthof280nm.Aserialofstandard dilu-tionsofcapsaicin(0.25,0.125,0.0625,0.0312,0.0156mg/ml)were preparedforobtainingtheregressioncurve.Theethanolicextracts weremeasuredandaccordingtotheabsorbanceobtainedforthem, regressionanalysiswasperformedforcalculationofconcentration ofcapsaicinintheextracts.
Cytotoxicitymethods
TheviabilityofB104cellsaftertreatmentwithcapsaicinor Cap-sicumextractswasassessedusingMTTmethod.Fordetermination ofthecelldeath,theLDHmethodwasused.
MTTassay
TheMTT assayinvolvesthe conversionofthewater soluble MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bro-mide)toaninsolubleformazan.Theformazanisthensolubilized anditsconcentrationwasdeterminedbyopticaldensityat570nm (Mosmann, 1983). MTT cell proliferation assay was performed accordingtotheprotocolgivenbythemanufacturerRoche Diag-nosticsGmbHforCellProliferationKitI.Inbrief,B104cellswere seededin96-wellcellcultureplates(2×104cells/well)and sub-sequentlytreatedwithcapsaicin(0.5,1,10,100and250mol/l) ordifferentethanolicCapsicumextractsat37◦Cfor1,6and24h, respectively.
LDHassay
TheLDHassayoffersasimplewaytomeasureplasma mem-branedamage,basedonthereleaseoflactatedehydrogenase(LDH), astablecytoplasmicenzymepresentinmanycells (Chouetal., 2009).Assaywasperformedaccordingtothemanufacturers pro-tocol (RocheDiagnostics GmbH)given for theLDH cytotoxicity detectionkit. Inbrief,afteradherenceof thecells tothewells, testsubstances(capsaicinindifferentconcentrationsandethanolic extracts)dilutedinanappropriateassaymedium(DMEM),were titratedinaseparatemicroplatebyseveraldilutions(final vol-umeupto200l/well). Then, theassaymedium wasremoved and100lfreshassaymediumwasaddedtoeachwell.100l ofthetestsubstancedilutionsweretransferredinto correspond-ingwellscontainingtheadherentcells.Thereafter,100lofthe supernatantwereremovedfromthewellscarefullyandtransferred intocorrespondingwellsofanopticallyclear96-wellflatbottom microplate.To determinethe LDH activityin thesupernatants, 100lofthereaction mixture(freshlyprepared)wasaddedto eachwellandincubatedfor30minat+15to+25◦Cinadark atmo-sphere.ThereactionmixturewaspreparedbymixingtheCatalyst (Diaphorase/NAD+mixture)andDyesolution(INTandsodium lac-tate).
Absorbanceofthesampleswasmeasuredat492nmbyanELISA platereader.ThelossofintracellularLDHanditsreleaseintothe culturemediumisanindicatorofirreversiblecelldeathduetocell membranedamage.A0.1%TritonXsolutionwasusedaspositive controlandDMEM(assaymedium)asnegativecontrol.
Voltammetricmethods:cyclicvoltammetryandsquarewave voltammetry
Electrochemicaldeterminationoftheantioxidativepotentialof theextractsanda mixtureofstandard solutionwasperformed bymeansofsquare-wavevoltammetry(SWV),aftershort electro-chemicalcharacterizationoftheelectrochemicalfeaturesbycyclic voltammetry(CV),ataglassycarbonworkingelectrode. Experi-mentsincyclicvoltammetrywereconductedoverapotentialrange from−0.200to1.000V, withascan rateof
v
=10mV/s.Experi-mentalconditionsforSWVwere:potentialstepdE=0.001V,pulse height(SWamplitude) Esw=0.050Vand frequency of f=10Hz. Priortoeachelectrochemicalexperimenttheworkingelectrode waspolishedbyusingAlCl3onapolishingcloth,followedbyrinsing oftheelectrodewithwaterandacetoneanddryinginair.
Statistics
VarianceanalyseswereperformedbyusingaGraphPadPrism 6.0.ProcessingoftheresultswasdonebyonewayANOVA,for threegroupsofresults(differenttimeofexposition),inwhicheach treatmentofthecellswascomparedbythetreatmentwith neg-ativecontroland/orpositivecontrol.Allgraphsrepresentmeans andstandarddeviationsfortriplicatesamplesfromeachofthree independentexperiments(n=9).Resultswhicharestatistically sig-nificantareshowedonthegraphs,p<0.01.Theregressionanalysis wasperformedusingtheprogramGraphPadPrism6.0,XYanalyses, linearregression.
Results
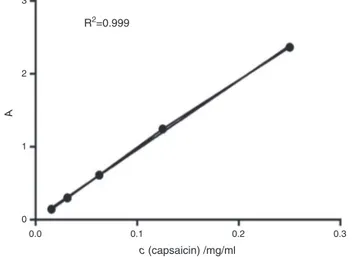
Determinationofthecapsaicinconcentration
Spectrophotometricmeasurementoftheconcentrationof cap-saicinintheethanolicoleoresinswasdeterminedonthebasisof standardsolutionsofcapsaicin.Theconcentrationofcapsaicinin
3
2
A
R2=0.999
1
0
0.0 0.1 0.2
c (capsaicin) /mg/ml
0.3
Fig.1.CalibrationcurveconstructedforstandardsolutionsofcapsaicinbyUVVIS.
thesampleswerecalculatedusingtheregression curveand lin-earityequation(Fig.1).Becausetheextractswereobtainedwith 96%ethanol,wedilutedtheextractswithDMEMinratio1:100,in ordertoescapeanycytotoxiceffectsofethanolonthecells. Addi-tionally,thedilutionaffectedthecoloroftheextracts.Thecolor comesfromthehighconcentrationofpigments,anditvanished afterthedilution.Therefore,toobviatetheprobabilityofgiving falseresults,extractsweredilutedtoappropriateconcentrations fortreatmentofthecellsgiveninTable1.Bombonagenotype con-tainedthehighestconcentrationofcapsaicinwith2.10mM,forthis extract.Concentrationsofcapsaicincalculatedasmg/gDWarein linewiththeresultsfoundintheliterature(Tilahunetal.,2013).
Effectofcapsaicinoncellviabilityandcelldeath
Asshown in Fig.2, capsaicin present in low concentrations (0.5–10M)didnotsignificantlyinfluencethecellviability.The concentrationrequired to inhibit 50%of the cells viability was foundtobe61.9M,whichisinaccordancewiththeprevious find-ingsforcapsaicin(Richeuxetal.,1999).Inconcentrationof100 and250Mitinhibited theviabilityofB104cells comparedto theeffectsof0.1%TritonXsolution,usedaspositivecontroland DMEM(assaymedium)asnegativecontrol.Theincubationof1h resultedinamoderateinhibitionfor100M(29±11.5% inhibi-tion,p>0.05),whereastheeffectsweremorepronouncedaftera longerincubationperiodorata higherconcentration.Capsaicin (100M)incubatedforaperiodof6hresultedin94±1% inhibi-tion,whileafteranincubationperiodof24h,theinhibitionwas 95±0.1%(p<0.01).Higher concentrationof capsaicin(250M)
Table1
ConcentrationofcapsaicininstandardsolutionandextractsofCapsicumusedfor
treatmentofB104cellsinMTTandLDHassays,(extractsweredilutedwithDMEM
inratio1/100).
Standard solutions
Concentration ofcapsaicin [M]
Diluted extractsfor treamentof cells
Concentration ofcapsaicinin extracts[mM]
St.solution1 0.5 Solutionof
Vezena
0.51
St.solution2 1 Solutionof
Feferona
0.78
St.solution3 10 Solutionof
Bombona BBBombona
2.1
St.solution4 100 Solutionof
Sivrija
0.65
120 1h incubation
6h incubation
24h incubation
100
80
60
Vibaility of cells (% of control)
40
20
0
DMEM 0.5 Triton X
#
** ** ** ** **
Concentrations of capsaicin (μm)
1 10 100 250
Fig.2.Effectofdifferentcapsaicinconcentrationsoncellviability(MTTassay)on B104neuroblastomacellsincubatedfor1,6or24h.Thereisasignificantdifference intheresultsfortheeffectof100Mcapsaicinfor1hcomparedtotheeffectfor 6h.Significance:#(p<0.05),**(p<0.01)vs.DMEM.
100
80
60
40
20
Viability of cells (% of control) 0
DMEM 0.5 1
Concentrations of capsaicin (μm)
10 100
1h incubation 6h incubation 24h incubation
250
Fig.3. Capsaicinsuppressescellviabilityinatimeanddosedependentmanner. Therewasasignificantcytotoxicityofhigherconcentrationsofcapsaicin(100and 250M),after6and24hincubation.IC5061.9Mfor6handIC5061.6Mfor24h.
resultedin much higher inhibition ofcell viabilityeven for1h
incubation(93±0.5%,p<0.01)andfor6hand24h(94±1%and
95±0.2%,p<0.01,respectively).
TheresultsfromtheMTT assay(Fig.3)showedthatthereis
significantdifferenceintheviabilityofculturedcellswhenthey weretreatedwithcapsaicinfor1h,and6or24h.Thecalculated IC50 valuefor6h(IC5061.9M)wasalmostthesameasfound after 24h incubation period (IC50 61.6M) indicating that the effectofcapsaicinwasnotpronouncedwithinalongerincubation period.
TheresultobtainedfromtheLDHassayispresentedinFig.4.and itconfirmedtheresultsobtainedfromtheMTTassay.Capsaicin inlowconcentrations(0.5–10M)didnotsignificanlyinfluence theLDHrelease.Inconcentrationof100and250Mitresulted inhighLDHreleasefromB104cells,indicatingcelldeath.Results werecomparedtotheeffectsof0.1%TritonX solution,usedas positivecontrolandDMEM(assay medium)asnegativecontrol. Concentrationofcapsaicinof100Mincubatedfor1hresultedina moderateLDHrelease(13±6%,p>0.05),whereastheeffectswere morepronouncedafteralongerincubationperiodoratahigher concentration.Thesameconcentrationofcapsaicin(100M)fora periodof6hincubationresultedin96±2.3%celldeath,whilefor 24htreatment,thecelldeathwasincresedto58±5.1%,(p<0.01). Higher concentration of capsaicin (250M) resulted in much higher cytotoxicity of cells even for 1h incubation (100±2.7%, p<0.01),aswellasfor6hand24hconsequently(90±1.38%and 98±6.9%,p<0.01).
120
100
80
60
% of cell death 40
20
0
DMEM 0.5 1 10 100 250
Concentrations of capsaicin (μm) 1h incubation ∗ ∗
∗∗
# ∗∗
∗∗ ∗∗
6h incubation 24h incubation
Fig.4.Effectofcapsaicinindifferentconcentration(0.5,1,10,100,250M),
exam-inedbyLDHassayonB104neuroblastomacellsfor1,6or24hofincubation.Treating
oftheneuroblastomacellswithcapsaicin(100and250M)for6or24hresulted
inhighincreaseinLDHreleaseindicatingcelldeath.(#p<0.05;*p<0.01).Values
aremeans±SEcomparedwithDMEMasnegativecontrol.n=9foreachgroup.
Ethanolic capsicum extracts
DMEM
120
100
80
60
40
Viability of cells (% of control)
20
0
V
ez
ena
F
ef
erona
Bombona
Sivr
ija
T
riton X
1h incubation 6h incubation 24h incubation
Fig.5. EffectofethanolicCapsicumextracts(obtainedfromCapsicumgenotypes:
Vezena,Feferona,Bombona,Sivrija)oncellviability(MTTassay)treatedfor1,6or
24h.Viabilityofthecellswascomparedtonegativecontrol(DMEM).
EffectofethanolicCapsicumextractsoncellviabilityandcell death
Incontrasttocapsaicin,theethanolicCapsicumextractsdidnot influencesignificantlyneitherthecellviabilitynorthecelldeath.In theMTTassay(Fig.5)therewasnosignificantinhibitionofthecell viabilityaftertreatmentofthecellswiththeextracts.The incu-bationtimeperiod hadnot effectonthecellviability.TheLDH assay(Fig.6)confirmedtheresultsofMTTassay.Noeffectsonthe LDHreleasewasfound,indicatingthattheextractdidnothaveany cytotoxiceffects.
Antioxidativepotentialofcapsaicinandotherco-extracted bioactivecompoundspresentintheethanolicCapsicumextracts
Abriefelectrochemicalcharacterizationofcapsaicin,vitaminE, ascorbicacidandquercetinhasbeenperformedbymeansofcyclic voltammetry(resultsnotshown),whiletheirpotentialsynergistic antioxidativeeffecthasbeenanalyzedbymeansofsquare-wave voltammetry.TypicalnetSWvoltammogramsofcapsaicin, vita-min E, quercetin, and ascorbic acid (each at concentration of 10mol/l)recordedataglassycarbonelectrodeinabuffer solu-tionatpH=7.0aregiveninFig.7.ThenetSWpeaksofvitaminE andquercetinarecloselypositionedatpotentialsofEp,net=0.146V
Ethanolic capsicum extracts
DMEM
120
100
80
60
40
% of cell death
20
0
Vezena Feferona
Bombona
Sivrij
a
1h incubation 6h incubation 24h incubation
T
riton
X
Fig.6.EffectofethanolicCapsicumextractsoncelldeath(LDHassay)treatedfor
1,6or24h.TherewasnosignificantdifferencewithethanolicCapsicumextracts
comparedtothenegativecontrol(DMEM).
5.0
4.5
4.0
3.5
3.0
I
/
μ
A
E/V (v.s Ag/AgCI) c
d b
a e
2.5
2.0
1.5
–0.20 –0.10 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70
Fig.7.Square-wavevoltammogramof:(A)vitaminE;(B)quercetin,(C)ascorbicacid
and(D)capsaicin(10M),(E)equimolarmixturecontainingvitaminE,quercetin,
ascorbicacidandcapsaicinatconcentrationof10M,recordedataglassy
car-bonelectrodeinabuffersolutionatpH=7.1.Instrumentalparameterswere:step
potentialdE=0.001V,square-waveamplitudeEsw=0.05Vandfrequencyof10Hz.
locatednearbythecapsaicinpotential(atEp,net=0.409Vfor
ascor-bicacidand Ep,net=0.352V forcapsaicin).VitaminEelevatesto
thehighestnetpeak current(Ip,net=1.894A) comparedtothe maximalpeakcurrentsofquercetin,ascorbicacidandcapsaicin (0.580Aforquercetin,0.193Aforascorbicacidand0.086A forcapsaicin).Theobtainedsquare-wavevoltammogramsforthe equimolar mixture of 10mol/l of each compound show that thevoltammetricresponseconsistsofasingleSWVpeakatthe potentialabout0.128V,whichisbetweenthetypicalpeakof Vita-minE and quercetin. Themeasured netpeak current obtained for the mixture of all four compounds was Ip,net=3.313A, Fig.7(e).
Discussion
It is well known that capsaicin has different carcinogenic effects onneuronal and non-neuronalcells (Chouet al., 2009). However, capsaicin induced cytotoxicity on pancreatic neu-roendocrine tumor cells Skrzypski et al. (2014), human skin fibroblasts Kim et al. (2004), human gastric adenocarcinoma cell line (Yi-Ching et al., 2005). Ethanolic extracts of several spices,inwhichchillipepperwasincluded,inhibitedcellgrowth at concentrations of 0.2–1mg/ml in vitro (Unnikrishnan and
4
3.5 y=0.304x+0.368
R2=0.987 3
2.5
2
1 1.5
I
pa/
μ
A
0.5
0
0 2 4
Equimolar concentration of all compounds in mixture μmol/I
6 8 10 12
Fig.8. Dependenceontheanodicpeakcurrentandtheconcentrationofequimolar
mixturescontainingvitaminE,quercetin,ascorbicacidandcapsaicinat
increas-ingconcentrationfrom1to10M,inaphosphatebufferatpH=7.1(dE=0.001V,
Esw=0.050Vandfrequencyof10Hz).
Ramadasan,1988).Otherauthorsconfirmedthatethanolic Cap-sicum extracts (0.01–1000g/ml) didnot alter endothelial cell survival(Chularojmontrietal.,2010).
Ourresultsshowedthatthecytotoxicactivityofcapsaicinon neuroblastomaB104cellswaspronouncedatconcentrationsof100 and200M.TheIC50valueswerefoundtobe61.9Mfor incuba-tiontimeperiodof6hand61.6Mfor24h,respectively,indicating thattheefficiencyunderthegivenconditionsdidnotchangewhen theincubationtimeperiodwasextendedform6hto12h. Previ-ousfindingsofcapsaicincytotoxicityhaveshowna similarIC50 valuebutit wasobtainedfor5daystreatmentofthecells with capsaicin(Richeuxetal.,1999).Lowerconcentrationsofcapsaicin (Figs.2and4)didnotshowanycytotoxicactivity.Interestingly,the extractsobtainedfromdifferentgenotypes(Figs.5and6)which containedevenhigherconcentrationofcapsaicindidnotinduce theexpectedcytotoxicity.
we could report that vitamin E and quercetin can eventually neutralizethe ROS produced bycapsaicin atthe mitochondrial membrane.
Huetal.(2008)haveshownthatTRPV1receptorisincluded incapsaicininducedCa2+influxbygenerationofreactiveoxygen species(ROS),depolarizationofthemitochondrialmembrane,and ultimatelycelldeathonthesynovialfibroblastsinrats.Huangetal. (2009)havedemonstratedthattheapoptoticprocesson hepatocel-lularcancerwasalsoaccompaniedbyincreasingoftheintracellular Ca2+level,increasedproductionofROS,anddisruptionof mito-chondrialmembranepotential.Thisapoptoticmechanismwasalso confirmedformanyothertypesofcancer.
Therefore,weassumethathighantioxidativepotentialofthese co-extractedcompoundspresentintheethanolicextractscould haveanantagonisticeffecttocapsaicincytotoxicmechanism.This hypothesis enforced us toconsider that thesynergistic antiox-idativeeffectofthecomplexcompositionofhotpepperfruitsis responsiblethatethanolicCapsicumextractshavenotshown cyto-toxicactivity,besideitshighconcentrationofcapsaicin.
Conclusions
Thisstudyexhibitedthatcapsaicincanactascytotoxicagent inneruoblastomacellsinadosedependentmanner.Knowingthat capsaicincanbeeasilyextractedandisolatedfromCapsicumfruits offersthechance for discovering a phytochemicalagent which possessesastrongpharmacologicalactivityinantitumortherapy. Incontrast,Capsicumextractsdidnotshowanyanti-proliferative activity.Therefore,additionalelectrochemicalexperimentswere performedtoexplainthesynergisticeffectsbetweencapsaicinand vitaminE,quercetinandascorbicacid,presenttogetherina com-plexmixture.Asshownintheliterature,acommonmechanismof capsaicincytotoxicityisachievedthroughproductionofreactive oxygenspeciesoncellularlevel.Thisleadstodisruptionof mito-chondrialmembranepotential,activationofcaspase-3activityand successiveapoptosis.Weassumedthatthisphenomenonof syner-gismbetweenthestudiedcompoundscouldbeapossiblereasonfor antagonisticeffectsoftheotherco-extractedphytochemicalsfrom thehotpepperfruitsonthecytotoxicityofcapsaicin.Inorderto ensureinthishypothesisfurtherexperimentsareneededtoobtain moredetailedresultsofthemechanismofcytotoxicityofcapsaicin whenitisinamixturewithotherbioactivecompoundsfoundin peppers.
Ethicaldisclosures
Protection of human subjects and animals in research.The authorsdeclarethatnoexperimentswereperformedonhumans oranimalsforthisstudy.
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirworkcenter onthe publicationof patientdata.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authorcontributions
VM(PhDstudent)contributedincollectionandidentification ofthepepperfruits,preparationoftheextracts,runningthe lab-oratory experiments in (spectrophotometry, cytotoxic analyses and voltammetry), analysesof the dataand preparation ofthe manuscript.LKGcontributedintheUVanalysesandsupervised theextractionprocedures.RGdesignedthestudyofthe
antioxida-tiveanalysesinvoltammetryandsupervisedallthevoltammetric experimentandresults.KNdesignedthestudyofcytotoxicity anal-yses and supervised allthe experimentsperformed onthecell cultures.Allcoauthorscontributedwiththeircritical readingof themanuscript.Alltheauthorshavereadthefinalmanuscriptand approveditforpublication.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
WethanktoDAADorganizationforcollaborationbetweenGoce DelcevUniversity,Stip,RepublicofMacedoniaandUniversityof Leipzig,Leipzig, Germany,throughtheprojectMatCatNet, 2013. Someexperiments were performedin theframe of studystay, whichwasfinanciallysupportedwithascholarshipbyDAAD foun-dation.
References
Asnin,L.,Park,S.W.,2015.IsolationandanalysisofbioactivecompoundsinCapsicum
peppers.Crit.Rev.FoodSci.Nutr.55,254–289.
Baek,Y.M.,Hwang,H.J.,Kim,S.W.,Hwang,H.S.,Lee,S.H.,Kim,J.A.,Yun,J.W.,2008.
Acomparativeproteomicanalysisforcapsaicin-inducedapoptosisbetween humanhepatocarcinoma(HepG2)andhumanneuroblastoma(SK-N-SH)cells. Proteomics8,4748–4767.
Barros, L.,Falcao, S., Baptista, P., Freire, C., Vilas-Boas, M.,Ferreira, I., 2008.
AntioxidantactivityofAgaricussp.mushroomsbychemical,biochemicaland electrochemicalassays.FoodChem.111,61–66.
Bode,A.M.,Dong,Z.,2011.Thetwofacesofcapsaicin.CancerRes.71,2809–2814.
Buczkowska,H.,Dyduch,J.,Agnieszcka,N.,2013. Capsaicinoidsin hotpepper
dependingonfruitmaturitystageandharvestdate.ActaSci.Pol.Hortorum Cultus.12,183–196.
Chou, C.C., Wu, Y.C., Wang, Y.F., Chou, M.J., Kuo, S.J., Chen, D.R., 2009.
Capsaicin-induced apoptosisin human breast cancerMCF-7 cells through caspase-independentpathway.Oncol.Rep.21,665–671.
Chularojmontri,L.,Suwatronnakorn,M.,Wattanapitayakul,K.S.,2010.Influenceof
Capsicumextractandcapsaicinonendothelialhealth.J.Med.Assoc.Thai.93, 92–100.
Clark,R.,Ho-Lee,S.,2016.Anticancerpropertiesofcapsaicinagainsthumancancer.
AnticancerRes.36,837–844.
Daood,H.G.,Vinkler,M.,Markus,F.,Hebshi,E.A.,Biacs,P.A.,1996.Antioxidant
vita-mincontentofspiceredpepper(paprika)asaffectedbytechnologicaland varietalfactors.FoodChem.55,365–372.
Díaz-Laviada,I.,Rodríguez-Henche,N.,2014.Thepotentialantitumoreffectsof
cap-saicin.Prog.DrugRes.68,181–208.
Dobes,J.,Zitka,O.,Sochor,J.,Ruttkay-Nedecky,B.,Babula,P.,Beklova,M.,Kynicky,
J.,Hubalek,J.,Klejdus,B.,Kizek,R.,Adam,V.,2013.Electrochemicaltoolsfor
determinationofphenoliccompoundsinplants.Areview.Int.J.Electrochem. Sci.8,4520–4542.
Hu,F.,Sun,W.W.,Zhao,X.T.,Cui,Z.J.,Yang,W.X.,2008.TRPV1mediatescelldeathin
ratsynovialfibroblaststhroughcalciumentry-dependentROSproductionand mitochondrialdepolarization.Biochem.Biophys.Res.Commun.369,989–993.
Huang,S.P.,Chen,J.C.,Wu,C.C.,Chen,C.T.,Tang,N.Y.,Ho,Y.T.,Lo,C.,Lin,J.P.,Chung,
J.G.,Lin,J.G.,2009.Capsaicin-inducedapoptosisinhumanhepatomaHepG2
cells.AnticancerRes.29,165–174.
Kim,S.J.,Won,Y.H.,Kim,J.K.,2004.Cytotoxicityofcapsaicinonculturedhumanskin
fibroblast.Exp.Dermatol.13,588.
Krinsky,N.I.,2001.Carotenoidsasantioxidants.Nutrition17,815–817.
Krinsky,N.I.,1994.Thebiologicalpropertiesofcarotenoids.PureAppl.Chem.66,
1003–1010.
Materska,.M.,Perucka,I.,2005.Antioxidantactivityofthemainphenoliccompounds
isolatedfromhotpepperfruit(CapsicumannuumL.).J.Agric.FoodChem.53, 1750–1756.
Matsufuji,H.,Nakamura,H.,Chino,M.,Takeda,M.,1998.Antioxidantactivityof
capsantinandthefattyacidestersinpaprika(Capsicumannuum).J.Agric.Food Chem.46,3468–3472.
Mosmann,T.,1983.Rapidcolorimetricassayforcellulargrowthandsurvival:
appli-cationtoproliferationandcytotoxicityassays.J.Immunol.Methods65,55–63.
Palevitch,D.,Craker,L.E.,1995.Nutritionalandmedicinalimportanceofredpepper
(Capsicumspp.).J.HerbsSpicesMed.Plants3,55–83.
Perucka,I.,Oleszek,W.,2000.Extractionanddeterminationofcapsaicinoidsinfruit
ofhotpepperCapsicumannuumL.byspectrophotometryandhigh-performance liquidchromatography.FoodChem.71,287–291.
Pohanka,M.,Hynek,D.,Kracmarova,A.,Kruseova,J.,Ruttkay-Nedecky,B.,Sochor,J.,
assayforassessmentofoxidativestresslinkedpathologiesinbraintumor suf-feredchildhoodpatients.Int.J.Electrochem.Sci.7,11978–11992.
Pramanik,K.C.,Boreddy,S.R.,Srivastava,S.K.,2011.Roleofmitochondrialelectron
transportchaincomplexesincapsaicinmediatedoxidativestressleadingto apoptosisinpancreaticcancercells.PLoSONE6,e20151.
Rafajlovska,V.,Slaveska-Raicki,R.,Koleva-Gudeva,L.,Klopceska,J.,2007.Spice
paprikaoleoresinextractionunderdifferentconditionsinvolvingacetoneand ethanol.J.FoodAgric.Environ.5,65–69.
Richeux,F.,Cascante,M.,Ennamany,R.,Saboureau,D.,Creppy,E.E.,1999.
Cytotoxic-ityandgenotoxicityofcapsaicininhumanneuroblastomacellsSHSY-5Y.Arch. Toxicol.73,403–409.
Sanchez,A.M.,Sanchez,M.G.,Malagarie-Cazenave,S.,Olea,N.,Diaz-Laviada,I.,2006.
InductionofapoptosisinprostatetumorPC-3cellsandinhibitionofxenograft prostatetumorgrowthbythevanilloidcapsaicin.Apoptosis11,89–99.
Skrzypski,M.,Sassek,M.,Abdelmessih,S.,Mergler,S.,Grötzinger,C.,Metzke,D.,
Wojciechowicz,T.,Nowak,K.W.,Strowski,M.Z.,2014.Capsaicininduces
cyto-toxicityinpancreaticneuroendocrinetumorcellsviamitochondrialaction.Cell. Signal.26,41–48.
Sukrasno,N.,Yeoman,M.M.,1993.Phenylpropanoidmetabolismduringgrowthand
developmentofCapsicumfrutescensfruits.Phytochemistry32,839–844.
ThePlanList,www.theplantlist.org(accessedFebruary,2015).
Tilahun,S.,Paramaguru,P.,Rajamani,K.,2013.Capsaicinandascorbicacidvariability
inchilliandpaprikacultivarsasrevealedbyHPLCanalysis.J.PlantBreed.Genet. 1,85–89.
Unnikrishnan,M.C.,Ramadasan,K.,1988.Cytotoxicityofextractsofspicesto
cul-turedcells.Nutr.Cancer11,251–257.
Uttara,B.,Singh,V.A.,Zamboni,P.,Mahajan,R.T.,2009.Oxidativestressand
neu-rodegenerativediseases:areviewofupstreamanddownstreamantioxidant therapeuticoptions.Curr.Neuropharmacol.7,65–74.
Won,H.C.,Hyun,J.L.,Yoon,J.C.,Joo,H.O.,Han,S.K.,Hwan,W.S.C.,2013.Capsaicin
inducesapoptosisinMG63humanosteosarcomacellsviathecaspasecascade andtheantioxidantenzymesystem.Mol.Med.Rep.8,1655–1662.
Yi-Ching,L.,Yuan-Chieh,Y.,I-Chen,W.,Fu-Chen,K.,Chi-Ming,L.,Hao-Wei,W.,
Chao-Hung,K.,Jeng-Yi,W.,Deng-Chyang,W.,2005.Capsaicin-inducedcell
deathinahumangastricadenocarcinomacellline.WorldJ.Gastroenterol.11, 6254–6257.
Zhang,R.,Humphreys,I.,Sahu,R.P.,Shi,Y.,Srivastava,S.K.,2008.Invitroand