LIST OF FIGURES
...…………...………...
V
LIST OF TABLES
...………...
VI
LIST OF APPENDICES ...
VII
LIST OF ABBREVIATIONS & ACRONYMS ...
IX
DEFINITIONS ...
XI
AGRADECIMENTOS ...
XIV
ABSTRACT
...………...
XV
RESUMO
...………...
XVI
STRUCTURE OF DISSERTATION ...
XVII
INTRODUCTION ..
.………...
1
OBJECTIVES ...
5
I. General Objective ...……….……….………...
6
II. Specific Objectives ....……...………….……….………...
6
LITERATURE REVIEW ...
7
Chapter I
: The Big Picture on Antibacterial Resistance ...
8
I. Antibacterial Resistance ....……….……….………...
9
1. Why is Antibacterial Resistance a Public Health Concern? …...
9
2. Origins of Antibacterial Resistance ...
10
3. Types of Antibacterial Resistance ...
11
4. Antibacterial Modes of Action and Resistance Mechanisms …...
12
4.1. Inhibition of Cell Wall Synthesis ...
14
4.2. Inhibition of Protein Synthesis ...
16
4.3.Inhibition of Metabolic Pathways & Interference with Nucleic Acid Metabolism
18
5. Genetics of Antibacterial Resistance Transfer ……...
20
6. Pharmacodynamics and Pharmacokinetics of Antibacterials ...
24
6.1. Time-dependent vs. Concentration-dependent Bacterial Decimation …...
27
7. Laboratory Detection of Antibacterial Resistance ……...
28
7.1. Antibacterial Susceptibility Breakpoints ...
28
31
7.5. Test Methods in Antibacterial Resistance Detection …...
34
7.5.1. Broth Dilution Methods ...
34
7.5.2. Disk Diffusion Method …...
35
7.5.3. Antibacterial Gradient Diffusion Method ...
36
7.5.4. Automated Antibacterial Susceptibility Testing Systems ...
37
7.5.5. Current Test Methods and Future Directions ...
38
II. The Use of Antibacterials ..……….……….……...…...
39
8. The Use of Antibacterial Agents in Human Populations ……...
39
8.1. The Relationship Between Antibacterial Use and Resistance ……...
39
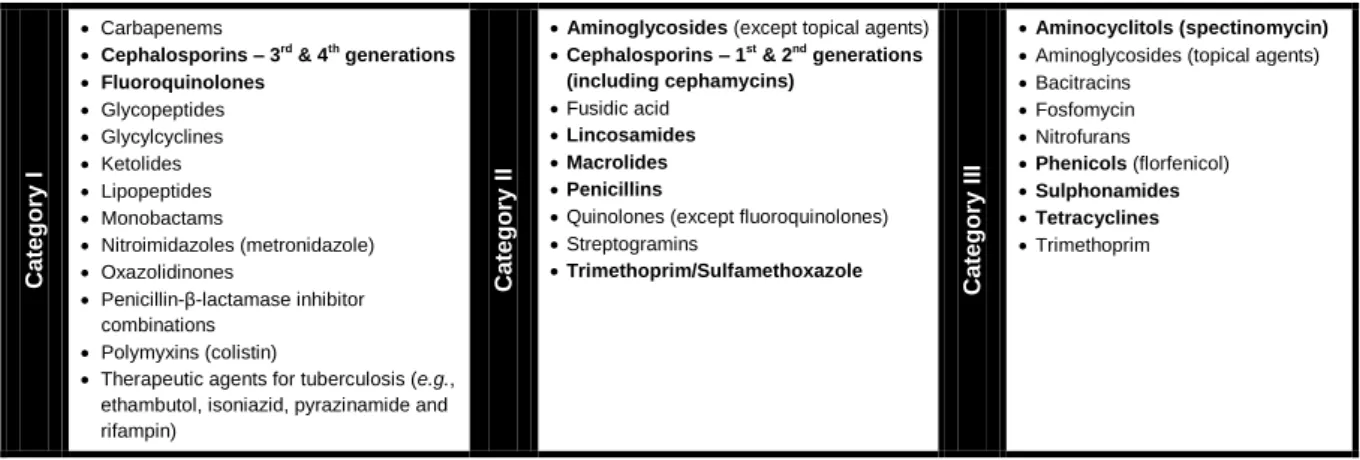
8.2. Critically Important Antibacterials for Human Medicine ……...
40
8.3. The Current Use of Antibacterials in Europe ……...
41
8.4. The Current Use of Antibacterials in Portugal …...
46
9. The Use of Antibacterials in Animal Husbandry …...
48
9.1. Definitions of Antibacterial Use ...
49
9.2. Pharmacodynamics and Pharmacokinetics of Antibacterial Use in Animal Husbandry …...
50
9.3. Regulation and Authorization of Antibacterial Use in the EU ...
51
9.4. The Current Use of Antibacterials in Europe ...
52
9.5. The Current Use of Antibacterials in Portugal ...
55
9.6. Applications of Antibacterials in Animal Husbandry Operations …...
58
9.6.1. Antibacterial Use in Dairy Production Systems ...
59
III. Dissemination and Transfer of Resistant Bacteria and Resistance
Genes from Animals to Humans ...
63
10. Sources and Routes for ABR Dissemination and Transfer ...
64
11. Bacteria of Public Health Concern ...
66
11.1. Foodborne Pathogens (Salmonella & Campylobacter) …...
66
11.2. Indicator (Commensal) Organisms ...…...
69
11.2.1. Enterococci …...…...
70
11.2.2. Escherichia coli …...…...
73
11.3. Other Gram-negative Bacteria ...
76
11.4. Staphylococcus aureus ...…...
76
and Interventions – The One Health Approach ...
80
12. Surveillance Systems to Track Antibacterial Use and Resistance ...
81
12.1 Surveillance of Antibacterial Resistance …...
82
12.2 Surveillance of Antibacterial Usage …...
83
12.3 Combined Surveillance …...
83
13. Reducing Antibacterial Use in Humans ...
84
13.1 Promoting Rational Antibacterial Use …...
84
13.2 Infection Prevention and Control in Health-Care Facilities …...
85
13.3 Fostering Innovation ...
85
14. Reducing Antibacterial Use in Animal Husbandry ...
87
14.1 Regulations to Restrict the Use of Antibacterials in Food-Producing Animals
87
14.2 Financial Incentives …...90
14.3 Prudent use Guidelines and Education ...
90
14.4 Improving Animal Health ...
90
14.5 Improving Hygiene in Food Production ...
91
14.6 Applying Advances in Data Management Technology …...
91
Chapter II: Antibacterial Resistance of Mastitis Pathogens ...
92
I. Mastitis in Dairy Production Operations ...………...
93
1. Introduction …...
93
2. Mastitis Pathogens …...
95
3. Current Approaches for Mastitis Diagnosis ...
97
II. Mastitis Antibacterial Therapy and the Use of Susceptibility
Profiles for Treatment Decisions ...………....
98
4. Assessing Efficacy …...
98
5. Pharmacological Considerations …... 101
6. Susceptibility Testing for Mastitis Pathogens …... 102
6.1. Determination and Validation of Susceptibility Breakpoints for Mastitis Pathogens ...
102
6.1.1. Limited Availability of MIC Values for Mastitis Pathogens ...
103
6.1.2. Incomplete PK/PD Data for Lactating Dairy Cows ...
103
6.1.3. Inadequate Number of Field Studies Validating Susceptibility Breakpoints ...
103
6.2. Test Methods …...
104
6.3.1. Validity of Developing a ‘‘Herd Profile’’ for Susceptibility ...
106
6.3.2. Validity of Selecting the Antibacterial with the Lowest In Vitro MIC Value ...
106
6.3.3. Validity of Assumption that all Antibacterials Within a Class have Identical MIC Values ...
107
6.3.4. Effect of Milk on MIC Values ...
107
6.3.5. Deleterious Effects of Antibacterials on Normal Mammary Defense Mechanisms ...
108
6.3.6. Distribution of Antibacterials in an Inflamed Mammary Gland ...
108
7. Calculation of Antibacterial Dosage …... 109
III. Resistance Patterns of Mastitis Pathogens ...
112
8.Trends on Resistance Patterns Over Time in Response to Antibacterial
Usage ...
112
MATERIALS & METHODS ...
115
I. Criteria for Selection of Cases ……...………...
116
II. Sample Collection and Microbiology ……...………...
116
III. In vitro Antibacterial Susceptibility Testing …....………...
117
IV. Tested Antibacterials ...……….……….………...
117
V. Selection of Pathogens ...…..……….………...
117
VI. Data Analysis ...………….……….………...
118
RESULTS ...
119
DISCUSSION ...
128
I. Novelty Aspects of this Study ……...………...
129
II. Antibacterial Resistance Pattern and Trend Analysis ……...
129
III. Data Analysis …....………...
132
IV. Limitations of the Study ...……….……….…………...
133
V. Improvement Suggestions for Future Similar Research ...
134
VI. Further Research Ideas and Recommendations ...
134
CONCLUSIONS ...
136
REFERENCES ...
139
APPENDICES
…...
i - xl
____________________________________________________________________________________
Figure 1: Broad depiction of major ABR mechanisms ... 13
Figure 2: Protein synthesis. Aminoacyl-tRNA molecules are formed in the cytoplasm and bind to the cognate triplicate codon of mRNA at the ribosome. Peptide bond formation links the new amino acid to the growing polypeptide chain. The ribosome migrates to free the A site for the next aminoacyl-tRNA molecule and the cycle repeats until a stop codon is encountered and translation is terminated ... 17
Figure 3: Activity of protein synthesis inhibitors. Schematic of the bacterial ribosome and the sites of action of select antibacterials that inhibit polypeptide biosynthesis ... 18
Figure 4: Schematic of multiple antibacterial resistance accumulation on a plasmid ... 22
Figure 5: Schematic representation of the complexity of interactions between patient, pathogen and antibacterial agent ... 25
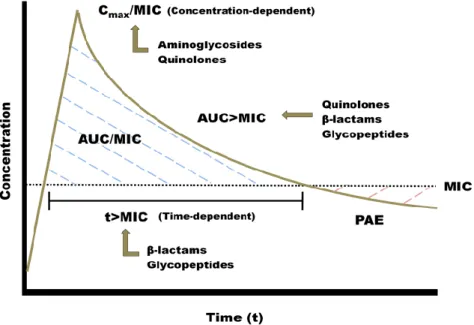
Figure 6: Concentration-versus-time curve with MIC superimposed and pharmacokinetic and pharmacodynamic markers ... 26
Figure 7: MIC distributions for four microorganism-antibacterial pairs. In each case, the wild type appears as the log-normally distributed population at the lower MICs. COWT – calculated wild-type cutoff value ... 33
Figure 8: A broth microdilution susceptibility panel containing 98 reagent wells and a disposable tray inoculator ... 35
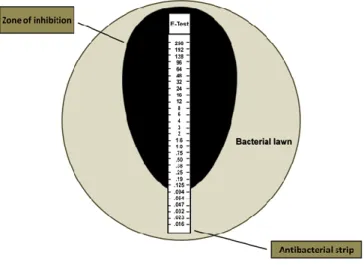
Figure 9: Antibacterial susceptibility testing by disk diffusion. On this agar plate, a bacterial isolate is tested for resistance to each of four different antibacterials ... 36
Figure 10: Antibacterial susceptibility testing by E-Test. On this agar plate, a bacterial isolate is tested for resistance to a specific antibacterial ... 37
Figure 11: Vitek® 2 System – BioMérieux, France ... 38
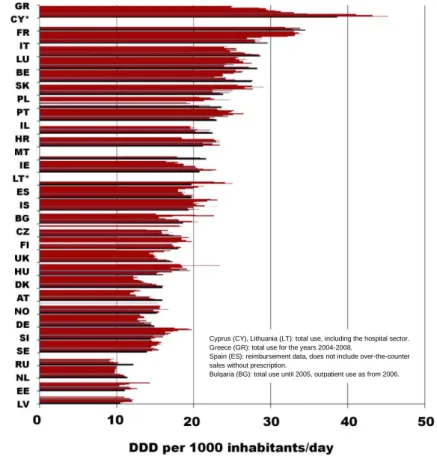
Figure 12: Total outpatient antibacterial use in 2009 in Europe ... 42
Figure 13: Boxplotted distribution of outpatient antibacterial use between 1999 and 2009 among the participating European countries ... 42
Figure 14: Trends of total outpatient antibacterial use in Europe from 1997 to 2009. Dark bars correspond to the year 2009 ... 44
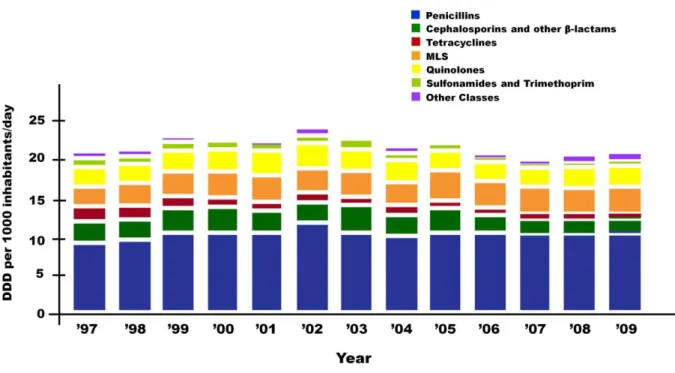
Figure 15: Outpatient antibacterial use in 2009 subdivided into the major antibacterial classes according to ATC classification ... 44
Figure 16: Hospital use of antibacterials for systemic use in 2009 (N= 22 countries) ... 46
Figure 17: Distribution of antibacterial classes in ambulatory (A) and hospital (B) care sectors in Portugal in 2009 ... 47
Figure 18: Trends of antibacterial usage in ambulatory care sector in Portugal ... 47
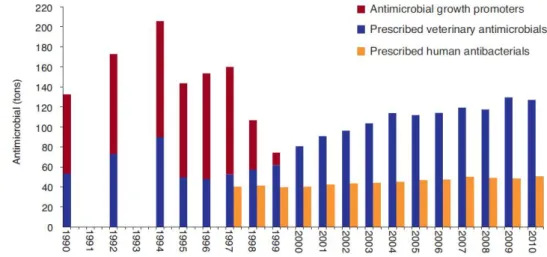
Figure 19: Annual antibacterial/antimicrobial use for human and veterinary practice in Denmark ... 48
Figure 20: PCU (in 1.000 tons) of the major food-producing animal species in 2009, by country ... 53
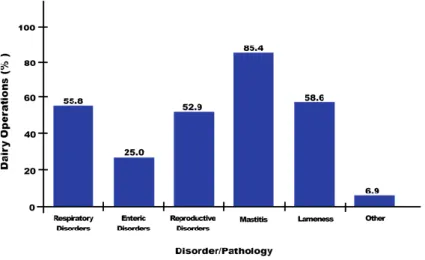
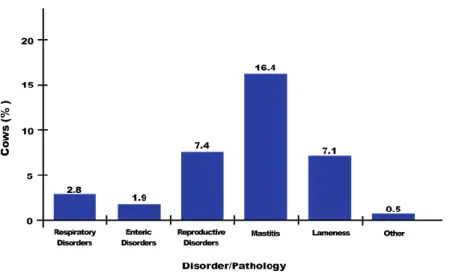
Figure 21: Proportion of US dairy operations in 2007 that treated cows with any antibacterial for the main diseases/disorders ... 59
Figure 22: Proportion of US adult dairy cows treated with antibacterials for the main diseases/disorders in 2007 ... 60
Figure 23: Proportion of preweaned and weaned heifers treated with antibacterials in 2007 for the main diseases/disorders ... 60
Figure 24: Possible routes of transmission of antibacterial-susceptible or -resistant gastrointestinal pathogens or normal intestinal flora between animals and humans ... 64
Figure 25: Reservoirs of ABR bacteria causing human infections. Schematic overview of some of the most important ABR pathogens and the overlap between the different reservoirs ... 65
Figure 26: Trend and number of reported confirmed human campylobacteriosis cases by month, in the EU and EEA/EFTA countries, 2006–09 ... 66
Figure 27: Trend and number of reported confirmed human salmonellosis cases by month, in the EU and EEA/EFTA countries, 2006–09 67 Figure 28: (A) E. faecalis: trends of high-level resistance to aminoglycosides by country, 2007–2010. (B) E. faecium: Trends of resistance to vancomycin by country 2007–2011 ... 71
Figure 29: S. aureus: Trends of resistance to methicillin (MRSA) by country, 2007–2011 ... 78
Figure 30: ECDC promotional One Health poster ... 80
Figure 31: Discovery timeline of new antibacterial classes (1930s to 2000s) ... 86
Figure 32: Macrolide use and resistance among enterococci in swine, Denmark ... 88
Figure 33: Cephalosporin resistance in poultry industry in Quebec, Canada ... 89
Figure 34: Reduction in antibacterial use after the introduction of vaccination in aquaculture in Norway ... 91
Figure 35: Sliding scale for contagious and environmental origin of mastitis pathogens, based on insights from molecular epidemiology .... 95
Table 1: Estimated annual burden due to selected antibacterial-resistant bacteria in EU-Member States, Iceland and Norway, 2007 ... 9
Table 2: Evolution of resistance to major antibacterials ...….……....………... 10
Table 3: Mechanisms of action of main antibacterial agents ...….……....………... 12
Table 4: Mechanisms of ABR …...………...….……....………... 13
Table 5: World organizations with published breakpoints …...………...….……....…………... 31
Table 6: Summary of the antibacterial classes included in the three categories of Critically Important Antimicrobials for Human Medicine 41 Table 7: Outpatient antibacterial use in 2009 subdivided into the major antibacterial classes according to ATC classification …... 43
Table 8: Hospital use of antibacterials for systemic use in 2009 (N= 22 countries) …...………...….……... 45
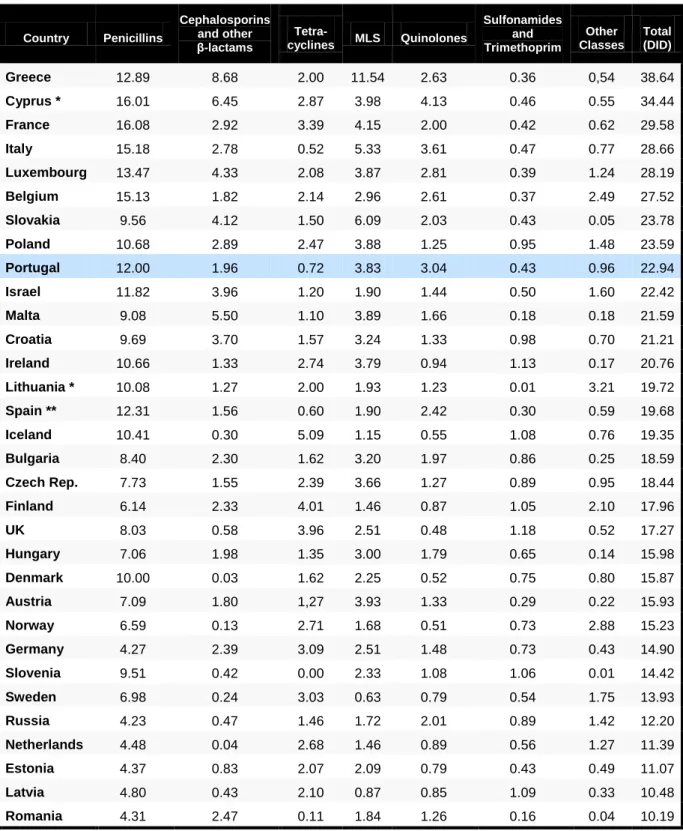
Table 9: Total sales of veterinary antibacterial agents (active ingredient) and PCU (1000 tons) in eight European countries (Switzerland not included) …...………...….……....………... 52
Table 10: Sales normalized by PCU (mg/PCU) for the years 2005-2009 …...……...….……....………... 53
Table 11: Difference between 2009 and 2005 sales, expressed as tons of active ingredient and as mg/PCU, for eight European countries (Switzerland not included) …...……...….……....………... 54
Table 12: Total 2010 sales of veterinary antibacterial agents (active ingredient, in tons and %) by antibacterial class in Portugal …... 56
Table 13: Distribution of active ingredients (in tons) in each animal species, in Portugal in 2010 …...……... 57
Table 14: Distribution of the antibacterial dosage forms in each animal species, in Portugal in 2010 …...……... 58
Table 15: Examples of diseases on the different food-producing animal species including organ, pathogen and type of treatment …... 62
Table 16: Salmonella serotypes most frequently reported from human salmonellosis cases in the EU and EEA/EFTA countries and percentage change, 2008-09 …... 67
Table 17: Number of invasive E. faecalis and E. faecium isolates and proportion of high-level aminoglycoside-resistant E. faecalis and vancomycin-resistant E. faecium (%R), including 95% CI, reported per country in 2011 ... 70
Table 18: Number and proportion of invasive E. coli isolates resistant to aminopenicillins, 3rd -generation cephalosporins, fluoroquinolones, aminoglycosides and multi-drug resistant (%R), including. 95% CI, reported per country in 2011 ... 74
Table 19: Number of invasive E. coli isolates resistant to 3rd-generation cephalosporins (CREC) and proportion of ESBL-positive (%ESBL) among these isolates, as ascertained by the participating laboratories in 2011 ... 74
Table 20: Overall resistance and resistance combinations among invasive E. coli isolates tested against aminopenicillins, fluoroquinolones, 3rd-generation cephalosporins and aminoglycosides (n= 49847) in Europe, 2011 ... 75
Table 21: Number and proportion of invasive S. aureus isolates resistant to methicillin (MRSA) and rifampin (RIF), including 95% CI, reported per country in 2011 …...……...….……....……... 77
Table 22: ABR surveillance networks for common bacterial pathogens in the WHO Regions …... 82
Table 23: Prevalence of mastitis pathogens in dairy herds from Northwestern Portugal, between 2005 and 2008 ... 96
Table 24: Current SCC measuring methods and alternatives for detection of mastitis ... 98
Table 25: Summary of three-compartment model to target mastitis pathogens ... 101
Table 26: MIC data for several bacterial isolates from mastitic milk samples from the MAHDL, 1999-2001 ... 111
Table 27: Conclusions from short- to long-term studies on the effect of antibacterials on resistance of mastitis pathogens worldwide ... 113
Table 28: Antibacterial agents used for susceptibility testing of 47,413 bacterial pathogen isolates obtained from dairy cow milk samples and submitted for bacterial culture between January 2004 and September 2012 ... 120
Table 29: Number of major bacterial pathogens isolated along the study period (2004-2012) ... 120
Table 30: Results of resistance in antibacterial susceptibility testing, by antibacterial agent, of major mastitis bacterial pathogens ... 121
Table 31: Results of logistic regression analysis to determine, for the isolated bacterial pathogens, whether the percentage of isolates resistant to the various antibacterial agents changed with year ... 122
Table 32: S. aureus resistance proportions, among each tested antibacterial agent, along each tested year (n = 28,126 isolates) ... 123
Table 33: S. agalactiae resistance proportions, among each tested antibacterial agent, along each tested year (n = 4,589 isolates) ... 123
Table 34: S. uberis resistance proportions, among each tested antibacterial agent, along each tested year (n = 5,799 isolates) ... 124
Table 35: S. dysgalactiae resistance proportions, among each tested antibacterial agent, along each tested year (n = 1,231 isolates) ... 125
Table 36: Enterococcus spp. resistance proportions, among each tested antibacterial agent, along each tested year (n = 979 isolates) ... 125
Table 37: E. coli resistance proportions, among each tested antibacterial agent, along each tested year (n = 5,916 isolates) ... 126
____________________________________________________________________________________
Appendix 1:
Table 39: Summary of some of the pertinent literature on the ABR of S. aureus isolated in milk from cows with mastitis worldwide ... ii
Table 40: Summary of some of the pertinent literature on the ABR of CNS isolated in milk from cows with mastitis worldwide ... iii
Table 41: Summary of some of the pertinent literature on the ABR of environmental Streptococcus sp. isolated in milk from cows with
mastitis worldwide ... iv
Table 42: Summary of some of the pertinent literature on the ABR of E. coli isolated in milk from cows with mastitis worldwide ... v
Table 43: Summary of some of the pertinent literature on the ABR of other Gram-negative bacteria (besides E. coli) isolated in milk from
cows with mastitis worldwide ... vi
Table 44: Summary of some of the pertinent literature on the ABR of S. uberis and S. dysgalactiae isolated in milk from cows with mastitis
worldwide ... vii
Table 45: Summary of some of the pertinent literature on the ABR of S. agalactiae, esculin-positive Streptococcus sp. and Enterococcus
spp. isolated in milk from cows with mastitis worldwide ... viii
Appendix 2:
Figure 36: Resistance proportions of S. aureus isolates, among each tested antibacterial agent, along each tested year (2004-2012) ... ix
Figure 37: Resistance proportions of S. aureus isolates, among each tested antibacterial agent, along each tested year. Asterisks
represent statistically significant changes (p<0.05) ... x
Figure 38: Resistance proportions of S. agalactiae isolates, among each tested antibacterial agent, along each tested year (2004-2012) .. xi
Figure 39: Resistance proportions of S. agalactiae isolates, among each tested antibacterial agent, along each tested year. Asterisks
represent statistically significant changes (p<0.05) ... xii
Figure 40: Resistance proportions of S. uberis isolates, among each tested antibacterial agent, along each tested year (2004-2012) ... xiii
Figure 41: Resistance proportions of S. uberis isolates, among each tested antibacterial agent, along each tested year. Asterisks
represent statistically significant changes (p<0.05) ... xiv
Figure 42: Resistance proportions of S. dysgalactiae isolates, among each tested antibacterial, along each tested year (2004-2010) ... xv
Figure 43: Resistance proportions of S. dysgalactiae isolates, among each tested antibacterial agent, along each tested year. Asterisks
represent statistically significant changes (p<0.05) ... xvi
Figure 44: Resistance proportions of Enterococcus spp. isolates, among each tested antibacterial, along each tested year (2004-2010) ... xvii
Figure 45: Resistance proportions of Enterococcus spp. isolates, among each tested antibacterial agent, along each tested year.
Asterisks represent statistically significant changes (p<0.05) ... xviii
Figure 46: Resistance proportions of E. coli isolates, among each tested antibacterial agent, along each tested year (2004-2012) ... xix
Figure 47: Resistance proportions of E. coli isolates, among each tested antibacterial agent, along each tested year. Asterisks represent
statistically significant changes (p<0.05) ... xx
Figure 48: Resistance proportions of K. pneumoniae isolates, among each tested antibacterial agent, along each tested year (2004-2012) xxi
Figure 49: Resistance proportions of K. pneumoniae isolates, among each tested antibacterial agent, along each tested year. Asterisks
represent statistically significant changes (p<0.05) ... xxii
Table 46: SPSS outputs for logistic regression analysis to determine, for the S. aureus isolates, whether the percentage of isolates
resistant to the various antibacterial agents changed throughout the study period ... xxiii
Table 47: SPSS outputs for logistic regression analysis to determine, for the S. agalactiae isolates, whether the percentage of isolates
resistant to the various antibacterial agents changed throughout the study period ... xxiv
Table 48: SPSS outputs for logistic regression analysis to determine, for the S. uberis isolates, whether the percentage of isolates
resistant to the various antibacterial agents changed throughout the study period ... xxv
Table 49: SPSS outputs for logistic regression analysis to determine, for the S. dysgalactiae isolates, whether the percentage of isolates
resistant to the various antibacterial agents changed throughout the study period ... xxvi
Table 50: SPSS outputs for logistic regression analysis to determine, for the Enterococcus spp. isolates, whether the percentage of
isolates resistant to the various antibacterial agents changed throughout the study period ... xxvii
Table 51: SPSS outputs for logistic regression analysis to determine, for the E. coli isolates, whether the percentage of isolates resistant
to the various antibacterial agents changed throughout the study period ... xxviii
Table 52: SPSS outputs for logistic regression analysis to determine, for the K. pneumoniae isolates, whether the percentage of isolates
Rocha, B.; Mendonça, D.; Niza-Ribeiro, J. (2013) "Trends in Antibacterial Resistance of Major Bovine Mastitis Pathogens in Portugal" ... xxx
Appendix 4:
Slide presentation of short communication presented by the author:
"Evolução de Padrões de Resistência a Antibióticos em Agentes Etiológicos da Mastite Bovina em Portugal". II Conferência Anual do
Conselho Português de Saúde do Úbere (CPSU). Santarém, Portugal – February 23rd
, 2013 ... xxxviii
Appendix 5: Poster presentation:
Rocha, B., Mendonça, D., Niza-Ribeiro, J. (2013). “Evolução de Padrões de Resistência a Antibióticos em Agentes Etiológicos da Mastite Bovina em Portugal”. XV Jornadas da Associação Portuguesa de Buiatria. Ílhavo, Portugal – May 24th
to 26th
____________________________________________________________________________________
3GCREC – E. coli isolates resistant to 3rd-generation cephalosporins
ABR – Antibacterial Resistance AI - Active Ingredient
AMP – Ampicillin
ATC – Anatomical Therapeutic Chemical (Classification System) ATCC – American Type Culture Collection
AUC – Area under the (concentration–time) curve AUG – Amoxicillin/Clavulanic acid
AHVLA – Animal Health and Veterinary Laboratories Agency bp – Base pair
BSAC – British Society for Antimicrobial Chemotherapy BTSCC – Bulk tank somatic cell count
CATs – Chloramphenicol acetyltransferases CDS-AST – CDS disk diffusion method - Australia CFP – Cefoperazone
CFU – Colony Forming Unit CI – Confidence Interval
CLSI – The Clinical and Laboratory Standards Institute CM – Clinical Mastitis
Cmax – Peak concentration
CN – Gentamicin
COWT – Calculated wild-type cutoff value
CSF – Cerebrospinal fluid
CSN - Coagulase-negative staphylococci
CVMP – Committee for Medicinal Products for Veterinary Use DDD – Defined Daily Dose
DGAV – Direção Geral de Alimentação e Veterinária DID – Defined Daily Doses per 1000 inhabitants per day DIN – Deutsches Institut für Normung
DNA – Deoxyribonucleic Acid
EARS-Net – The European Antimicrobial Resistance Surveillance Network EEA – European Economic Area
EFTA – European Free Trade Association EMA – European Medicines Agency
ESAC-Net – European Surveillance of Antimicrobial Consumption Network ESBL – Extended Spectrum β-Lactamase
ESVAC – European Surveillance of Veterinary Antimicrobial Consumption Project EU – European Union
EU-MS – European Union Member State
EUCAST – European Committee of Antimicrobial Susceptibility Testing FAO – Food and Agriculture Organization of the United Nations HACCP – Hazard Analysis and Critical Control Point
HHPM – Herd Health and Production Management HGT – Horizontal gene transfer
IMI – Intramammary Infection IMM – Intramammary IS – Insertion sequence
LA-MRSA – Livestock-associated MRSA LPS – Lipopolysaccharides
LR – Logistic Regression
mg/PCU – Milligram of antimicrobial per Population Correction Unit MIC – Minimum Inhibitory Concentration
MIS – Management Information Systems mL – Milliliter
MLS (group) – Macrolides, Lincosamides and Streptogramins Group MRSA – Methicillin-resistant Staphylococcus aureus
mRNA – Messenger RNA NAG – N-acetylglucosamine
NAHMS – National Animal Health Monitoring System of the USDA NAM – N-acetylmuramic acid
NMC – National Mastitis Council
OIE – Office International des Epizooties (World Organization for Animal Health) OMPs – Outer Membrane Proteins
P – Penicillin G
PABA – p-aminobenzoic acid PAE – Post-antibiotic effect PBPs – Penicillin-binding proteins PCU – Population Correction Unit PD – Pharmacodynamic PK – Pharmacokinetic
PNCUM – Plano Nacional de Controlo e Utilização de Medicamentos PRK – People's Republic of Korea
PRSP – Penicillin G-resistant S. pneumoniae QACs – Quaternary ammonium compounds QRDR – Quinolone resistance-determining region rRNA – Ribossomal RNA
RNA – Ribonucleic Acid S – Streptomycin
SCAN – European Union’s Scientific Committee on Animal Nutrition SCC – Somatic Cell Count
SCM – Subclinical Mastitis
SFM – Société Française de Microbiologie SGI – Salmonella Genomic Island SHV – Sulphydryl variable
SRGA – Swedish Reference Group for antibiotics ST398 – Sequence type 398
t – ton
TEM – From Temoniera, the name of the patient from whom the original β-lactam resistant isolate was acquired TMP-SMX – Trimethoprim/Sulfamethoxazole
tRNA – Transfer RNA
URI – Upper Respiratory Infections
USDA – United States Department of Agriculture VMP – Veterinary Medicinal Products
VRE – Vancomycin-resistant enterococci VRTI – Viral Respiratory Tract Illness WFE – Whole Fish Equivalent WHO – World Health Organization
____________________________________________________________________________________
DEFINITIONS
Anatomical Therapeutic Chemical (ATC) Classification System: This system is used for the classification of drugs. It is controlled by the WHO Collaborating Centre for Drug
Statistics Methodology (WHOCC), and was first published in 1976. This pharmaceutical
coding system divides drugs into different groups according to the organ or system on which they act and/or their therapeutic and chemical characteristics. 2
Antibacterial (agents): Synthetic (chemotherapeutics) or natural (antibiotics) substances that destroy bacteria or suppress bacterial growth or reproduction. 3
Antimicrobial (agents): This broad term normally covers antibacterial, antiviral, coccidiostatic and antimycotic agents. 4
Bactericidal (activity): Refers to a substance or a condition capable of killing bacteria. 5
Bacteriologic cure: Failure to isolate the same pathogen from affected quarter within a specific time interval. 6
Bacteriostatic (activity): Refers to a substance or condition that inhibit or prevent further growth of bacteria. 5
Breakpoints: Breakpoints are specific MIC values used to assign bacteria to one of three categories – susceptible, intermediate and resistant – using recommendations for testing veterinary pathogens from organizations created specifically to set those breakpoints (e.g., EUCAST or CLSI). 7
Clinical cure: Return to visibly normal secretion in a specific time interval. 6
Control: Administration of an antimicrobial to animals, usually as a herd or flock, in which morbidity and/or mortality has exceeded baseline norms. 8
Defined Daily Dose (DDD): Unit of measurement that corresponds to the assumed average maintenance dose per day for a drug used for its main indication in adults. Drug consumption data presented in DDDs only give a rough estimate of consumption and not an exact picture of actual use. The DDD provide a fixed unit of measurement independent of price and dosage form (e.g., tablet strength) enabling the researcher to assess trends in drug
consumption and to perform comparisons between population groups. Doses for individual patients and patient groups will often differ from the DDD and will necessarily have to be based on individual characteristics (e.g., age and weight) and pharmacokinetic considerations. 2
Dry cow: A cow that is not lactating or secreting milk after it has completed a lactation period following calving. 9
Fresh cow: A cow that has recently given birth to a calf. 9
Growth promotion: Administration of an antimicrobial, usually as a feed additive, over a period of time, to growing animals that results in improved physiological performance. 8
High-level ABR: Increased antibacterial potency within the clinically susceptible range. 10
Level of antibacterial resistance: Percentage of resistant isolates from the tested isolates.11
L-forms: Antibacterial-induced variants of S. aureus that lack a cell wall. 12
Low-level ABR: Diminished antibacterial potency within the clinically susceptible range. 10
Minimum Inhibitory Concentration (MIC): Corresponds to the lowest antibacterial concentration (expressed in g/mL or mg/L) that under defined in vitro conditions prevents the growth of bacteria within a defined period of time. 7 It is generally accepted that MIC values are very repeatable. Statistics such as MIC50 (the median MIC for all isolates) and MIC90 (the MIC value that exceeds or equals the MIC for 90% of the isolates) are frequently used to summarize population data. 13
Minimum Bactericidal Concentration (MBC): Corresponds to the lowest concentration of an antibacterial that under defined laboratory conditions reduces by 99.9% (three-log reduction) the number of organisms in a medium containing a defined bacterial inoculum within a defined period of time. 7
Multidrug-resistant bacteria: Bacteria with resistance to three or more antibacterial drug classes. 14
____________________________________________________________________________________ Population Correction Unit (PCU): The amounts of veterinary antimicrobial agents sold in the different countries are, among others, linked to the animal demographics in each country, which may vary over time. In this dissertation's section 9.4., the annual sales figures in each country were divided by the estimated weight at treatment of livestock and of slaughtered animals in the corresponding year. The PCU is therefore used as the term for the estimated weight and is purely a technical unit of measurement used only to estimate temporal trends in individual countries and across countries. 1 PCU = 1 kg of different categories of livestock and slaughtered animals. 15
Prevention/prophylaxis: Administration of an antimicrobial to exposed healthy animals considered to be at risk, but before expected onset of disease and for which no etiological agent has yet been cultured. (Metaphylaxis is a term sometimes used when there is clinical disease in some animals, but all are treated). 8
Somatic Cell Counts (SCC): Somatic cells are present in a certain normal physiological amount in milk. These consist of various cell types and their relative proportions depend on the health status of the udder. In a healthy lactating mammary gland, the major proportion of somatic cells is constituted by leukocytes. 16 These are primarily macrophages and lymphocytes, but a small fraction consists of neutrophils and epithelial cells. 17, 18 Microbial infection results in rapid accumulation of large numbers of somatic cells in the udder, and these are predominantly neutrophils. 16-18 The increase in SCC constitutes an important part of the cow’s immune response, and SCC is, therefore, along with bacterial culture examination, a widely used indicator of mastitis. According to the International Dairy
Federation, a threshold of 200×103 cells/mL of milk is suggested for subclinical mastitis. 19
Therapy: Administration of an antimicrobial to an animal or group of animals, which exhibit frank clinical disease. 8
Tripartite Concept Note: FAO, OIE and WHO issued, in 2010, this document that sets a
strategic common direction and proposes a long-term basis for international collaboration
aimed at coordinating global activities to address health risks at the human-animal-ecosystems interfaces. New synergies between the three partners include
normative work, public communication, pathogen detection, risk assessment and management, technical capacity building and research development. 20
Withdrawal period: Length of time needed to allow an antimicrobial to be removed from edible tissue. 10
AGRADECIMENTOS
À minha família, por todo o apoio e incentivo, sem os quais não teria podido levar a bom termo esta dissertação.
Ao Professor Doutor João Niza Ribeiro pelo interesse que me incutiu neste tema, bem como pela orientação e apoio prestados.
À Professora Doutora Denisa Mendonça, pela preciosa ajuda prestada na análise estatística e interpretação dos dados.
À Segalab - Laboratório de Sanidade Animal e Segurança Alimentar, S.A., pela disponibilização dos dados necessários.
Ao Departamento de Serviços Técnicos da Segalab, nomeadamente Dr.ª Helena Madeira, Dr.ª Abigail Barbosa, Eng.ª Paula Santos e Eng.ª Marta Barbosa, pela disponibilização dos dados e pela ajuda em questões de natureza técnica.
____________________________________________________________________________________
RESUMO
O incremento de resistências a compostos antibacterianos tem-se revelado, ao longo dos anos, alvo de crescente preocupação a nível Mundial, quer do ponto de vista da Saúde Pública, quer na perspectiva da Segurança Alimentar. A mastite bovina é a causa mais frequente de utilização destes fármacos em efectivos leiteiros, sendo o seu uso apontado como factor selectivo na ecologia bacteriana do úbere bovino. Os padrões de resistências antibacterianas em agentes etiológicos de mastite têm, deste modo, vindo a suscitar um crescente interesse por parte da comunidade científica veterinária. A monitorização e análise destes padrões tem fornecido informações de grande utilidade na escolha de antibacterianos no tratamento de animais afectados, especialmente no que diz respeito a agentes etiológicos isolados em diferentes regiões geográficas.
Esta dissertação pretende dar a conhecer os padrões de resistência a sete fármacos antibacterianos disponíveis no mercado Português e a sua evolução, em 47.413 testes de susceptibilidade antibacteriana. Analisaram-se os principais agentes etiológicos da mastite –
Staphylococcus aureus, Streptococcus agalactiae, Streptococcus uberis, Streptococcus dysgalactiae, Enterococcus spp. (E. faecium e E. faecalis), Escherichia coli e Klebsiella pneumoniae. Os isolados foram obtidos a partir de amostras de leite provenientes de
explorações leiteiras das regiões Litoral Norte, Centro e Sul de Portugal, entre 2004 e 2012. Os testes de susceptibilidade foram executados através do método de difusão em disco. Os fármacos utilizados foram penicilina, amoxicilina/ácido clavulânico, cloxacilina, cefazolina, cefquinoma, gentamicina e trimetoprim/sulfametoxazol.
Os resultados obtidos dos níveis de resistência não diferem significativamente dos de estudos semelhantes. Porém, a avaliação da evolução desses padrões, ao longo dos nove anos do estudo, revelou tendência para um aumento estatisticamente significativo dos níveis de resistência entre certos agentes testados. Estudos adicionais devem ser elaborados no esforço de se encontrar a origem destes aumentos, de modo a serem tomadas medidas concretas na inversão destas tendências.
ABSTRACT
Worldwide, the development of resistance to antibacterial agents has proved to be a growing concern over the years, from both public health and food safety perspective. Bovine mastitis reports as the most common pathology in dairy herds, and therefore, to which these drugs are mostly used. This use accounts as a selective factor in the bacterial ecology of the bovine udder. Antibacterial susceptibility patterns in mastitis pathogens have, for this reason, accordingly raised a growing interest in the veterinary scientific community. The long-term monitoring and analysis of these patterns has provided useful information in guiding the selection of antibacterial therapy of affected animals, especially with regard to etiologic agents isolated from different geographic regions.
This dissertation aims to provide patterns of resistance to seven antibacterial drugs available in the Portuguese market and their evolution over time, in 47,413 antibacterial susceptibility tests. The major etiological agents of mastitis isolated were: Staphylococcus aureus, Streptococcus
agalactiae, Streptococcus uberis, Streptococcus dysgalactiae, Enterococcus spp. (E. faecium
and E. faecalis), Escherichia coli and Klebsiella pneumoniae. Isolates were obtained from milk samples from dairy farms in the northwestern, central and southern regions of Portugal, between 2004 and 2012. Susceptibility testing was performed by the disk diffusion method. Tested antibacterials were penicillin, amoxicillin/clavulanic acid, cloxacillin, cefazolin, cefquinome, gentamicin and trimethoprim/sulfamethoxazole.
The overall antibacterial resistance levels found for these mastitis isolates were, in most cases, comparable to what other relevant similar studies have reported worldwide. When assessing the evolution of these patterns over the nine years of data, significant increases were determined among certain agents tested. Further research should be addressed in an effort to find the source of these increases and take actual steps in reversing these trends in the region.
The current dissertation was organized into two main parts. The first part consists of an introduction, the objectives of the dissertation and a literature review. The second part describes the respective research project.
In the introduction, the author sets up the research topic, as well as its scope and significance; followed by the definition of the general and specific objectives. In order to contextualize the dissertation, a literature review was prepared, consisting of two chapters with relevant information concerning the subject in question: Antibacterial resistances by
bacteria and the impact on animal and human health, with emphasis on the dairy environment, especially to mastitis pathogens.
The first chapter of the review corresponds to an introductory chapter, with the aim of covering the subject in its generality, and is divided into four subchapters. The first subchapter focuses on the origins and types of antibacterial resistance, as well as modes of action and resistance mechanisms by bacteria; pharmacodynamics and pharmacokinetics of the different antibacterial agents; and a section on laboratory detection breakpoints and diverse test methods used in identifying antibacterial resistances. The second subchapter describes the usage statistics of antibacterials among both humans and animals, mainly in Europe and Portugal. The third subchapter centers on the potential sources and routes of dissemination and transfer of resistant bacteria and resistance genes from animals to humans, describing the diverse bacteria of public health concern. The fourth subchapter focuses on the strategies and interventions available nowadays to monitor and control antibacterial resistance, with the One Health approach taking centre-stage.
Chapter II covers a more specific and detailed approach on antibacterial resistance, as it pertains to the dairy production systems. This chapter exposes the reader to an introduction on mastitis, mastitis pathogens and antibacterial therapy, as well as the use of susceptibility profiles for treatment decisions. Still in this chapter, a review on relevant available literature regarding resistance patterns of mastitis pathogens is made.
The second main part of this dissertation concerns the research project per se, describing the methodologies, results and conclusions obtained in its completion.
Since the launch of large-scale production in the 1940s, antibacterial agents are conceivably the foremost medical advance in history, having become imperative tools in decreasing morbidity and mortality associated with a multitude of infectious diseases in both humans and animals. In addition to the considerable contribution to human wellbeing and quality of life, their use in food-producing animal agriculture has resulted in healthier and more productive animals, yielding high-quality and low-cost food products for human consumption. 10, 21-23
Even though most antibacterial agents currently remain effective in both humans and animals, their intensive and often inappropriate use over the years has triggered an increase in the emergence and dissemination of antibacterial resistance, consequently reducing their efficiency. In fact, resistance mechanisms have been reported in every major bacterial pathogen for all known antibacterial agents currently available for clinical use in human and veterinary medicine. 24-27
The evolution of bacteriology over the past seven decades has made us much better equipped to recognize that we were clearly unaware of the implications associated with the indiscriminate use of these compounds and underestimated the genetic flexibility of the targeted microorganisms. Over the last decades, serious public health concerns about antibacterial resistance from hospital-acquired, community-acquired and foodborne pathogens have been raised at international levels. The World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO) and the World Organization for Animal Health (OIE) consider antibacterial resistance in zoonotic pathogens as a growing global public health threat and recognize that documented emerging resistance phenotypes may be the outcome of the administration of therapeutic and subtherapeutic levels of antibacterial agents to food-producing animals. 20 Although this issue has taken center stage over the years, there is still, however, no complete agreement among researchers on the significance of these deliberations. This is mainly due to major gaps in information and evidence regarding the development and dissemination of bacterial resistance to antibacterial agents in various animal production settings. The understanding of the complete effects of these compounds in the animal production environment is particularly difficult because the involved ecosystems are extremely complex, containing hundreds of bacterial genera and species in constant interaction and adaptation to countless variables, in addition to the antibacterial selection pressure itself. 10,21,23,28
Regardless of the enduring debate, the facts are clear: bacterial pathogens of animal and human origin are becoming increasingly resistant to most vanguard antibacterials, including last-generation cephalosporins, aminoglycosides and even fluoroquinolones. If current resistance trends persist, we may certainly encounter, in the near future, bacterial pathogens that are impervious to the effects of all current therapeutic antibacterials to a point
____________________________________________________________________________________ where therapeutic options could become very limited. This reality has pushed for important changes in awareness and priorities of government agencies worldwide with regard to antibacterial management. Hence the One Health concept. This concept embraces a worldwide strategy for expanding interdisciplinary collaborations (e.g., FAO-OIE-WHO collaboration, through the Tripartite Concept Note) and communications in all aspects of healthcare for humans, animals and the environment. Only with such a holistic approach can we better understand why the problem of antibacterial resistance is currently so pervasive and how we should best intervene to improve the situation. 20,27
Monitoring of resistance in bacteria circulating in animals should focus on bacteria of public health importance from food animals, considering that the food chain is a major route for transfer of bacteria from animals to humans. Bacterial species included in the surveys are foodborne pathogens and foodborne commensal organisms, preferably isolated from healthy animals at slaughter. To represent foodborne commensal organisms, sentinel or indicator organisms are used that may be a source for transmission of resistance determinants to pathogens. E. coli and enterococci are included as indicator for Gram-negative and -positive bacteria, respectively. The inclusion of animal pathogens is of indirect importance for public health, but still very relevant. These pathogens usually represent a worst-case scenario because they are isolated from diseased animals that were treated with antibacterials. Therefore the surveillance of these strains in animal husbandry can be used for early warning purposes regarding the detection of new emerging resistances. 1, 28-30
Although contamination of carcasses at slaughter and meat products at retail are considered the major routes of transmission, other important routes may exist. Routes such as contamination of the environment, direct contact and contaminated products like milk or eggs may contribute to the dissemination of bacteria and their genes. 1,10,29,30
The dairy industry, over the last century, and in response to broad economic forces ultimately driven by the price elasticity of consumer demand, has turned out to be a major segment in the food production sector. The top goal of modern dairy operations is to produce maximum quantities of high-quality milk with a longer shelf-life. However, the intensification of this industry has brought vast advantages, but also great challenges when it comes to disease control and management, with the dairy producer relying on increasingly advanced veterinary diagnostic methods, treatment protocols and management strategies in an attempt to control and prevent herd pathology, consequently increasing animal performance. As a result, antibacterial agents are, nowadays, used extensively in the dairy industry for the prophylaxis/metaphylaxis and treatment of a variety of bacterial diseases affecting calves/heifers and adult females in the different rearing and milk production stages, respectively. In general, most of these antibacterials belong to the same general classes as
those used in human medicine, and in many cases even if they are not exactly the same compounds, their mode of action is identical. 31
Bovine mastitis accounts as the most common pathology in dairy operations worldwide and also the one on which antibacterials are mostly employed on. In addition to mastitis therapy in lactating animals, one of the more consistent uses of antibacterials in dairy herds takes place at drying-off. Dry cow therapy, which is in fact, a mastitis prophylactic measure, consists in the application of high levels of intramammary antibacterials in a slow release base, following the last milking of lactation in adult cattle. 32
Resistance to antibacterial agents in mastitis pathogens discloses three relevant aspects: 1) Reduction in cure rates after treatment of mastitis cases; 2) Potential risk of transmission of resistant bacteria to humans via the food chain; and 3) Potential source of resistance genes between mastitis pathogens and other environmental pathogens, which may consequently, through other routes, affect humans. 33-35
At present, most authors consider that, although antibacterial resistance does occur, the advantages of using antibacterials for mastitis treatment far outweigh the disadvantages. Consequently, the ultimate challenge will be to find a balance between antibacterial use and its risk outcomes (e.g., antibacterial residues, antibacterial resistances, etc.) always having in mind that the implications of this challenge are far reaching and include aspects like public health, animal welfare, and the impact on food quantity, quality, costs, among others. 21,31
I.
General Objective
The aim of the present dissertation was to determine whether antibacterial resistance patterns of major mastitis pathogens isolated from milk samples from dairy cattle of northwestern, central and southern Portugal have changed over time.
II.
Specific Objectives
In order to accomplish the referred general objective, the specific objectives were, firstly, to establish antibacterial resistance patterns of major bovine mastitis pathogens isolated from submitted milk samples from dairy herds in northwestern, central and southern Portugal in a nine-year period and, in consequence, determine if those patterns have changed over the referred period, presumably in response to antibacterial use among the herds in those regions.
The Big Picture on Antibacterial
Resistance
____________________________________________________________________________________
I. Antibacterial Resistance
1. Why is Antibacterial Resistance a Public Health Concern?
There are several reasons why bacterial resistance should be of great concern for the medical community. First, resistant bacteria, particularly staphylococci, enterococci,
Klebsiella pneumoniae and Pseudomonas spp. are becoming increasing threats in
health-care institutions. 36-42 In addition, many of those microorganisms have been identified with the potential to employ simultaneous multiple resistance mechanisms towards different antibacterials, rendering combination treatments ineffective. 22
Bacterial resistance makes it difficult and more expensive to treat a variety of common infections, causing delays in effective treatment, or in worst cases, failure to provide appropriate therapy, which can have serious consequences, especially in critically ill patients. Estimates from Europe indicate that the excess mortality due to multidrug-resistant bacterial hospital infections exceeds 25 000 each year (Table 1) and the death rate is presumed to be higher in other parts of the world. Apart from additional patient morbidity/mortality, the attributable health-care costs and productivity losses are estimated to be at least €1.5 billion annually. This reality places an added burden on health-care management, especially with the cost-containment pressures of today’s health-care environment. 43
Table 1: Estimated annual burden due to selected antibacterial-resistant bacteria in EU-Member States, Iceland
and Norway, 2007 (Adapted from ECDC/EMA, 2009). 43
Antibacterial-Resistant Bacteria No. Cases of
Infection* No. Extra Deaths No. Extra Hospital Days Gr a m
+ Methicillin-resistant S. aureus (MRSA) 171.200 (12%) 5.400 (37%) 1.050.000 (16%)
Vancomycin-resistant E. faecium (VRE) 18.100 (9%) 1.500 (28%) 111.000 (22%)
Gr
a
m
– 3
rd
generation cephalosporin-resistant E. coli 32.500 (27%) 5.100 (52%) 358.000 (27%)
3rd generation cephalosporin-resistant K. pneumoniae 18.900 (27%) 2.900 (52%) 208.000 (27%)
Carbapenem-resistant P. aeruginosa 141.900 (3%) 10.200 (7%) 809.000 (3%)
* Bloodstream infections, lower respiratory tract infections, skin and soft tissue infections, and urinary tract infections. Numbers in parentheses indicate percentage of bloodstream infections.
Resistant bacteria may also spread and become broader infection-control problems, not only within health-care institutions, but in communities too. Clinically important bacteria, such as Methicillin-resistant S. aureus (MRSA) 39,44 and extended-spectrum β-lactamase (ESBL)–producing E. coli 45,46 are increasingly isolated in the community. Infected individuals, including children, often lack identifiable risk factors for MRSA, and appear to have acquired their infections in a variety of community settings. 47,48 Laboratory reports show increasing resistance among pneumonia causing bacteria, which kills about 1.8 million children annually. 49 Community-associated MRSA strains are typically less resistant to antibacterial agents than health-care-associated MRSA, but are more likely to produce toxins, such as
Panton-Valentine leukocidin. 47 The spread of resistant bacteria within the community poses obvious additional problems for infection control, not just in long-term care facilities but also among sport teams, military recruits, and even children attending day care centers – a task that is complicated by the increased population mobility. 14
Finally, a recent development and cause for concern is an apparent shift in the burden of ABR which may be occurring between the main classes of pathogenic bacteria. This shift, from Gram-positive to Gram-negative pathogens, could further stretch the already limited resources of health-care services as the infections due to resistant Gram-negative organisms will likely outweigh recent achievements in the control of Gram-positive pathogens. 50 The evolution of ABR (Table 2), coupled with a shortage of new antibacterials in the pipeline, raises the possibility that untreatable multidrug-resistant infections will become more and more common. It is particularly worrisome that once it develops, ABR is either irreversible or very slow to reverse, despite the introduction of ABR containment and stewardship programs. 4
Table 2: Evolution of resistance to major antibacterials (Adapted from Palumbi, 2001). 51
Antibacterial Agent Year Deployed Year of Observed Resistance Sulfonamides 1930s 1940s Penicillin 1943 1946 Streptomycin 1943 1959 Chloramphenicol 1947 1959 Tetracycline 1948 1953 Erythromycin 1952 1988 Vancomycin 1956 1988 Methicillin 1960 1961 Ampicillin 1961 1973 Cephalosporins 1960s Late 1960s
2. Origins of Antibacterial Resistance
Antibacterial resistance, via specific genes and mechanisms, represents an outcome of bacterial evolution that characterizes to perfection Darwin’s biological principle of ‘survival of
the fittest’. This phenomenon has been, thus, occurring in nature for thousands of years, long
before the introduction of commercial antibacterials. 52 In fact, Dancer et al. (1999) have isolated resistant bacteria estimated to be over 2 000 years old from deep within glaciers in Canada’s high Arctic regions. 53 Another study detected TEM-type β-lactamases from a metagenomic library in cold-seep sediments of Edison Seamount (south of Lihir Island, Papua New Guinea), estimated to be approximately 10 000 years old. 54 Furthermore, resistance to sulfadiazine, spectinomycin and tetracycline has been identified among E. coli isolates collected before the introduction of large-scale commercial antibacterials. 52 Barlow &
____________________________________________________________________________________ Hall (2002) also showed that ampC ß-lactamase genes recovered from Citrobacter freundii strains collected prior to the clinical use of antibacterials, in the 1920s, were shown to be as effective at providing lactam-resistance in E. coli as were the plasmid-borne alleles from ß-lactam-resistant clinical isolates. 55
ABR is likely to have had its origin when originally susceptible organisms managed to acquire protection from the competitive attack of antibiotic-producing organisms. In addition, these organisms had to obtain protection from their own toxic products as well. 10,52,53,56-58 This presumption has been substantiated by the finding of aminoglycoside-modifying enzymes in aminoglycoside-producing organisms that display marked homology to modifying enzymes found in aminoglycoside-resistant bacteria. 59 Also, the essential genetic determinants associated with resistance to vancomycin – vanA, vanH, and vanX – appear to be very similar to the self protection mechanism employed in the vancomycin producing strains of Actinomyces spp. 60 Moreover, researchers have shown that several antibacterial preparations employed for human and animal use were found to be contaminated with chromosomal DNA of antibiotic-producing organisms, including identifiable ABR gene sequences. 61,62 It was further postulated that this presence of DNA encoding ABR in antibacterial preparations has been a factor in the rapid development of multiple resistance byprovidingtheresistancesequencesthatcanthenbetakenupbythecausativepathogen.22,26 With this said, it is even so imperative to recognize that the bacterial evolutionary response has not been limited to the acquisition of resistance genes. Bacteria have also developed means for stabilizing the resistance phenotype, thus dashing initial hopes of reversing resistance by merely reducing antibacterial usage. 23
Resistance to natural antibacterial agents (antibiotics) and synthetic derivatives has been observed in a collection of soil-dwelling actinomycetes, with some displaying resistance mechanisms not usually observed in clinical bacterial pathogens. Unexpectedly, researchers found that every isolate tested displayed resistance to at least six different antibacterial agents and in other cases, as many as twenty. The use of novel resistance mechanisms by these organisms, coupled with the fact that these soil microorganisms are not as intensively exposed to antibacterial selective pressures as are the clinical pathogens, emphasizes the fact that resistance is not a new phenomenon. Additionally, the identification of this new reservoir of resistance genes highlights the possibility of future horizontal transfer of novel ABR determinants to bacteria of human and veterinary significance. 58
3. Types of Antibacterial Resistance
Bacteria can display one of three fundamental ABR phenotypes: susceptibility, intrinsic
phenomenon that is displayed by all strains of a species and is a function of the physiological or biochemical makeup of that species. Of greater concern are cases of acquired resistance, where initially susceptible populations of bacteria become resistant to an antibacterial and proliferate in exposed host-animal populations under the selective pressure of usage of that agent. 10 This type of resistance may be consequent to the mutation of regulatory or structural genes (vertical evolution), to the acquisition of foreign resistance genes (horizontal
evolution), or to a combination of these two mechanisms and is present not in the entire
speciesbutonlywithinacertainlineageofbacteriaderivedfromasusceptibleparent.14, 26, 59, 63 ABR defined in this way is a microbiological phenomenon, which may have clinical implications, depending on pharmacokinetic and pharmacodynamic parameters as they apply to specific antibacterial agents. Nevertheless, even low-level resistance is worth mentioning since it may be a first step towards clinical resistance. 10 These considerations have always been important in definitions of rational antibacterial therapy, 64 and have been reemphasized by recent calls for prudent therapy in human and veterinary medicine. More on this matter will be described in detail in upcoming sections.
4. Antibacterial Modes of Action and Resistance Mechanisms
Antibacterial agents function as selective toxins that inhibit enzymes that are either unique to the prokaryotic cell or sufficiently different such that toxicity to the mammalian host is minimal. 25 Most antibacterials may be classified according to their main mechanisms of action (Table 3): 1) inhibition of cell wall synthesis, 2) inhibition of protein synthesis, 3) interference with nucleic acid synthesis, and 4) inhibition of a metabolic pathway. Although less well characterized, some authors consider the disruption of bacterial phospholipid membrane structure to be a fifth major mechanism of action. 14, 65-67
Table 3: Mechanisms of action of main antibacterial agents (Adapted from Tenover, 2006). 68
Mechanisms of Action of Main Antibacterial Agents
I. Inhibition of cell wall synthesis
β-lactams: penicillins, cephalosporins, carbapenems, monobactams
Glycopeptides: vancomycin, teicoplanin
II. Inhibition of protein synthesis
Binding to 50S ribosomal subunit: macrolides, chloramphenicol, clindamycin, linezolid,
lincomycin, quinupristin-dalfopristin
Binding to 30S ribosomal subunit: aminoglycosides, tetracyclines
Binding to bacterial isoleucyl-tRNA synthetase: mupirocin
III. Interference with nucleic acid metabolism
Inhibition of DNA synthesis: quinolones (fluoroquinolones)
Inhibition of RNA synthesis: rifamycins (rifampicin)
IV. Inhibition of metabolic pathways: sulfonamides (trimethoprim/ormetoprim), folic acid analogues
____________________________________________________________________________________ In spite of these mechanisms of action and even with a great variety of currently available antibacterial agents for human and veterinary clinical use, ABR mechanisms are known to exist for them all. 25,26 The most common mechanisms by which resistance occurs are listed and illustrated in Table 4 and Figure 1, respectively. Resistance generally develops through one or more of these mechanisms. 22 In the first example of resistance (Table 4), the antibacterial is either degraded or modified by enzymatic activity before it can reach the target site and damage the cell. A very large number of drug-modifying enzymes have been identified for numerous antibacterial agents. For example, there are now more than ninety TEM-type β-lactamases and more than 25 SHV-type enzymes identified that are capable of hydrolyzing penicillins and cephalosporins. 69 In the second resistance mechanism, changes in the target molecule occur as a result of spontaneous mutation, resulting in decreased affinity of the target for the antibacterial. Both decreased uptake and increased efflux mechanisms, due to their non-specific nature, are normally responsible for the creation of multiple ABR phenotypes. 70 Lastly, organisms may acquire antibacterial-insensitive enzymes that circumvent a specific metabolic pathway that antibacterial sensitive enzymes drive, allowing the microorganism to survive and continue growth in the presence of the antibacterial. 25
Figure 1: Broad depiction of major ABR mechanisms (Adapted from www.scq.ubc.ca).
Table 4: Mechanisms of ABR (Adapted from McDermott, 2003). 25
Mechanisms of Antibacterial Resistance
I. Modification of the antibacterial agent
Aminoglycosides, chloramphenicol, β-lactams, streptogramins
II. Mutation at target site
Aminoglycosides, β-lactams, macrolides, quinolones, rifampicin, trimethoprim, tetracyclines, mupirocin
III. Decreased antibacterial accumulation
Decreased uptake: Numerous antibacterials
Increased efflux: Tetracyclines, macrolides, chloramphenicol, quinolones
IV. Bypass of antibacterial-sensitive step through acquisition of drug-insensitive enzymes: