www.revportcardiol.org
Revista
Portuguesa
de
Cardiologia
Portuguese
Journal
of
Cardiology
REVIEW
ARTICLE
Adjuvant
antithrombotic
therapy
in
ST-elevation
myocardial
infarction:
A
narrative
review
Daniel
Caldeira
a,b,c,d,∗,
Hélder
Pereira
a,baCardiologyDepartment,HospitalGarciadeOrta,Almada,Portugal
bCentroCardiovasculardaUniversidadedeLisboa,CCUL,FaculdadedeMedicina,UniversidadedeLisboa,Portugal cLaboratóriodeFarmacologiaClínicaeTerapêutica,FaculdadedeMedicina,UniversidadedeLisboa,Portugal dInstitutodeMedicinaMolecular,FaculdadedeMedicina,UniversidadedeLisboa,Portugal
Received2January2018;accepted13May2018
KEYWORDS Myocardialinfarction; Antiplatelet; Anticoagulant; Antithrombotic; Adjuvant
Abstract Thisarticlereviewsthemajorpharmacologicfeaturesof,andclinicalevidenceon, adjuvantmedicaltherapyinpatientswithST-elevationmyocardialinfarction(STEMI)treated withprimarypercutaneouscoronaryintervention.Thesedrugsincludeoralantiplatelets(aspirin and P2Y12 inhibitors suchas clopidogrel,prasugrel andticagrelor),intravenous antiplatelet
agents(theP2Y12inhibitorcangrelor,GPIIb/IIIainhibitorssuchasabciximab,eptifibatideand
tirofiban),andintravenousanticoagulantagents(unfractionatedheparin,lowmolecularweight heparinandbivalirudin).
© 2019SociedadePortuguesade Cardiologia.Publishedby ElsevierEspa˜na,S.L.U.Allrights reserved. PALAVRAS-CHAVE Enfartedomiocárdio; Antiagregante; Anticoagulante; Antitrombótico; Adjuvante
Terapêuticaantitrombóticaadjuvantenoenfartedomiocárdio comsupra-desnivelamentodosegmentoST:revisãonarrativa
Resumo Nesteartigoforamanalisadasasprincipaiscaracterísticasfarmacológicasea evidên-cia clínica douso de terapêuticamédica adjuvante em doentes comenfarte do miocárdio comsupradesnivelamentodosegmentoSTtratadoscomangioplastiaprimária.Essesfármacos incluemantiagregantesorais(ácidoacetilsalicílicoeinibidoresdeP2Y12,taiscomoclopidogrel, prasugrel,ticagrelor),antiagregantesendovenosos(oinibidorP2Y12cangrelor,inibidores GpI-IbIIIa,comooabciximab,eptifibatideetirofiban)eagentesanticoagulantesdeusoendovenoso (heparinanãofracionada,heparinadebaixopesomolecularebivalirudina).
©2019SociedadePortuguesa deCardiologia.PublicadoporElsevierEspa˜na,S.L.U.Todosos direitosreservados.
∗Correspondingauthor.
E-mailaddress:dgcaldeira@hotmail.com(D.Caldeira).
https://doi.org/10.1016/j.repc.2018.05.020
Introduction
The acute standard of care for ST-elevation myocardial
infarction (STEMI)1---3 includes activation of a STEMI care
network,administrationof adjuvantmedicaltherapy,and
reperfusionbyprimarypercutaneouscoronaryintervention
(PCI).
Developmentsin organizationalaspectsofhealth care,
suchastheStentforLifeinitiative,andinreperfusion
ther-apy,haveestablishedprimaryPCIasthecurrentfirstoption
forthetreatmentofpatientswithSTEMI,duetoitsimpact
onprognosis.4,5
The improvements nowseen in STEMIcare areclosely
relatedtoadvancesinadjuvantmedicaltherapyinrecent
decades,particularlyantithrombotictherapy.
Inthisarticle,wepresentanarrativereviewdescribing
themainpharmacologiccharacteristicsof,andclinical
evi-denceon,adjuvantmedicaltherapyusedintheacutephase
ofSTEMI.
Review
Aspirin(AcetylsalicylicAcid)
Aspirin acts as a platelet inhibitor at lower doses
(75-325mg),buthasantipyreticandanti-inflammatoryeffects
athigherdoses.
Thelandmarktrialforantiplatelettreatmentinpatients
withacutemyocardialinfarction(MI)wasthe1988Second
InternationalStudyofInfarctSurvival(ISIS-2).6
Inthis trial, theadministrationof162.5 mgaspirin for
30daysachieveda21%relativeriskreduction(RRR)in
vas-cular mortality compared with placebo (Figure 1), which
wassimilartotheeffectofstreptokinasealone(RRR23%).
Areductionof39%invascularmortalitywasobservedwhen
aspirinwasaddedtostreptokinase.6Overall,aspirin
treat-mentshowedanabsoluteriskreductioninvascularmortality
of2.4%andanumberneededtotreatof42patientswithin
fiveweekstopreventone vascular death, comparedwith
placebo.
Duringthe acutepresentation of STEMI,the guidelines
recommendaloadingdoseofaspirinof150-300mgorallyor
80-150mgintravenouslywithlysineacetylsalicylateinorder
toensureinhibitionofserumthromboxaneB2synthesis.1,3,7
The issue ofaspirin dosingwasfurtheranalyzed inthe
Clopidogrel and Aspirin Optimal Dose Usage to Reduce
RecurrentEvents−SeventhOrganizationtoAssessStrategies
in Ischemic Syndromes 7 (CURRENT-OASIS 7) trial, which
included 25 086 patients with acute coronary syndromes
(29%ofwhom,about7000patients,presentedwithSTEMI).8
Patientsrandomizedtohigh-doseaspirin(300-325mgdaily)
forsevendaysshowedsimilarratestothelow-dosegroup
(75-100mgdaily)forthecompositeoutcomeof30-day
car-diovascularmortality,non-fatalMIornon-fatalstroke.The
secondaryoutcomeswerealsosimilarbetweenthe
interven-tions,withtheexceptionofalowerincidenceofrecurrent
ischemiainthehigh-doseaspirinarm.Minorbleedingevents
wereincreasedwithhigh-doseaspirinbuttheriskofmajor
bleedingwassimilarwiththedifferentdoses.8
P2Y12inhibitors:clopidogrel,prasugrel,ticagrelor andcangrelor
Clopidogrel
Thebenefitsoftheadditionofasecondantiplateletagent
inadditiontoaspirininSTEMIpatientsareclearly
demon-strated by clopidogrel.Clopidogrelis a second-generation
memberofthethienopyridinedrugclass,whichinhibit
bind-ingofADPtoP2Y12 receptorsinplateletsandthusprevent
ADP-relatedglycoprotein(GP)IIb/IIIareceptoractivation.
Clopidogrelis a prodrug and requires a two-step
acti-vation process in the liver in which it is converted by
cytochrome P450 (CYP) enzymes into an intermediate
metabolite and a final active metabolite. CYP2C19 is
one of the most important CYPs involved in clopidogrel
metabolism. The concomitant use of CYP2C19 inhibitors
(omeprazole, fluoxetine, orazole antifungalagents) leads
todecreasedlevelsoftheactivemetabolite,buttheclinical
relevanceofthisdecreasehasnotbeenclearlydetermined.9
Thus, alternative drugs should be considered before
pre-scribingCYP2C19inhibitors.
ClopidogrelasAdjunctiveReperfusionTherapy-
Throm-bolysis In Myocardial Infarction (CLARITY-TIMI 28) was a
placebo-controlled trial designed to determine whether
clopidogrel added to standard antiplatelet therapy with
aspirin (loading dose plus maintenance dose of
75-162mg/day)wouldfurtherimprovetheprognosisof adult
patients (18-75 years old) managed with fibrinolysis.10
A total of 3491 patients presenting within 12 hours of
MI intended for treatment with a fibrinolytic agent were
enrolledandrandomizedtoreceiveeitheraspirinaloneor
aspirinplusclopidogrel(300mgloadingdosefollowedby75
mg/day).Theadditionofclopidogreltoaspirinshowedan
RRRof36%foroccludedinfarct-relatedartery,deathorMI
bythetimeofangiography,anda20%RRRfor
cardiovascu-larmortality,MIorurgentrevascularizationduetorecurrent
ischemiaat30days.10
The PCI-CLARITY study was a subgroup analysis of
CLARITY-TIMI28,with1863patientsundergoingPCIamedian
ofthreedaysafterrandomization. Allpatientsinthis
sub-group received a loading dose of 300 mg, then 75 mg
oncedaily of open-labelclopidogrel beforePCI.
Pretreat-mentwithclopidogrelafterfibrinolytictherapyandbefore
PCI resulted in a46% RRR of the compositeof
cardiovas-cular death, recurrent MI or stroke compared to placebo
(Figure1).11
The clopidogrel arm of ClOpidogrel and Metoprolol in
Myocardial Infarction Trial/Second Chinese Cardiac Study
(COMMIT-CCS2)12enrolled45852patientswithMI(93%with
STEMIor MI withleftbundle branchblock)randomizedto
receiveeitherclopidogrelorplaceboinadditiontoaspirin
162mg/dailyuntildischargeoruptofourweeksinhospital.
Clopidogrelsignificantlyreducedall-causemortalityandthe
composite outcome of death, reinfarction or stroke, with
RRRs of 7% and 9%, respectively, after a meantreatment
periodoftwoweeks.
The rates of major bleeding found in these two trials
(CLARITY-TIMI28 andCOMMIT-CCS2)were notsignificantly
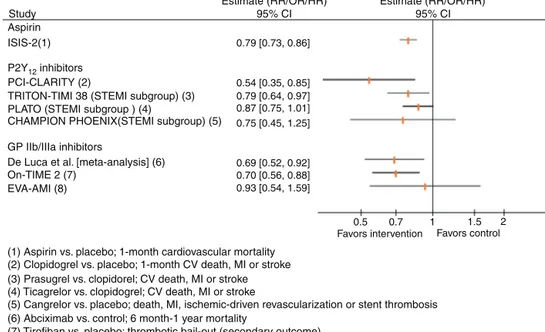
Study
Estimate (RR/OR/HR) Estimate (RR/OR/HR)
95% CI 95% CI
Aspirin ISIS-2(1) P2Y12 inhibitors
PCI-CLARITY (2)
TRITON-TIMI 38 (STEMI subgroup) (3) PLATO (STEMI subgroup ) (4)
CHAMPION PHOENIX(STEMI subgroup) (5) GP IIb/IIIa inhibitors
De Luca et al. [meta-analysis] (6) On-TIME 2 (7)
EVA-AMI (8)
(1) Aspirin vs. placebo; 1-month cardiovascular mortality (2) Clopidogrel vs. placebo; 1-month CV death, MI or stroke (3) Prasugrel vs. clopidorel; CV death, MI or stroke (4) Ticagrelor vs. clopidogrel; CV death, MI or stroke
(5) Cangrelor vs. placebo; death, MI, ischemic-driven revascularization or stent thrombosis (6) Abciximab vs. control; 6 month-1 year mortality
(7) Tirofiban vs. placebo; thrombotic bail-out (secondary outcome) (8) Eptifibatide vs. abciximab; death, MI or TVR (secondary outcome)
0.79 [0.73, 0.86] 0.54 [0.35, 0.85] 0.79 [0.64, 0.97] 0.87 [0.75, 1.01] 0.75 [0.45, 1.25] 0.69 [0.52, 0.92] 0.70 [0.56, 0.88] 0.93 [0.54, 1.59] 0.5
Favors intervention Favors control
0.7 1 1.5 2
Figure1 ResultsofselectedtrialsassessingantiplateletagentsinpatientswithST-segmentelevationmyocardialinfarction.CI: confidenceinterval;CV:cardiovascular;GP:glycoprotein;HR:hazardratio;MI:myocardialinfarction;OR:oddsratio;RR:relative risk;STEMI:ST-segmentelevationmyocardialinfarction;TVR:targetvesselrevascularization.
Clopidogrel loading doses (600 mg vs. 300 mg) were
comparedin 201STEMIpatientsundergoingprimaryPCIin
theAntiplatelettherapy forReductionofMYocardial
Dam-ageduringAngioplasty-MyocardialInfarction(ARMYDA-6MI)
trial.13 The primary outcome, the area under the curve
ofcardiacbiomarkersasasurrogateofmyocardialinfarct
size,wassignificantlylowerwiththe600mgloadingdose
thanwiththe300mgloadingdose.Therewerealsofewer
30-day major adverse cardiovascular events but bleeding
complications were similarbetween the different loading
doses.13
The CURRENT-OASIS 7 trial (see section on aspirin,
above),witha2-by-2factorialdesign,alsoassessedthe
clin-icalimpactofhigh-doseclopidogrel(loadingdose600mg,
sixdaysat 150 mg/day, followedby 75 mg/day)vs.
stan-darddose(loadingdose300mg,followedby75mg/day).8
Overall, high-dose clopidogrel didnot reduce 30-day
car-diovascularmortality,MIor stroke,but inthesubgroupof
patientswhounderwentPCI(STEMIandnon-STEMI)therisk
ofthesemajoradversecardiovasculareventswas
significan-tlydecreased,mainlyduetothereductionofMI.High-dose
clopidogrelincreasedtheriskofmajorbleeding.8
Prasugrel
Prasugrel,anothermemberofthethienopyridinedrugclass,
is an irreversible P2Y12 inhibitor that overcomes some
of clopidogrel’s limitations, particularly the degree of its
antiplateleteffectandtimetoonset.
Prasugrel is rapidly hydrolyzed toa thiolactonein the
intestine,andthenafterabsorptionisconvertedbyCYP3A4
andCYP2B6andtoalesserextentbyCYP2C9andCYP2C19to
theactivemetabolite,whichreachespeakplasma
concen-tration30minafteradministration.
Thebenefitsofprasugrel(loadingdose60mg,followed
by10 mgoncedaily)instead ofclopidogrel inadditionto
aspirin(atanydosefrom75to162mg)wereshownbythe
TRITON-TIMI38 trial,which enrolled 13608 patients with
acutecoronarysyndromesmanagedinvasivelywithPCI.14
Prasugrelreducedtherelativeriskofthecomposite
out-come of cardiovascular death, non-fatal MI and non-fatal
strokeby19%comparedwithclopidogrel.Theresultswere
mainlydrivenby reduced riskfor non-fatal MI (RRR 24%),
andtheoverallclinicalimpactofprasugrelwasnot
differ-entwithinthe subgroup of 3534STEMI patients (p=NSfor
interaction);theRRRinthissubgroupwas21%.Therewas
nomortality benefit withprasugrel and the bleeding risk
wassignificantlyhigheraccordingtothestudydefinitionof
majorbleeding(TIMImajorbleedingnotrelatedtocoronary
artery bypassgrafting [CABG]).14 The risk of death,
non-fatalMI,non-fatalstrokeandnon-CABG-relatedTIMImajor
bleedingwassignificantlyhigherinpatients withahistory
ofstrokeortransientischemicattack,andthusprasugrelis
notindicatedforthesepatients.Othersubgroupsforwhich
specificconcernswereraised weretheelderly(≥75years
old)andlow-weightindividuals(<60kg),particularly
regard-ingtheirbleedingrisk with10mgprasugreldaily.Current
regulatorydocumentsrecommendacarefulindividual
ben-efit/riskassessmentandifprescribedthemaintenancedose
shouldbe5mgdailyforsuchpatients.
Ticagrelor
Ticagrelor is an oral cyclopentyltriazolopyrimidine that
reversiblyandnon-competitivelyinhibits ADP-induced
sig-naling downstream of P2Y12 receptors. Compared to
clopidogrel,ticagrelorshowedafavorableprofileregarding
timetoonsetanddegreeofantiplateleteffect.
Ticagrelorreaches peakplasma levelsbetween 90 min
andthreehoursandpeakplateletinhibitionintwohours.
ThepivotaltrialofticagrelorinACSwasPLATO(aStudy
antiplatelettreatmentwithaspirinandticagrelorwas
com-paredwithaspirinandclopidogrel.Thetrialincludedatotal
of18624patientswithACS.BothP2Y12inhibitorsweregiven
inaloadingdose(ticagrelor180mg;clopidogrel300-600mg)
andamaintenancedose(ticagrelor90mgtwicedaily;
clopi-dogrel 75 mgonce daily). Ticagrelorshowed a significant
16%RRRincardiovascularmortality,MIandstroke,dueto
decreasesincardiovascularmortalityandMI.Overallmajor
bleedingriskwasnotsignificantlyincreased,but
non-CABG-relatedmajorbleedingwassignificantlyincreased.
This trial included 7544 patients with STEMI, and the
subgroup’sresultwassimilartothe non-STEMIpopulation
(p=0.29forinteraction).Itisnoteworthythatticagrelorhad
lesseffectinNorthAmericathanintherestoftheworld.15
Otherthanchancealoneoratrueregionaldifference,one
possibleexplanationisthefactthattheaspirinmaintenance
dosewasonaveragehigherinNorthAmerica.Asignificant
statisticalinteractionwasobservedfavoringtheinteraction
oflow-doseaspirinwithticagrelor.Therecommendeddose
ofaspirintobetakenwithticagreloris75-150mg,
prefer-ablylowerdoses.
Themain adverseevents associatedwithticagrelorare
dyspneaand bradyarrhythmias. The incidence of dyspnea
intheticagrelorarm in thePLATOtrial was13.8%.It was
usually non-severe, transient and not related to cardiac,
pulmonaryormetabolicfactors.
Ventricularpausesof>3s,assessedbyHoltermonitoring,
weremore frequent withticagrelorthan withclopidogrel
intheacute phaseofthe coronaryevent(duringthefirst
week), but not at 30 days after randomization. The risk
ofheart block,syncope andpacemakerimplantation with
ticagrelorwasnotsignificantlydifferentfromclopidogrel.
In addition to P2Y12 signal blockade, ticagrelor also
inhibits equilibrative nucleoside transporter-1 (ENT-1),
which is implicated in the cellular uptake of endogenous
adenosine.16---18 The above adverse drug reactions maybe
relatedtothismechanism.
The Administration of Ticagrelor in the Cath Lab or
inthe Ambulancefor NewST ElevationMyocardial
Infarc-tiontoOpentheCoronaryArtery(ATLANTIC)trialassessed
the potential benefits of the prehospital administration
of ticagrelor compared with in-hospital administration.19
In this study involving 1862 STEMI patients, the median
timefromrandomizationtocoronaryangiogramwas48min
andthemediantimedifferencebetween pre-hospitaland
in-hospitaladministrationoftheticagrelorloadingdosewas
31min.Theprimaryoutcomes,surrogatesofantithrombotic
efficacyrelatedtopre-PCIcoronaryreperfusion(resolution
ofST-segmentelevationandtheproportionofcoronaryTIMI
flow<3),werenotimprovedbyearlypre-hospitalticagrelor
administration. The risk of definite stent thrombosis was
lowerwithpre-hospitalticagreloradministration.19
Cangrelor
Cangreloris an intravenousreversibleP2Y12 inhibitor.The
mainadvantagesofthisdrugarerapidonset(within2min
ofabolusfollowedbyaninfusion)andoffset(onehourafter
stoppingdruginfusion)ofantiplateleteffects.20
The drugismetabolizedin theplasma intoaninactive
metabolitewhichiseliminatedthroughrenalanddigestive
routes.Thus,cangrelordoesnotrequiredose adjustments
inrenalorliverimpairment.
Cangrelorhasbeenapprovedforpatientswithcoronary
disease undergoingPCI whodid notreceive an oral P2Y12
inhibitor priortotheprocedure andin whomcurrentoral
therapywithP2Y12inhibitorsisnotfeasible(e.g.absenceof
oralroute)ordesirable(e.g.bridgingtoCABGsurgery).20
ThepivotalCHAMPIONPHOENIX(Cangrelorversus
Stan-dard TherapytoAchieve OptimalManagement of Platelet
Inhibition PHOENIX) trial comparedcangrelorand placebo
in 10 942 patients treated witha 300- or 600-mg loading
dose ofclopidogrelbeforePCI.21 This trialshowedanRRR
of22%and15%inthecompositeendpointofmortalityand
ischemic events (MI, ischemia-driven revascularization or
stent thrombosis) at 48 hours and 30 days of follow-up,
respectively. This benefit was mainly driven by a
signifi-cantRRRof38%instentthrombosis(intraproceduralstent
thrombosisindependentlyadjudicatedbytheangiographic
corelaboratorycommittee,ordefinitestentthrombosisas
defined by the Academic ResearchConsortium criteria22).
CHAMPIONPHOENIXincluded1992patientswithSTEMIand
no interaction was observed regarding treatment effect
accordingtoclinicalpresentation(STEMIandnon-STEMI).21
Thebenefitsof cangrelorarelikelyrelatedtoitsrapid
antiplateletactioncomparedtootheroralP2Y12 inhibitors
suchasclopidogrel.
GlycoproteinIIb/IIIainhibitors
Thisclassincludesthreedrugs,administeredintravenously,
thatdisablefibrinogenandvonWillebrandfactorbybinding
totheGPIIb/IIIareceptor:abciximab,tirofibanand
eptifi-batide.
Abciximab
Abciximabisanantigen-bindingfragmentofachimeric
mon-oclonal antibody that binds to GPIIb/IIIa and vitronectin
receptors. Abciximab’santiplatelet effect is seen 10 min
after bolus administration and platelet function typically
recovers24-48hoursafterdrugwithdrawal.23---25
Mostof the evidence supporting the use of GP IIb/IIIa
inhibitorsinSTEMIarisesfromtrialswithabciximabinthe
eraofballoonangioplastyandbeforetheroutine
implemen-tationofdualantiplatelettherapyinthesepatients.DeLuca
etal.publishedasystematicreviewontheclinicalimpact
of abciximabinSTEMIpatients treatedwithfibrinolysisor
primaryPCI.26Inthisreviewabciximabreducedboth30-day
andlong-term(sixmonthstooneyear)all-causemortalityby
31%and32%,respectively.Nosignificantincreaseinmajor
bleedingrisks,includingintracranialhemorrhage,wasnoted
(Figure1).26
Themainadverseeventratherthanbleeding(majoror
minor)isthrombocytopenia,which usuallydevelopsinthe
first24hours.Theincidenceofthrombocytopeniais2.9%for
firstadministrationand5%forrepeatadministrations.27,28
Tirofiban
Tirofibanisanon-competitivenon-peptideGPIIb/IIIa
recep-torantagonist.Thisdrug’santiplateleteffectbegins15min
ofplateletaggregationis<50%fourhoursaftercessationof
druginfusion.30
IntheOn-TIME2trial,936STEMIpatientswere
random-ized to high-dosetirofiban or placebo in the pre-hospital
setting,ontopofaspirin500mg,clopidogrel600mg,and
unfractionated heparin 5000 IU.31 The trial was designed
to assess ST-elevation resolution, and tirofiban
significan-tlydecreased theextent of residualST-segmentdeviation
one hour after PCI. The rates of the composite of
death,recurrentMIortargetvesselrevascularization,and
major bleeding were not significantly different between
interventions.Therewassignificantlylessneedfor
throm-boticbail-outduetoTIMIflowgrade0-2andabruptclosure
of the culprit vessel in the tirofiban arm (Figure 1).
In-hospitalthrombocytopeniawasnotsignificantlyhigher(2.0%
withtirofibanand1.8%withplacebo).
Eptifibatide
Eptifibatide is a synthetic heptapeptide that reversibly
andcompetitivelyinhibitsGPIIb/IIIareceptors.Itis given
throughan initial double intravenous bolus (separatedby
10min)of180 g/kgfollowedbyacontinuousinfusionof
2 g/kg/min for up to72 hours. The antiplatelet effects
areobservedafterthebolusandplateletfunctionrecovers
fourhoursaftercessationofinfusion.Because50%of
epti-fibatide clearance is kidney-dependent, the infusion dose
shouldbehalvedinpatientswithmoderatechronickidney
disease(estimatedglomerularfiltrationrate30-50ml/min).
Themaindataoneptifibatideuseinacutecoronary
syn-dromescome fromthePURSUITtrial, whichenrolledonly
patients without persistent ST-segment elevation.32
PUR-SUIT was a randomized, double-blind assessment of the
efficacyandsafetyofeptifibatideversusplacebofor
redu-cingmortality and MI in patients with unstable angina or
non-Q-wave MI and included 13.8% of patients with
non-persistent ST segment elevation or persistent ST-segment
elevationof0.5-0.9mm.ThistrialshowedaRRRof10%in
mortalityor MIat 30days.Asignificant increase inmajor
and/or minor bleedingevents wasobserved in the
eptifi-batidearm.Theriskofthrombocytopeniawitheptifibatide
wasnotsignificantlydifferentfromplacebo.
InthecontextofSTEMI,theEptifibatideVersusAbciximab
in Primary PCI for Acute Myocardial Infarction (EVA-AMI)
trialrandomized427STEMIpatientstoeptifibatide(double
bolusand24-hourinfusion)orabciximab(bolusand12-hour
infusion) as adjuncts to primary PCI.33 The primary
out-comewascompleteST-segmentresolution60minafterPCI.
Theelectrocardiographicandclinicalimpactofeptifibatide
wassimilartoabciximab(Figure1).Non-randomizedstudies
showsimilarfindings.34,35
Heparins
Unfractionatedheparin
Unfractionatedheparin(UFH)isapolysaccharidethatbinds
to plasma proteins, including antithrombin, enhancing its
action,whichincludes inactivationofthrombinandfactor
Xa. The heparin-antithrombin complex also inhibits other
coagulation factors including VIIa, XIa and XIIa. UFH also
enhancesendogenousfibrinolysis.UFHisusuallygiven
intra-venously,includinginthecontextofSTEMI.
This drug hastwo independentclearancemechanisms:
asaturable pathwayin which rapid depolymerization
fol-lows binding to endothelial cells, macrophages and local
proteins;andanon-saturablerenalclearancepathway.The
half-lifeofUFHis90-120 mindepending onthe clearance
mechanism.The existenceofthesetwoclearance
mecha-nismscanleadtointra-andinter-individualvariabilityinthe
pharmacodynamiceffectsofUFH,whichisalimitationonits
useduetotheunpredictabilityofitsanticoagulanteffects,
whichnecessitatemonitoringbymeasurementofactivated
partialthromboplastintimeoractivatedclottingtime.
Although therehave been noplacebo-controlledtrials,
thevalueofUFHwashighlightedbytheOASIS-6trialon
fon-daparinux,inwhich UFHwasadministeredtosomeofthe
controlgroup.36InthesubgroupofpatientswithSTEMIwho
underwentprimaryPCI,UFHreducedtheriskofthrombotic
bailout or catheter thrombosis,none of the latter
occur-ringwithUFH.Therewasalsoatrendtowardsanincreased
riskofdeathorreinfarctioninprimaryPCIpatientstreated
withfondaparinuxcomparedtoUFHat30daysand90-180
days,withasignificantsubgroupinteraction(primaryPCIvs.
non-primaryPCI,trendingforafavorableeffectofUFHin
theprimaryPCIsubgroup,andasignificantfavorableeffect
of fondaparinux in the non-primary PCI subgroup).36 For
thesereasonsfondaparinuxisnotrecommendedforpatients
withSTEMIundergoingPCI,butisavalidanticoagulantfor
STEMIpatients treatedwithfibrinolysis.3 UFHisan option
forparenteralanticoagulantsinthissettingaccordingtothe
EuropeanSocietyofCardiology(ESC)guidelines.1
Regarding dosage of UFH, the initial bolus should be
weight-adjusted, and should be as follows according to
expectationoftheuseofGPIIb/IIIainhibitors:UFH70-100
IU/kgwhennoGPIIb/IIIainhibitorisplanned,orUFH50-60
IU/kgwhentheuseofGPIIb/IIIainhibitorsisexpected.1
The main safetyissuesarebleedingevents and
throm-bocytopenia. For bleeding events, protamine can be
administeredto reverse the anticoagulant effectof UFH.
The incidenceof heparin-inducedthrombocytopenia (HIT)
ranges from 0.1% to 5.0% and at least one quarter of
thesepatientsdevelopthromboticcomplications.InHIT,the
plateletcount fall is 50% or more and the nadiris above
20×109/l.37 It usually occurs after 5-10 days of exposure
toUFHor soonerifthe patientwasexposedinthe
previ-ous30days.37 BothHIT andHIT-relatedthromboticevents
arerelatedtothedevelopmentofimmunoglobulinsagainst
complexesofplateletfactor4andUFH.37
Lowmolecularweightheparins
Lowmolecularweightheparins(LMWH)aresmall
antithrom-bin polysaccharides derived from UFH. LMWH have more
anti-Xa than anti-IIa activity and enhance the release of
tissuefactor pathway inhibitors. These drugs donot bind
to endothelial cells or macrophages, and hence have a
predictabledose-effectrelationshipusingbolus
administra-tions.Theirbioavailabilityisalsohigher,enablingeffective
subcutaneous administration. Regarding safety issues,the
rate of HIT is lower with LMWH than with UFH, but in
bleedingconditions the antidote protamine is less
effec-tive, sinceit only partially neutralizesthe anti-Xa effect
ofLMWH.Thenewanti-factorXaantidoteandexanetalfa
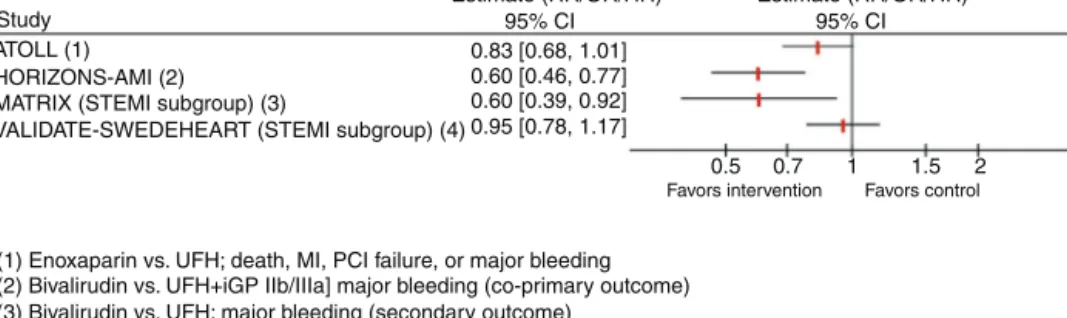
Study ATOLL (1) HORIZONS-AMI (2) MATRIX (STEMI subgroup) (3)
VALIDATE-SWEDEHEART (STEMI subgroup) (4)
(1) Enoxaparin vs. UFH; death, MI, PCI failure, or major bleeding (2) Bivalirudin vs. UFH+iGP IIb/IIIa] major bleeding (co-primary outcome) (3) Bivalirudin vs. UFH; major bleeding (secondary outcome)
(4) Bivalirudin vs. UFH; death, MI, or major bleeding
Estimate (RR/OR/HR) Estimate (RR/OR/HR)
95% CI 0.83 [0.68, 1.01] 0.60 [0.46, 0.77] 0.60 [0.39, 0.92] 0.95 [0.78, 1.17] 0.5 0.7 1 1.5 2
Favors intervention Favors control
95% CI
Figure2 ResultsofselectedtrialsassessinganticoagulantagentsinpatientswithST-segmentelevationmyocardialinfarction. CI:confidenceinterval;CV:cardiovascular;GP:glycoprotein;HR: hazard ratio;MI:myocardialinfarction; OR:odds ratio;PCI: percutaneouscoronaryintervention;RR:relative risk;STEMI: ST-segmentelevationmyocardialinfarction; UFH:unfractionated heparin.
patientsrequiringrapidhemostasis.38However,the
Andex-anetAlfa,aNovelAntidotetotheAnticoagulationEffectsof
FXAInhibitors4(ANNEXA-4)trialonlyenrolledfourpatients
with major bleeding treated with enoxaparin (out of
67patientstreatedwithfactorXainhibitors).38
ThemainclinicalevidencefortheuseofLMWHinSTEMI
patients derives from the Acute STEMI Treated with
pri-mary angioplasty and intravenous enoxaparin Or UFH to
Lowerischemicandbleedingeventsatshort-andLong-term
follow-up study (ATOLL).39 Compared to UFH, enoxaparin
(intravenousbolusof0.5mg/kg followedbysubcutaneous
injections every 12 hours) did not significantly (p=0.06)
reduce theprimary compositeoutcome of death,
compli-cation of MI, procedure failure, or major bleedingin 910
STEMI patients (Figure 2). However, secondary outcomes
includingdeath, recurrent acute coronarysyndrome, and
urgentrevascularizationweresignificantly decreased(41%
RRR) with enoxaparin.39 No differences were observed in
majorbleeding.Basedonthesefindings,theESCguidelines
statethatLMWHmaybeconsideredinsteadofUFH(classIIb
recommendation).1AnotheradvantageofLWMHoverUFHis
thelowerriskofHIT.
Bivalirudin
Bivalirudin is a polypeptide that is a direct thrombin
inhibitor,bindingbothcirculatingandclot-boundthrombin.
The Harmonizing OutcomesWith Revascularizationand
Stents in Acute Myocardial Infarction (HORIZONS-AMI)
trial compared UFH plus a GP IIb/IIIa inhibitor with
bivalirudinalone(0.75mg/kgintravenousbolusfollowedby
1.75mg/kg/hour infusion) inpatients undergoing primary
PCI.40
In 3602 STEMI patients undergoing primary PCI,
bivalirudin,comparedtoUFHplusGPIIb/IIIainhibitors(52%
abciximab,46%tirofiban),showedanRRRof24%within30
daysinnetadverseclinicalevents,acompositeof
cardio-vasculardeath,MI,targetvesselrevascularization,strokeor
majorbleeding,mostlyduetothe40%RRRinmajor
bleed-ing(Figure2).40Anincreasedriskofstentthrombosiswithin
24hourswasobservedinthebivalirudingroup.
The European Ambulance Acute Coronary Syndrome
Angiography(EUROMAX) trial studied 2218 STEMI patients
transportedfor primaryPCIandrandomizedtobivalirudin
(bolus and infusion for at least four hours after PCI at
0.25 mg/kg/hour)or UFH(and optional use of GP IIb/IIIa
inhibitors).41 The primary outcome of death and major
bleedingwassignificantlydecreased,duetothe57%RRRin
majorbleeding.41 SimilarlytotheHORIZONS-AMItrial,the
riskofacutestentthrombosiswassignificantlyhigher.
TheMinimizingAdverseHemorrhagicEventsby
Transra-dial Access Site and Systemic Implementation of Angiox
(MATRIX) trial also compared bivalirudin with UFH (with
GPIIb/IIIainhibitorsonlyinbail-outcircumstances).42This
trial included 7213 ACS patients, 4010 (56%) with STEMI.
The results for the STEMI group were not different from
the overall study results.43 The risk of death, non-fatal
MI and non-fatal stroke was similar between groups, but
there was a significant 40% RRR in major bleeding with
bivalirudinintheSTEMIgroup(Figure2).TheMATRIXstudy
also assessed the potential impactof post-PCI bivalirudin
infusion;thisstrategydidnotimproveanyoutcome,
includ-ingstentthrombosis.42
TheBivalirudin versus Heparin inST-Segmentand
Non-ST-Segment ElevationMyocardial Infarction in Patientson
Modern Antiplatelet Therapy in the Swedish Web System
forEnhancementandDevelopmentofEvidence-basedCare
in Heart Disease Evaluated according to Recommended
TherapiesRegistryTrial(VALIDATE-SWEDEHEART)wasa
ran-domized open-labelregistry-basedcontrolled clinicaltrial
that compared bivalirudin and UFH monotherapy in 6006
patientswithMI(3001 withSTEMI).The clinicalimpactof
bivalirudinwassimilartoUFHinmonotherapyinthe
over-all cohort as well as in the STEMI subgroup in terms of
the compositeprimaryendpoint of all-causemortality, MI
or majorbleedingduring180daysoffollow-up(Figure2).
Nosignificantdifferenceswereobservedinanyofthe
sec-ondaryendpoints.
Discussion
STEMIis an acutethrombus-drivenevent.The importance
ofthispathophysiologicallinkishighlightedinthisreview,
inwhichthemainpharmacokinetic,pharmacodynamicand
clinicaldataonantithromboticdrugsinSTEMIarereviewed.
These adjuvant medical agents, particularly antiplatelet
drugs, have significantly improved the prognosis of this
patientgroup.Afteraspirin(withISIS-2),6thebreakthrough
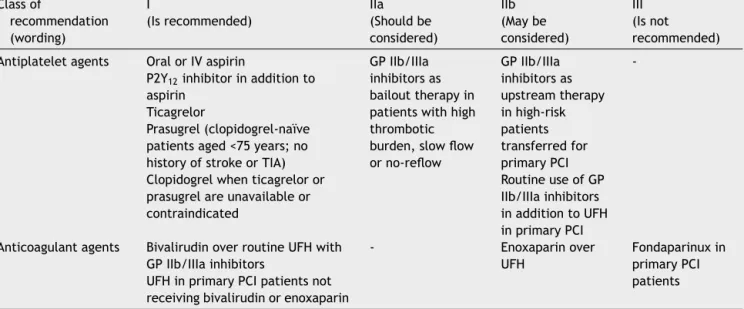
Table 1 2017 European Society of Cardiology recommendations for antithrombotic therapy in patients with ST-elevation myocardialinfarctionundergoingprimarypercutaneousintervention.3
Classof recommendation (wording) I (Isrecommended) IIa (Shouldbe considered) IIb (Maybe considered) III (Isnot recommended) Antiplateletagents OralorIVaspirin
P2Y12inhibitorinadditionto aspirin
Ticagrelor
Prasugrel(clopidogrel-naïve patientsaged<75years;no historyofstrokeorTIA) Clopidogrelwhenticagreloror prasugrelareunavailableor contraindicated
GPIIb/IIIa inhibitorsas bailouttherapyin patientswithhigh thrombotic burden,slowflow orno-reflow GPIIb/IIIa inhibitorsas upstreamtherapy inhigh-risk patients transferredfor primaryPCI RoutineuseofGP IIb/IIIainhibitors inadditiontoUFH inprimaryPCI
-Anticoagulantagents BivalirudinoverroutineUFHwith GPIIb/IIIainhibitors
UFHinprimaryPCIpatientsnot receivingbivalirudinorenoxaparin
- Enoxaparinover
UFH
Fondaparinuxin primaryPCI patients
GP:glycoprotein;IV:intravenous;PCI:percutaneouscoronaryintervention;TIA:transientischemicattack;UFH:unfractionatedheparin.
IntheSTARStrialthecombinationofticlopidineandaspirin
instentedpatients(patientswithrecentMIwereexcluded)
showedadecreaseinstentthrombosis-relatedevents
com-paredtoaspirinaloneandaspirincombinedwithwarfarin.44
In the ISAR trial the combination of aspirin and
ticlopi-dine (24%of the patientsin this grouphad hadacuteMI)
wassuperior to aspirin withphenprocoumon (a vitamin K
antagonist).45The formerreducedthecompositeendpoint
of cardiovascular mortality, non-fatal MI or
revasculariza-tion,aswellasthromboticstentocclusion.45Consequently,
theadventofGPIIb/IIIainhibitorscontributedsignificantly
totheperception that dualantiplatelet therapywas
nec-essary in acute coronary syndromes. The process of PCI,
includinginterventioninSTEMIandthe evolutionto
drug-elutingstentsandradialaccess,andinhibitionoftheP2Y12
receptorpathwaywithclopidogrel,prasugrelandticagrelor,
have sincebeen furtherstudied andoptimized.Nowadays
themainstayforantiplatelettherapyinSTEMIisaspirinand
apotentP2Y12inhibitor(usuallyticagrelororprasugrel).GP
IIb/IIIainhibitorsarereservedforbailoutsituations,while
cangrelormayplayaroleduetoitsrapidantiplateletaction
incaseswherethepharmacodynamiceffectsofthenewer
andfaster P2Y12 inhibitorsarenot optimal. InSTEMI, the
fibrin-richthrombi that occludearteries arethe resultof
plateletactivationandalsoofthereleaseoftissuefactor
bythedamagedvesselthatleadstoactivation,amplification
andpropagationofthecoagulationcascade.Anticoagulants
inthissettingaregiventopreventexpansionand
enhance-mentofthrombi.Furthermore,contemporarymanagement
withprimary PCI requires a suitable antithrombotic
envi-ronment to prevent catheter thrombosis, which may be
achievedwithUFH,enoxaparinorbivalirudin.Fondaparinux
was found to decrease the risk of death or MI,
particu-larly in patients managed medically or in those receiving
fibrinolysis (mostly streptokinase). In patients undergoing
primary PCI, fondaparinux increased the risk of catheter
thrombosis and no clinical benefit was noted regarding
otheroutcomes.Fondaparinuxisthereforecontraindicated
in patients undergoing primary PCI. Regarding the other
anticoagulants,UFHremainsthegoldstandardintravenous
anticoagulant,althoughitsanti-ischemicefficacywas
chal-lenged by enoxaparin in one trial (ATOLL39), and despite
concerns of increased risk of stent thrombosis the major
bleedingriskwasshowntobelowerwithbivalirudin.
The2017EuropeanSocietyofCardiology recommendationsforantithrombotictherapyin patientswithST-elevationmyocardialinfarction undergoingprimarypercutaneousintervention3 Antiplateletdrugs
- Aspirinisrecommended(classI).
- A P2Y12 inhibitor is recommended inaddition toaspirin
(classI),particularly ticagrelor(classI)or prasugrel(in
clopidogrel-naïvepatientsaged<75yearsorwithno
his-toryofstrokeortransientischemicattack---classI).
- GP IIb/IIIa inhibitors should be considered for bailout
therapy in patients with high thrombotic burden or
complications,includingslowfloworno-reflow(classIIa).
- GPIIb/IIIainhibitorsmaybeconsideredforupstream
ther-apyinhigh-risk patientsundergoingtransferfor primary
PCI(classIIb),orasroutineadjunctantiplatelettherapy
inpatientstreatedwithUFH(classIIb).
Anticoagulantdrugs
- Bivalirudin is recommended over routine UFH with GP
IIb/IIIainhibitors(classI).
- EnoxaparinmaybepreferredtoUFH(classIIa).
- UFHshouldbeusedinSTEMIpatientsundergoingprimary
PCInotreceivingbivalirudinorenoxaparin(classI).
- FondaparinuxiscontraindicatedinSTEMIpatients
Table 1 summarizes the recommendations and their
classes regarding antithrombotic therapy in patients with
STEMItreatedwithprimaryPCI.
Conclusions
Advancesin antithrombotic treatment have led to
signifi-cantimprovementsintheprognosisofpatientswithSTEMI
treatedby primaryPCI. The growingbody ofevidence on
eachofthesetherapeuticoptionsposesnewchallengesin
termsofdrugcombinations.
Conflicts
of
interest
Theauthorshavenoconflictsofinteresttodeclare.
References
1.TaskForceonthemanagementofST-segmentelevationacute myocardial infarction of the European Societyof Cardiology (ESC),StegPG,JamesSK,AtarD,etal.ESCGuidelinesforthe managementofacutemyocardialinfarctioninpatients present-ingwithST-segmentelevation.EurHeartJ.2012;33:2569---619.
2.Yanamala CM, Bundhun PK, Ahmed A. Comparing mortal-ity between fibrinolysis and primary percutaneous coronary intervention in patients with acute myocardial infarction: a systematic review and meta-analysis of 27 randomized-controlledtrials including11429patients.CoronArtery Dis. 2017;28:315---25.
3.IbanezB,JamesS,AgewallS,etal.ESCGuidelinesforthe mana-gementofacutemyocardialinfarctioninpatientspresenting withST-segmentelevation:TheTaskForceforthemanagement ofacutemyocardialinfarctioninpatientspresentingwith ST-segmentelevationoftheEuropeanSocietyofCardiology(ESC). EurHeartJ.2017:2017.
4.PereiraH,PintoFJ,CaleR,etal.StentforLifeinPortugal:this initiativeisheretostay.RevPortCardiol.2014;33:363---70.
5.KaifoszovaZ,KalaP,WijnsW.TheStentforLifeInitiative:quo vadis?EuroIntervention.2016;12:14---7.
6.Randomised trial of intravenous streptokinase, oral aspirin, both,orneitheramong17,187casesofsuspectedacute myocar-dial infarction: ISIS-2. ISIS-2 (Second International Study of InfarctSurvival)CollaborativeGroup.Lancet.1988;2:349---60.
7.BuerkeM,PittroffW,MeyerJ,etal.Aspirintherapy:optimized platelet inhibition with different loading and maintenance doses.AmHeartJ.1995;130:465---72.
8.CURRENT-OASIS7Investigators,MehtaSR,BassandJP, Chrolavi-ciusS, etal. Dose comparisonsofclopidogreland aspirinin acutecoronarysyndromes.NEnglJMed.2010;363:930---42.
9.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or withoutomeprazoleincoronaryarterydisease.NEnglJMed. 2010;363:1909---17.
10.SabatineMS,CannonCP,GibsonCM,etal.Additionof clopido-greltoaspirinandfibrinolytictherapyformyocardialinfarction withST-segmentelevation.NEnglJMed.2005;352:1179---89.
11.SabatineMS,CannonCP,GibsonCM,etal.Effectof clopido-grelpretreatmentbeforepercutaneouscoronaryintervention inpatientswithST-elevationmyocardialinfarctiontreatedwith fibrinolytics:thePCI-CLARITYstudy.JAMA.2005;294:1224---32.
12.ChenZM,JiangLX,ChenYP,etal. COMMIT(ClOpidogreland MetoprololinMyocardialInfarctionTrial)collaborativegroup. Additionofclopidogreltoaspirinin45,852patientswithacute myocardial infarction: randomised placebo-controlled trial. Lancet.2005;366:1607---21.
13.PattiG,BarcziG,OrlicD,etal.Outcomecomparisonof 600-and300-mgloadingdosesofclopidogrelinpatients undergo-ingprimarypercutaneouscoronaryinterventionforST-segment elevationmyocardialinfarction:resultsfromtheARMYDA-6MI (AntiplatelettherapyforReductionofMYocardialDamage dur-ingAngioplasty-MyocardialInfarction)randomizedstudy.JAm CollCardiol.2011;58:1592---9.
14.WiviottSD, BraunwaldE,McCabeCH, etal.Prasugrel versus clopidogrelinpatientswithacutecoronarysyndromes.NEngl JMed.2007;357:2001---15.
15.MahaffeyKW, Wojdyla DM, Carroll K, et al. Ticagrelor com-pared withclopidogrel bygeographic regionin the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544---54.
16.ArmstrongD,SummersC,EwartL,etal.Characterizationofthe adenosinepharmacologyofticagrelor revealstherapeutically relevantinhibition ofequilibrative nucleoside transporter1. JCardiovascPharmacolTher.2014;19:209---19.
17.vanGiezenJJ,SidawayJ,GlavesP,etal.Ticagrelorinhibits adenosineuptakein vitro and enhancesadenosine-mediated hyperemiaresponsesinacaninemodel.JCardiovascPharmacol Ther.2012;17:164---72.
18.NylanderS, Femia EA, Scavone M, et al. Ticagrelor inhibits humanplateletaggregationviaadenosineinadditiontoP2Y12 antagonism.JThrombHaemost.2013;11:1867---76.
19.MontalescotG, van’tHofAW, LapostolleF,etal.Prehospital ticagrelorinST-segmentelevationmyocardialinfarction.NEngl JMed.2014;371:1016---27.
20.Marcano AL, Ferreiro JL. Role of new antiplatelet drugs on cardiovascular disease: update on cangrelor. Current AtherosclerosisReports.2016;18:66.
21.BhattDL,StoneGW,MahaffeyKW,etal.Effectofplatelet inhi-bitionwithcangrelorduringPCIonischemicevents.NEnglJ Med.2013;368:1303---13.
22.ApplegateRJ,SacrintyMT,LittleWC,etal.Incidenceof coro-narystentthrombosisbasedonacademicresearchconsortium definitions.AmJCardiol.2008;102:683---8.
23.Tcheng JE, Ellis SG, George BS, et al. Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet anti-body Fab 7E3 in high-risk coronary angioplasty. Circulation. 1994;90:1757---64.
24.Simoons ML, de Boer MJ, van den Brand MJ, et al., Euro-peanCooperativeStudyGroup.RandomizedtrialofaGPIIb/IIIa plateletreceptorblockerinrefractoryunstableangina. Circu-lation.1994;89:596---603.
25.MadanM,Berkowitz SD,Christie DJ, etal. Determinationof platelet aggregationinhibition duringpercutaneous coronary interventionwiththeplateletfunctionanalyzer PFA-100.Am HeartJ.2002;144:151---8.
26.De LucaG, Suryapranata H, Stone GW, et al. Abciximab as adjunctivetherapytoreperfusioninacuteST-segmentelevation myocardial infarction:a meta-analysis of randomized trials. JAMA.2005;293:1759---65.
27.Kereiakes DJ, Berkowitz SD, Lincoff AM, et al. Clinical correlates and course of thrombocytopenia during percu-taneous coronary intervention in the era of abciximab plateletglycoproteinIIb/IIIablockade.AmHeartJ.2000;140: 74---80.
28.TchengJE, Kereiakes DJ,Lincoff AM,et al. Abciximab read-ministration:resultsoftheReoProReadministrationRegistry. Circulation.2001;104:870---5.
29.Summary of Product Characteristics of Tirofiban - Aggras-tat50 mg/mlSolution for infusion. https://www.medicines. org.uk/emc/product/566[accessedApril2018].
30.Kereiakes DJ, Kleiman NS, Ambrose J, et al. Random-ized,double-blind, placebo-controlled dose-ranging study of tirofiban (MK-383) platelet IIb/IIIa blockade in high risk
patientsundergoingcoronary angioplasty.J AmCollCardiol. 1996;27:536---42.
31.Van’tHofAW, Ten BergJ, Heestermans T, etal. Prehospital initiation of tirofiban in patients with ST-elevation myocar-dialinfarctionundergoingprimary angioplasty(On-TIME2):a multicentre,double-blind,randomisedcontrolledtrial.Lancet. 2008;372:537---46.
32.PlateletGlycoproteinIIb/IIIainUnstableAngina:Receptor Sup-pressionUsingIntegrilinTherapy(PURSUIT)TrialInvestigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998;339:436---43.
33.ZeymerU,MargenetA,HaudeM,etal.Randomizedcomparison ofeptifibatideversusabciximabinprimarypercutaneous coro-naryinterventioninpatientswithacuteST-segmentelevation myocardialinfarction:resultsoftheEVA-AMITrial.JAmColl Cardiol.2010;56:463---9.
34.Akerblom A, James SK, Koutouzis M, et al. Eptifibatide is noninferior to abciximab in primary percutaneous coro-naryintervention:resultsfrom theSCAAR (SwedishCoronary Angiography and Angioplasty Registry). J Am Coll Cardiol. 2010;56:470---5.
35.Singh HS, Dangas GD, Guagliumi G, et al. Comparison of abciximab versus eptifibatide during percutaneous coronary intervention in ST-segment elevation myocardial infarction (from the HORIZONS-AMI trial). Am J Cardiol. 2012;110: 940---7.
36.YusufS,MehtaSR,ChrolaviciusS,etal.Effectsoffondaparinux onmortalityandreinfarctioninpatientswithacuteST-segment elevationmyocardialinfarction:theOASIS-6randomizedtrial. JAMA.2006;295:1519---30.
37.WarkentinTE.ThinkofHIT.HematologyAmSocHematolEduc Program.2006:408---14.
38.ConnollySJ,MillingTJJr,EikelboomJW,etal.Andexanetalfa foracutemajorbleedingassociatedwithfactorXainhibitors. NEnglJMed.2016;375:1131---41.
39.MontalescotG,ZeymerU,SilvainJ,etal.Intravenous enoxa-parin or unfractionated heparin in primary percutaneous coronaryintervention for ST-elevationmyocardialinfarction: theinternationalrandomisedopen-labelATOLL trial.Lancet. 2011;378:693---703.
40.StoneGW,WitzenbichlerB,GuagliumiG,etal.Bivalirudin dur-ingprimaryPCIinacutemyocardialinfarction.NEnglJMed. 2008;358:2218---30.
41.Steg PG, van’tHof A, Hamm CW, et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. 2013;369:2207---17.
42.ValgimigliM,FrigoliE,LeonardiS,etal.Bivalirudinor unfrac-tionatedheparininacutecoronarysyndromes.NEnglJMed. 2015;373:997---1009.
43.Leonardi S, Frigoli E, Rothenbuhler M, et al. Bivalirudin or unfractionated heparinin patientswith acute coronary syn-dromes managed invasively with and without ST elevation (MATRIX):randomisedcontrolledtrial.BMJ.2016;354,i4935.
44.LeonMB,Baim DS, PopmaJJ,et al. Aclinicaltrial compar-ingthreeantithrombotic-drugregimens aftercoronary-artery stenting.StentAnticoagulationRestenosisStudyInvestigators. NEnglJMed.1998;339:1665---71.
45.Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084---9.