Contents lists available atScienceDirect
Infection, Genetics and Evolution
journal homepage:www.elsevier.com/locate/meegid
Research paper
Genetic diversity of
Giardia duodenalis
circulating in three Brazilian biomes
Beatriz Coronato Nunes
a, Deiviane Aparecida Calegar
a, Márcio Galvão Pavan
a,b,
Lauren Hubert Jaeger
a, Kerla Joeline Lima Monteiro
a, Elis Regina Chaves dos Reis
a,
Marli Maria Lima
c, Márcio Neves Bóia
d, Filipe Anibal Carvalho-Costa
a,e,⁎aLaboratório de Epidemiologia e Sistemática Molecular, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz. Av. Brasil, 4365 - Manguinhos, Rio de Janeiro, RJ 21040-900,
Brazil
bLaboratório de Mosquitos Transmissores de Hematozoários, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz. Av. Brasil, 4365 - Manguinhos, Rio de Janeiro, RJ
21040-900, Brazil
cLaboratório de Ecoepidemiologia da Doença de Chagas, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz. Av. Brasil, 4365 - Manguinhos, Rio de Janeiro, RJ 21040-900,
Brazil
dLaboratório de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz. Av. Brasil, 4365 - Manguinhos, Rio de
Janeiro, RJ 21040-900, Brazil
eEscritório Regional Fiocruz Piauí, Fundação Oswaldo Cruz. Rua Magalhães Filho, 519
–Centro (Norte), Teresina, PI, 64000-128, Brazil
A R T I C L E I N F O
Keywords: Giardia duodenalis
Triosephosphate isomerase Glutamate dehydrogenase
β-Giardin Genotypes Brazil
A B S T R A C T
Giardia duodenalishas a wide genetic variety, and its characterization helps in the understanding of its trans-mission dynamics and in the development control strategies. This study aimed to assess the genetic diversity of
G. duodenalisobtained in different Brazilian biomes and estimate their phylogenetic relationships. Three surveys including 944 participants were carried out in the municipalities of Russas (RSS,Caatingasemiarid biome), Santa Isabel do Rio Negro (SIRN, Amazon rainforest biome) and Nossa Senhora de Nazaré (NSN,Cerrado-Caatinga
transition biome).G. duodenalis-positive fecal samples were submitted to amplification of gene fragments en-codingβ-giardin (βG, N = 71), glutamate dehydrogenase (GDH, N = 42), and triosephosphate isomerase (TPI, N = 27). Overall detection rates of assemblage A inG. duodenalis-positive samples throughβG, GDH and TPI were 22/71 (31%), 13/42 (31%), and 13/27 (48.1%), respectively. Concerning assemblage B, rates with distinct genetic markers were 49/71 (69%), 29/42 (69%), and 14/27 (51.9%), respectively. In the Amazon, assemblage B was more prevalent (77.8%, 71.8% and 65% throughβG, GDH and TPI, respectively), while in the Cerrado biome assemblage A predominated (50%, 66.6%, and 85.7%, throughβG, GDH and TPI, respectively). In Caatinga biome assemblage A also predominated (71.4%, throughβG). Thirty new sub-assemblages are de-scribed for assemblage B (24βG and six TPI), as well as three new sub-assemblages are described for assemblage A (one GDH and 2 TPI). Higher genetic diversity of assemblage B in the Amazon may be related to demographic concentration leading to a more complex transmission network within a poorer sanitation background. The high genetic divergence between assemblages A and B (5.5–6.3%) support the proposal of taxon separation in distinct
species.
1. Introduction
Intestinal parasitism has clear social and environmental determi-nants, with higher prevalence rates in areas with poor sanitation, lim-ited access to clean water and poor living conditions (Carvalho-Costa et al., 2007a, 2007b;Brown et al., 2015). Among the protozoan para-sites, Giardia duodenalis is present in distinct socio-environmental conditions (Genser et al., 2015;Einarsson et al., 2016). In some coun-tries, reporting of giardiasis is mandatory, as in the United States,
where in 2012 approximately 15,000 cases were diagnosed (Painter et al., 2015). Prevalence rates present great variation, mainly between developed and developing countries (Feng and Xiao, 2011), e.g. 1.3% in the UK and 40.7% in Uganda (Davies et al., 2009; Johnston et al., 2010). In Brazil, studies reveal infection rates ranging 8–12% in the
northern region, 4–22% in the southeast, and 24% in the south of the
country (Valverde et al., 2011;Bóia et al., 2006;Carvalho-Costa et al., 2007a, 2007b;Nunes, 2011;Oliveira-Arbex et al., 2016;Basso et al., 2008).
https://doi.org/10.1016/j.meegid.2018.02.001
Received 10 July 2017; Received in revised form 10 January 2018; Accepted 1 February 2018
⁎Corresponding author at: Laboratório de Epidemiologia e Sistemática Molecular, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz. Av. Brasil, 4365 - Manguinhos, Rio de Janeiro, RJ 21040-900, Brasil.
E-mail address:guaratiba@ioc.fiocruz.br(F.A. Carvalho-Costa).
Available online 02 February 2018
1567-1348/ © 2018 Published by Elsevier B.V.
G. duodenaliscysts are transmitted both directly and indirectly by the fecal-oral route. Water used for recreation or consumption is par-ticularly important in the transmission, as it may be contaminated with cysts excreted by humans and animals (Efstratiou et al., 2017;Kumar et al., 2016;Adam et al., 2016).
G. duodenalisexhibits great genetic diversity with isolates belonging to eight major genetic groups (assemblages A to H) (Heyworth, 2016). Different loci have been used for genotyping this species, including gene fragments encoding β-giardin (βG), glutamate dehydrogenase (GDH), triosephosphate isomerase (TPI), elongation factor 1-αand the small subunit rRNA.G. duodenalissub-assemblages are clusters in which isolates with minor differences are grouped within the assemblages. The descriptions of sub-assemblages are based in new differences be-tween isolates, being described until now: A-I, which encompasses isolates found in humans and other animals, and A-II, which consists only of human isolates. Assemblage B isolates are mainly found in humans (Thompson et al., 2000) and present much greater genetic variation, thus their categorization in sub-assemblages is difficult.
The Northern and Northeastern regions of Brazil present the worst sanitation and highest proportion of people living in poverty (Hummell et al., 2016;Paim et al., 2011). In these areas, extremely diverse en-vironmental and climatic conditions are observed. In the Northeast region, the rainfall levels are very low, constituting theCaatingabiome, which is characteristically semiarid. In the North, the humid equatorial climate of the Amazon rainforest biome, predominates high rainfall. There is an area of transition in the mid-north, dominated by the Cer-rado-Caatingabiome, with intermediate rainfall (Coutinho, 2006).
There has been no study conducted in Brazil to assess the genetic diversity and phylogenetic relationships ofG. duodenalisfrom different Brazilian biomes. Since the infection is endemic in both the north and the northeast of Brazil, we hypothesized that genotype distribution and intraspecific genetic diversity ofG. duodenalis would be different in these distinct demographic, climatic and socio-environmental sce-narios, in which the potential for zoonotic transmission may also be diverse.
2. Materials and methods
2.1. Identification of Giardia duodenalis-positive fecal samples
G. duodenalis-positive fecal samples were identified in three Brazilian biomes: I)Caatinga, represented by the municipality of Russas (RSS) in the State of Ceará; II) Amazon, represented by Santa Isabel do Rio Negro (SIRN) in the State of Amazonas; III) Cerrado-Caatinga transition biome, represented by Nossa Senhora de Nazaré (NSN), State of Piauí (Fig. 1). General abiotic conditions of each site,G. duodenalis prevalence and main population characteristics in study areas are presented inTable 1.G. duodenalis-positive fecal samples were identi-fied through parasitological methods (Ritchie's modiidenti-fied ethyl acetate centrifugation technique) in cross-sectional surveys performed pre-viously in order to assess prevalence and associated factors of giardiasis in the studied localities. Approximately 2 g of theG. duodenalis-positive fecal samples were cryopreserved and transported to the Laboratory of Epidemiology and Molecular Systematics/Fiocruz in Rio de Janeiro (for additional data on epidemiology ofG. duodenalisin the studied areas accessCoronato-Nunes et al. (2016)andCoronato-Nunes et al. (2017).
2.2. Molecular and computational analyses
DNA extraction was performed in G. duodenalis-positive samples using ZR Fungal/Bacterial DNA kit (ZymoResearch, Irvine-USA). Three genetic targets were used to conventional PCR theβ-giardin gene (βG); and nested PCR the glutamate dehydrogenase (GDH) and triosepho-sphate isomerase (TPI) genes. PCR conditions and primers as described inCacciò et al. (2002),Read et al. (2004), andSulaiman et al. (2003), respectively, with modifications to primer optimization.
Reconstructive DNA polymerization was performed on samples with successive amplification failures, accordingGolenberg et al. (1996).
PCR products were purified with Illustra-GFX kit (GE Healthcare, Pittsburgh, PA-USA) and sequencing with ABI-BigDye Terminator kit (Applied Biosystems, Foster City, CA-USA) in an ABI 3730 automated DNA sequencer (Applied Biosystems). Samples presents overlapping peaks were cloned using pGEM1T-Easy (Promega, Madison, WI-USA).
Bioedit-7.1 (Hall, 1999) and Mega-6.0 (Tamura et al., 2013) were used to edit and align sequences. All sequences obtained were deposited in GenBank (accession numbers:βG: KY612242-49; GDH: KY612230-41; and TPI: KY612211-29). TheβG sequences of the Amazon biome were published in Coronato-Nunes et al. (2016), accession numbers KU504701-38,
Phylogenetic trees were inferred using BEAST-1.8 (Bayesian) and PhyML-3.0 (Maximum-Likelihood, or ML). The best-fit evolutionary model for the dataset were Tamura-Nei with four gamma categories, elected with Akaike and Bayesian Information Criteria in jMODELT-EST-2 (Darriba et al., 2012). Twenty-eight orthologous sequences re-presenting the diversity ofG. duodenaliswere retrieved from GenBank and added to the analyses.
3. Results
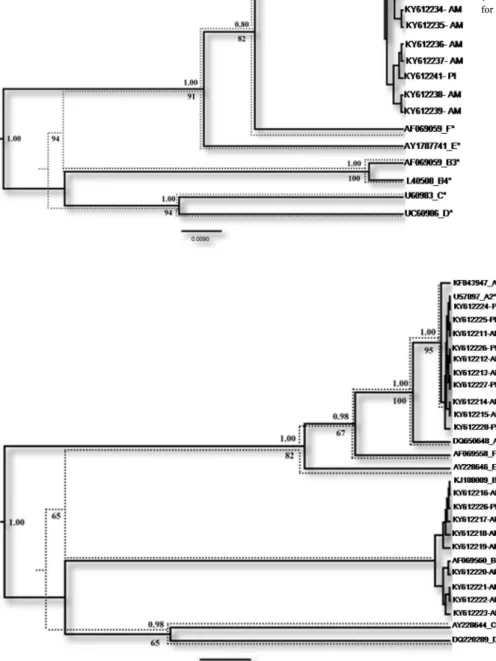
As presented inTable 2, overall detection rates of assemblage A in G. duodenalis-positive samples throughβG, GDH and TPI were 22/71 (31.0%), 13/42 (31.0%), and 13/27 (48.1%), respectively. Concerning assemblage B, rates with distinct genetic markers were 49/71 (69.0%), 29/42 (69.0%), and 14/27 (51.9%), respectively. In Amazon biome, assemblage B was more frequent (77.8%, 71.8% and 65.0% through βG, GDH and TPI, respectively), while in the Cerrado biome assemblage A predominated (50.0%, 66.6%, and 85.7%, throughβG, GDH and TPI, respectively). In Caatinga biome assemblage A also predominated (71.4%, throughβG). Phylogenetic consensus trees were reconstructed for each target gene and the isolates clustered into well-supportedG. duodenalisclades (Figs. 2,3and4).
Sub-assemblage distribution is presented in Table 2 and Fig. 1. ConsideringβG genetic target, Amazon biome presents II (n = 2), A-III (n = 10), B-A-III (n = 3), 23 previously undescribed assemblage B sequences and 16 sequences could not be defined (poor quality se-quences); while Caatinga presented A-II (N = 3), two previously un-described assemblage A sequences, one previously unun-described assem-blage B sequence and one undefined; and Cerrado A-II (N = 3), two undefined A and five undefined B. Considering GDH genetic target, Cerrado and Amazon biomes demonstrated two and nine sub-assem-blage A-II, respectively. Regarding TPI genetic target, Cerrado and Amazon biomes present four andfive sub-assemblage A-II and one and two sub-assemblage B-III, respectively (Table 2). Interestingly, 30 new sub-assemblages are described for assemblage B (24 toβG and six to TPI), as well as, three new sub-assemblages are described for assem-blage A (one to GDH and 2 to TPI). Most of the new sub-assemassem-blages are from samples from the Amazon biome.
Genotyping by the three target genes was possible in 20 samples. It was observed that most samples in which the assemblage was char-acterized with more than one gene, there was concordance among the different targets, with the exception of two samples, residents of the same household, which presented characterization in assemblage B withβG and A-II with TPI, suggesting mixed infections.
4. Discussion
assemblage B when compared to assemblage A. Predominance of as-semblage B over asas-semblage A has been described by other authors in Europe, Brazil and Thailand (Wielinga and Thompson, 2007; Sprong et al., 2009;Kosuwin et al., 2010;Durigan et al., 2014).
Outside Amazon, in the driest regions of the Northeast Brazil, as-semblage A was more prevalent than asas-semblage B, both in Ceará and Piauí states, even considering the failure to sequence GDH and TPI genes in RSS, situated in the Caatinga biome. Sub-assemblages dis-tributions demonstrated that A-III was detected only in Amazon. In the Cerrado and Caatinga biome only A-II was found. In some samples, it was not possible to define sub-assemblages as these had overlapping peaks, suggesting mixed infections (Messing, 1993). Mixed infections by assemblages A and B were observed in two residents of the same house, where each assemblage was identified by a different target gene. This phenomenon has also been confirmed byCaccio and Ryan (2008). Mixed infections are frequently reported in the literature, but their prevalence is higher in developing countries (Gelanew et al., 2007; Cooper et al., 2010;Ryan and Caccio, 2013).
It should be taken into account, as a limitation of the study, that the three biomes were not evaluated simultaneously, nor in the same season. However, the climatic characteristics of the regions studied are stable throughout the year, in order to allow comparisons between
them. Another important aspect to be emphasized is the urban nature of the population sampled in the Amazon region, in contrast to the rural characteristics of the populations studied in the states of Piauí and Ceará. In this context, a higher population density associated with higher precipitation rates may favor environmental contamination and increase transmissibility, which may be associated to the greater ge-netic diversity observed in this region, where the highest prevalence of giardiasis was observed.
Few assemblage B samples were characterized as previously de-scribed sub-assemblages, mostly due to failed identity matching with nucleotide sequence data repositories. One sample from northeast (Caatinga) was identical to an Amazonian isolate. All other haplotypes were different, showing the high genetic variation of the assemblage B. Assemblages A and B were grouped into well-defined clades. Samples characterized as assemblage A through GDH and TPI markers maintained the phylogenetic characteristics observed with βG gene. The high genetic divergence between assemblages A and B (5.5–6.3%)
support the proposal of taxon separation in distinct species,G. duode-nalisandG. enterica, as previously proposed (Monis et al., 2009). The greater genetic variation ofG. duodenalisassemblage B suggests that it is potentially associated with zoonotic transmission, where multiple hosts can maintain environmental contamination, mainly in the
Fig. 1.Map of the study areas in Brazil. Santa Isabel do Rio Negro (Amazonas) in the Amazon biome, Russas (Ceará) in the Caatinga biome and Nossa Senhora de Nazaré (Piauí) in the Cerrado-Caatinga transition biome. The assemblages obtained by the sequencing of theβ-giardin gene are shown in the graphs. Map were created using DIVA-GIS program v. 7.5 (http:// www.diva-gis.org).
Table 1
Characteristics of the three studied localities.
Characteristic Russas (Ceará) Nossa Senhora de Nazaré
(Piauí)
Santa Isabel do Rio Negro (Amazonas)
Biome Caatinga Cerrado-Caatinga Amazon
Rainfall 775.6 mm/yearly 1.412 mm/yearly 2.280 mm/yearly
Temperature (minimum, maximum) 27–29 °C 26–34 °C 26–32 °C
Population (no. of inhabitants) 75,018 4,747 22,404
No. of families living in poverty 751 30 402
Proportion of population performing open evacuation (%) 47 42,3 –
Main drinking water source Harvested rain water stored in cisterns
Artesian wells Negro River and artesian wells
Communities/districts included 4 8 6
Localization of districts Rural Rural and urban Urban
Participants included 213 298 433
Year 2013 2014 2011
Giardia duodenalispositive persons and prevalence (Coronato-Nunes et al., 2016, 2017)
Amazon biome (Durigan et al., 2017).
Among samples for which it was possible to obtain DNA sequences of the three genes, 60% presented overlapping peaks in electro-pherograms. This exemplifies the difficulty of performing concatenated sequence analysis of different genes using a multilocus method. We observed a pattern of amplification failure in samples derived from inhabitants from the same families, supporting the hypothesis raised by Robertson et al. (2007) that the phenomenon may be related to hap-lotypes that have accumulated mutations (insertions, deletions or
substitutions) in the primer binding area.
5. Conclusions
G. duodenalischaracterized as belonging to assemblage B was more prevalent in the Amazon region, while assemblage A predominated in Northeastern states. Assemblage B showed higher genetic variation when compared to A, especially in the Amazon. Great demographic and socioenvironmental differences can be observed in the studied regions.
Table 2
Distribution ofGiardia duodenalisassemblages and sub-assemblages according to study areas in three different biomes.
Assemblages/sub-assemblages/genetic loci Santa Isabel do Rio Negro (Amazonas)/Amazon Nossa Senhora de Nazaré (Piauí)/Transitional Cerrado Russas (Ceará)/Caatinga
β-giardin(βG) N = 54 N = 10 N = 7
Assemblage A 12/54 (22.2%) 5/10 (50.0%) 5/7 (71.4%)
Sub-assemblage A-II 2/12 (16.7%) 3/5 (60.0%) 3/5 (60.0%)
Sub-assemblage A-III 10/12 (83.3%) – –
Undefined sub-assemblage A – 2/5 (40.0%) 2/5 (40.0%)
Assemblage B 42/54 (77.8%) 5/10 (50.0%) 2/7 (28.6%)
Sub-assemblage B-III 3/42 (7.1%) – –
New sub-assemblage B 23/42 (54.8%) – 1/2 (50.0%)
Undefined sub-assemblage B 16/42 (38.1%) 5/5 (100%) 1/2 (50.0%)
Glutamate dehydrogenase(GDH) N = 39 N = 3 –
Assemblage A 11/39 (28.2%) 2/3 (66.6%) –
Sub-assemblage A-II 9/11 (81.8%) 2/2 (100%) –
Sub-assemblage A-III – – –
New sub-assemblage A 1/11 (9.1%) – –
Undefined Sub-assemblage A 1/11 (9.1%) – –
Assemblage B 28/39 (71.8%) 1/3 (33.3%) –
Triosephosphate isomerase(TPI) N = 20 N = 7 –
Assemblage A 7/20 (35%) 6/7 (85.7%) –
Sub-assemblage A-II 5/7 (71.4%) 4/6 (66.6%) –
Sub-assemblage A-III – – –
New sub-assemblage A 1/7 (14.3%) 1/6 (16.7%) –
Undefined sub-assemblage A 1/7 (14.3%) 1/6 (16.7%) –
Assemblage B 13/20 (65%) 1/7 (14.3%) –
Sub-assemblage B-III 2/13 (15.4%) 1/1 (100%) –
New sub-assemblage B 6/13 (46.1%) – –
Undefined sub-assemblage B 5/13 (38.5%) – –
These conditions can be related to the difference in prevalence, genetic diversity andG. duodenalisassemblage distribution. Higher genetic di-versity of assemblage B in the Amazon may be related to demographic concentration leading to a more complex transmission network within a poorer sanitation background. The high genetic divergence between assemblages A and B support the proposal of taxon separation in dis-tinct species.
Acknowledgements
This work was supported by the Oswaldo Cruz Foundation (Fiocruz) and the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES)/Brazilian Ministry of Education and
Ministry of Social Development, in the context of the CAPES-Fiocruz-Brazil without Poverty Plan.
References
Adam, E.A., Yoder, J.S., Gould, L.H., Hlavsa, M.C., Gargano, J.W., 2016. US giardiasis outbreaks, 1971–2011. Epidemiol. Infect. 144, 2790–2801.
Basso, R.M.C., Silva-Ribeiro, R.T., Soligo, D.S., Ribacki, S.I., Callegari-Jacques, S.M., Zoppas, B.C.A., 2008. Evolução da prevalência de parasitoses intestinais em escolares em Caxias do Sul, RS. Rev. Soc. Bras. Med. Trop. 41, 263–268.
Bóia, M.N., Carvalho-Costa, F.A., Sodré, F.C., Eyer-Silva, W.A., Lamas, C.C., Lyra, M.R., Pinto-Junior, V.L., Cantalice Filho, J.P., Oliveira, A.L., Carvalho, L.M., Gross, J.B., Sousa, A.L., Moraes, T.I., Bermudez-Aza, E.H., Martins, E.B., Coura, J.R., 2006. Mass treatment for intestinal helminthiasis control in an Amazonian endemic area in Brazil. Rev. Inst. Med. Trop. São Paulo. 48, 189–195.
Fig. 3.Majority-rule Bayesian consensus tree of GDH sequences from Russas-CE, Nossa Senhora de Nazaré-PI e Santa Isabel do Rio Negro-AM. Dotted-line tree depict thefinal topology found in ML analysis when trees were discrepant in any node. Posterior probabilities (Bayesian reconstruction) > 0.9 and bootstrap values (ML tree) > 70 are shown above and below the nodes, respectively. GenBank acces-sion numbers are shown in the terminal branches and reference se-quences are marked with asterisks. Abbreviation of each state (Amazonas: AM, Russas: CE and Piauí: PI) follows accession numbers for identification of provenance of each sample.
Brown, J., Cumming, O., Bartram, J., Cairncross, S., Ensink, J., Holcomb, D., Knee, J., Kolsky, P., Liang, K., Liang, S., Nala, R., Norman, G., Rheingans, R., Stewart, J., Zavale, O., Zuin, V., Schmidt, W.P., 2015. A controlled, before-and-after trial of an urban sanitation intervention to reduce enteric infections in children: research pro-tocol for the Maputo Sanitation (MapSan) study, Mozambique. BMJ Open 5, e008215.
Caccio, S.M., Ryan, U., 2008. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 160, 75–80.
Cacciò, S., De Giacomo, M., Pozio, E., 2002. Sequence analysis of theβ-giardin gene and development of a polymerase chain reaction-restriction fragment length poly-morphism assay to genotypeGiardia duodenaliscysts from human faecal samples. Int. J. Parasitol. 32, 1023–1030.
Carvalho-Costa, F.A., Gonçalves, A.Q., Lassance, S.L., de Albuquerque, C.P., Leite, J.P., Bóia, M.N., 2007a. Detection ofCryptosporidiumspp and other intestinal parasites in children with acute diarrhea and severe dehydration in Rio de Janeiro. Rev. Soc. Bras. Med. Trop. 40, 346–348.
Carvalho-Costa, F.A., Gonçalves, A.Q., Lassance, S.L., Silva Neto, L.M., Salmazo, C.A., Bóia, M.N., 2007b.Giardia lambliaand other intestinal parasitic infections and their relationships with nutritional status in children in Brazilian Amazon. Rev. Inst. Med. Trop. Sao Paulo 49, 147–153.
Cooper, M.A., Sterling, C.R., Gilman, R.H., Cama, V., Ortega, Y., Adam, R.D., 2010. Molecular analysis of household transmission ofGiardia lambliain a region of high endemicity in Peru. J. Infect. Dis. 202, 1713–1721.
Coronato-Nunes, B., Pavan, M.G., Jaeger, L.H., Monteiro, K.J., Xavier, S.C., Monteiro, F.A., Bóia, M.N., Carvalho-Costa, F.A., 2016. Spatial and molecular epidemiology of Giardia intestinalisdeep in the Amazon, Brazil. PLoS One 11, e0158805.
Coronato-Nunes, B., Calegar, D.A., Monteiro, K.J.L., Jaeger, L.H., Reis, E.R.C., Xavier, S.C.C., Carpp, L.N., Lima, M.M., Bóia, M.N., Carvalho-Costa, F.A., 2017.Giardia in-testinalisinfection associated with malnutrition in children living in northeastern Brazil. JIDC. 11, 563–570.
Coutinho, M.L., 2006. O conceito de bioma. Acta Bot. Bras. 20, 13–23.
Darriba, D., Taboada, G.L., Doallo, R., Posada, D., 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772.
Davies, A.P., Campbell, B., Evans, M.R., Bone, A., Roche, A., Chalmers, R.M., 2009. Asymptomatic carriage of protozoan parasites in children in day care centers in the United Kingdom. Pediatr. Infect. Dis. J. 28, 838–840.
Durigan, M., Abreu, A.G., Zuccenhi, M.I., Franco, R.M., de Souza, A.P., 2014. Genetic diversity ofGiardia duodenalis: multilocus genotyping reveals zoonotic potential be-tween clinical and environmental sources in a metropolitan region of Brazil. PLoS One 9, e115489.
Durigan, M., Ciampi-Guillardi, M., Rodrigues, R.C., Greinert-Goulart, J.A., Siqueira-Castro, I.C., Leal, D.A., Yamashiro, S., Bonatti, T.R., Zucchi, M.I., Franco, R.M., de Souza, A.P., 2017. Population genetic analysis ofGiardia duodenalis: genetic diversity and haplotype sharing between clinical and environmental sources.
Microbiologyopen. 6 (424).
Efstratiou, A., Ongerth, J.E., Karanis, P., 2017. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011–2016. Water Res. 114, 14–22.
Einarsson, E., Ma'ayeh, S., Svärd, S.G., 2016. An up-date onGiardiaand giardiasis. Curr. Opin. Microbiol. 34, 47–52.
Feng, Y., Xiao, L., 2011. Zoonotic potential and molecular epidemiology ofGiardiaspecies and giardiasis. Clin. Microbiol. Rev. 24, 110–140.
Gelanew, T., Lalle, M., Hailu, A., Pozio, E., Cacciò, S.M., 2007. Molecular characterization of human isolates ofGiardia duodenalisfrom Ethiopia. Acta Trop. 102, 92–99. Genser, L., Poitou-Bernert, C., Brot-Laroche, E., 2015. AsymptomaticGiardia intestinalis
infection and Roux-en-Ygastric bypass. Surg. Obes. Relat. Dis. 11, 1182–1183. Golenberg, E.M., Bickel, A., Weihs, P., 1996. Effect of highly fragmented DNA on PCR.
Nucleic Acids Res. 24, 5026–5033.
Hall, T.A., 1999. BioEdit: a user-friendly biological sequence alignment editor and ana-lysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. Heyworth, M.F., 2016.Giardia duodenalisgenetic assemblages and hosts. Parasite 23, 1–5. Hummell, B.M.L., Cutter, S.L., Emrich, C.T., 2016. Social vulnerability to natural hazards
in Brazil. Int. J. Disaster Risk Sci. 7, 111–122.
Johnston, A.R., Gillespie, T.R., Rwego, I.B., McLachlan, T.L., Kent, A.D., Goldberg, T.L., 2010. Molecular epidemiology of cross-speciesGiardia duodenalistransmission in western Uganda. PLoS Negl. Trop. Dis. 4, e683.
Kosuwin, R., Putaporntip, C., Pattanawong, U., Jongwutiwes, S., 2010. Clonal diversity in Giardia duodenalisisolates from Thailand: evidences for intragenic recombination and purifying selection at the beta giardin locus. Gene 449, 1–8.
Kumar, T., Abd Majid, M.A., Onichandran, S., Jaturas, N., Andiappan, H., Salibay, C.C., Tabo, H.A., Tabo, N., Dungca, J.Z., Tangpong, J., Phiriyasamith, S., Yuttayong, B., Polseela, R., Do, B.N., Sawangjaroen, N., Tan, T.C., Lim, Y.A., Nissapatorn, V., 2016. Presence ofCryptosporidium parvumandGiardia lambliain water samples from Southeast Asia: towards an integrated water detection system. Infect. Dis. Poverty. 5 (3).
Messing, J., 1993. M13 cloning vehicles. Their contribution to DNA sequencing. Methods Mol. Biol. 23, 9–22.
Monis, P.T., Caccio, S.M., Thompson, R.C., 2009. Variation inGiardia: towards a taxo-nomic revision of the genus. Trends Parasitol. 25, 93–100.
Nunes, B.C., 2011. Parasitoses intestinais na Ilha da Marambaia, Mangaratiba, RJ e intervenção educativa [Dissertação de Mestrado]. Universidade Federal Fluminense, Niterói (149 pp.).
Oliveira-Arbex, A.P., David, E.B., Oliveira-Sequeira, T.C., Bittencourt, G.N., Guimarães, S., 2016. Genotyping ofGiardia duodenalisisolates in asymptomatic children at-tending daycare centre: evidence of high risk for anthroponotic transmission. Epidemiol. Infect. 144, 1418–1428.
Paim, J., Travassos, C., Almeida, C., Bahia, L., Macinko, J., 2011. O sistema de saúde brasileiro: história, avanços e desafios. Lancet 1, 11–31.
Painter, J.E., Gargano, J.W., Collier, S.A., Yoder, J.S., 2015. Giardiasis surveillance— United States, 2011–2012. MMWR Surveill. Summ. 64, 1–5.
Read, C.M., Monis, P.T., Thompson, R.C., 2004. Discrimination of all genotypes ofGiardia duodenalisat the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 4, 125–130.
Robertson, L.J., Forberg, T., Hermansen, L., Gjerde, B.K., Langeland, N., 2007. Molecular characterisation ofGiardiaisolates from clinical infections following a waterborne outbreak. J. Infect. 55, 79–88.
Ryan, U., Caccio, S.M., 2013. Zoonotic potential ofGiardia. Int. J. Parasitol. 43, 943–956. Sprong, H., Cacciò, S.M., van der Giessen, J.W., ZOOPNET network and partners, 2009. Identification of zoonotic genotypes ofGiardia duodenalis. PLoS Negl. Trop. Dis. 3, e558.
Sulaiman, I.M., Fayer, R., Bern, C., Gilman, R.H., Trout, J.M., Schantz, P.M., Das, P., Lal, A.A., Xiao, L., 2003. Triosephosphate isomerase gene characterization and potential zoonotic transmission ofGiardia duodenalis. Emerg. Infect. Dis. 9, 1444–1452. Tamura, K., Stecher, G., Peterson, D., Filipski, A., Kumar, S., 2013. MEGA6: molecular
evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. Thompson, R.C., Hopkins, R.M., Homan, W.L., 2000. Nomenclature and genetic
group-ings ofGiardiainfecting mammals. Parasitol. Today 16, 210–213.
Valverde, J.G., Gomes-Silva, A., De Carvalho Moreira, C.J., Leles De Souza, D., Jaeger, L.H., Martins, P.P., Meneses, V.F., Bóia, M.N., Carvalho-Costa, F.A., 2011. Prevalence and epidemiology of intestinal parasitism, as revealed by three distinct techniques in an endemic area in the Brazilian Amazon. Ann. Trop. Med. Parasitol. 105, 413–424. Wielinga, C.M., Thompson, R.C., 2007. Comparative evaluation ofGiardia duodenalis