I
Resumo
Os estuários são dos ecossistemas aquáticos mais produtivos do planeta, sendo contudo, também dos mais impactados pela vida humana. Estes são cruciais na dinâmica populacional de muitas espécies de peixes. Muitos peixes marinhos utilizam-no durante breves períodos, especialmente nas fases iniciais do seu ciclo de vida, o que se deverá a uma maior abundância de presas, redução de risco de predação e redução da competição com indivíduos adultos, enquanto outros são verdadeiros residentes estuarinos podendo completar todo o seu ciclo de vida dentro do estuário. Os estuários oferecem ainda uma grande diversidade de habitats diferentes passíveis de ser usados pelas espécies de peixes. A perda destes habitats pode originar numa perda de biodiversidade do estuário, e em reduzidas abundâncias das espécies que o usam nos seus ciclos de vida. Isto torna-se especialmente importante devido ao facto dos estuários servirem como áreas de viveiro para muitas espécies de peixes comercialmente importantes. Assim, torna-se essencial arranjar metodologias eficazes para determinar a importância de um determinado habitat para o estuário e no ciclo de vida dos peixes que os habitam e consequentemente, estabelecer prioridades de conservação destes mesmos habitats. Os parasitas são considerados como eficazes como marcadores naturais, sendo usados em estudos de diferenciação populacional, filogenéticos, migratórios, de ecologia alimentar e, mais recentemente, na distinção do uso de habitat por peixes. Consequentemente o presente estudo tem como objectivo verificar se os parasitas são úteis como ferramenta de distinção do uso de habitat por peixes em estuários da costa Portuguesa.
A tese é dividida em três capítulos, em que um é um artigo científico produzido para responder a estes objectivos. Este artigo é precedido por um primeiro capítulo que corresponde a uma introdução geral, e sucedido por um capítulo que integra as considerações finais e perspectivas futuras.
Na introdução geral, capítulo 1, são abordados os processos gerais que levam a este estudo, com especial atenção à importância dos estuários e dos seus habitats no ciclo de vida das espécies de peixes que os habitam. É indicado, também, a importância dos estudos espaço-temporais na determinação da importância dos habitats estuarinos, sendo mencionados os principais meios de determinação dos padrões de movimentação dos peixes e da conectividade entre habitats. São referidas as vantagens dos marcadores naturais face aos artificiais, em especial as vantagens do uso de parasitas como marcadores naturais, assim como o princípio subjacente ao seu
II
uso como marcadores. É referida a importância dos parasitas e dos estudos parasitológicos para melhor compreender a ecologia das espécies de parasitas e hospedeiros, e os factores que influenciam a comunidade de parasitas nos diferentes habitats. Os estudos anteriores que usam as diferenças na comunidades de parasitas para a distinção do uso de habitat por diversos peixes são também indicados, assim como os objectivos específicos do presente trabalho.
O capítulo 2 corresponde a um artigo científico em que são estudadas as comunidades de parasitas do caboz, Pomatoschistus microps em três habitats físicos distintos em cinco estuários portugueses: plataformas vazosas intertidais, sapais e canais subtidais. É demonstrado a importância destes habitats para as espécies de peixes estuarinos, em particular para o P. microps. Pretendeu-se com este estudo caracterizar as comunidades de parasitas nestes habitats para os cinco estuários portugueses e perceber se as comunidades de parasitas podem ser utilizadas como ferramenta para indicar o uso dos habitats estuarinos por P. microps. Os exemplares de P. microps foram recolhidos nos meses de Julho e Outubro, nos estuários da Ria de Aveiro, Tejo, Sado, Mira e Guadiana. Em cada estuário foram amostrados os três habitats (plataformas vazosas, sapais e intertidais) em duas localizações similares do estuário recorrendo ao uso de um arrasto de vara ou chincha dependendo do habitat amostrado. Os endoparasitas do P. microps foram recolhidos e foram calculados os índices parasitológicos prevalência e abundância média correspondentes a cada habitat amostrado no estuário. Subsequentemente usaram-se duas análises de correspondência (CA) com base nestes índices parasitológicos, para evidenciar possíveis diferenças entre as comunidades de parasitas. O P. microps apresentou dez taxa de parasitas diferentes, 2 pertencentes ao filo Nematoda e as restantes 8 pertencentes a sub-classe Digenea, sendo os parasitas mais representados nos 5 estuarios: Lecithochirium musculus, Hemiurus appendiculatus, Timoniella sp., Diplostomum sp. e um diplostomidae não identificado, sendo o parasita L. musculus e H. appendiculatus os únicos destes parasitas descritos a serem encontrados em estado adulto. Os parasitas encontrados apresentaram algumas afinidades por alguns habitats, podendo estar associados a esses habitats, tais como o nematode Raphidascaris acus e um diplostomidae não identificado. De facto, a CA efectuada com os dados de abundância média demonstrou que existiam várias diferenças nas comunidades de parasitas, dos diferentes habitats e diferentes estuarios. Alguns habitats demonstraram-se bem isolados no diagrama da analise o que indicou que as comunidades de parasitas nesses habitats eram diferentes dos restantes. As diferenças encontradas entre as comunidades de parasitas de habitats diferentes
III
foram maioritariamente entre sapais e as plataformas vazosas, demonstrando que o uso que os P. microps fazem desses habitats é diferente. No entanto, outras diferenças emergiram da análise, que demonstrou que há diferenças entre alguns locais de amostragem, nomeadamente nos estuários do Tejo, Sado e Ria de Aveiro. Estas diferenças entre locais devem-se a diferenças acentuadas de certas espécies de parasita que podem ser devido a diferenças na abundância dos seus hospedeiros intermediários, ou devido às condições físico-químicas não serem ideais para a transmissão dos estádios de vida livre destes parasitas. Já a análise de correspondência efectuada com os dados da prevalência de parasitas em cada habitat estuarino, demonstra que os estuários são agrupados independentemente, não havendo no entanto uma grande diferenciação entre os habitats e entre os locais de amostragem, com a devida excepção das amostras do Sapal do Tejo de Outubro que conseguem uma boa diferenciação devido à diferença acentuada na prevalência de R. acus, e das amostras do local de amostragem da Gâmbia no Sado devido à prevalência de Z. viviparous. Comprova-se com este artigo que com base nas diferenças parasitológicas há diferenças no uso dos diferentes habitats pelos indivíduos da espécie de P.microps, sugerindo assim, que os parasitas podem ser usados como tags naturais de uso de habitats por peixes estuarinos.
No capítulo 3, são evidenciadas as perguntas que permaneceram em aberto após este estudo e a importância dos estudos de diferenciação de utilização de habitat por peixes estuarinos. É evidenciada a necessidade de confirmar este estudo com outros que utilizem diferentes técnicas para estudar as diferenças espaço-temporais dos peixes estuarinos nos diversos habitats, entre as quais o uso de marcadores artificiais, o uso de medições de isótopos estáveis e a microquímica de otólitos. Refere-se, também a necessidade deste estudo ser continuado a longo prazo, de modo a contrariar e prever quais os efeitos sazonais nas comunidades de parasitas que infectam os peixes estuarinos, bem como, a necessidade de se replicar este estudo investigando os padrões de uso destes habitats por outras espécies de peixes que usem o estuário durante o seu ciclo de vida, principalmente espécies com elevado interesse comercial. É referido também, a necessidade de complementarmente a um estudo dos macroendoparasitas existir um estudo da abundância e diversidade das comunidades das principais presas dos peixes estudados.
Palavras-chave: Parasitas; Marcadores naturais; Diferenciação de habitat; Conectividade; Ecologia estuarina;
IV
Abstract
Estuaries are one of the most valuable ecosystems on earth, being crucial in the development of many fish species. They consist of a fine mixture of intrinsically connected habitats that can be used by fish. Any loss of these habitats is reflected in loss of diversity and abundance of estuarine species. Therefore there is a growing need to develop tools that can be used to manage the conservation of these habitats. Parasites have long been considered good natural tags for stock discrimination, philogenetic, migration and feeding ecology studies. More recently, they have been found to be usefull as a reliable natural tag of habitat use by some fish species. In order to assess if parasites are natural tags of habiat use in estuaries, the parasite assemblages of a typical estuarine species, Pomatoschistus microps, were investigated in five Portuguese estuaries (Ria de Aveiro, Tejo, Sado, Mira, Guadiana). There, in July and October, P. microps specimens were captured in three different habitats (Intertidal mudflat, Saltmarshes and Subtidal channel) in two similar locations of the estuary. P. microps, was infected with ten different parasite taxa, eight Digenea species and two Nematoda species. Correspondence analyses of both parasite prevalence and mean abundance suggested that there are habitat related differences in P.microps parasites communities, and therefore that P. microps uses the three sampled habitats differently. With the present study it is suggested that parasites can be a good tool to assess habitat preference of estuarine fishes.
Key-words: Parasites; Natural tags; Habitat differentiation; Conectivity; Estuarine ecology;
Table of contents
Resumo ... I
Abstract ... IV
Chapter 1 ... 1
General introduction ... 3
Chapter 2... 7
Parasite assemblages as a natural tag of the habitat use of P. microps in five
estuaries in the Portuguese Coast ... 9
Abstract ... 9
Introduction ... 10
Material and methods ... 11
Results ... 14
Discussion ... 18
Chapter 3... 23
Final remarks ... 25
References ... 26
Agradecimentos ... 33
1
3
General introduction
Estuaries are very important in nutrient recycling, food production, recreation and as habitat for many species (Costanza et al., 1997), supporting very rich and dense fish and invertebrate communities. Fishes use estuarine systems very differently, although very few species use it during their entire life-cycle (Elliott & Dewailly, 1995). Estuaries are especially important as nursery grounds for many commercially important fish species due to higher prey abundance, lowered predation risk and lower competition with adult individuals of the same species (Haedrich, 1983; Miller et al., 1985; Lenanton & Potter, 1987; Beck et al., 2001). Diversity and abundance of species in an estuary are dependent of its geographical location, relation with adjacent marine and freshwater habitats and dimension and depth, all conditioning the environmental characteristics of the estuary such as salinity, turbidity, dissolved oxygen and temperature (Mclusky & Elliott, 2004; Nicolas et al., 2010). Another very important condition that influences all estuarine communities is habitat availability and its extension. Estuaries are composed of a variety of habitats associated with each other (Pihl et al., 2002; França et al., 2009) such as saltmarshes, mangrove forests, seagrass meadows, oyster reefs and nonvegetated areas. Any loss in these habitats can lead to a loss in diversity and abundance of the organisms that use them, and can even lead to a decrease in the abundance of commercially important fishes in the adjacent coastal areas as some habitats are important nursery grounds for many commercially important fish species (Connelly, 1994; Jenkins et al., 1997). Previous studies have already evidenced the importance of specific habitats within an estuary, showing that some support higher densities and diversity of fishes than others. Vegetated habitats, such as seagrass meadows or saltmarshes are known to support higher diversity and abundance of organisms than adjacent nonvegetated areas (Sogard & Able, 1991; Connelly, 1994; Jenkins et al., 1997; Rozas & Minello, 1998) although some nonvegetated habitats support higher densities of some particular species (Castellanos & Rozas, 2001).
Estuaries in the coast of Portugal have long been appointed as very important in the life-cycles of some fish species that use them as nurseries (e.g. Cabral & Costa, 1999; Pombo et al., 2002; Veiga et al., 2006; Cabral et al., 2007; Martinho et al., 2007; Vasconcelos et al., 2010). França et al. (2009) found clear differences in the densities and abundances of fishes in different habitats in estuaries along the Portuguese coast, suggesting that fish use available habitats differently. In addition to their physical and chemical differences, these estuaries are subjected to different anthropogenic impacts
4 that can also influence their use by fishes. Although a wide scientific knowledge of the functioning and importance of Portuguese estuaries has been acquired, it has not yet been applied in the management of these systems (Vasconcelos et al., 2007). The importance of understanding how fish select and use these habitats has also been recognized and fish connection with their habitats is known to be very dynamic and complex (França et al., 2009). Both artificial and natural tags have been used thoroughly in order to assess fish spatio-temporal movements. Artificial tags, ranging from the traditional t-bar tags to the more sophisticated internal tags and chemical tags, have the easy recognition of the tagged organism as a principle. Although very useful, artificial tags have limitations that make them unreliable to use in some cases, either because they are very expensive and time consuming (many of these techniques involve capture and recapture of fishes) or because they are unsuitable to use in some fishes (e.g. very small fish). Many studies have sought to overcome these problems by employing techniques using naturally occurring variations in the individuals caused by different environmental conditions in the locations that fishes inhabited during their life -cycles. These natural tags, such as otolith elemental signatures, stable isotopes and parasitological infections, have been successfully employed on fish movement and connectivity studies, presenting a good trade-off between the effort/time and the information obtained (Gillanders et al., 2003).Fishes are usually parasitized to some extent and using parasites as natural tags has been very important to study fish origin, migrations, feeding ecology, phylogeny and population structure (e.g. Williams et al., 1992; Mackenzie, 1999, 2002; Chambers & Dick, 2005; Marques et al., 2006; Muñoz et al., 2006; Durieux et al., 2007). The use of parasites as natural tags relies on the fact that fishes can only be infected with a parasite if they move to an area suitable for its transmission (Mackenzie, 2005). Parasites are affected by the different conditions of the habitats their hosts use. Physicochemical conditions such as salinity, temperature, pH and pollutants affect all the free-living stages of parasites, generally leading to increased or decreased abundances of these parasites (Pietrock & Marcogliese, 2003). But one of the most important factors leading to differences in the abundance and prevalence of parasites is the distribution (densities and abundances) of their intermediary and final hosts, which may allow distinguishing the way that fishes use available habitats. Previous studies focusing coral reefs (Grutter, 1998) and coastal areas (Durieux et al., 2010) suggested that parasites were promising tools to study habitat use. However, parasitological studies to distinguish habitat use within an estuary are still lacking. The present study assesses differences in the composition of parasitic communities of fish in different estuarine habitats, and if these differences are strong enough for parasites to be used as a tool when assessing habitat preference by
5 estuarine fishes, contributing to diminish the gap in studies using parasites in small-scale studies.
7
9
Parasite assemblages as a natural tag of the habitat use of P. microps in
five estuaries in the Portuguese Coast
Abstract
In order to study the usefulness of parasites as natural tags of habitat use of P. microps three different habitats (Intertidal mudflat, Saltmarshes, Subtidal Channel) were sampled in five estuaries (Ria de Aveiro, Tejo, Sado, Mira, Guadiana) in the Portuguese Coast in July and October of 2009. The class Digenea and phylum Nematoda were found, with the digenetic trematoda L. musculus and an unidentified metacercaria being the most prevalent attaining a maximum of 67% and 57% respectively. The same unidentified metacercária was also the most abundant taxa reaching a max of 7.33 parasites per fish in a mudflat of the Guadiana estuary. Some parasites showed different mean abundances and prevalences between different habitats. Diplostomum sp. appeared to be related with mudflats while R. acus and an unidentified Diplostomidae appear to be related with saltmarshes. Correspondence analysis (CA) of prevalence shown differences between estuaries sampled, with little evidence of habitat differentiation, while the CA of squared root mean abundance was more informative, showing that habitat habitat differentiation was possible. These differences between habitats were explained by different intermediary hosts abundances. Overall, P. microps was found to use estuarine habitats differently and parasites were found to be good natural tags of habitat use.
10
Introduction
Estuaries are one of the most productive and valuable aquatic ecosystems on Earth (Costanza et al., 1997), with upmost importance for many fish species. Fish may use it permanently (estuarine species), occasionally (freshwater and marine adventitious species), transitionally (catadromous or anadromous species), or as nursery grounds (Franco et al., 2008). Estuaries comprise various habitats, intrinsically connected to each other (Pihl et al., 2002). Habitat selection by fishes is related with habitats availability, structural complexity, environmental conditions and predator and prey abundance and consequently some species attain high densities in a specific habitat (Blaber & Blaber, 1980). In order to better manage and prioritize habitat protection is therefore crucial to understand how fish select and use those habitats.
The common goby, Pomatoschistus microps (Krøyer, 1838) is one of the most abundant species in European waters. It is common in coastal lagoons, estuaries and shores occurring in shallow waters on sandy or muddy bottoms, in which it plays a major role as a benthic predator (Miller, 1986) and as an important prey of commercially important fishes and piscivorous birds (Costa, 1988). França et al. (2009) found that P. microps attained the highest population densities in tidal freshwater, soft-bottom subtidal and saltmarsh areas, suggesting some degree of habitat preference. To study fish spatio-temporal dynamics many methods have been employed each presenting advantages and limitations. Artificial tags have been widely used in some situations but are too expensive and time consuming or unsuitable to use in some fish. Natural tags (otolith microchemistry, stable isotopes and parasites), despite their limitations (e.g. otolith chemichal signatures must be stable overtime, turnover time for some stable isotopes may be too quick to track movements, parasitic communities must be different in the habitats sampled and the infection must be maintained for a long period to produce significant differences), have been found to be well suited to study fish movements within different habitats (Gillanders et al., 2003). Amongst natural tags, parasites have been commonly used to distinguish large scale variations in fish assemblages, such as stock discrimination (e.g. Mackenzie, 1990; McClelland et al., 2005; Marques et al., 2006; Durieux et al., 2007), population structure (e.g. Marques et al., 2005) and depth related differences (e.g. Oliva et al., 2004). The underlying principle of using parasites as tags relies on the fact that fishes are only infected with a parasite if they move to an area suitable for its transmission (Mackenzie, 2005). Although playing a major role in intertidal ecosystems, where they shape the composition and structure of animal communities (Mouritsen & Poulin,
11 2002), only a few studies have considered parasites as indicators of small scale variations (e.g. Grutter, 1998; Vignon et al., 2008) and only recently were they used to measure patterns of habitat use by juveniles of Solea solea (Linnaeus, 1758) in coastal areas of the Bay of Biscay, France (Durieux et al., 2010).
A study conducted by Freitas et al. (2009) on the parasite fauna of P. microps in five Portuguese estuaries evidenced differences in macroparasites assemblage composition and infection levels between the sampled areas and between Portuguese waters and those of northern seas (Baltic and North seas). These results pointed out host’s ecology and the different environmental conditions in the sampled estuaries as the major factors accounting for the differences found. Hence, the present study aims to: 1) characterize the macroparasite assemblages of the common goby within different habitat in five estuaries along the Portuguese coast (Ria de Aveiro, Tejo, Sado, Mira and Guadiana); 2) assess if the differences in the parasite assemblages are related to differences in the extension and composition of the habitats; and 3) understand if these habitat related differences in macroparasite assemblages can be used as natural tags of habitat use by P. microps.
Material and methods
Sample collection
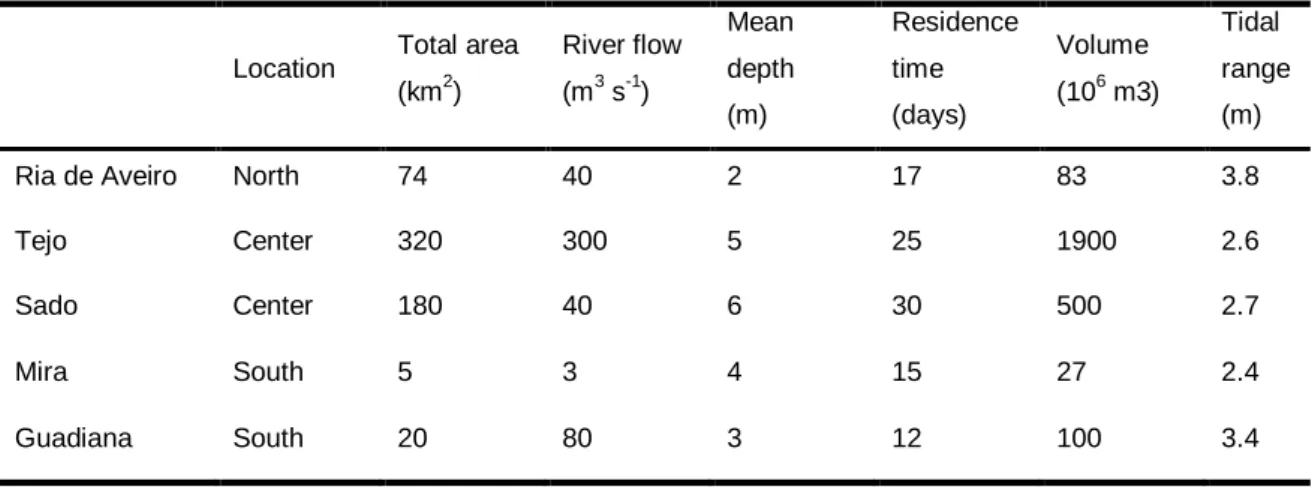
Five estuaries were sampled in this study (Fig. 1): Ria de Aveiro, Tejo, Sado, Mira and Guadiana, each presenting different morphological and hydrologic characteristics (Table 1). Ria de Aveiro is the northernmost estuary consisting of a shallow coastal lagoon with large intertidal areas. Tejo and Sado estuaries are the largest estuarine systems along the Portuguese coast (with areas of 320 km2 and 180km2 respectively) with Tejo estuary being the one with the highest river flow and volume, whereas Sado presents the highest residence time. Mira estuary is the smallest estuary included in the study and has the lowest river flow when compared to the other estuaries (3 m3 s-1). Guadiana is the southernmost estuary being located in the southern coast of Portugal, and has a river flow of 80 m3 s-1.
12
Figure 1 – Estuarine systems sampled along the Portuguese coast: Ria de Aveiro, Tejo, Sado, Mira and
Guadiana. Sampling locations within each estuary are also shown (marked as (a) and (b))
According to the classification of França et al. (2009), three habitats within the estuary were sampled: mudflat (intertidal unvegetated areas with predominance of fine silts), saltmarsh (intertidal, sediment based, macrophyte dominated and saline influenced areas) and subtidal channel (permanently subtidal areas, unvegetated with soft to hard substrate). These habitats were sampled in two similar locations of the estuary (indicated as a) and b) in Figure 1) in order to assess if identical habitats in different parts of the estuary support similar parasite assemblages.
Table 1 – Main hydrological and morphological characteristics of the 5 estuarine systems sampled along the Portuguese coast (adapted from França et al., 2009).
Location Total area (km2) River flow (m3 s-1) Mean depth (m) Residence time (days) Volume (106 m3) Tidal range (m) Ria de Aveiro North 74 40 2 17 83 3.8
Tejo Center 320 300 5 25 1900 2.6 Sado Center 180 40 6 30 500 2.7
Mira South 5 3 4 15 27 2.4
13 Habitat area proportion varies greatly between the estuaries sampled. Subtidal habitat has the highest area proportion in all estuaries, corresponding to more than 60% of all estuarine area. Intertidal mudflats are also largely represented in all estuaries, especially in Tejo and Sado reaching almost 50% of the estuarine area. Saltmarshes occupy a relatively small area, when compared to other habitats, although in Ria de Aveiro and Guadiana estuary, they represent approximately 30% and 15% of the total area, respectively.
Specimens of P. microps were collected in July and October 2009 using a beam trawl or a purse seine net, according to the habitat being sampled. The collected fish were measured and weighted and their digestive tracts were then removed, tagged and immediately frozen (-20ºC) for posterior parasitological examination under a stereomicroscope.
Parasitological and statistical analysis
Since macroendoparasites reflect the local availability of their intermediary hosts (Mackenzie & Abaunza, 1998) and, therefore, habitat related differences in fish diet and prey abundance (Marques et al., 2010), these were selected for the present analyses. Macroendoparasites from each sample (P. microps specimens collected in a habitat within a location in an estuary in a given month) were identified to the lowest possible taxonomic level, counted and preserved in 70% ethanol. For each macroendoparasite taxon infecting each sample, the parasitological indices prevalence (the percentage of infected hosts) and mean abundance (mean number of parasites per host) were calculated according to Bush et al. (1997). These two indices were chosen as they better characterize the composition of the macroparasite assemblages within each host sample.
To evaluate the relationships between host samples in different habitats and macroparasite assemblages, a correspondence analysis (CA) was performed in Canoco 4.5 software (ter Braak & Smilauer, 2002) using the two parasitological indices calculated. The purpose of CA is to evaluate the relationship between categories (samples) and variables (parasite species prevalence or mean abundance) in a low dimensional space, usually a plot defined by the first two canonical functions. Similar categories are plotted close to each other and the distance between them are related to the similarity between variables. To discard potential accidental infections only parasites with at least 10% of prevalence in any estuary were used.
14
Results
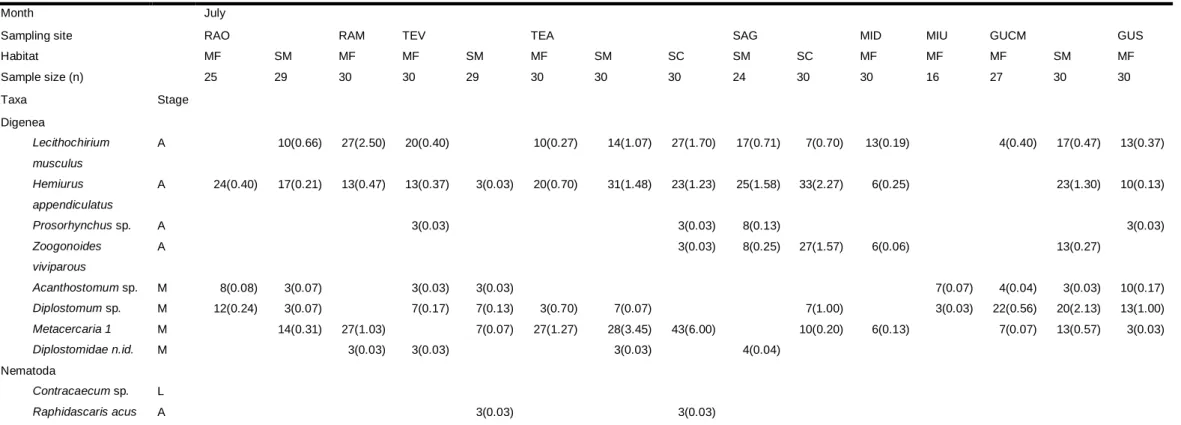
P. microps were infected with 10 different parasite taxa (Table 1), belonging to Digenea and Nematoda. Among the adult Digenea only Lecithochirium musculus (Looss, 1907) and Hemiurus appendiculatus (Rudolphi, 1802) were found in all estuaries in both sampling months. Zoogonoides viviparous (Olsson, 1868) was also found in all estuaries but only occurred in both months in Tejo, Sado and Guadiana; in Mira it occurred only in July and in Ria de Aveiro only in October. Considering the metacercariae, only Diplostumum sp. and Metacercaria 1 were found in all estuaries. The Nematoda Contracaecum sp. only infected specimens of P. microps collected in Guadiana estuary in July whereas Raphidascaris acus (Bloch, 1779) was present in Ria de Aveiro (only in October), Tejo and Sado estuaries. The prevalence and mean abundance of macroendoparasites varied much between the estuaries as well as between the two months, generally attaining higher values in October. L. musculus, H. appendiculatus, Diplostomum sp. and Metacercaria 1 were usually the most prevalent and abundant. As for differences of prevalence and abundance between habitats, Diplostomum sp. appeared to be more abundant in mudflats and could be associated to these habitats; while the unidentified Diplostomidae and R. acus appeared to be closely related to saltmarshes, since they were more prevalent and abundant in this habitat. Samples from the two sampled locations in Tejo estuary (Vila Franca de Xira and Alcochete) showed notably different prevalence and mean abundance of some parasite species: Acanthostomum sp. is more prevalent in Vila Franca de Xira samples, while Metacercaria 1 is more prevalent and abundant in Alcochete samples.
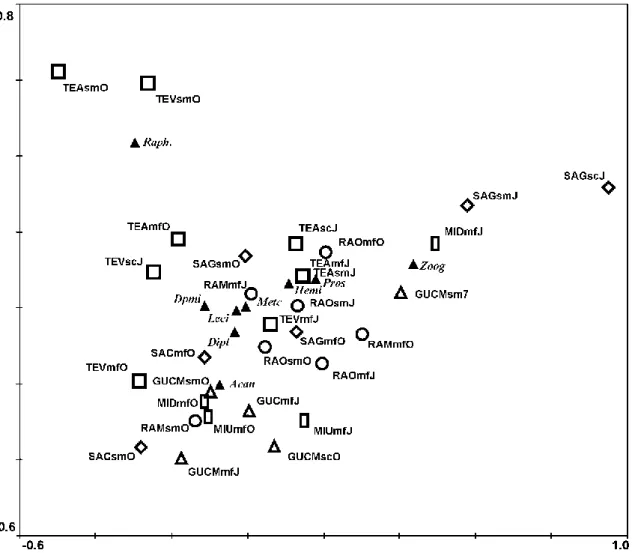
In the correspondence analysis using prevalence data (Fig. 2), 45.8% of the variance was explained by the first two axes. Independently of the month sampled, Guadiana and Mira samples were grouped in the bottom of the diagram (related with the prevalence of Acanthostomum sp. and Diplostomum sp.), and Ria de Aveiro in the centre of the diagram (related with the prevalence of Hemiurus appendiculatus and Diplostomum sp.), all with few scattering. Whereas Tejo samples appeared spread in the top left, mostly due to the prevalence of R. acus, Sado showed some degree of seasonal differentiation with samples collected in Gâmbia in July located in the far right of the diagram, mostly due to the higher prevalence of Zoogonoides viviparous. As for habitats, only the saltmarshes of Tejo estuary in October appeared well isolated in the top left of the ordination due to high prevalence of R. acus when compared to the other habitats.
15
Table 1 - Prevalence (%) and mean abundance (between brackets) of macroendoparasites found for Pomatoschistus microps in July and October within each sampling site
Month July
Sampling site RAO RAM TEV TEA SAG MID MIU GUCM GUS
Habitat MF SM MF MF SM MF SM SC SM SC MF MF MF SM MF Sample size (n) 25 29 30 30 29 30 30 30 24 30 30 16 27 30 30 Taxa Stage Digenea Lecithochirium musculus A 10(0.66) 27(2.50) 20(0.40) 10(0.27) 14(1.07) 27(1.70) 17(0.71) 7(0.70) 13(0.19) 4(0.40) 17(0.47) 13(0.37) Hemiurus appendiculatus A 24(0.40) 17(0.21) 13(0.47) 13(0.37) 3(0.03) 20(0.70) 31(1.48) 23(1.23) 25(1.58) 33(2.27) 6(0.25) 23(1.30) 10(0.13) Prosorhynchus sp. A 3(0.03) 3(0.03) 8(0.13) 3(0.03) Zoogonoides viviparous A 3(0.03) 8(0.25) 27(1.57) 6(0.06) 13(0.27) Acanthostomum sp. M 8(0.08) 3(0.07) 3(0.03) 3(0.03) 7(0.07) 4(0.04) 3(0.03) 10(0.17) Diplostomum sp. M 12(0.24) 3(0.07) 7(0.17) 7(0.13) 3(0.70) 7(0.07) 7(1.00) 3(0.03) 22(0.56) 20(2.13) 13(1.00) Metacercaria 1 M 14(0.31) 27(1.03) 7(0.07) 27(1.27) 28(3.45) 43(6.00) 10(0.20) 6(0.13) 7(0.07) 13(0.57) 3(0.03) Diplostomidae n.id. M 3(0.03) 3(0.03) 3(0.03) 4(0.04) Nematoda Contracaecum sp. L Raphidascaris acus A 3(0.03) 3(0.03)
Life-Stage: M, metacercaria; A, Adult; L, L3 larvae; Sampling site: RAO, Ria de Aveiro – Ovar channel; RAM, Ria de Aveiro – Mira channel; TEV, Tejo – Vila franca de Xira; TEA, Tejo – Alcochete; SAG, Sado – Gâmbia; SAC, Sado – Carrasqueira; MID, Mira – Downstream; MIU, Mira – upstream; GUCM, Guadiana – Castro Marim; GUC, Guadiana – Carrasqueira. Habitat: MF, mudflat; SM, salt-marsh; SC, subtidal channel
16
Table 1 (cont.)
Month October
Sampling site RAO RAM TEV TEA SAG SAC MID MIU GUCM
Habitat MF SM MF SM MF SM MF SM MF SM MF SM MF MF MF SC Sample size (n) 30 30 30 30 30 32 27 30 30 14 27 29 11 15 30 10 Taxa Stage Digenea Lecithochirium musculus A 10(0.33) 23(1.00) 26(0.52) 30(0.70) 33(2.57) 31(2.63) 15(1.11) 34(1.97) 40(1.83) 21(0.64) 67(3.53) 45(1.45) 47(2.60) 10(0.70) Hemiurus appendiculatus A 20(0.60) 17(0.77) 3(0.03) 7(0.26) 27(0.73) 20(0.47) 13(1.34) 15(0.41) 28(1.52) 13(1.23) 7(0.50) 27(1.13) 27(4.00) 13(0.77) Prosorhynchus sp. A 10(0.10) 7(0.10) 4(0.04) 7(0.17) Zoogonoides viviparous A 10(0.10) 3(0.03) 3(0.03) 3(0.10) 7(0.11) 3(0.03) 10(0.50) Acanthostomum sp. M 7(0.10) 7(0.07) 3(0.03) 22(0.37) 7(0.07) 3(0.03) 4(0.04) 7(0.20) 21(0.29) 27(4.00) 36(1.18) 23(0.63) 30(1.30) Diplostomum sp. M 27(0.83) 33(1.13) 7(2.13) 7(0.80) 26(1.67) 10(0.63) 20(1.07) 25(0.81) 30(0.81) 7(3.07) 23(1.70) 7(1.43) 33(0.53) 55(5.27) 17(0.37) 10(0.10) Metacercaria 1 M 13(0.63) 33(1.80) 3(0.03) 7(0.07) 10(0.33) 47(2.83) 31(2.53) 30(1.44) 17(0.66) 33(5.13) 7(0.20) 45(3.18) 57(7.33) 40(5.70) Diplostomidae n.id. M 3(0.10) 7(0.33) 3(0.13) 3(0.07) 7(0.07) 10(0.13) 19(0.50) 7(0.22) 31(3.14) 13(0.23) 21(0.29) 13(0.13) 9(0.09) 3(0.03) Nematoda Contracaecum sp. L 3(0.03) 10(0.10) Raphidascaris acus A 7(0.10) 7(0.07) 27(1.10) 13(0.13) 41(2.22) 3(0.07)
Life-Stage: M, metacercaria; A, Adult; L, L3 larvae; Sampling site: RAO, Ria de Aveiro – Ovar channel; RAM, Ria de Aveiro – Mira channel; TEV, Tejo – Vila franca de Xira; TEA, Tejo – Alcochete; SAG, Sado – Gâmbia; SAC, Sado – Carrasqueira; MID, Mira – Downstream; MIU, Mira – upstream; GUCM, Guadiana – Castro Marim; GUC, Guadiana – Carrasqueira. Habitat: MF, mudflat; SM, salt-marsh; SC, subtidal channel;
17
Fig. 2 – Correspondence analysis (CA) ordination diagram based on parasite prevalence at the different
habitats in the 5 sampled estuaries. Macroendoarasite taxa are represented by filled triangles: Leci – Lecithochirium musculus; Hemi – Hemiurus appendiculatus; Pros – Prosorhynchus sp.; Zoog –
Zoogonoides viviparous; Acan – Acanthostomum sp.; Metc – Metacercaria 1; Dipl – Diplostomum sp.;
Dpmi – Diplostomidae n.id.; Raph. – Raphidascaris acus; Sampling sites are indicated by: open circles (RAO, Ria de Aveiro – Ovar channel; RAM, Ria de Aveiro – Mira channel); open squares (TEV, Tejo – Vila franca de Xira; TEA, Tejo – Alcochete); open diamonds (SAG, Sado – Gâmbia; SAC, Sado – Carrasqueira); open rectangles (MID, Mira – Downstream; MIU, Mira – upstream); and open triangles (GUCM, Guadiana – Castro Marim; GUC, Guadiana – Carrasqueira). Habitat: MF, mudflat; SM, saltmarsh; SC, subtidal channel; Months: J – July; O – October.
The correspondence analysis performed using square root transformed macroendoparasites mean abundance (Fig. 3), revealed that 44.6% of the data variance was explained by the first two ordination axes. Contrary to the previous analysis, some seasonal, sampling location and habitat related differences were evidenced despite great sample scattering. Aveiro samples were mostly grouped in the centre of the ordination diagram with only Mira channel in October being apart, in the bottom right. Tejo samples showed some differentiation between Alcochete and Vila Franca de Xira as these appeared in opposite sides of the diagram: Alcochete in the
18 left and Vila Franca de Xira in the right. Analysis also suggested some degree of habitat differentiation since most saltmarsh and intertidal samples were grouped together in the upper and central parts of the diagram, respectively. R. acus was, again, related to the differentiation of saltmarsh samples from Tejo. For Sado estuary, seasonal variation was suggested from the location of October samples (more central) relatively to July samples (bottom of the diagram), influenced by the higher mean abundance of Z. viviparous in July. Habitat related differences in parasite mean abundance were also pointed out in Sado since mudflat and saltmarsh samples collected in October appeared close together in the centre-right and centre-left of the diagram mostly related with differences in the abundance of Metacercaria 1 between these habitats. Seasonal differentiation was also evidenced in Guadiana estuary as the samples from each month appeared in opposite sides of the diagram; possible similarities between the mudflats of both sampling sites were also suggested given that these samples were close together in the right half of the diagram. Seasonal differences of Guadiana samples seem to be related with lower Diplostomum sp. and Diplostomidae n.id mean abundance and an increased abundance of Metacercaria 1 in October. Mudflats were grouped together due to their higher mean abundance of Metacercaria 1. Mira samples, although grouped relatively close to each other, showed some degree of season and sampling locations differences in July.
Discussion
Parasite assemblages of P. microps found in this study differed somewhat of the assemblages found by Freitas et al. (2009). Nevertheless, as reported by these authors, L. musculus and Prosorhynchus sp. were also found in all estuaries with L. musculus being one of the most prevalent species found in all estuaries. Seven other macroendoparasites were found in the present study namely, Hemmiurus appendiculatus, Zoogonoides viviparus, Acanthostomum sp., Diplostomum sp. and a non-identified Diplostomidae (Digenea) and Raphidascaris acus and Contracaecum sp. (Nematoda). Previous studies conducted on P. microps in the Baltic Sea (Zander et al., 2004) found Hemiurus communis (Odhner, 1905) whereas this study found H. appendiculatus. One possible reason for this differentiation is the geographic distribution patterns of these parasites: H. communis has a Boreal distribution while H. appendiculatus has a Lusitanian distribution being primarily a parasite of clupeids, especially Alosa spp. (Gibson & Bray, 1986).
19
Fig. 3 – Correspondence analysis (CA) ordination diagram based on square root transformed mean abundance at the different habitats in the 5 sampled estuaries. Macroendoarasite taxa are represented by filled triangles: Leci – Lecithochirium musculus; Hemi – Hemiurus appendiculatus; Pros – Prosorhynchus sp.; Zoog – Zoogonoides viviparous; Acan – Acanthostomum sp.; Metc – Metacercaria 1; Dipl –
Diplostomum sp.; Dpmi – Diplostomidae n.id.; Raph. – Raphidascaris acus; Sampling sites are indicated
by: open circles (RAO, Ria de Aveiro – Ovar channel; RAM, Ria de Aveiro – Mira channel); open squares (TEV, Tejo – Vila franca de Xira; TEA, Tejo – Alcochete); open diamonds (SAG, Sado – Gâmbia; SAC, Sado – Carrasqueira); open rectangles (MID, Mira – Downstream; MIU, Mira – upstream); and open triangles (GUCM, Guadiana – Castro Marim; GUC, Guadiana – Carrasqueira). Habitat: MF, mudflat; SM, saltmarsh; SC, subtidal channel; Months: J – July; O – October
Although it was not possible to identify Timoniella sp. individuals present in Portuguese estuaries, the geographic distribution of Timoniella balthicum (Reimer, Hildebrand, Scharberth & Walter, 1996),found for P. microps in the North and Baltic Seas (Zander, 2004), suggest the ones found in the present study belong to a different species. Z. viviparous is the only species of Zoogonoides reported in the Northeast Atlantic region, and is commonly found as an adult in flatfishes, blennies and gobies (Bray & Gibson, 1986). It usually has echinoderms as intermediary hosts but polychaetes have also been described as important intermediary hosts (Bray & Gibson, 1986) and these have
20 been described as an important prey of P. microps in Portuguese estuaries (Salgado et al., 2004; Leitão et al., 2006). Whereas Freitas et al. (2009) reported a faint presence of Anisakis sp. in Mira and Guadiana estuaries, this study found Contracaecum sp. and R. acus in Ria de Aveiro, Tejo, Sado and Guadiana estuaries (Contracaecum sp. only in Guadiana) but with low infection levels. In fact, Zander (2004) reported these three genera as parasites of Pomatoschistus spp. in the Baltic Sea. Differences in the assemblages of parasites of P. microps in the Portuguese coast can be due to different environmental conditions related to seasonal and inter annual differences, which play an important role in the establishment of a parasite community (Kesting et al., 1996). However, and given the low prevalence of the Nematoda found in the present study, they might correspond to accidental infections and, therefore, were not detected in the study of Freitas et al. (2009).
Overall, and as reported by Freitas et al. (2009), the parasite assemblages reported in the estuaries of the Portuguese coast were distinct from those found in the Baltic Sea (Kesting et al., 1996; Zander, 2003, 2004) and North Sea (Zander, 1998, 2005) and from those of Southern Wales (Malek, 2001). Hemiurids (L. musculus and H. appendiculatus), which are important parasites of P. microps, are mainly transmitted by planktonic copepods. Zander (2004) reported that the success of infection by this family for the Baltic Sea was very low compared to the number of ingested copepods. In Portuguese estuaries, and although the feeding ecology of P. microps differs from that reported by Zander (2004) in the Baltic Sea, copepods are also found to be a prey of P. microps (Salgado et al., 2004) and the transmission of hemiurids appears to be successful, which is probably due to the different environmental conditions and/or hosts ecology in Portuguese estuaries.
Correspondence analysis of prevalence data suggested the differentiation of the five estuaries, and a low degree of habitat and collection month-related differences, with only the samples from Tejo’s saltmarsh being separated from the others collected within the same estuary. This was mostly due to differences in the prevalence of R. acus which might be related to intermediary host abundance. Moravec (1996) reported gammarids as intermediary hosts of R. acus and insects as paratenic hosts. As these are reported to be abundant in saltmarshes of Tejo estuary (Salgado et al., 2007) and as a prey of P. microps in these same habitats (Salgado et al., 2004), insect consumption might explain the high values of R. acus in P. microps from Tejo saltmarshes. The fact that they only appeared to be highly prevalent in October may be due to variations in the abundance of insect hosts and their life cycle. Although it was not possible to identify Acanthostomum sp. metacercariae to the species level,
21 Hydrobia sp. has been reported as the first intermediary host of Acanthostomum imbutiforme (Molin, 1859). Hydrobia spp. are deposit feeders that inhabit intertidal areas of costal lagoons, and estuaries in the coasts of Europe (Graham, 1988). According to Silva et al. (2006), in a six-year study conducted in Tejo estuary, the abundance of Hydrobia sp. peaks in summer/autumn months. This is in agreement with the higher prevalence and mean abundance of Acanthostomum sp. found in a great range of samples of mudflat and saltmarsh habitats, mostly in October, suggesting a relationship between the high mean abundances of this parasite and the distribution and ecology of Hydrobia sp.
When analyzing the results of parasite mean abundance some differences within estuaries emerged. Seasonal patterns were identified in Ria de Aveiro, Mira and Guadiana estuary, with July and October samples being usually widely separated in the diagram. These seasonal patterns evidenced in Guadiana were mostly related to Diplostomum sp., Diplostomidae n.id, and Metacercaria 1, the first two showing a decreased abundance in October, and the last showing a remarked increase, as is also evidenced in Table 1. Because parasites could not be identified to its specific level, their first intermediary hosts cannot be precisely known. However, and since Digenea (and the Diplostomidae) usually have mollusks as their first intermediary host, and some cercarial stages are affected by different environmental conditions, such as temperature, salinity, pH or punctual environmental pollution (Pietrock & Marcogliese, 2003), these differences might be related to the lack of optimal conditions for cercariae infection. In Mira estuary, seasonal differences are mostly due to increased parasite diversity in October in the sampling locations (6 parasite species in each sampling site, Table 1), which might be due to different intermediary hosts (prey) consumption between the two sampling sites. In Ria de Aveiro, seasonal differences were related with an increased mean abundance of metacercarial stages. These may be due to increased abundance of first intermediary hosts (such as Hydrobia ulvae), or better environmental conditions for cercarial stages to infect P.microps.
Within Tejo and Sado estuaries, habitat-related differences in parasite mean abundances were evidenced, especially in October, with mudflat and saltmarsh samples being well apart from each other. Whereas in Tejo estuary the main factor influencing this differentiation was the infection level of R. acus, in Sado estuary this seemed to be due to the mean abundance of Metacercaria 1 (Table 1). Moreover, in Tejo estuary mudflats mean abundances differ greatly between sampling locations (Vila Franca de Xira and Alcochete), which might be due to different hydrological and morphological characteristics and anthropogenic impacts that may be determinant to
22 the establishment of intermediary hosts. Vila Franca de Xira has a higher mean depth, lower and highly variable salinity while Alcochete is shallower, more saline and with lower variability (Cabral, 1998; Cabral & Costa, 1999; Vinagre et al., 2008). As parasites development is also influenced by pollutants and anthropogenic disturbances, either by disturbing the parasite itself, its intermediary hosts distribution or by imunodepressing fish hosts (Lafferty, 1997; Marcogliese, 2005), it is also important to acknowledge that these locations are differently impacted. While Vila Franca de Xira is located in an area that receives substantial quantities of industrial and urban sewage, Alcochete is located in a less impacted area and has no important adjacent industries (Vale, 1986; Hampel et al., 2009).
The present study suggests that P. microps use habitats differently, as suggested by França et al. (2009). It also comes in agreement with previous studies that correctly used parasites as tags for small scale spatial distribution (Grutter, 1998; Vignon et al., 2008; Durieux et al., 2010). However, it is necessary to access if seasonality is an issue when using parasitic communities for small-scale studies since parasitic communities are known to have seasonal patterns in brackish water environments (Zander & Kesting, 1998; Zander, 2004). Results pointed out differences in the prevalence and mean abundance P. microps macroendoparasites between estuaries and habitats, showing that sampling areas have to be carefully selected to minimize the effects of the different environmental conditions associated with different locations, rather than different habitats. If similar locations are sampled, it is possible to distinguish some differences in the use of the different habitats by fish. Nevertheless, to validate these differences further studies need to be conducted considering the availability of intermediary hosts, habitat connectivity and environmental contamination of those habitats.
23
25
Final remarks
Certain habitats within an estuary are considered to be crucial to the development of many species. The present study confirms that parasites can be considered use as natural tags of habitat use for P. microps, and therefore are promising as useful tools to assess the importance of habitats for fishes. It is, however, very important to confirm this study with other methodologies and tags already known to be reliable in the study of fishes spatio-temporal patterns, such as artificial tags, stable isotopes analysis and otolith element signatures.
Long term parasitological studies of the communities of fish and its prey should also be considered in order to better predict seasonal changes, and assess if seasonality is an issue when using parasites as natural tags. Also, parasites supra and infracommunity studies should also be done, in order to better understand which are the mechanisms that drive parasites occurrence, prevalence and abundances within their hosts.
Since many commercially important fishes use estuaries as nursery grounds, it would be very important to replicate this study and assess if there are habitat preferences in these species, in order to investigate which habitats are crucial in the development of these fishes. This would be very useful to better manage estuarine systems, and appoint which habitats have the highest conservation priority.
26
References
Beck, M.W., Heck, K.L., Able, K.W., Childers, D.L., Eggleston, D.B., Gillanders, B.M., Halpern, B., Hays, C.G., Hoshino, K., Minello, T.J., Orth, R.J., Sheridan, P.F., Weinstein, M.P., 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51(8):633-641.
Blaber, S.J.M., Blaber, T.G., 1980. Factors affecting the distribution of juvenile estuarine and inshore fish. Journal of Fish Biology 17, 143–162.
Bray, R.A., Gibson, D.I., 1986. The Zoogonidae (Digenea) of fishes from the north-east Atlantic. Bulletin of the British Museum (Natural History) Zoology 51(2):127-206.
Bush, A. O., Lafferty, K. D., Lotz, J. M., Shostak, A. W., 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83: 575–583.
Cabral, H.N., Costa, M.J., 1999. Differential use of nursery areas within the Tagus estuary by sympatric soles, Solea solea and Solea senegalensis. Environmental Biology of Fishes 56: 389–397
Cabral, H.N., Vasconcelos, R.P., Vinagre, C., França, S., Fonseca, V., Maia, A., Reis-Santos, P., Lopes, M., Ruano, M., Campos, J., Freitas, V., Reis-Santos, P.T., Costa, M.J., 2007. Relative importance of estuarine flatfish nurseries along the Portuguese coast. Journal of Sea Research 57: 209–217.
Castellanos, D.L., Rozas, L.P., 2001. Nekton use of submerged aquatic vegetation,marsh, and shallow unvegetated bottom in the Atchafalaya River Delta, a Louisiana tidal freshwater ecosystem. Estuaries 24: 184–197.
Chambers, C.A.., Dick, T.A., 2005. Trophic structure of one deep-sea benthic fish community in the eastern Canadian Arctic: application of food, parasites and multivariate analysis. Environmental Biology of Fishes 74:365–378.
Connolly, R.M., 1994. A comparison of fish assemblages from seagrass and unvegetated areas of a southern Australian estuary. Australian Journal of Marine and Freshwater Research 45: 1033–1044.
27 Costa, M. J., 1988. Écologie alimentaire des poissons de l’estuaire du Tage. Cybium
12: 301–320.
Costanza, R., dArge, R., deGroot, R., Farber, S., Grasso, M., Hannon, B., Limburg, K., Naeem, S., ONeill, R.V., Paruelo, J., Raskin, R.G., Sutton, P., van den Belt, M., 1997. The value of the world’s ecosystem services and natural capital. Nature 387: 253–260
Durieux, E.D.H., Marques, J.F., Sasal, P., Begout, M-L., Cabral, H.N., 2007. Comparison of Solea solea macroparasites between two nursery-continental Shelf systems in the Bay of Biscay and the Portuguese coast. Journal of Fish Biology 70: 1921–1930.
Durieux, E.D.H., Bégout, M-L, Pinet, P., Sasal, P., 2009. Digenean metacercariae parasites as natural tags of habitat use by 0-group common sole Solea solea in nearshore coastal areas: A case study in the embayed system of the Pertuis Charentais (Bay of Biscay, France). Journal of Sea Research 64: 107– 117
Elliott, M., Dewailly, F., 1995. The structure and components of European estuarine fish assemblages. Netherlands Journal of Aquatic Ecology 29: 397–417.
França, S., Costa, M.J., Cabral, H.N., 2009. Assessing habitat specific fish assemblages in estuaries along the Portuguese coast. Estuarine, Coastal and Shelf Science 83: 1–12.
Franco, A., Elliot, M., Franzoi, P., Torricelli, P., 2008. Life strategies of fishes in European estuaries: the functional guild approach. Marine ecology progress series 354: 219-228.
Gibson, D.I.; Bray, R.A., 1986. The Hemiuridae (Digenea) of fishes from the north-east Atlantic. Bulletin of British Museum of (Natural History) Zoology 51:1–125.
Gillanders, B.M., Able, K.W., Brown, J.A., Eggleston, D.B., Sheridan, P.F., 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247: 281–295.
28 Graham, A.F.R.S., 1988. Molluscs: prosobranch and pyramidellid gastropods. In: Kermack, D.M., Barnes, S.K. (Eds.), Synopses of the British Fauna (New Series), vol. 2. The Linnean Society of London and the Estuarine and Brackish-Water Science Association, London. 662 pp.
Grutter, A.S., 1998. Habitat-related diVerences in the abundance of parasites from a coral reef fish: an indication of the movement patterns of Hemigymnus melapterus. Journal of Fish Biology 53: 49–57
Haedrich, R.L., 1983. Estuarine fishes. In Ketchum B (ed) Ecosystems of the world, Estuarine and Enclosed Seas, Book 26. Elsevier, Amsterdam, Netherlands, p 183-207.
Hampel, M., Canário, J., Branco, V., Vale, C., Blasco, J., 2009. Environmental levels of Linear alkylbenzene Sulfonates (LAS) in sediments from the Tagus estuary (Portugal): environmental implications. Environmental Monitoring and Assessment 149:151-161.
Jenkins, G.P., May, H.M.A., Wheatley, M.J., Holloway, M.G., 1997. Comparison of fish assemblages associated with seagrass and adjacent invegetated habitats of Port Philip Bay and Corner Inlet, Victoria, Australia, with emphasis on commercial species. Estuarine, Coastal and Shelf Science 44: 569–588.
Kesting, V., Gollasch, S., Zander, C. D., 1996. Parasite communities of the Schlei Fjord (Baltic coast of northern Germany). Helgol. Meeresunters. 50: 477–496.
Lafferty, K.D., 1997. Environmental parasitology: What can parasites tell us about human impacts on the environment? Parasitology Today 13: 251–255.
Leitão, R., Martinho, F., Neto, J. M., Cabral, H., Marques, J. C., Pardal, M. A., 2006. Feeding ecology, population structure and distribution of Pomatoschistus microps (Krøyer, 1838) and Pomatoschistus minutus (Pallas, 1770) in a temperate estuary, Portugal. Estuarine Coastal Shelf Science 66: 231–239.
Lenanton, R.C.J., Potter, I.C., 1987. Contribution of estuaries to commercial fisheries in temperate Western Australia and the concept of estuarine dependence. Estuaries 10: 28-35.
Mackenzie, K., 1990. Cestode parasites as biological tags for mackerel (Scomber scombrus L.) in the Northeast Atlantic. ICES Journal of Marine Science 46: 155-166.
29 Mackenzie, K., 1999. Parasites as pollution indicators in marine ecosystems: A
proposed early warning system. Marine Pollution Bulletin 38: 955–959.
Mackenzie, K., 2002. Parasites as biological tags in population studies of marine organisms: an update. Parasitology 124: 153–163.
MacKenzie, K., 2005. Parasites as Biological Tags. In: Rhode, K. (Ed.), Mar. Parasitol. CABI Publishing, Oxon, pp. 351–355.
Malek, M., 2001. Effects of the digenean parasites Labratrema minimus and Cryptocotyle concavum on the growth parameters of Pomatoschistus microps and P. minutus from Southwest Wales. Parasitology Research 87: 349–355.
Marcogliese, D.J., 2005. Parasites of the superorganism: are they indicators of ecosystem health? International Journal for Parasitology 35: 705-716.
Marques, J.F., Santos, M.J., Costa, J.L., Costa, M.J., Cabral,H.N., 2005. Metazoan parasites as biological indicators of population structure of Halobatrachus didactylus on the Portuguese coast. Journal of applied Ichthyology 21: 220-224.
Marques, J.F., Teixeira, C.M., Cabral, H.N., 2006. Differentiation of economically important flatfish populations along the Portuguese coast: evidence from morphology and parasitology. Fisheries Research 81: 293–305.
Marques, J.F., Santos, M.J., Cabral, H.N., 2010. Aggregation patterns of macroendoparasites in phylogenetically related fish hosts. Parasitology 137: 1671-1680.
Martinho, F., Leitão, R., Neto, J.M., Cabral, H.N., Marques, J.C., Pardal, M.A., 2007. The use of nursery areas by juvenile fish in a temperate estuary, Portugal. Hydrobiologia 587: 281–290.
McClelland, G., Melendy, J., Osborne, J., Reid, D., Douglas, S., 2005. Use of parasite and genetic markers in delineating populations of winter flounder from the central and south-west Scotian Shelf and north-east Gulf of Maine. Journal of Fish Biology 66:1082–1100.
McLusky, D.S., Elliott, M., 2004. The Estuarine Ecosystem: ecology, threats and management. Oxford University Press, Oxford.
30 Miller, J.M., Crowder, L.B., Moser, M.L., 1985. Migration and utilization of estuarine nurseries by juvenile fishes: an evolutionary perspective. Contributions in Marine Science 27(Suppl):338-352.
Miller, P.J., 1986. Gobiidae. In: Fishes of the north-eastern Atlantic and Mediterranean, Vol. III. P. J. P. Whitehead; M. L. Bauchot; J. C. Hureau; J. Nielsen; E. Tortonese (Eds). UNESCO, Paris, pp. 1360–1361.
Moravec, F., 1998. The Amphipod Gammarus fossarum as a Natural True Intermediate Host of the Nematode Raphidascaris acus. The Journal of Parasitology 82: 668-669.
Mouritsen, K. N., Poulin, R., 2002. Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 124: 101-117.
Muñoz, G., Grutter, A.S., Cribb, T.H., 2006. Endoparasite communities of five fish species (Labridae: Cheilininae) from Lizard Island: how important is the ecology and phylogeny of the hosts? Parasitology 132: 363–374.
Nicolas, D., Lobry, J., Lepage, M, Santour, B., Le Pape, O., Cabral, H., Uriarte, A., Boët, P., 2010. Fish under influence: A macroecological analysis of relations between fish species richness and environmental gradients among European tidal estuaries. Estuarine, Coastal and Shelf Science 86: 137-147.
Oliva, M.E., Gonzalez, M., Acuña, E., 2004. Metazoan parasite fauna as a biological tag for the habitat of the flounder Hippoglossina macrops from the northern chile, in a depth gradient. Journal of Parasitology 90:1374-1377.
Pietrock, M., Marcogliese, D.J., 2003. Free-living endohelminth stages: at the mercy of environmental conditions. Trends in Parasitology 19: 293-299.
Pihl, L., Cattrijsse, A., Codling, I., Mathieson, S., McLusky, D.S., Roberts, C., 2002. Habitat use by fishes in estuaries and other brackish areas. In: Elliott, M., Hemingway, K.L. (Eds.), Fishes in Estuaries. Blackwell Science.
Pombo, L., Rebelo, J.E., 2002. Spatial and temporal organization of a coastal lagoon fish community - Ria de Aveiro, Portugal. Cybium 26: 185–196.
Salgado, J.P.; Cabral, H.N.; Costa, M.J., 2004: Feeding ecology of the gobies Pomatoschistus minutus (Pallas, 1770) and Pomatoschistus microps (Krøyer, 1838) in the upper Tagus estuary, Portugal. Scientia Marina 68: 425–434.
31 Salgado, J.P., Cabral, H.N., Costa, M.J., 2007. Spatial and temporal distribution Patterns of the macrozoobenthos assemblage in the salt marshes of Tejo estuary (Portugal). Hydrobiologia 587:225–239
Silva, G., Costa, J.L., Almeida, P.R., Costa, M.J., 2006. Structure and dynamics of a benthic invertebrate community in an intertidal area of the Tagus estuary, western Portugal: a six year data series. Hydrobiologia 555:115-118.
ter Braak, C.J.F., Smilauer, P., 2002. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination. Version 4.5. Microcomputer Power, Ithaca, New York, USA 500 pp.
Vale, C. 1986: Distribuição de metais e matéria particulada em suspensão no sistema estuarino do Tejo. Instituto Nacional de Investigacão Pesca, Dissert. Invest. Lisboa, 153pp.
Vasconcelos, R.P., Reis-Santos, P., Fonseca, V., Maia, A., Ruano, M., França, S., Vinagre, C., Costa, M.J., Cabral, H., 2007. Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: a multi-metric index and conceptual approach. Science of the Total Environment 374:199–215.
Vasconcelos, R.P., Reis-Santos, P., Maia, A., Fonseca, V, França, S., Wouters, N., Costa, M. J., Cabral, H.N., 2010. Nursery use patterns of commercially important marine fish species in estuarine systems along the Portuguese coast. Estuarine, Coastal and Shelf Science 86: 613–624
Veiga, P., Vieira, L., Bexiga, C., Sá, R., Erzini, K., 2006. Structure and temporal variations of fish assemblages of the Castro Marim salt marsh, southern Portugal. Estuarine, Coastal and Shelf Science 70: 27–38.
Vignon, M., Morat, F., Galzin, R., Sasal, P., 2008. Evidence for spatial limitation of the bluestripe snapper Lutjanus kasmira in French Polynesia from parasite and otolith shape. Journal of Fish Biology 73: 2305–2320.
Vinagre, C., Fonseca, V., Maia, A, Amara, R., Cabral, H.N., 2008. Habitat specific growth rates and condition indices for the sympatric soles Solea solea (Linnaeus, 1758) and Solea senegalensis Kaup 1858, in the Tagus estuary, Portugal, based on otolith daily increments and RNA-DNA ratio. Journal of Applied Ichthyology 24:163–169.
32 Williams, H.H., Mackenzie, K., McCarthy, A.M., 1992. Parasites as biological indicators of the population biology, migrations, diet and phylogenetics of fish. Reviews in Fish Biology and Fisheries 2: 144–176.
Zander, C.D.; Kesting, V., 1998. Colonization and seasonality of goby (Gobiidae, Teleostei) parasites from the southwestern Baltic Sea. Parasitology Research 84: 459–466.
Zander, C.D., Reimer, L.W., Barz, K., Dietel, G., Strohbach, U., 2000. Parasite communities of the SalzhaV (Northwest Mecklenburg, Baltic Sea). II. Guild communities, with special regard to snails, benthic crustaceans, and small-sized fish. Parasitology Research 86:359–372.
Zander, C.D., Reimer, L. W., 2002. Parasitism at the ecosystem level in the Baltic Sea. Parasitology 124: 119–135.
Zander, C.D., 2002. The influence of eutrophication on parasite communities in the Baltic Sea. Proceedings of 10th International Congress of Parasitology: 247– 253.
Zander, C.D., 2003. Four-year monitoring of parasite communities in gobiid fishes of the south-western Baltic: I. Guild and component community. Parasitology Research 90:502–511
Zander, C.D., 2004. Four-year monitoring of parasite communities in gobiid fishes of the south-western Baltic II. Infracommunity. Parasitology Research 93:17–29.
Zander, C.D., 2005. Comparative studies on goby (Teleostei) parasite communities from the North and Baltic seas. Parasitology Research 96: 62–68.
33
Agradecimentos
Esta tese é dedicada a todos os que, directa ou indirectamente, contribuíram para o desenvolvimento deste trabalho. Gostava assim de deixar o meu mais profundo e sincero agradecimento:
Ao Professor Henrique Cabral, por me ter acolhido na sua equipa de investigação e me ter introduzido ao fantástico mundo da ecologia estuarina; pela orientação neste trabalho desde o seu inicio.
À Doutora Joana Marques, sobretudo pela incasável paciência com que me orientou e ajudou em todas as fases de desenvolvimento desta tese; pelo facto de me ter mostrado que os parasitas, embora por vezes desprezados, são tão importantes e úteis como qualquer outro grupo animal.
A toda a equipa de investigação do Professor Henrique Cabral, por me terem acolhido de braços abertos e por se mostrarem sempre disponíveis para ajudar e com os quais pude compartilhar alguns dias (e noites) de arrastos e dragas, particularmente: Rita, Susana, Patrick, Marisa, Inês, Vanessa, Suzanne, Noémie; de igual modo ao Miguel e Sofia por sempre se mostrarem disponíveis a ajudar e pelos mergulhos que sempre me ajudaram a descontrair dos vários dias na lupa na identificação dos parasitas; à companhia permanente do laboratório do C8 pelas conversas e companhia: Marina, companheira neste mesmo barco que é a Tese; à Célia, Joana Oliveira e Maria Paula.
À Patrícia, por estar sempre pronta para me ajudar, pela motivação e todo o seu apoio incondicional.
Por fim, e não menos importante, aos meus pais, sempre presentes e que sempre me suportaram em todas as minhas escolhas, sem os quais a elaboração desta tese nunca teria sido possível.