Paulo José Pyles Cicchi1,4, Marco Aurélio de Sena2, Denise Maria Peccinini-Seale2, Marcelo Ribeiro Duarte3
Biota Neotropica v7 (n2) – http://www.biotaneotropica.org.br/v7n2/pt/abstract?inventory+bn03907022007
Recebido em 19/06/06 Versão Reformulada recebida em 08/05/07
Publicado em 05/07/07
1Universidade Estadual Paulista – UNESP, Instituto de Biociências, Departamento de Zoologia, Laboratório de Herpetologia, Campus Botucatu, Rubião Junior, s/n, CEP 18618-000, Botucatu, SP, Brasil
2Departamento de Genética e Biologia Evolutiva, IBUSP 3Laboratório de Herpetologia, Instituto Butantan, São Paulo, Brazil
4Autor para correspondência: Paulo José Pyles Cicchi, e-mail: paulocicchi@yahoo.com.br
Abstract
Cicchi, P.J.P.; De Sena, M.A. Peccinini-Seale, D.M. and Duarte, M.R. Snakes from coastal
islands of State of São Paulo, Southeastern Brazil. Biota Neotrop. May/Aug 2007 vol. 7, no. 2.
http://www.biotaneotropica.org.br/v7n2/pt/abstract?inventory+bn03907012007. ISSN 1676-0603.
There are relatively few studies on snake fauna from coastal islands of the State of São Paulo (SSP), Southeastern Brazil and the number of species housed in Brazilian institutional zoological collections is relatively limited. In Brazil, for the first time, a snake inventory for eighteen islands of coastal SSP is presented. Here we record data from sampling on eleven islands as well information on vouchered species in the main herpetological collections. Thirty-six species from four families: one Boidae, thirty Colubridae, one Elapidae and four Viperidae from eighteen islands are listed as well as the thirteen new island records for snakes. Relative abundance categories were used for species rarity: common, infrequent and rare; 44.4% of the snakes with voucher specimens were considered rare. The most common species in twelve of the eighteen islands was Micrurus corallinus; in eleven of
the eighteen islands were Bothrops jararaca and Liophis miliaris; in ten of the eighteen islands were B. jararacussu
andChironius bicarinatus. The most common snake species on coastal islands were Micrurus corallinus which
was found in twelve of the eighteen islands, followed by Bothrops jararaca and Liophis miliaris found on eleven
of the eighteen islands and B. jararacussu and Chironius bicarinatus which were found in ten of the eighteen
islands studied. There are seven new records of snake species for Cardoso Island (25° 05’ S and 047° 59’ W): C. bicarinatus,C. multiventris,Dipsas petersi,Echinanthera bilineata,E. cephalostriata,Helicops carinicaudus
andXenodon neuwiedii; three new records for Comprida Island (24° 54’ S and 47° 48’ W): B. jararacussu, C. bicarinatus and H.carinicaudus; one for Anchieta Island (23° 32’ S and 045° 03’ W): Spilotes pullatus; one for Couves Island (23° 25’ S and 44° 52’ W): L. miliaris; one for Porcos Island (23° 23’ S and 44° 54’ W), B. jararaca. The endemic species B.alcatraz from Alcatrazes Island and B.insularis from Queimada Grande Island
are considered endangered species by IUCN. Snake fauna on Monte de Trigo Island are extinct. The fragility of insular snake fauna needs more attention for environmental conservation, since 52.0% of snake species preys on amphibians, highlighting the importance of forest conservation.
Keywords: snakes, coastal islands, inventory, conservation, diversity.
Resumo
Cicchi, P.J.P.; De Sena, M.A. Peccinini-Seale, D.M. and Duarte, M.R. Serpentes das ilhas
costeiras do Estado de São Paulo, Sudeste do Brasil. Biota Neotrop. May/Aug 2007 vol. 7, no. 2.
http://www.biotaneotropica.org.br/v7n2/pt/abstract?inventory+bn03907012007. ISSN 1676-0603.
Há poucos estudos sobre a fauna de serpentes em ilhas costeiras do Estado de São Paulo, Sudeste do Brasil e um baixo número de espécies depositadas em coleções zoológicas. No Brasil, pela primeira vez, foi realizado um inventário em 18 ilhas do litoral paulista a partir de pesquisa de registros nas coleções herpetológicas do Sudeste do Brasil. Também foram realizadas coletas de campo em onze ilhas. Trinta e seis espécies de quatro famílias foram registradas: uma espécie de Boidae, trinta de Colubridae, uma de Elapidae e quatro de Viperidae. Os dados de campo apresentaram treze ocorrências novas de espécies sem registro nas coleções. Para estimar a raridade das espécies utilizaram-se categorias de abundância relativa: comum, não-freqüente e rara. Das espécies amostradas, 44,4% foram consideradas raras. As espécies mais comuns foram Micrurus corallinus, presente em doze ilhas; Bothrops jararaca e Liophis miliaris, presentes em onze ilhas, B. jararacussu e Chironius bicarinatus, presentes em 10 ilhas. Foram efetuados sete novos registros para a Ilha do Cardoso (25° 05’ S e 47° 59’ W): C. bicarinatus, C. multiventris,Dipsas petersi,Echinanthera bilineata,E. cephalostriata,Helicops carinicaudus e Xenodon neuwiedii; três para Ilha Comprida (24° 54’ S e 47° 48’ W): B. jararacussu, C. bicarinatus e H.carinicaudus; um
para Ilha Anchieta (23° 32’ S e 45° 03’ W): Spilotes pullatus; um para a Ilha das Couves (23° 25’ S e 44° 52’ W):
L. miliaris; um para a Ilha dos Porcos (23° 23’ S e 44° 54’ W): B. jararaca.B. alcatraz e B. insularis, endêmicos
segundo IUCN. Foi registrada a extinção da fauna de serpentes na Ilha Monte de Trigo. Os ecossistemas insulares, mais vulneráveis que os continentais, carecem de uma proteção mais efetiva. A maioria destas espécies (cerca de 52%) preda anfíbios, reforçando a necessidade de conservação das florestas.
Palavras-chave: serpentes, ilhas, inventário, diversidade, conservação.
Introduction
Studies on island herpetological fauna are relevant since there is a significant human impact on these threatened environments, mainly by mismanagement of natural resources and predatory tourism. Few islands are unaltered by human action, and often anthropogenic altera-tions have taken place on a scale even greater than in most continental systems (Vitousek et al. 1995).
The coastal area of the State of São Paulo (SSP), southeastern Brazil, encompasses 106 islands isolated from the mainland by distances which vary from a few meters up to 38 km (Ângelo 1989), with a wide range of anthropogenic alterations.
As commented by Ângelo (1989), less than 10% of the insular environment in SSP has some official environmental protection. In fact, the relatively low representation of island species in the lists of threatened and endangered species around the world is emblematic (Vitousek et al. 1995). In Brazil, snake inventories on this set of coastal islands has still not been addressed, except for some studies made on a relatively small number of coastal islands of SSP (Ihering 1897; Amaral 1921; Luederwaldt 1923; Hoge 1950; Mertens 1955; Hoge et al. 1959; Müller 1968; Vanzolini 1973; Duarte et al. 1995, Marques et al. 2002; Centeno 2003; Cicchi 2004) which addressed specific bio-logical aspects rather than snake inventories. As in Rodrigues (2005), except for the Crocodylia, the richness of the Brazilian reptile fauna is still underestimated because of insufficient inventories and too few taxonomists. The study of these insular faunas, comparing the fauna among themselves and with those from the adjacent mainland must, therefore, afford insight into several evolutionary mechanisms that have determined the formation of complex tropical biota (Vanzolini 1973). This study presents a first general approach for snakes living on coastal islands of Sao Paulo, based on fieldwork on nine different islands of SSP in Brazil and the lists of vouchered specimens of snake species from eighteen islands in the main herpetological collections of Brazilian Institutions. The fragility of insular snake fauna needs more attention for environmental conservation.
Material and Methods
Our inventory of the snakes inhabiting coastal islands of the SSP (between the latitudes 23° 20’ S to 25° 15’ and longitudes 44° 45’ W to 48° 00’, Figure 1; Climatic data Figure 2) was based on literature,
SP
MG 44° 45' W 48° 00' W
25° 15' S
23° 20' S RJ
PR
Figure 1. Map of Study Area. Coast of the State of São Paulo, Brazil.
Figura 1. Mapa da área de estudo. Costa do Estado de São Paulo, Brasil.
360
180
0
M
Climate
M
J J S N
0 10
T
(°C)
20 30
Rainfall (mm)
Ru Rc Tu Tc
Figure 2. The graph presents monthly average rainfall and temperature
(1980-1988) in extreme sites of the study area: north — Ubatuba and south — Ca-nanéia. Ru: rainfall in Ubatuba, Rc: rainfall in Cananéia, Tu: temperature in Ubatuba, Tc: temperature in Cananéia. Modified from Marques et al. 2000.
Figura 2. O gráfico apresenta a média mensal de temperatura e precipitação (1980-1988) em locais extremos da área de estudo: norte – Ubatuba e Sul – Cananéia. Ru: precipitação em Ubatuba, Rc: precipitação em Cananéia, Tu: temperatura em Ubatuba, Tc: temperatura em Cananéia. Modificado de Marques et al. 2000.
Figure 3. Armação da Baleia, Bom Abrigo Island. Photo: Marcelo R. Duarte.
Figura 3. Armação da Baleia, Ilha do Bom Abrigo. Foto: Marcelo R. Duarte.
Figure 6. Cardoso Island. Photo: Marcelo R. Duarte.
Figura 6. Ilha do Cardoso. Foto: Marcelo R. Duarte.
Figure 9. Queimada Grande Island. Photo: Marcelo R. Duarte.
Figura 9. Ilha da Queimada Grande. Foto: Marcelo R. Duarte.
Figure 5. Alcatrazes Island. Photo: Marcelo R. Duarte.
Figura 5. Ilha de Alcatrazes. Foto: Marcelo R. Duarte.
Figure 8. Vitória Island. Photo: Marcelo R. Duarte.
Figura 8. Ilha de Vitória. Foto: Marcelo R. Duarte.
Figure 4. Erosion, Bom Abrigo Island. Photo: Marcelo R. Duarte.
Figura 4. Erosão. Ilha do Bom Abrigo. Foto: Marcelo R. Duarte.
Figure 7. Búzios Island. Photo: Marcelo R. Duarte.
Figura 7. Ilha de Búzios. Foto: Marcelo R. Duarte.
island inhabitants. Relative abundance categories (RAC) were used for species rarity: Common: occurrence on more than five islands; Infrequent: occurrence on four distinct islands and Rare: occurrence on three or less islands. The RAC for snake species were compared with data from the SSP Atlantic Forest mainland available in the literature. Supplementary information on species occurrence on the islands was provided by experienced herpetologists.
Results
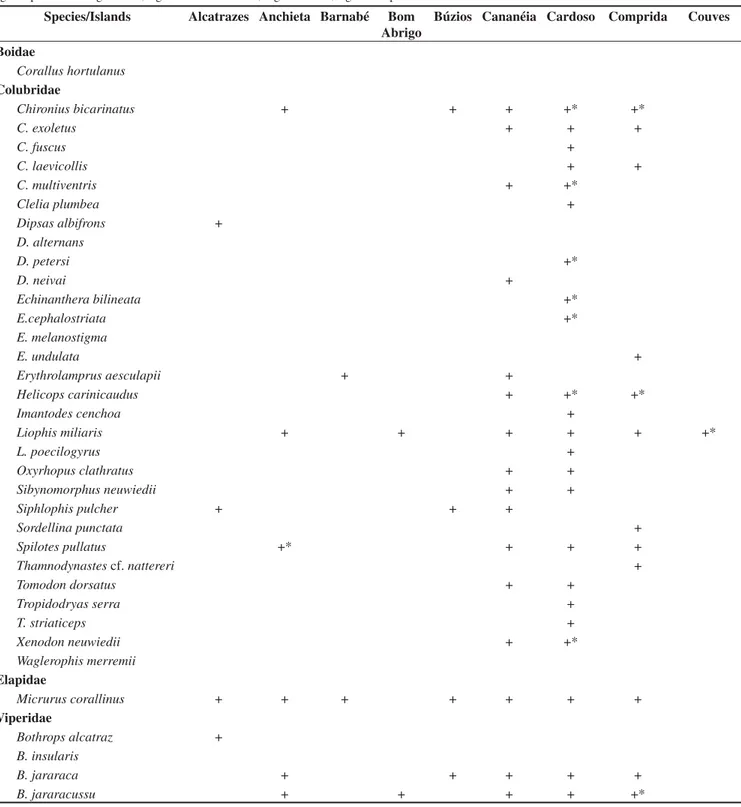
aster-Table 1. Thirty-six species of snakes from 18 islands from SSP (23° 20’ S to 25° 15’ and 44° 45’ W to 48° 00’) with an asterisk representing a new island record. Species with an asterisk representing a new island record. Figures 3-9, some islands visited; Figures 11-41, some species collected.
Tabela 1. Trinta e seis espécies de serpentes em 18 ilhas no SSP (23° 20’ S até 25° 15’ e 44° 45’ W até 48° 00’). Espécies com asterisco representam novo registro para a ilha. Figuras 3-9, algumas ilhas visitadas; Figuras 11-41, algumas espécies coletadas.
Species/Islands Alcatrazes Anchieta Barnabé Bom
Abrigo
Búzios Cananéia Cardoso Comprida Couves
Boidae
Corallus hortulanus
Colubridae
Chironius bicarinatus + + + +* +*
C. exoletus + + +
C. fuscus +
C. laevicollis + +
C. multiventris + +*
Clelia plumbea +
Dipsas albifrons +
D. alternans
D. petersi +*
D. neivai +
Echinanthera bilineata +*
E.cephalostriata +*
E. melanostigma
E. undulata +
Erythrolamprus aesculapii + +
Helicops carinicaudus + +* +*
Imantodes cenchoa +
Liophis miliaris + + + + + +*
L. poecilogyrus +
Oxyrhopus clathratus + +
Sibynomorphus neuwiedii + +
Siphlophis pulcher + + +
Sordellina punctata +
Spilotes pullatus +* + + +
Thamnodynastes cf. nattereri +
Tomodon dorsatus + +
Tropidodryas serra +
T. striaticeps +
Xenodon neuwiedii + +*
Waglerophis merremii
Elapidae
Micrurus corallinus + + + + + + +
Viperidae
Bothrops alcatraz + B. insularis
B. jararaca + + + + +
B. jararacussu + + + + +*
isk marks each one of the 13 new records of snakes. There are seven new records of snake species for Cardoso Island (25° 05’ S and 47° 59’ W): C. bicarinatus,C. multiventris,Dipsas petersi,Echinanthera bilineata, E. cephalostriata, Helicops carinicaudus and Xenodon neuwiedii; three new records for Comprida Island (24° 54’ S and 47°
48’ W): B. jararacussu, C. bicarinatus and H.carinicaudus; one for
Anchieta Island (23° 32’ S and 45° 03’ W): Spilotes pullatus; one for
Couves Island (23° 25’ S and 44° 52’ W): L. miliaris; one for Porcos Island (23° 23’ S and 44° 54’ W), B. jararaca.
Species/Islands Mar Virado Monte de
Trigo1
Porchat2 Porcos Queimada
Grande
Santo Amaro
São Sebastião
São Vicente
Vitória
Boidae
Corallus hortulanus +
Colubridae
Chironius bicarinatus + + + + +
C. exoletus + + + +
C. fuscus + + +
C. laevicollis + +
C. multiventris + + +
Clelia plumbea + +
Dipsas albifrons + +
D. alternans +
D. petersi + + +
D. neivai +
Echinanthera bilineata
E.cephalostriata + + +
E. melanostigma + +
E. undulata +
Erythrolamprus aesculapii + +
Helicops carinicaudus + + +
Imantodes cenchoa
Liophis miliaris + + + + +
L. poecilogyrus + +
Oxyrhopus clathratus + +
Sibynomorphus neuwiedi + + +
Siphlophis pulcher + + +
Sordellina punctata
Spilotes pullatus + + + +
Thamnodynastes cf. nattereri +
Tomodon dorsatus + +
Tropidodryas serra +
T. striaticeps +
Xenodon neuwiedii + +
Waglerophis merremii +
Elapidae
Micrurus corallinus + + + + +
Viperidae
Bothrops alcatraz +
B. insularis +
B. jararaca + +* + + + +
B. jararacussu + + + + +
1Snake population of Monte de Trigo Island extirpated by locals (MFD Furtado, pers. communication); and 2Presumed Porchat Island (error; originally
vouchered Paranapuã Island, name of the continental beach beside Porchat Island).
1População de serpentes da Ilha do Monte de Trigo dizimada pela população humana local. (MFD Furtado, com. pess.); e 2Suposta Ilha Porchat (erro:
origi-nalmente tombada como Ilha de Pararnapuã, nome dado a uma praia continental próxima a Ilha Porchat).
Table 1. Continued...
correlation, (r = 0.72; p = 0.007; n =18). Data on distances from the continent and snake species richness (Figure 42) showed a negative correlation, (r = –0.39; p = 0.1; n =18).
From the total of snake species with voucher specimens depos-ited in institutional collections, 44.4%were considered rare, 25.0%
Figure 10. Corallus hortulanus, Family Boidae. Photo: Marcelo R. Duarte.
Figura 10. Corallus hortulanus, Família Boidae. Foto: Marcelo R. Duarte.
Figure 14. Chironius multiventris, Family Colu-bridae. Photo: Marcelo R. Duarte.
Figura 14. Chironius multiventris, Família Colubridae. Foto: Marcelo R. Duarte.
Figure 18. Echinanthera bilineata, Family Co-lubridae. Photo: Marcelo R. Duarte.
Figura 18. Echinanthera bilineata, Família Colubridae. Foto: Marcelo R. Duarte.
Figure 13. Chironius laevicollis, Family Colu-bridae. Photo: Marcelo R. Duarte.
Figura 13. Chironius laevicollis, Família Colu-bridae. Foto: Marcelo R. Duarte.
Figure 17. Dipsas petersi, Family Colubridae.
Photo: Antônio Bordignon.
Figura 17. Dipsas petersi, Família Colubridae.
Foto: Antônio Bordignon.
Figure 21. Erythrolamprus aesculapii, Family Colubridae. Photo: Marcelo R. Duarte.
Figura 21. Erythrolamprus aesculapii, Família Colubridae. Foto: Marcelo R. Duarte.
Figure 11. Chironius bicarinatus, Family Colu-bridae. Photo: Marcelo R. Duarte.
Figura 11. Chironius bicarinatus, Família Colu-bridae. Foto: Marcelo R. Duarte.
Figure 15. Dipsas albifrons, Family Colubridae.
Photo: Marcelo R. Duarte.
Figura 15. Dipsas albifrons, Família Colubridae.
Foto: Marcelo R. Duarte.
Figure 19. Echinanthera cephalostriata, Family
Colubridae. Photo: Ricardo J. Sawaya.
Figura 19. Echinanthera cephalostriata, Família
Colubridae. Foto: Ricardo J. Sawaya.
Figure 20. Echinanthera undulata, Family
Co-lubridae. Photo: Marcelo R. Duarte.
Figura 20. Echinanthera undulata, Família
Colubridae. Foto: Marcelo R. Duarte.
Figure 16. Dipsas neivai, Family Colubridae.
Photo: Antônio Bordignon.
Figura 16. Dipsas neivai, Família Colubridae.
Foto: Antônio Bordignon.
Figure 12. Chironius fuscus, Family Colubridae. Photo: Antônio Bordignon.
Figure 24. Liophis miliaris, Family Colubridae. Photo: Antônio Bordignon.
Figura 24. Liophis miliaris, Família Colubridae. Foto: Antônio Bordignon.
Figure 28. Siphlophis pulcher, Family Colubri-dae. Photo: Antônio Bordignon.
Figura 28. Siphlophis pulcher, Família Colubri-dae. Foto: Antônio Bordignon.
Figure 32. Tropidodryas serra, Family Colubri-dae. Photo: Marcelo R. Duarte.
Figura 32. Tropidodryas serra, Família Colubri-dae. Foto: Marcelo R. Duarte.
Figure 22. Helicops carinicaudus, Family Co-lubridae. Photo: Marcelo R. Duarte.
Figura 22. Helicops carinicaudus, Família Colubridae. Foto: Marcelo R. Duarte.
Figure 26. Oxyrhopus clathratus, Family Colu-bridae. Photo: Antônio Bordignon.
Figura 26. Oxyrhopus clathratus, Família Colu-bridae. Foto: Antônio Bordignon.
Figure 30. Spilotes pullatus, Family Colubridae. Photo: Marcelo R. Duarte.
Figura 30. Spilotes pullatus, Família Colubridae. Foto: Marcelo R. Duarte.
Figure 23. Imantodes cenchoa, Family Colubri-dae. Photo: Marcelo R. Duarte.
Figura 23. Imantodes cenchoa, Família
Colu-bridae. Foto: Marcelo R. Duarte.
Figure 27. Sibynomorphus neuwiedi, Family Colubridae. Photo: Antônio Bordignon.
Figura 27. Sibynomorphus neuwiedi, Família
Colubridae. Foto: Antônio Bordignon.
Figure 31. Tomodon dorsatus, Family Colubri-dae. Photo: Marcelo R. Duarte.
Figura 31. Tomodon dorsatus, Família
Colubri-dae. Foto: Marcelo R. Duarte.
Figure 25. Liophis poecilogyrus, Family
Colu-bridae. Photo: Marcelo R. Duarte.
Figura 25. Liophis poecilogyrus, Família
Colu-bridae. Foto: Marcelo R. Duarte.
Figure 29. Sordellina punctata, Family
Colubri-dae. Photo: Marcelo R. Duarte.
Figura 29. Sordellina punctata, Família
Colu-bridae. Foto: Marcelo R. Duarte.
Figure 33. Tropidodryas striaticeps, Family
Colubridae. Photo: Marcelo R. Duarte.
Figura 33. Tropidadryas striaticeps, Família
Figure 34. Waglerophis merremii, Family Colu-bridae. Photo: Marcelo R. Duarte.
Figura 34. Waglerophis merremii, Família Colu-bridae. Foto: Marcelo R. Duarte.
Figure 35. Xenodon neuwiedii, Family Colubri-dae. Photo: Antônio Bordignon.
Figura 35. Xenodon neuwiedii, Família Colubri-dae. Foto: Antônio Bordignon.
Figure 36. Micrurus corallinus, Family Elapidae. Photo: Antônio Bordignon.
Figura 36. Micrurus corallinus, Família Elapi-dae. Foto: Antônio Bordignon.
Figure 37. Bothrops alcatraz, Family Viperidae.
Photo: Marcelo R. Duarte.
Figura 37. Bothrops alcatraz, Família
Viperi-dae. Foto: Marcelo R. Duarte.
Figure 38. Bothrops insularis, Family Viperidae.
Photo: Marcelo R. Duarte.
Figura 38. Bothrops insularis, Família Viperidae.
Foto: Marcelo R. Duarte.
Figure 39. Bothrops jararaca, Family Viperidae.
Photo: Antônio Bordignon.
Figura 39. Bothrops jararaca, Família Viperidae.
Foto: Antônio Bordignon.
Figure 40. Bothrops jararacussu, Family Vi-peridae. Photo: Luís Coelho.
Figura 40. Bothrops jararacussu, Família Vi-peridae. Foto: Luís Coelho.
Figure 43. The most common species in twelve of the eighteen islands was Micrurus corallinus; in eleven of the eighteen islands Bothrops jararaca and Liophis miliaris were found; in ten of the eighteen B. jararacussu and Chironius bicarinatus were found.
Table 2. Area of the islands of the SSP with vouchered snakes in herpetological collections or in the literature, distance from mainland (km), threats and category of use.
Tabela 2. Área das ilhas no SSP com as espécies de serpentes tombadas nas coleções herpetológicas ou na literatura, distância das ilhas ao continente (km), ameaças e categoria de uso.
Island (number of species) Area (ha) Distance from
mainland (km)
Threats2 Category of use
Alcatrazes (n = 4) 135 33.40 D Inhabited
Anchieta (n = 6) 828 0.49 E State Park
Barnabé (n = 2) 173.4 0.01 Wide
Bom Abrigo (n = 2) 154 3.55 A,B,C Populated
Búzios (n = 4) 755 24.09 A,B,C Populated
Cananéia (n = 15) 13.7 0.24 A,B,C,G Wide
Cardoso (n = 23) 22,500 0.08 A,B,F State Park
Comprida (n = 12) 20,000 0.31 A,B,C,G Wide
Couves (North) (n = 1) 64.5 2.53 A,B,C Populated
Mar Virado (n = 2) 119 2.00 B Populated
Monte de Trigo (*) 130 10.20 Populated
Porchat1 (n = 10) 15 0.23 Wide
Porcos (n = 1) 24.2 0.74 A,B Populated
Queimada Grande (n = 2) 430 34.80 A Inhabited
Santo Amaro (n = 21) 14,000 0.05 A,B,G Wide
São Sebastião (n = 20) 33,600 1.76 A,B,E,G State Park/Wide
São Vicente (n = 23) 6,000 0.12 Wide
Vitória (n = 4) 221.3 37.97 A,B Populated
1Presumed Porchat Island (error; originally voucher specimens recorded as from Paranapuã Island, name of the continental beach beside Porchat Island);
2Threat categories: A) Deforestation & Fire B) Domestic animals C) Erosion D) Military target E) Wild exotic fauna introduced F) Indians harvest G) Urban expansion ) Extremely impacted; and *Snake population of Monte de Trigo Island extirpated by locals (MFD Furtado, pers. communication).
Discussion
In fact, the SSP coast was one of the gateways to continental explorations since the beginning of the 15th century (Dean 1996).
Deforestation and fire are commonplace on several islands both for
25
20
15
Species richness
5 10
0
0 10
Distance continent (Km)
20 30 40
Figure 42. The graph shows a negative correlation between the distance island-mainland and the number of species.
Figura 42. O gráfico demonstra a correlação negativa entre a distância ilha-continente e o número de espécies.
Figure 41. The graph shows a positive correlation between island areas and the number of species collected.
Figura 41. O gráfico demonstra a correlação positiva entre a área das ilhas e o número de espécies coletadas.
25
20
15
Species richness
5 10
0
0 10000 20000
Area (ha)
30000 40000
veg-Table 3. SSP Islands species, habitat, preys, and relative abundance categories.
Tabela 3. Espécies das ilhas do SSP, hábitats, presas e categorias de abundância relativa.
Species Habitat Preys* Relative abundance
(SSP Islands)
Relative abundance (SSP Shore)* Boidae
Corallus hortulanus Arboreal Rodents and birds Rare Rare
Colubridae
Chironius bicarinatus Arboreal Amphibians Common Common
C. exoletus Arboreal Amphibians Common Common
C. fuscus Arboreal Amphibians Infrequent Common
C. laevicollis Arboreal Amphibians Infrequent Infrequent
C. multiventris Arboreal Amphibians Common Infrequent
Clelia plumbea Terrestrial Lizards & Snakes Rare Rare
Dipsas albifrons Terr/Arb Snails Infrequent Absent
D. alternans Arboreal Snails Rare Rare
D. petersi Arboreal Snails Infrequent Rare
D. neivai Arboreal Snails Rare Rare
Echinanthera bilineata Terrestrial Amphibians Rare Rare
E. cephalostriata Terrestrial Amphibians Infrequent Rare
E. melanostigma Terrestrial Amphibians Rare Rare
E. undulata Terrestrial Amphibians Rare Common
Erythrolamprus aesculapii Terrestrial Snakes Infrequent Common Helicops carinicaudus Aquatic Amphibians & Fishes Common Common
Imantodes cenchoa Arboreal Lizards & Amphibians Rare Rare
Liophis miliaris Aqua/Terr Amphibians & Fishes Common Common
L. poecilogyrus Terrestrial Amphibians Rare Common
Oxyrhopus clathratus Terrestrial Rodents & Lizards Infrequent Common
Sibynomorphus neuwiedi Terrestrial Snails Common Common
Siphlophis pulcher Arboreal Lizards & Snakes Common Rare
Sordellina punctata Aqua/Terr Earthworms Rare Rare
Spilotes pullatus Arboreal Rodents, Birds, Eggs, Bats Common Common Thamnodynastescf. nattereri Terrestrial Rodents & Amphibians? Rare Rare
Tomodon dorsatus Terrestrial Snails Infrequent Common
Tropidodryas serra Arboreal Rodents & Lizards Rare Infrequent
T. striaticeps Arboreal Rodents & Lizards Rare Infrequent
Xenodon neuwiedii Terrestrial Amphibians Infrequent Common
Waglerophis merremii Terrestrial Amphibians Rare Rare
Elapidae
Micrurus corallinus Fossorial Amphisbaenians & Snakes Common Common
Viperidae
Bothrops alcatraz Terrestrial Centipedes & Amphibians Rare/Endemic Absent B. insularis Arboreal Birds & Amphibians Rare/Endemic Absent
B. jararaca Terrestrial Rodents Common Common
B. jararacussu Terrestrial Rodents & Amphibians? Common Common
Marques et al., 2001; Marques & Sazima, 2003.
etation. Probably the only exception is where snake fauna extinction is definitive (e.g., Monte de Trigo Island) and where several wild carnivore predators were deliberately introduced and probably caused extinction of some representative species (e.g., Anchieta Island).
Studies of global extinctions of reptiles during the last 10,000 years demonstrate two clear patterns: the majority of extinc-tions occurred on islands and usually are associated with anthropic
Table 4. Sample effort.
Tabela 4. Esforço amostral.
Islands Trips Work
Group
Days in field
Total hours in the field
Alcatrazes 1 4 people 4 36
Anchieta 12 1-9 people 62 1877
Bom Abrigo 5 1-4 people 9 104
Búzios 2 4-5 people 5 146
Cananéia 30 3-6 people 39 1386
Cardoso 15 1-6 people 110 1516
Comprida 27 3-6 people 27 768
Couves 1 5 people 4 40
Ilhabela 2 3-6 people 7 386
Queimada Grande 29 3-13 people 50 2260
Vitória 1 5 people 1 20
Figure 44. Positive correlation between total sampling effort, hours of field-work, and the number of the new records of species.
Figura 44. correlação positiva entre esforço amostral total, horas de trabalho de campo e número de novos registros de espécies.
Species (n = 36)
Number of islands (n = 18)
Mcor Lmil Bjar Bjus Cbic Spul Cexo Hcar Sipu Clae Cmul Sneu Cfus Dpet Ecep Eesc Ochl Tdor Xneu Cplu Dalb Lpoe Dnei Emel Eund Tnat Tser Tstr Balc Bins Chor Dalt Ebil Icen Spun Wme
18 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0
Figure 43. Frequency of 36 snakes species on the eighteen islands of the state of São Paulo. Mcor = Micrurus corallinus;Lmil = Liophis mil-iaris;Bjar =Bothrops jararaca;Bjus = Bothrops jararacussu;Cbic=
-Chironius bicarinatus;Spul = Spilotes pullatus;Cexo = Chironius exoletus;
Hcar= Helicops carinicaudus;Sipu = Siphlophs pulcher;Clae = Chironius laevicollis;Cmul=Chironius multiventris;Sneu = Sibynomorphus neuwiedii; Cfus=Chironius fuscus;Dpet = Dipsas petersi;Ecep = Echinanthera cepha-lostriata;Eesc=Erythrolamprus aesculapii;Ochl =Oxyrhopus clathratus; Tdor=Tomodon dorsatus;Xneu = Xenodon neuwiedii;Cplu=Clelia plumbea;Dalb = Dipsas albifrons;Lpoe = Liophis poecilogyrus;Dnei=
-Dipsas neivai;Emel= Echinanthera melanostigma;Eund =Echinanthera undulata;Tnat=Thamnodynastes cf. nattereri;Tser = Tropidodryas serra;
Tstr =Tropidodryas striaticeps;Balc = Bothrops alcatraz;Bins =Bothrops insularis;Chor = Corallus hortulanus;Dalt = Dipsas alternans;Ebil= -Echinanthera bilineata;Icen = Imantodes cenchoa; Spun = Sordellina punctata;Wme =Waglerophis merremii.
Figura 43. Freqüência das 36 espécies nas 18 ilhas no Estado de São Paulo.
Mcor = Micrurus corallinus; Lmil = Liophis miliaris; Bjar = Bothrops jara-raca; Bjus = Bothrops jararacussu; Cbic = Chironius bicarinatus; Spul = Spilotes pullatus; Cexo = Chironius exoletus; Hcar = Helicops carinicaudus; Sipu = Siphlophs pulcher; Clae = Chironius laevicollis; Cmul = Chironius multiventris; Sneu = Sibynomorphus neuwiedii; Cfus = Chironius fuscus; Dpet = Dipsas petersi; Ecep = Echinanthera cephalostriata; Eesc = Erythrolam-prus aesculapii; Ochl = Oxyrhopus clathratus; Tdor = Tomodon dorsatus; Xneu = Xenodon neuwiedii; Cplu = Clélia plumbea; Dalb = Dipsas albifrons; Lpoe = Liophis poecilogyrus; Dnei = Dipsas neivai; Emel = Echinanthera melanostigma; Eund = Echinanthera undulata; Tnat = Thamnodynastes cf. nattereri; Tser = Tropidodryas serra; Tstr = Tropidodryas striaticeps; Balc = Bothrops alcatraz; Bins = Bothrops insularis; Chor = Corallus hortulanus; Dalt = Dipsas alternans; Ebil = Echinanthera bilineata; Icen = Imantodes cenchoa; Spun = Sordellina punctata; Wme = Waglerophis merremii.
coast and is a host for some protozoarians (Lainson & Paperna 1999), helminths (Anjos et al. 2005) and mites (Rivera et al. 2003) (category E). There are cases of parasite transport from reptiles to the islands (Goldberg & Bursey 2000; Rocha & Vrcibradic 2003). And finally there is the loss or degradation of habitat corresponding to categories A, C, D of Table 2. Corke (1987) documented the local extinction of
Clelia clelia and Liophis ornatus in the Lesser Antilles.
As expected from the classical theory of island biogeography (MacArthur & Wilson 1967), coastal islands of São Paulo State with a larger area tend to have a larger number of species. However, the data showed that the number of snake species decreased significantly with the distance of the island from mainland.
Thirteen species of snakes given as new records were sampled during the exiguous period, eventhough the capture effort has not been homogeneous for eleven of the field surveyed islands. Many other snake species are likely to exist and our findings should be considered preliminary, since during the brief fieldwork period we vouchered a specimen of Bothrops jararacussu from Bom Abrigo Island in the
IBSP collection forty three years after the last specimen was recorded. Five of the six more common species of the eighteen islands had a wide range of distribution on the continent also. At the present time two insular species are endemic and threatened, Bothrops alcatraz
(Marques et al. 2002) and Bothrops insularis (Duarte et al. 1995).
An interesting disjunct distribution occurs for Dipsas albifrons
which is present on Alcatrazes, São Sebastião and Queimada Grande Island. The known distribution of D.albifrons includes a large gap
along most of the continental portion of the State of São Paulo. Considering that São Paulo State has one of the largest populations in Brazil and that the Instituto Butantan has been receiving constant from São Paulo for more than a hundred years, it is likely that the disjunct distribution is not an artifact of poor sampling (Passos et al. 2005). Porto & Fernandes (1996) found the same pattern for Dipsas neivai, and suggested that natural events that caused regional
extinc-tions, were caused by transgression of the sea level in the Quaternary (cf. Müller 1969; Vanzolini 1973).
Similarly, two voucher specimens of Waglerophis merremii
were recorded in two different herpetological collections (IBSP and
0
New records
MZUSP) from Santo Amaro Island during the 1930´s. This colubrid snake is typical of open areas, but cannot be found in this kind of landscape along the southeastern coast of Brazil. This is not the case for some open area species along the coast of Bahia State, northeastern Brazil that inhabits areas close to the seashore (Crotalus durissus cascavella from Itaparica Island), Boa constrictor constrictor (Argôlo
2004; MRDuarte, pers. observation) and Waglerophis merremii.
A single specimen of Boidae (Corallus hortulanus) was recorded
for SSP islands. Conversely, Corallus hortulanus and Boa constric-tor are species commonly found on Ilha Grande (Grande Island) in Rio de Janeiro State (Soares et al. 1987). Two voucher specimens of
Boa constrictor from Santos (São Vicente Island) (IBSP 8.641 and
17.428) in 1934 and 1958 respectively, were excluded because they were collected in the neighborhood of the largest harbor of South America and passive transportation must be considered.
The integrity and preservation of SSP island herpetofauna is limited since, with few exceptions (e.g., Queimada Grande and Alcatrazes Islands), many of these island environments are under uncontrolled human occupation and few of them are under legal protection (see Table 2). A striking appeal for SSP island preserva-tion is the fact that 52% of the voucher specimens of snake species inhabiting these islands preys on amphibians that are sensitive to environmental disturbance.
Acknowledgments
We thank FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo) Programa BIOTA (Proc. 99/08291-5); IBAMA-RAN, Proc. 02027.004018/01-26; Instituto Florestal, Proc. 40.128. Antonio Bordignon, Luís Coelho and Ricardo J. Sawaya for photographies. Hussam Zaher, MZUSP; Francisco Luis Franco, IBSP, for the acc-cess to herpetological collections and Lígia Pizzato, ZUEC, for the ZUEC research. People of Cananéia: family Pedro Cardoso; Roberto Cardoso; family Rafael Xavier; family Carlos Lacerda Nunes, Cláudio e Selmo Bernardo; people Ilha Comprida: South, Santino Cardoso, Sítio Trincheira; “Paraíba”, do restaurante Morretinho; Juan Carlos Machado, “Rambo”; Glauco e Maurício do Boqueirão Norte. Directors of PEIA, Parque Estadual da Ilha Anchieta, and PEIC, Parque Estadual da Ilha do Cardoso, Viviane Buchianeri and Marcos Campolim respec-tively. Staff of PEIC, Romeu Mário Rodrigues, Ilso Neves, Nanqui, Sr. Roberto, Lair; IO-USP,Base Cananéia, for colaboration. Valmir Augusto from Instituto de Biociências da USP; Elaine Cardoso de Sá; Antonio Bordignon, Ricardo J. Sawaya, Eduardo Nakano, Luís Coelho, Paulo Sérgio Garrubo, Fernanda da Cruz Centeno and Herbert Serafim de Freitas for help with fieldwork. Wayne Seale, IF-USP, for help in english version. Comunities of Itacuruçá, family Junior Neves and fam-ily João Cardoso, Comunity Foles,Gelson and Comunities Cambriú, Marujá, Pontal do Leste, Enseada da Baleia from Ilha do Cardoso.
References
AMARAL, A. 1921. Contribuição para o conhecimento dos ophidios do Brasil. A Parte II. Biologia de uma nova espécie. Anexos das Memórias do Instituto Butantan, 1:39-44.
ÂNGELO, S. 1989. Ilhas do litoral paulista. Série Documentos. Secretaria do Meio Ambiente – SMA, São Paulo.
ANJOS, L.A.; ROCHA, C.F.D.; VRCIBRADIC, D. & VICENTE, J.J. 2005. Helminths of the exotic lizard Hemidactylus mabouia from a rock outcrop area in southeastern Brazil. Journal of Helminthology. 79:307-313. ARGÔLO, A.J.S. 2004. Serpentes dos cacauais do sudeste da Bahia. Ilhéus,
Bahia. Editus, 260p.
CASE, T, J., BOLGER, D. T. & RICHMAN, A., 1992. Reptilian Extinctions: The last ten thousand years. In: Conservation Biology: the theory and
practice of nature conservation, preservation, and management. (P. L. Fiedler & S. K. Jain, eds.). Chapman & Hall, New York. p.91-125. CENTENO, F.C. 2003. Serpentes de Ilhabela: taxonomia, composição
faunís-tica e história natural. São Bernardo do Campo: UMESP. Unpublished monograph.
CICHI, P.J.P. 2004. Composição faunística de serpentes das ilhas do litoral do Estado de São Paulo. São Bernardo do Campo: UMESP. Unpublished monograph.
CORKE, D. 1987. Reptile Conservation on the Maria Islands (St Lucia, West Indies). Biological Conservation. 40:263-279.
DEAN, W. 1996. A ferro e fogo: a história e a devastação da Mata Atlântica brasileira. São Paulo, Companhia das Letras. 484p.
DUARTE M.R.; PUORTO, G. & FRANCO, F.L. 1995. A biological survey of the pitviper Bothrops insularis Amaral (Serpentes, Viperidae): an endemic and threatened offshore island snake of southeastern Brazil. Studies on Neotropical Fauna and Environment. 30(1):1-13.
GOLDBERG, S. R. & BURSEY, C. R. 2000. Transport of helminths to Hawaii via the brown anole, Anolis sagrei (Polychrotidae). J. Parasitol. 86(4):750-755.
HOGE, A.R. 1950. Notas erpetológicas 7. Fauna erpetológica da Ilha de Queimada Grande. Mem. Inst. Butantan. 22:151-172.
HOGE, A.R., BELLUOMINI, H.E., SCHREIBER, G. & PENHA, A.M. 1959. Sexual abnormalities in Bothrops insularis (Amaral, 1921). Mem. Inst. Butantan. 29:17-88.
IHERING, H. von. 1897. A Ilha de São Sebastião. Ver. Mus. Paul. 2:129-171.
LAINSON, R. & PAPERNA, I. 1999. Re-descriptions of Isospora ameivae
Carini, 1932 in the Teiid Lizard Ameiva ameiva and Isospora hemidac-tyli Carini,1936 in the Gecko Hemidactylus mabouia, with Particular Reference to their Endogenous Stages. Mem Inst Oswaldo Cruz, Rio de Janeiro. 94(4):459-466.
LUEDERWALDT, H. 1923. A Ilha de Alcatrazes. Rev. Mus. Paul. 13:441-512.
MAC ARTHUR, R. H. & WILSON, E. O. 1967. The theory of island bioge-ography. Princeton University Press. Princeton, New Jewrsey. MARQUES, O.A.V., ETEROVIC, A. & SAZIMA, I. 2001. Serpentes da
Mata Atlântica: Guia ilustrado para a Serra do Mar. Ribeirão Preto: Holos. 184p.
MARQUES, O.A.V., ETEROVIC, A. & ENDO, W. 2000. Seasonal activity of snakes in the Atlantic forest in southeastern Brazil. Amphibia-Reptilia, 22:103-111.
MARQUES, O.A.V., MARTINS, M. & SAZIMA, I. 2002. A new insular species of pitviper from Brazil, with comments on evolutionary biology and conservation of the Bothrops jararaca group (Serpentes, Viperidae).
Herpetologica. 58(3):303-312.
MARQUES, O.A.V. & SAZIMA, I. 2004. História Natural dos Répteis da Estação Ecológica da Itatins. In: Estação Ecológica da Juréia-Itatins: ambiente físico, Flora e Fauna. (Otávio AV Marques & Wânia Duleba, eds.). Ribeirão Preto: Holos Editora. p.257-277.
MERTENS, R. 1955. Eine brasilianische Inselfahrt. 1. Queimada Grande, die Insel der Giftschlangen. Natur u. Volk. 85(10):305-314.
MÜLLER, P. 1968. Die Herpetofauna der Insel von São Sebastião (Brasilien). Saarbrücken Zeitung, Verlag und Druckerei, Germany.
MÜLLER, P. 1969. Vertebratenfaunen brasilianischer inseln als indikatoren für glaziale und postglaziale vegetationsfluktuationen. Zool. Anz., Suppl. 33:97-107.
OLMOS, F., SÃO BERNARDO, C.S. & GALETTI, M. 2004. O impacto dos Guarani sobre Unidades de Conservação em São Paulo. In: Terras indígenas e unidades de conservação da natureza – O desafio das sobre-posições. (Ricardo Fanny, ed.), Editora Socioambiental.
PASSOS, P., FERNANDES, R. & PORTO, M. 2005. Geographical varia-Geographical varia-tion and taxonomy of the snail-eating snake Dipsas albifrons (Sauvage, 1884), with comments on the systematic status of Dipsas albifrons cavalheiroi Hoge, 1950 (Serpentes: Colubridae: Dipsadinae). Zootaxa,
PORTO, M. & FERNANDES, R. 1996.Variation and natural history of the snail-eating snake Dipsas neivai (Colubridae: Xenodontinae). Journal of
Herpetology. 30(2):269–271.
RIVERA, C.C., NEGRÓN, A. G., BERTRAND, M. & ACOSTA; J. 2003.
Hemidactylus mabouia (Sauria: Gekkonidae), host of Geckobia hemidac-tyli (Actinedida: Pterygosomatidae), throughout the Caribbean and South America. Caribbean Journal of Science. 39(3):321-326.
ROCHA, C.F.D & VRCIBRADIC, D. 2003. Nematode assemblages of some insular and continental lizards hosts of the genus Mabuya Fitzinger (Rep-tilia, Scincidae) along Brazilian coast. Rev. Bras. Zool. 20(4):755-759. RODRIGUES, M.T. 2005. The conservation of Brazilian reptiles: Challenges
for a Megadiverse Country. Conservation Biology. 19(3):659-664. SOARES, M.A., PORTO, M.A., RANGEL, S. & CARAMASCHI, U. 1987.
Levantamento preliminar da herpetofauna da Ilha Grande (Angra dos
Reis, RJ). XIV Congresso Brasileiro de Zoologia, Juiz de Fora, MG. p.256. Abstract 724.
VANZOLINI, P.E. 1973. Distribution and differentiation of animals along the coast and in continental islands of the State of São Paulo, Brasil. I. Introduction to the area and problems. Pap. Av. Zool. 26(24):281-294. VIEITAS, C.F. 1995. Análise ambiental das ilhas da região de Ubatuba (SP) e
proposta de manejo para a ilha do Mar Virado. Dissertação de Mestrado. Universidade de São Paulo, São Paulo.
VITOUSEK, P.M., ADSERSEN, H. & LOOPE, L.L. 1995. 1 Introduction – Why focus on Islands? In: Islands: biological diversity and ecosystem function. pp. 1-4. (PM Vitousek, LL Loope & H Adsersen., eds.) Ecologi-cal Studies, v.115. Berlin: Springer-Verlag.
WHITTAKER, R. J., 1998. Island Biogeography: Ecology, evolution, and conservation. Oxford Univerd Press, New York.
BOIDAE
1. Corallus hortulanus Santo Amaro Island IBSP62926
COLUBRIDAE
Chironius bicarinatus: Anchieta Island IBSP 16023. Búzios
IslandIBSP 56236, 56951. MZUSP3941-44, 4831-75. Cananéia IslandIBSPField no. 2791. *Cardoso Island IBSP Field no. 1873. *Comprida Island IBSP 70529 IBSP Field no. 2740. Mar Virado Island (Vieitas, 1995). Porchat Island1IBSP 14265, 14435. Santo
Amaro Island IBSP 22250, 68184. São Sebastião Island IBSP 56009, 58723. São Vicente Island IBSP 15850, 18761.
Chironius exoletus: Cananéia Island IBSPField no. 0671. Car-doso Island IBSP 55876, 70748, 72186, 72187 IBSP Field no. 1873, 0095-96, 1247. Comprida Island IBSP 11472-73, 11479, 31887. Mar Virado Island (Vieitas, 1995). Porchat Island1.IBSP 14436, 14439,
14544-46. Santo Amaro Island IBSP 22386, 22392, 28106, 29519, 29542, 29691. MZUSP5611. São Vicente Island IBSP 704, 13934-35, 18113934-35, 22090-91, 22249, 22571, 37375, 57389, 58363, 70043.
Chironius fuscus: Cardoso Island IBSP 43488, 43626, 70749,
71393, 71711, 72188 IBSPField no. 1873, 3693, 0098. Porchat Island1IBSP 14437-38. Santo Amaro Island IBSP 8285, 21488,
23977, 26309, 40870, 42456, 43632, 44125, 44334. MZUSP3177. São Vicente Island IBSP 2588, 13932, 13936, 15481, 23847, 27990, 30960, 32671.
Chironius laevicollis: Cardoso Island IBSP 56135, 71001 IBSP
Field no. 2454, 1247. MZUSP8866. Comprida Island IBSP 11468-69, 55973. São Sebastião Island IBSP 54190, 57433. ZUEC1061. São Vicente Island IBSP 63635.
Chironius multiventris: Cananéia Island IBSPField no. 4458,
0671. *Cardoso Island IBSP 71710, 71855, 72185, 72190 IBSP Field no. 3692, 0093-94. Santo Amaro Island IBSP 22042-43, 22106, 22387, 22571, 24326-28, 24590, 25349, 40870, 42707, 45115, 45913, 58409. São Sebastião Island IBSP 54190, 57096, 57353. ZUEC 1062. São Vicente Island IBSP 8069, 22089, 29085, 33459.
Clelia plumbea: Cardoso Island IBSPField no. 955. São
Se-bastião Island IBSP 20420, 21696, 26977, 43981, 56932. São Vicente IslandIBSP 21996, 22497, 29852.
Dipsas albifrons: Alcatrazes Island IBSP 13029, 62175.
Quei-mada Grande Island IBSP 11488, 15808, 15809, 17151, 17213, 18426-27, 29727, 30088-90, 30092, 30094-95, 52670, 55723. São Sebastião Island IBSP 60345.
Dipsas alternans: São Sebastião Island IBSP 55951.
Dipsas petersi: *Cardoso Island IBSP Field no. 3171. Santo
Amaro Island IBSP 24165, 24478-80, 24636, 25934, 26040, 26091, 42458, 43999, 49231, 57302, 64909. São Sebastião Island IBSP 53647, 57090. São Vicente Island IBSP 9244, 10391, 13938-40, 25934, 41093, 55345, 55946-47.
Dipsas neivai: Cananéia Island IBSP 41999. São Sebastião Island
IBSP 41027, 55877, 56628.
Echinanthera bilineata: *Cardoso Island IBSPField no. 1247.
Echinanthera cephalostriata: *Cardoso Island IBSPField no.
2103. Santo Amaro Island IBSP 22572, 44127. São Sebastião Island IBSP 56933, 57089. São Vicente Island IBSP 22251, 54814.
Echinanthera melanostigma: Santo Amaro Island IBSP 42336. São Vicente Island IBSP 22572.
Echinanthera undulata: Comprida Island IBSP 52204. São
Vicente Island IBSP 56343.
Erythrolamprus aesculapii: Barnabé Island IBSP 28268. Ca-nanéia Island IBSP Field no. 3367. São Sebastião Island IBSP 12902, 13584. São Vicente Island IBSP 22215, 28968, 30043.
Helicops carinicaudus: Cananéia Island IBSPField no. 2726,
2021, 2531. *Cardoso Island IBSPField no. 3371. *Comprida Island IBSPField no. 0670. Porchat Island1IBSP 14267. Santo Amaro
IslandIBSP 43013, 45160, 63032, 67814, 68295. São Vicente Island IBSP 800, 14267, 22094, 30965, 32484-86, 34095, 66996, 68118, 68307.
Imantodes cenchoa: Cardoso Island IBSP Field no. 152.
Liophis miliaris: Anchieta Island IBSP 15818-21. Bom Abrigo
Island 22723-24. Cananéia Island IBSP Field no. 0260, 0671, 1863, 1869, 2531, 2677, 2796, 3106. Cardoso Island IBSP 56129, 71020 IBSPField no. 2454, 2521, 3171. MZUSPdata missing. Comprida IslandIBSP 11477-78, 71175, 71313 IBSPField no. 2740, 3035, 3171. *Couves Island (North coast) IBSP 70127. Porchat Island1
IBSP 14263, 14337-40, 14422-34, 14548, 59220. Santo Amaro IslandIBSP 18287, 20856, 23992, 28001, 30234, 67349, 68401.
MZUSPdata missing. São Sebastião Island IBSP 11611, 27541.
São Vicente Island IBSP 2607, 13933, 14263-64, 14337-40, 14422-35, 14548-49, 19723, 22072-73, 22076-78, 61395, 68306, 69189.
MZUSP 4072; 4155-56, 4594-95, 4597, 4570-71, 788-9. Vitória
IslandIBSP 15822-26.
Liophis poecilogyrus: Cardoso Island IBSPField no. 955. Santo Amaro Island IBSP 5304, 73190. São Vicente Island IBSP 7284.
MZUSP4596.
Oxyrhopus clathratus: Cananéia Island IBSPField no. 3265,
1877. Cardoso Island IBSP 62463 IBSP Field no. 3171. São Sebastião IslandIBSP 10064, 26877, 57724, 68430, 69919. São Vicente Island IBSP 5778.
Sibynomorphus neuwiedi: Cananéia Island IBSPField no. 2781,
4246, 4458, 0258, 2793, 2799. Cardoso Island IBSP 56134 IBSP Field no. 3371. Santo Amaro Island IBSP 18288, 22044, 70108. São Sebastião Island IBSP 31157, 56245, 56440, 55950, 67857, 69129. São Vicente Island IBSP 19029, 43235.
Siphlophis pulcher: Alcatrazes Island IBSP 13030. Búzios Island
MZUSP 3945-46.ZUEC 2226. Cananéia Island IBSPField no.
2799. Santo Amaro Island IBSP 22398, 22465, 33119, 51902, 56637, 63235. São Sebastião Island IBSP 58492, 58621, 62854. São Vicente IslandIBSP 10449, 13937, 41222, 45793.
Sordellina punctata: Comprida Island IBSP 40851.
Spilotes pullatus: *Anchieta Island IBSP 74439, 74440. Cananéia IslandIBSPField no. 2025, 2041. Cardoso Island IBSP 29199 IBSP Field no. 1259, 1131, 2454, 3171, 0099. MZUSP10254. Comprida IslandIBSP 11470-71, 67752-53 IBSPField no. 3266. Porchat Island1IBSP 14460-61. Santo Amaro Island IBSP 8492, 19660.
São Sebastião Island IBSP 12237, 57535. São Vicente Island IBSP 13260, 13942, 13962-63, 14907, 15416, 15418, 30905.
Thamnodynastes cf. nattereri: Comprida Island IBSP 45873,
52203, 69182. São Sebastião Island IBSP 23677,40921,40926, 40927, 42945, 44776, 44778, 53736, 54215, 55118, 57092, 57494, 57725, 62020, 64084, 68482, 70741.
Tomodon dorsatus: Cananéia Island IBSPField no. 0673.
Car-doso Island IBSPField no. 1143. Porchat Island1IBSP 14440-48.
São Vicente Island IBSP 7892, 14342, 14460-61, 15686, 18425, 22095-96, 56208, 67558.
Tropidodryas serra: Cardoso Island IBSP 56209, 56521, 57423, 59463IBSPField no. 3171, 4228. MZUSP10240. Santo Amaro IslandIBSP 27126, 28226, 31943, 42240, 47052, 47103.
Tropidodryas striaticeps: Cardoso Island IBSPField no. 3643.
Santo Amaro Island IBSP 18650.
Xenodon neuwiedii: Cananéia Island IBSPField no. 0263, 0671.
*Cardoso Island IBSPField no. 0699. Santo Amaro Island IBSP
68620. São Sebastião Island MZUSP2607.
Waglerophis merremii: Santo Amaro Island IBSP 8644. MZUSP 3594.
ELAPIDAE
Micrurus corallinus: Alcatrazes Island IBSP 62274-75. Anchieta IslandIBSP 417-18, 2653. Barnabé Island IBSP 29684. Búzios Island
IBSP 56223. MZUSP3935-39. Cananéia Island IBSP Field no. 2454.
Cardoso Island IBSP 70752 IBSPField no. 1873, 1844. MZUSP 9970, 10239. Comprida Island IBSP 7184, 8468, 11476, 31891, 41697, 42212, 42302, 42306, 42352, 42602, 42608, 42688, 42891, 42987, 43010, 43266, 43769, 43936, 45879, 46250, 46317, 49256, 50498, 71151IBSPField no. 1691, 0250. MZUSP8209, 12404. Porchat
Is-land1IBSP 14268, 14464-65. Santo Amaro Island IBSP 21329, 24396,
31892, 41831, 42174, 45435, 46379, 46557, 49484, 51563. MZUSP 3539, 10499-500, 11591. São Sebastião Island IBSP 12219, 12455, 12789-90, 12811, 12901, 13589, 14221, 25044, 42568, 45307, 49722, 56309, 57404, 60871, 61106, 62825, 68481. São Vicente Island IBSP 11312, 14268, 14341, 14464-65, 14614, 15415, 22498, 22526, 23654, 23831, 24164, 29444, 30948, 30979, 32260, 32974, 37322, 40263, 40400, 42359, 42373, 42985, 44261, 45983, 47674, 50697, 53394, 53402, 55725, 62383, 62434, 68684. Vitória Island MZUSP3947.
VIPERIDAE
Bothrops alcatraz (Endemic): Alcatrazes Island IBSP 584-88,
13031-32, 13126, 13135, 13183, 16211-12, 56133, 55124, 55578-81, 55791, 57105, 57107, 62238, 62290, 62392. MZUSP1453. ZUEC 2224-25.
Bothrops insularis (Endemic): Queimada Grande Island IBSP n
> 500. ZUEC1869-72.
Bothrops jararaca: Anchieta Island IBSP 417-18, 655. Búzios
Island IBSP 13133, 26160-63, 56256, 56952, 57253. MZUSP 3862, 3934. ZUEC 2713. Cananéia Island IBSPField no. 3698-99, 2016, 2054, 273698-99, 3366. Cardoso Island IBSP 56302, 56388, 57106, 57475 IBSPField no. 1502, 2454, 3171, 3265. Comprida Island IBSP 11474. *Porcos Island (Ricardo Janini Sawaya, per-sonal communication). Porchat Island1IBSP14450-59, 14562-64.
Santo Amaro Island IBSP 55524, 55863-64, 57246, 64763, 69008.
MZUSP3583-84. São Sebastião Island IBSP n > 200. São Vicente
Island IBSP 10459, 13922, 14262, 14450-59, 14562-64, 15840, 18722, 58322, 61373, 62316, 62765. Vitória Island IBSP 18866-82.
MZUSP3949-52, 5577-85.
Bothrops jararacussu: Anchieta Island IBSP 16307-09, 62833,
64672, 64917-18. Bom Abrigo Island IBSP 22720-22 IBSPField no. 3370. Cananéia Island IBSPField no. 0671, 0673, 2531, 2544, 2740, 3699. Cardoso Island IBSP 53807, 56210, 56429, 56472, 70750-51, 71225 IBSPField no. 1259, 1845-46, 2017, 2031, 2788, 3371, 3908. *Comprida Island IBSPField no. 2723. Porchat Island1