www.jped.com.br
ORIGINAL
ARTICLE
Evaluation
of
factors
associated
with
vertical
HIV-1
transmission
夽
Matheus
Costa
da
Rosa,
Rubens
Caurio
Lobato,
Carla
Vitola
Gonc
¸alves,
Naylê
Maria
Oliveira
da
Silva,
Maria
Fernanda
Martínez
Barral,
Ana
Maria
Barral
de
Martinez,
Vanusa
Pousada
da
Hora
∗UniversidadeFederaldoRioGrande(FURG),RioGrande,RS,Brazil
Received22October2014;accepted22December2014 Availableonline28June2015
KEYWORDS
Human
immunodeficiency virustype1; Verticalinfection transmission; Pregnantwoman
Abstract
Objective: To compare the prevalenceand factorsassociated with vertical transmission of human immunodeficiency virus1(HIV---1) amongpregnant womentreatedinthe periodsof 1998---2004and2005---2011inareferenceserviceforthecareofHIV-infectedpatientsin south-ernBrazil.
Methods: Thiswasadescriptiveandanalyticalstudythatusedthedatabasesoflaboratories fromtheCD4andSTDs/AIDSViralLoadNationalLaboratoryNetworkoftheBrazilianMinistry ofHealth. HIV-1-infectedpregnantwomenwere selectedafter anactivesearch forclinical informationandobstetricandneonataldatafromtheirmedicalrecordsbetweentheyearsof 1998and2011.
Results: 102pregnantwomenwereanalyzed between1998and2004and251intheperiod between 2005 and2011, totaling 353 children born to pregnantwomen with HIV-1. Itwas observedthattheverticaltransmissionratewas11.8%between1998and2004and3.2%between 2005and2011(p<0.001).Theincreaseduseofantiretroviraldrugs(p=0.02),thedecreasein viralload(p<0.001),andtimeofmembranerupturelowerthan4h(p<0.001)wereassociated withthedecreaseofverticaltransmissionfactorswhencomparingthetwoperiods.
Conclusion: Itwasobserved adecreaseintherateofverticaltransmissioninrecentyears. Accordingtothestudiedvariables,issuggestedthattheriskfactorsforverticaltransmission ofHIV-1wereabsenceofantiretroviraltherapy,highviralloadinthepregnantwomen, and membranerupturetime>4h.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽
Pleasecitethisarticleas:daRosaMC,LobatoRC,Gonc¸alvesCV,daSilvaNM,BarralMF,deMartinezAM,etal.Evaluationoffactors associatedwithverticalHIV-1transmission.JPediatr(RioJ).2015;91:523---8.
∗Correspondingauthor.
E-mail:dahoravp@gmail.com(V.P.daHora).
http://dx.doi.org/10.1016/j.jped.2014.12.005
PALAVRAS-CHAVE
Vírusda
Imunodeficiência HumanaTipo1; TransmissãoVertical deInfecc¸ões; Grávidas
Avaliac¸ãodosfatoresassociadosàtransmissãoverticaldeHIV-1
Resumo
Objetivo: CompararaprevalênciaeosfatoresassociadosàtransmissãoverticaldeHIV-1entre grávidas tratadas nos períodos de 1998-2004 e 2005-2011 em um servic¸o de referência de cuidadodepacientescomHIVnosuldoBrasil.
Métodos: EstudodescritivoeanalíticoqueutilizouasbasesdedadosdelaboratóriosdaRede NacionaldeLaboratóriosdeCD4eCargaViraldeDSTs/AIDSdoMinistériodaSaúde.Asgrávidas comHIV-1foramselecionadasemumapesquisaativadeinformac¸õesclínicasedadosobstétricos eneonataisemseusprontuáriosmédicosentre1998-2011.
Resultados: 102grávidas foramanalisadasentre1998e 2004e 251entre2005-2011, total-izando353crianc¸asnascidasdegrávidascomHIV-1.Observou-sequeatransmissãoverticalfoi de11,8%entre1998e2004ede3,2%entre2005-2011(p<0,001).Omaiorusodemedicamentos antirretrovirais(p=0,02),areduc¸ãonacargaviral(p<0,001)eotempoderupturade mem-branasmenorde4h(p<0,001)foramassociadosàreduc¸ãonosfatoresdetransmissãovertical quandoosdoisperíodossãocomparados.
Conclusão: Observou-se uma reduc¸ão nataxa de transmissãovertical nos últimosanos. De acordocomasvariáveisestudadas,sugere-sequeosfatoresderiscodetransmissãovertical deHIV-1foramausênciadeterapiaantirretroviral,altacargaviraldasgrávidasetempode rupturamaiorde4h.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Themother-to-child transmission(MTCT) ofhuman immu-nodeficiencyvirus type1(HIV-1)canoccur inthreemajor periods:in utero,at birth, or during breastfeeding.1
HIV-1 can be transmitted in utero via transplacental cellular
transport,throughaprogressiveinfectionoftheplacenta’s
trophoblasts until the virus reaches the fetal circulation
or due to ruptures in the placental barrier followed by
microtransfusions that occur from mother to child.2 The
transmission during delivery occurs via the contact of
thefetus with infected maternal secretions while passing
throughthebirthcanal, throughascendinginfection from
vaginato fetal membranes and amniotic fluid or through
absorptionintheneonataldigestivetract.Inthepostpartum
period,themainformoftransmissionisbreastfeeding.3
TheverticaltransmissionrouteofHIV-1canbeinfluenced
byseveralfactors,suchasthedelivery mode,4 theuseof
antiretroviraltherapy,5oralinflammationsinthenewborn,6
prematurity, andhigh maternal viral load.7 Inaddition to
thesefactors,theviralgeneticdiversityappearstoplayan
importantroleinverticaltransmission.1,8
The epidemic of acquired immunodeficiency syndrome
(AIDS)isinprocessofstabilization;however,itstillpresents
highratesoftransmission,especiallyamongwomen,which
characterizesthefeminizationofthedisease.9 Therefore,
itisimportanttounderstandtheepidemiologicalprofileof
pregnantwomenandofMTCT,sincethechangesin
preva-lencedepend onfactorssuchastheuse ofantiretrovirals
and the adhesion to prenatal care. These and other
fac-torsmayleadtoa reductioninMTCT,therebyfacilitating
theadoptionofmoreeffectivepreventivemeasures.9,10 In
Brazil, from1980 to June of 2013, it was estimated that
718,230 peoplewere living with HIV/AIDS.11 According to
the Brazilian epidemiological bulletinof 2013,considered
information from 2010, the prevalence of HIV infection
in pregnantwomenwas0.38%.11 Vertical transmissionhas
becomeamajorchallengetopublichealth,as
epidemiolog-icaldatashow that80% ofHIV casesin children under13
yearshadMTCTastheformoftransmission.11
Due to the increasing number of infected pregnant
women, actions such as the development of
governmen-tal programsandthemonitoring ofpregnantwomenhave
been implemented since 2000 in Brazil; healthcare staff
havetheobligationtoreportcasesofinfectedwomenand
exposedchildren.11AccordingtodatafromtheNational
Dis-easeNotificationSystem(SistemadeInformac¸ãodeAgravos
deNotificac¸ão [SINAN]),11 77,066cases ofHIV inpregnant
womenwerereportedfrom2000to2013;TheSouthregion
ofBrazilisinsecondplacewith31.3%ofcases,behindonly
the Southeast region(41.7%), and followedby the
North-east(14.9%),North(6.3%),andMidwestregions(5.7%).The
detectionrateofAIDSinchildrenunder5years(the
indica-torusedinBraziltomonitortheverticaltransmissionofHIV)
between2012and2003presentedareductionof35.8%.11
This study aimed to compare the prevalence and
fac-tors associated with vertical transmission of HIV-1 among
women treatedfrom1998 to2004 andfrom2005 to2011
inareferenceserviceforthecareofHIV-infectedpatients
inSouthernBrazil,locatedattheUniversityHospitalofthe
FederalUniversityofRioGrande(HU-FURG),inthecity of
RioGrande,RS,Brazil.
Methods
1998---2004and251intheperiod2005---2011,withatotalof 353births.
Despite the fact that Brazilian governmental programs and monitoring of pregnant women were implementedin 2000,thecareforHIVpatientsinHU-FURGbeganin1994, withtesting andsubsequent observation of the high inci-denceofcasesintheregion.Suchattentionwasruledfrom normative of the Brazilian Ministry of Health and subse-quentlyeverycareprotocolsmetassuchrecommendations. Due tocertainchanges, such asthe higherprevalence of viralsubtypeC andthedifferenceof thetherapeutic and pharmacologicalmodelofthepatientwithHIVreferenced indifferentanalyzedperiods,itwasdecidedtostratifythe datasothatanalysiswouldbefeasible.Moreover,itcould reportthe effectiveness of care modelsrecommendedby BrazilianMinistryofHealth.8,12
Since 1998, all pregnant women attended to at
HU-FURGweresubjectedtoHIV/AIDStestsasrecommendedin
theguidelinesbytheBrazilianMinistryofHealth.Pregnant
womenwhopresentedtwopositiveserologictestsandone
confirmationtest,ortwoconsecutivestestswithdetectable
viral load, were classified as HIV-infected. The mothers
signedaninformedconsenttoparticipateinthisresearch,
which wasapproved by theresearch ethicscommittee of
theinstitution(ProtocolNo.23116001368/2003-44).
ThestudyoutcomewasMTCTofHIV-1innewborns,and
thestudied variableswere:useof highly-active
antiretro-viraltherapy (HAART)--- Biovir® (lamivudina+ zidovudina,
GlaxoSmithKline Brasil,RJ, Brazil)+ Kaletra® (Lopinavir e
Ritonavir,Abbot,USA)duringpregnancy,CD4+Tcellscount
inthelastthreemonthsofpregnancy,pregnantwomenviral
load, delivery mode, membrane rupture time,and
birth-weight(kg).Theuseofantiretroviraltherapywasclassified
as:a)complete,---whenthemotherreceivedantiretrovirals
duringpregnancyandinthemomentofdeliveryaswellas
thenewborn;andb)incomplete,whenatleastoneofthe
threeprocedureswereconductedorwhenthemotherdid
notuseantiretroviraltherapy.Socio-demographicvariables
werenotstandardizedbetweentheseperiods.Therefore,it
wasnotpossibletodescribethedemographicprofileofthe
populationinthisstudy.
DatawasanalyzedusingStataversion8.0(StataCorp
---CollegeStation,TX, USA).Ananalyticaldescriptive
analy-sisofnumericalvariableswasperformed accordingtothe
studiedperiods,whichwerepresentedbytheirfrequencies,
meanvalues,standard deviation,andasignificantp-value
of0.05inatwo-tailedtest.
Results
This study assessed 353 children born fromHIV-1 positive pregnantwomen,attendedtoatHU-FURG.
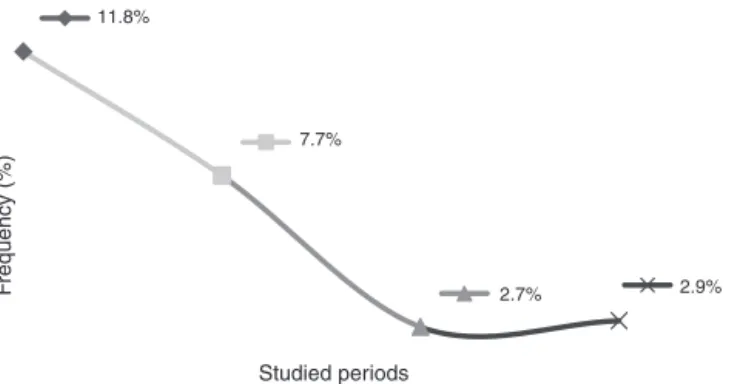
This study showed that the rates of mother-to-child transmissionobtainedbetween1998and2004andbetween 2005 and 2011 decreased significantly (p<0.001), from 11.8%to3.2%,respectively.Itidinterestingtoobservethe resultsindifferentperiods:thetransmissionratefrom1998 to2000 was11.8%;from2001to2004,theratewas7.7%; from2005to2008,2.7%;andfrom2009to2011the trans-missionratewas2.9%(Fig.1).
11.8%
7.7%
2.7% 2.9%
Studied periods
F
requency (%)
Figure1 Comparisonofthemother-to-childHIV-1 transmis-sionratesfrom1998to2011inareferenceservice.
For the analyzed variables in both studied periods (Table 1),it wasobservedthat between theperiods from
1998to2004,79.4%ofthepregnantwomenhadamembrane
rupture time longer than 4h. In contrast, for the period
between2005and2011,only10.8%ofpregnantwomenhad
amembranerupturetimelongerthan4h(p<0.001).
Therewasnosignificantdifferenceinthemodeof
deliv-ery between the twostudied periods, nor in the average
birthweight.The use of antiretroviraltherapy throughout
thegestationalperiodwasobservedin69.7%ofthepregnant
women.Between1998and2004,60.7%ofpregnantwomen
adheredtoHAARTand,betweentheyears2005and2011,
theadhesionratewas73.3%(p<0.02),suggestingthat
adhe-siontotheantiretroviraltherapybypregnantwomenisan
importantfactor in the reductionof MTCT.It was
consid-eredcompleteuseofantiretroviraltherapywhenmothers
reportedhavingusedthedrugduringtheantenatalperiod,
atdeliveryandhernewbornhavereceivedprophylaxiswith
oralsuspensionofzidovudine(AZT)forsixweeksafter
deliv-ery.TheuseofinjectableAZTandoralAZTwascheckedwith
thedrugdispensingcontrolspreadsheetsduring
hospitaliza-tions,accordingtotheprotocolestablishedbytheBrazilian
Ministryof Health. Since1998, PMTCTattention was
per-formedfollowingtherecommendationtoAZTmonotherapy.
In2001,tripletherapywasrecommended,insertingBiovir®
and Nelfinavir. In 2007, following a recommendation of
theBrazilian Ministryof Health,Kaletra® wasintroduced,
replacingNelfinavir.
Therewas anincrease in theCD4+ Tcells count(CD4+
Tcells>500)whencomparingbothstudiedperiods.Inthe
periodfrom1998to2004, 28.4% ofpregnant women
pre-sentedCD4+Tcellscounthigherthan500.Intheperiodfrom
2005to2011,thepercentageofpregnantwomenwithCD4+
Tcellscounthigherthan500increasedto57.3%(p<0.001).
Whenanalyzingthematernalviralload,itwasobserved
that the percentage of pregnant women with viral load
betweenundetectableandlogof2.9was68.0%intheperiod
from2005to2011and28.4%intheperiodfrom1998to2004
(p<0.001).
Discussion
Brazil aims to eliminate HIV-1 vertical transmission (less than1% of transmission)until2015.11 Studies have shown
Table1 Comparisonoffactorsassociated withmother-to-childHIV-1transmissionbetweentheperiods1998and2004and 2005---2011inareferenceservice.
Membranerupturetime p-value
Period n >4h % <4h % <0.001
1998---2004 102 81 79.4 21 20.6
2005---2011 251 27 10.8 224 89.2
Total 353 108 30.6 245 69.4
Deliverymode 0.67
n Cesarean % Normal %
1998---2004 102 39 38.2 63 61.7
2005---2011 251 90 35.8 161 64.8
Total 353 129 36.5 224 63.5
Mother-to-childtransmission <0.001
n HIV+ % HIV− %
1998---2004 102 12 11.8 90 88.2
2005---2011 251 8 3.2 243 96.8
Total 353 20 5.7 333 94.3
Antiretroviraltherapyduringpregnancy 0.02
n Incomplete % Complete %
1998---2004 102 40 39.3 62 60.7
2005---2011 251 67 26.7 184 73.3
Total 353 107 30.3 246 69.7
TCD4+cellcount
n cell0---199 % cell200---499 % Cell>500 %
1998---2004 95 18 18.9 50 52.6 27 28.4
2005---2011 251 13 5.2 94 37.5 144 57.3
p <0.001 <0.001 1.00
Maternalviralload---Log10
n 0---2.99 % 3.0---3.99 % 4.0---4.99 % ≥5 %
1998---2004 95 27 28.4 23 24.2 35 36.8 10 10.5
2005---2011 251 173 68.9 49 19.5 26 10.3 3 1.2
p <0.001 0.37 <0.001 <0.001
Newborngender 0.68
n Male % Female %
1998---2004 102 54 52.9 48 47.1
2005---2011 251 127 50.5 124 49.5
Total 353 181 51.3 172 48.7
whenHIV-positivepregnantwomenhavetimelyand appro-priateaccesstoprenatalcareandtoHAART.13Inthepresent
study,a5.7%rateofverticaltransmissionwasobservedfrom
atotalof353childrenborn fromseropositive mothersfor
HIV-1betweentheyears1998and2011.However,itis
inter-estingtoanalyzetherateofMCTC indifferentperiods.In
thisstudy,between1998and2000,theMTCTratewas11.8%;
from2001to2004,7.7%;from2005to2008,2.7%;andfrom
2009to2011,2.9%.ThisdemonstratesthattheMTCTrates
arerelativelylowerwhenanalyzedatdifferenttimes.There
wasasmallincreaseinMTCTratesbetweentheyears2005
and2008(2.7%)and2009---2011(2.9%),whichmaybe
justi-fiedduetothefactthataportionofHIV-positivepregnant
women still donot make use of chemoprophylaxis during
thepregnancy,especiallydrugusersandcurrentlytheuse
help identifyless-than-optimal adherence totreatment.14
GiventhestabilizationofMTCTrates observednotonlyin
this study,butthroughout in Brazil, theBrazilian Ministry
ofHealthimplementedin2012theuseofnevirapine(NVP;
TechnicalNoten.388/2012).InBrazil,theuseofAZT
asso-ciatedwithNPVhasbeenadvocatedfor thepreventionof
MTCT,sincearecentlypublishedstudy demonstratedthat
the oral treatment with a solution containingAZT during
sixmonthsassociatedtoanoralsuspensionwithNPV(three
dosesinthefirstweekoflife)significantlyreducestherate
ofMTCTfrompregnantwomenwhodidnotuse
chemopro-phylaxisduringpregnancy.11
Takingintoaccounttheaimofthisstudy,observingthe
ratesobtainedbetween1998and2004and2005---2011,a
sig-nificantdropinthetransmissionrates(from11.8%to3.2%,
respectively)wasobserved,clearlydemonstratinga
reduc-tion in MTCT rates. Comparing these results withstudies
conductedinthesameBrazilianregion,astudiedpublished
in 2006 observed a MTCT rate of 11.8% in infants born
between1998and2003.12Inanotherstudypublishedin2010
fromthe sameregion,a MTCT rateof 4.8%wasobserved
betweentheyears2003and2007.8Inthepresentstudy,the
MTCTratewasonly2.9%whenconsideringonlytheperiod
from2007to2011.Theseresultsclearlydemonstrate that
therehasbeenadecreaseintheMTCTratesofHIV-1,which
highlights theeffectiveness of the national policyfor the
controlofMTCT.
With the approval of Law No. 9313, onNovember 13,
1996,Brazil begantorelyin itslegal systemwitha
legis-lationthatensurestheaccesstoantiretroviralmedication
for individuals living with HIV/AIDS. Thus, Brazil became
the first emerging country to provide antiretroviral
ther-apy.In2009,theSecretaryofSubstituteHealthSurveillance
started touse fast HIV tests in pregnant women,
accord-ing to the authority conferred by the Article 45 of the
Decree No. 6860 of May 27, 20099,11 Therefore, it can
be suggested that these control measures had an
influ-ence in the decline of the HIV infection rates in infants
betweenthestudiedperiods,demonstratingtheimportance
of these control measures in public healthcare services.
Similardecreases in MTCT rates wereobserved in several
countriesthatadoptedcontrolmeasures,especiallytheuse
ofantiretroviraltherapybyHIV-positivepregnantwomen.15
Inthe present study,it wasobservedthat 69.7%of
moth-ers made use of antiretroviralmedication throughout the
gestationalperiod,suggestingadeclineinthevertical
trans-missionrate.Inastudypublishedin2011,itwasobserved
that, from 25 seropositive children, 9% were born from
mothers who received inadequate antiretroviral therapy
duringpregnancy,afactthatoccasionedarateofonly1.7%
ofverticaltransmission.16
Whenanalyzingthedifferentperiodsofthisstudy,itwas
observedthat,between1998and2004,60.7%ofpregnant
women adhered to antiretroviral therapy and, between
2005 and 2011, there was an increase in this adherence:
73.3%. This suggests a low viral load in pregnant women
andadecreasein verticaltransmissionratesbetween the
periodsfrom2005to2011,only3.2%.Theseresults
corrob-oratethosefromapreviousstudy,whichdemonstratedthat
the main risk factors for HIV transmissionwere failureof
antiretroviraltherapy,latematernaldiagnosis and,
conse-quently,highviralloadofpregnantwomenatdelivery.8The
useofantiretroviraltherapyduringpregnancyisextremely
importantinordertopreventverticaltransmission;itcanbe
usedduringanyperiod,regardlessoftheclinicalcondition
ofthemother.17Studieshavereportedthatahighviralload
andalowCD4+Tcellscountduringpregnancyaresignificant
factorsinMTCT.8,18
It is noteworthy that a membrane rupture timelower
than 4h is extremely important to reduce MTCT.19 In the
present study, observed a significant decrease (p<0.001)
in rupture time was observed when analyzing the
stud-iedperiods,since79.4%ofpregnantwomenhadarupture
timehigher than 4h in the period from1998 to 2004. In
contrast,from2005to2011,thisratewas10.8%.The
signif-icantdifferencebetween membranerupture timein both
studiedperiods is the resultof theupdate of care
proto-colsforHIV-infectedpregnantwomen.In2004,HIVtesting
duringprenatalcareandproperimplementationof
preven-tionactionsofverticaltransmissionofHIV wereinitiated,
andthefirstprotocolwaspublishedin2007.According to
theBrazilian guidelinefor prophylaxis of HIVtransmission
andantiretroviraltherapy inpregnant women, theactive
management of labor should occur to prevent prolonged
membrane rupture time, since a reduced time decreases
theriskofverticaltransmission.9
Despiteof theeffort toreduce MTCT,the residualrisk
ofthattransmissionisstillrelativelyhighincomparisonof
whatitisobservedincountrieswhoadoptHAART.20Thefact
that SouthernBrazil is characterized by having a highere
prevalenceofHIV-1subtypeC,whichismoretransmissible
inutero,8mayexplaintherateofMTCTfoundinthepresent
study.Additionally,lateentranceandlackofadherenceto
prenatalcare,especiallyindrugusers,favorMTCT.Astudy
demonstratedthatlowprenatalscreeningofmaternal HIV
infection,impairingmaternaltreatmentorprophylaxis,and
theincorrectuse ofthe rapidscreening test atadmission
fordeliveryareimpedimentstotheeffectivereductionof
MTCT.21 Aimingto increase the care for pregnant women
withlowadherencetoprenatal,especiallythosedrugusers,
thereferralservice ofHU-FURGconducts activesearchof
womeninfavoroftheeffectivenessofcompliancewithcare
protocolstoprenatalcareasrecommendedbytheBrazilian
MinistryofHealth.
Therefore,theresultsofthepresentstudysuggestthat
theincreaseofantiretroviraltherapyduringpregnancy,time
for membrane rupture lower than 4h, and low viralload
contributedtothe decline MTCT in both studied periods.
Theseresultsareinagreementwiththedataobtainedinthe
literature.2,7,22 However,morestudiesshouldbeconducted
toestablishwhichfactorsareinvolvedinMTCT.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
References
1.RenjifoB,GilbertP,ChaplinB,MsamangaG,MwakagileD,Fawzi W,etal.Preferentialin-uterotransmissionofHIV-1subtypeC ascomparedtoHIV-1subtypeAorD.AIDS.2004;18:1629---36.
2.NewellML.Mechanismsandtimingofmother-to-child transmis-sionofHIV-1.AIDS.1998;12:831---7.
3.Neilson JR, John GC, Carr JK, Lewis P, Kreiss JK, Jackson S, et al. Subtypes of human immunodeficiency virus type 1 and diseasestage among women in Nairobi,Kenya. J Virol. 1999;73:4393---403.
4.Jamieson DJ, Read JS, Kourtis AP, Durant TM, Lampe MA, Dominguez KL. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197:S96---100.
5.ZijenahLS,MoultonLH,IliffP,NathooK,MunjomaMW,Mutasa K,et al.Timingofmother-to-childtransmissionofHIV-1and infantmortalityinthefirst 6months oflifeinHarare, Zim-babwe.AIDS.2004;18:273---80.
6.EmbreeJE,Njenga S,DattaP,NagelkerkeNJ,Ndinya-Achola JO,MohammedZ,etal.Riskfactorsforpostnatalmother---child transmissionofHIV-1.AIDS.2000;14:2535---41.
7.JourdainG,MaryJY,CoeurSL,Ngo-Giang-HuongN,Yuthavisuthi P,LimtrakulA,etal.Riskfactorsforinuteroorintrapartum mother-to-childtransmissionofhumanimmunodeficiencyvirus type1inThailand.JInfectDis.2007;196:1629---36.
8.Tornatore M, Gonc¸alves CV, Mendoza-Sassi RA, Silveira JM, D’ávilaNE,MaasCG,etal.HIV-1verticaltransmission inRio Grande,SouthernBrazil.IntJSTDAIDS.2010;21:351---5.
9.Brasil.MinistériodaSaúde.SecretariadeVigilânciaemSaúde. ProgramaNacionaldeDSTeAids.Recomendac¸õespara profi-laxiadatransmissãoverticaldoHIVeterapiaantirretroviralem gestantes.Brasília:MinistériodaSaúde;2010(SérieManuais,n. 46).
10.Barral MF, de Oliveira GR, Lobato RC, Mendoza-Sassi RA, Martínez AM, Gonc¸alves CV. Risk factors of HIV-1 vertical transmission (VT)and theinfluence ofantiretroviraltherapy (ART) in pregnancy outcome. Rev Inst Med Trop Sao Paulo. 2014;56:133---8.
11.Brasil.MinistériodaSaúde.BoletimEpidemiológico---AidseDST, AnoII---n◦1---atésemanaepidemiológica.26a---Dezembrode 2013.
12.MartínezAM,Hora VP,SantosAL,Mendoza-SassiR, VonGroll A, Soares EA, et al. Determinants of HIV-1 mother-to-child
transmissioninSouthernBrazil.AnAcadBrasCienc.2006;78: 113---21.
13.UNAIDS. The United Nations Joint Programme on HIV/AIDS. [citedJan2013].Availablefrom:http://www.unaids.org
14.CruzML,CardosoCA,DarmontMQ,SouzaE,AndradeSD,D’Al FabbroMM,etal.Viralsuppressionandadherenceamong HIV-infectedchildren and adolescents onantiretroviral therapy: resultsofamulticenterstudy.JPediatr(RioJ).2014;90:563---71.
15.Plipat T, Naiwatanakul T, Rattanasuporn N, Sangwanloy O, Amornwichet P, Teeraratkul A, et al. Reduction in mother-to-child transmission of HIV in Thailand, 2001-2003: results from population-based surveillance in six provinces. AIDS. 2007;21:145---51.
16.EuropeanCollaborativeStudyinEuroCoordBaileyH,Townsend C,Cortina-BorjaM,ThorneC.Insufficientantiretroviral ther-apy in pregnancy: missed opportunities for prevention of mother-to-child transmission of HIV in Europe. AntivirTher. 2011;16:895---903.
17.BecquetR,EkoueviDK,Arrive E,StringerJS,MedaN,Chaix ML, et al. Universal antiretroviral therapy for pregnant and breast-feedingHIV-1-infectedwomen:towardstheelimination of mother-to-child transmission of HIV-1 in resource-limited settings.ClinInfectDis.2009;49:1936---45.
18.Tubiana R, LeChenadec J, Rouzioux C,Mandelbrot L, Ham-reneK,DollfusC,etal.Factorsassociatedwithmother-to-child transmission of HIV-1 despite a maternal viral load<500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1). Clin Infect Dis. 2010;50:585---96.
19.NishimotoTM,ElufNetoJ,RozmanMA.Mother-to-child trans-missionofhumanimmunodeficiencyvirus(HIV-I):evaluationof controlmeasures inthe cityofSantos. RevAssoc MedBras. 2005;51:54---60.
20.MofensonLM.Preventioninneglectedsubpopulations: preven-tionofmother-to-childtransmissionofHIVinfection.ClinInfect Dis.2010;50:S130---48.
21.Fernandes RC, Ribas GF, Pires e Silva D, Gomes AM, Medina-AcostaE.Persistentoperationalchallengesleadto non-reductioninmaternal-infanttransmissionofHIV.JPediatr(Rio J).2010;86:503---8.