w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Severe
infection
in
patients
with
rheumatoid
arthritis
taking
anakinra,
rituximab,
or
abatacept:
a
systematic
review
of
observational
studies
Vanderlea
Poeys
Cabral
a,
Carlos
Augusto
Ferreira
de
Andrade
b,∗,
Sonia
Regina
Lambert
Passos
b,
Maria
de
Fátima
Moreira
Martins
c,
Yara
Hahr
Marques
Hökerberg
baFundac¸ãoOswaldoCruz(Fiocruz),InstitutoNacionaldeInfectologiaEvandroChagas(INI),ComissãodeControledeInfecc¸ão
Hospitalar,RiodeJaneiro,RJ,Brazil
bFundac¸ãoOswaldoCruz(Fiocruz),InstitutoNacionaldeInfectologiaEvandroChagas(INI),LaboratóriodeEpidemiologiaClínica,
RiodeJaneiro,RJ,Brazil
cFundac¸ãoOswaldoCruz(Fiocruz),InstitutodeComunicac¸ãoeInformac¸ãoCientíficaeTecnológicaemSaúde,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11August2015 Accepted12July2016
Availableonline27October2016
Keywords:
Rheumatoidarthritis/therapy Anti-rheumaticdrugs/adverse effects
Infection
Biologicaltherapy/adverseeffects Reviewofliteratureastopic
a
b
s
t
r
a
c
t
Aquestionisraisedaboutanincreasedriskofsevereinfectionfromtheuseofbiological drugsinpatientswithrheumatoidarthritis.Thissystematicreviewofobservationalstudies aimedatassessingtheriskofsevereinfectionassociatedwiththeuseofanakinra, ritux-imab,andabataceptinpatientswithrheumatoidarthritis.Thefollowingdatabaseswere searched:PubMed,ScienceDirect,Scopus,WebofKnowledge,Scirus,Cochrane,Exerpta MedicaDatabase,Scielo,andLilacsuptoJuly2010.Severeinfectionsweredefinedasthose life-threateningonesinneedoftheuseofparenteralantibioticsorofhospitalization. Lon-gitudinalobservationalstudieswereselectedwithoutlanguagerestriction,involvingadult patientsdiagnosedwithrheumatoidarthritisandwhousedanakinra,rituximab,or abat-acept.Infourstudiesrelatedtoanakinra,129(5.1%)severeinfectionswererelatedin2896 patients,ofwhichthreedied.Withrespecttorituximab,twostudiesreported72(5.9%) severeinfectionsin1224patients,ofwhichtwodied.Abataceptwasevaluatedinonlyone studyinwhich25(2.4%)severeinfectionswerereportedin1046patients.Themainsiteof infectionforthesethreedrugswastherespiratorytract.Onepossibleexplanationforthe highfrequencyofsevereinfectionsassociatedwithanakinramaybethelongerfollow-up timeintheselectedstudies.Thehighfrequencyofsevereinfectionsassociatedwith ritux-imabcouldbecreditedtothelessstrictinclusioncriteriaforthepatientsstudied.Therefore, infectionmonitoringshouldbecautiousinpatientswithrheumatoidarthritisinuseofthese threedrugs.
©2016PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mails:carlosandrade07@gmail.com,carlos.andrade@ipec.fiocruz.br(C.A.Andrade). http://dx.doi.org/10.1016/j.rbre.2016.10.001
544
rev bras reumatol.2016;56(6):543–550Infecc¸ões
graves
em
pacientes
com
artrite
reumatoide
em
uso
de
anakinra,
rituximab
ou
abatacept:
revisão
sistemática
de
estudos
observacionais
Palavras-chave:
Artritereumatoide/terapia Antirreumáticos/efeitosadversos Infecc¸ão
Terapiabiológica/efeitos adversos
Revisãodeliteraturacomo assunto
r
e
s
u
m
o
Existeumquestionamentosobreaumentodoriscodeinfecc¸õesgravespelousode medica-mentosbiológicosporpacientescomartritereumatoide.Estarevisãosistemáticadeestudos observacionaisobjetivouavaliaroriscodeinfecc¸õesgravesassociadasaousodeanakinra, rituximabeabataceptempacientescomartritereumatoide.Forampesquisadasasbases PubMed, Science Direct, Scopus, Webof Knowledge, Scirus,Cochrane, Exerpta Medica Database, ScieloeLilacsatéjulho/2010.Infecc¸õesgravesforamdefinidascomoaquelas comderiscodevida,necessidadedeantibióticosparenteraisoudehospitalizac¸ão.Foram selecionadosestudosobservacionaislongitudinais,semrestric¸ãodeidioma,queenvolviam pacientesadultoscomdiagnósticodeartritereumatoidequeusaramanakinra,rituximab, abatacept.Emquatroestudosrelacionadosaoanakinra,foramrelatadas129(5,1%)infecc¸ões gravesem2.896pacientes,dosquaistrêsevoluíramparaóbito.Sobreorituximab,dois estu-dosrelataram72(5,9%)infecc¸õesgravesem1.224pacientes,dosquaisdoisevoluírampara óbito.Oabataceptfoiavaliadoemapenasumestudo,noqualforamrelatadas25(2,4%) infecc¸õesgravesem1.046pacientes.Oprincipalsítiodeinfecc¸ãoparaostrês medicamen-tosfoiotratorespiratório.Umapossívelexplicac¸ãoparaafrequênciaelevadadeinfecc¸ões gravesassociadasaoanakinrapodeseromaiortempodeacompanhamentonosestudos selecionados.Afrequênciaelevadadeinfecc¸õesgravesassociadasaorituximabpoderiaser creditadaaocritériomenosrestritodeinclusãodepacientes.Portanto,devesercautelosa a monitorac¸ãodeinfecc¸õesnospacientescomartritereumatoidequeusamessestrês medicamentos.
©2016PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Rheumatoidarthritis(RA)isachronicinflammatorydisease ofunknownetiologythat affects0.5–1% ofthe worldadult population,predominantlyinwomen,andwiththehighest incidenceintheage rangeof30–50years.1 Thesymmetric polyarthritis characteristicof RAcan cause pain and joint destructionanddeformity,especiallyinthejointsofthehands andwrists,aswellaspainandsystemicmanifestationssuch asfatigue,morningstiffness,andweightloss.2
RA patients may remain with active disease despite the use of synthetic (or “non-biological”) Disease Modify-ing Antirheumatic Drugs(DMARDs) such as methotrexate, leflunomide,azathioprine,andcyclosporine.Thus,since1997 new therapies, such as “biological DMARDs”, have been employed,demonstratinggreaterefficacyinthetreatmentof RA,3 amongwhich onecan mentiontumor-␣ necrosis
fac-tor(TNF-␣)-antagonists(infliximab,etanercept,adalimumab,
andgolimumab)andnon-TNF-␣antagonists(abatacept,
rit-uximab,anakinra,andtocilizumab)DMARDs.4
Somerandomizedcontrolledtrialshaveshownanincrease ininfectionwiththeuseofnon-TNF-␣antagonistDMARDsin
patientswithRA,bothwithrituximab(5.2/100patient-years formethotrexate/rituximab association vs. 3.7/100 patient-yearsformethotrexateonly),5aswellaswithabatacept(2.9% fortheassociationvs.1.9%formethotrexateonly).6However, other authors havenot foundthis increase forrituximab,7 abatacept,8oranakinra.9
Threesystematicreviews(SRs)thatevaluatednon-TNF-␣
antagonistDMARDswerepublished.4,10,11
Gartlehneretal.10haveexaminedthecomparativeefficacy and safety ofthreeTNF-␣ antagonists and ofa non-TNF-␣
antagonist(anakinra)forthetreatmentofrheumatoid arthri-tis in 18 observational and experimental studies, but the authorsdidnotpresentresultsoninfections.
Basedon12clinicaltrials,Salliotetal.11havesuggesteda trend(notstatisticallysignificant)forincreasedriskofsevere infection during the treatment with rituximab (odds ratio [OR]=1.45,confidenceinterval[CI]95%:0.56–3.73),abatacept (OR=1.35;95%CI:0.78–2.32),andanakinra(OR=2.75;95%CI: 0.91–8.35).
Storageetal.,4inasystematicreviewofeight(randomized andopen-label)clinicaltrials,identifiedsevereinfectioninup to2.3%incasesofexclusive useofabataceptandfrom1.3 to12.7%withtheuseofabataceptassociatedwithsynthetic DMARDs.
Although performing a meta-analysis of observational studiesonthesafetyofbiologicalDMARDs,Bernatskyetal.12 studied only TNF-␣ antagonists. Thus, we emphasize that
except for abatacept, systematic reviews of observational studiesontheriskofsevereinfectionassociatedwith non-TNF-␣ antagonists (anakinra, rituximab, and tocilizumab)
havenotyetbeencarriedout.
Method
The description of this systematic review was conducted accordingtoapre-specifiedprotocol,basedonthePreferred ReportingItemsforSystematicReviewsand Meta-Analyses (PRISMA)guideline.13Thefollowingarethemaindefinitions andstepsdescribed intheprotocol.Severeinfections were definedasthoseinwhichthereisariskforthepatient’slife, andthenecessityofparenteralantibioticsorof hospitaliza-tioninadultpatientswithRAusersofanakinra,rituximab, andabatacept.Onlylongitudinalobservationalstudieswere selected, including post-marketing evaluation studies and clinicaltrialfollow-upstudies(e.g.,open-labelclinicaltrials). Thefollowingelectronicdatabasesweresearched:PubMed, LiteraturaLatino-AmericanaedoCaribeemCiênciasdaSaúde (LILACS), Scopus, Web ofScience, Science Direct,Excerpta Medica Database (EMBASE), SciELO, and Scirus. For each base search, a strategy combining the following descrip-tors– infection, bacterial infections,antirheumatic agents, adverse effects, therapeuticuse, rheumatoid arthritis, and drugtherapy – were developed.There were no restrictions on language. The strategy used in Pubmed was: ((infec-tion [MeSH Terms] OR bacterial infections [MeSH Terms]) AND(antirheumaticagents/adverseeffects[MeSHTerms]OR antirheumaticAgents/therapeutic use [MeSHTerms])) AND (arthritis,rheumatoid/drugtherapy[MeSHTerms]).Equivalent strategieshavebeenformulatedfortheotherbases.TheScirus basealsocoveredgrayliterature.Searchesincludedstudies publisheduptoJuly2010,coveringpriorperiodswithout lim-itationofpublicationdate.Thereferencelistsofallarticles weremanuallyinvestigated,inapursuitofnewarticles (cross-references).
Adatabasefortheelectronicsearcheswascreatedwiththe helpoftheEndNoteX1program.Duplicatequotationswere deleted.Potentiallyrelevanttitlesandabstractswereselected independently by peer reviewers VPC/CAFA and VPC/SRLP, whoalsotookthereadingsofthefulltext,extracted informa-tion,andassessedthequalityofstudies.Disagreementswere resolvedbyconsensusand,wherenecessary,theopinionof thethirdauditor(externaltothepairofpeerreviewers)was requested.
Theextractionofindependentdatawasbasedon comple-tingastandardformwithrelevantdataabouteachstudy(full reference,countryofitsproduction,samplesize,design, dura-tion,clinicalanddemographiccharacteristicsofpatients,the resultsfortheriskofsevereinfection,andqualityassessment scores).Theauthorswerecontactedfordatarequestneeded andnotcontainedinthepublishedversionofarticles.
Astotheinterpretationofthefrequencyofsevere infec-tions,weusedtheclassificationproposedbyMeyboomand Egberts14foradversedrugreactions: commonly,whenthey happenin1–10%ofusers;unusually,0.1–1%;andrarely,inless than0.1%.
Toevaluatethequalityofobservationalstudies,an instru-mentadapted,basedontheNewcastle-OttawaScale,15was used.Threeitemswereconsidered;eachreceivedonepoint whenthe requiredstandardwas met:(1)mannerof selec-tionofparticipants–adescribedsample,representativeofthe targetpopulationbybeingcomplete,randomorsystematic;
(2)percentageoflosses–withdescription,andunder20.0%; and (3) manner of outcome assessment – an available or describedmeasuringinstrument.Inthosearticlesthatwere scoredforthethreeitems,thestudywasconsideredasbeing ofhighquality;articlesthatreceivedpointsfortwoitemswere consideredofintermediatequality;andwhenonlyoneitem wasscored,thestudywasconsideredoflowquality.
ThisstudywasapprovedbytheResearchEthics Commit-teeoftheInstitutoNacionaldeInfectologiaEvandroChagas, OswaldoCruzFoundation(Opinion017/2010).
Results
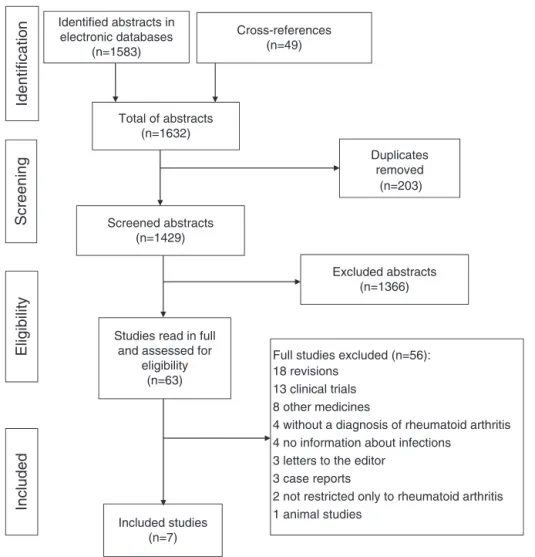
A flow chart(Fig. 1)describing the resultsof bibliographic search performedwas prepared.Initially, thesesearchesin eight databases provided1583 abstracts, ofwhich 19 were selectedforfull-articlereading.Apartfromtheseabstracts,we selected44ofthe49abstractsobtainedbycross-references, resultingin63fullstudies.Ofthesestudies,onlysevenwere approvedandincludedinthesystematicreview:five open-label articles (Nuki et al.,16 Fleischmann et al.,17 Keystone etal.,18Genoveseetal.,19andSchiffetal.20),onecohort-nested case–controlstudy,21andonecohortstudy22(Fig.1).
Table1presentsthegeneralcharacteristicsofeachstudy. Thesevenobservationalstudiesincludedinthisreviewhad atotaldurationoffollow-upperiodsfromsixto63months, wereconductedinEuropeancountries,intheUnitedStates (USA) and Mexico, and addressed5166 patients diagnosed withRAtreatedwithoneofthreedrugs(anakinra,rituximab, abatacept). A meta-analysiswas notperformed dueto the presenceofmultiplesourcesofheterogeneityamongstudies, andalsotothelimitednumberofpublicationsforthestudied drugs(differenttypesofstudydesigns,eligibilitycriteria,and medicines).
Anakinra
Intotal,2896patientsweretreatedwithanakinraand evalu-atedinfourstudieslastingfrom12to63months:onecohort study,22 two open-labelarticles,16,17 and one cohort-nested case–controlstudy21(Table1).
Among the evaluated patients, the female gender pre-vailed, with a mean age from 52.8 to 54.8 years,with RA durationrangingfromfourto13years,andwithaveryactive disease,accordingtothenumberofswollenorpainfuljoints, C-reactiveprotein,andadiseaseactivity indexbasedon28 joints (DAS28>5.1). In those two articles that reported on comorbidities,21,22theirauthorsobservedmainlychroniclung disease and diabetes mellitus.Concomitant use of cortico-steroidsrangedfrom45.9%to87.0%,andtheuseofvarious syntheticDMARDsrangedfrom71.4%to80.5%(Table2).
Brassard et al.21 onlyevaluated the riskoftuberculosis, adding demographic data from the use of three biological DMARDs(infliximab,etanercept,andanakinra). Despitethe establishmentofacontactwiththeauthor,therewereno spe-cificdemographicdataforanakinra,aswellasforthesitesof infection.
546
rev bras reumatol.2016;56(6):543–550Identified abstracts in electronic databases
(n=1583)
Cross-references (n=49)
Screened abstracts (n=1429)
Studies read in full and assessed for
eligibility (n=63)
Full studies excluded (n=56): 18 revisions
13 clinical trials 8 other medicines
4 without a diagnosis of rheumatoid arthritis 4 no information about infections
3 letters to the editor 3 case reports
2 not restricted only to rheumatoid arthritis 1 animal studies
Duplicates removed
Identification
Screening
Total of abstracts (n=1632)
Excluded abstracts (n=1366)
Eligibility
Included
Included studies (n=7)
(n=203)
Fig.1–Flowchartofselectionofstudiesforthesystematicreview.
treatedwithanakinra,alongwiththeirrespectiveincidences (frequenciesandrates).
Thefrequencyofinfectionforanakinra rangedfrom 1.3 to11.1%.Nuki etal.16 andBrassard etal.21 showedsimilar frequencies(1.3%).Fleischmannetal.17reportedpneumonia
(23.8%)andcellulitis(14.8%)asthemostfrequentinfections, but without detailing their clinical aspects. These authors weretheonlyoneswhoreporteddeathsrelatedtotheuseof anakinra.Nukietal.16didnotspecifythesitesofthefourcases ofsevereinfectionandalthoughtheseauthorshaveinformed
Table1–Descriptionofthesevenpublications(2002–2009)ontheriskofsevereinfectionsinpatientswithrheumatoid
arthritisassociatedwithuseofnon-anti-TNF-␣biologicalDMARDs.
Firstauthor,year Drug Design Place Samplesize Durationof
studyin months
Nuki,a200216 Anakinra Openlabel Europe(11countries) 309 12–18
Listing,200522 Anakinra Cohort Germany 70 18
Brassard,b200621 Anakinra Case–controlnestedincohort USA 1414 63
Fleischmann,c200617 Anakinra Openlabel USA 1103 36
Keystone,200718 Rituximab Openlabel – 1039 6
Genovese,200919 Rituximab Openlabel 9internationalstudies 185 12
Schiff,200920 Abatacept Openlabel USA,EuropeandMexico 1046 6
DMARDS,DiseaseModifyingAntirheumaticDrugs;USA,UnitedStatesofAmerica.
a 218inuseofanakinraand71withplacebointhefirstsixmonthsofthedouble-blindclinicaltrial,allincludedintheextensionphasewith
openlabel(12months).
b Theoutcomewastuberculosis.
r
e
v
b
r
a
s
r
e
u
m
a
t
o
l
.
2
0
1
6;
5
6(6)
:543–550
547
Firstauthor,year Drug Sample,n Women (%)
Meanage (SD)
Rheumatoid arthritis
activity
Yearsof disease(SD)
Comorbidities (%)
Corticosteroids, n(%)
Useof synthetic DMARDs,
n(%)
Useof synthetic DMARDs, n(SD)
Nuki,200216 Anakinra 309 75.1 52.8(13.0) NPJ33.5(13.1)
NSJ25.9(9.3)
CRP3.9(3.8)
4.0(2.4) – 142(45.9) 223c(72.2) 1.2c(1.0)
Listing,200522 Anakinra 70 77.1 54.3(11.6) 6.1(1.2)a 13.0(7.0–22.0)b Chroniclung
disease(12.9)
Diabetes(11.4)
Psoriasis(1.4)
61(87.0) 50d(71.4) 4.2(1.9)
Brassard,200621 Anakinra 1414 – – – – Diabetes.
Silicosis
CKD.Solidorgan
transplantand
carcinoma(...)
– – –
Fleischmann,e200617 Anakinra 1103 74.3 54.8(19–85)f NPJ22.2(0–68)
NSJ18.2(0–66)
CRP2.7(0.1–25.6)
10.3(0.2–59.5)f –g 654(59.3) 888d(80.5) –
Keystone,200718 Rituximab 1039 80.0 51.9(11.4) 6.8(1.0)a 11.2(8.2) –h – – 2.5e(1.6)
Genovese,200919 Rituximab 185 78.4 51.2(12.3) 7.0(0.9)a 11.9(9.2) – – – 4.0f(2.4)
Schiff,200920 Abatacept 1046 81.2 54.4(12.4) 6.2(0.7)a 11.6(9.5) – 611(58.4) 1003e(96.2) –
DMARDS,DiseaseModifyingAntirheumaticDrugs;SD,standard-deviation;NPJ,numberofpainfuljoints;NSJ,numberofswollenjoints;CRP,C-reactiveprotein;n,number.
a ArthritisrheumatoidactivitybyDAS28(SD)–indexofactivityofthediseasewith28joints.
b Interquartilerange.
c Prioruseofnon-biologicalDMARDs
d Concomitantuseofnon-biologicalDMARDs.
e Patientswhoparticipatedinthefirstsixmonthsofplacebo-controlledclinicaltrialwereincluded.
f (minimumandmaximum).
g Diabetesandcancerexcluded.
548
rev bras reumatol.2016;56(6):543–550Table3–Resultsrelativetotheriskofsevereinfectionsassociatedwiththeuseofnon-anti-TNF-␣biologicalDMARDSin
patientswithrheumatoidarthritis.
Firstauthor,year Drug Sample,n Infectedpatients (numberof
episodes)
Severeinfections %(95%CI)b
Rateofsevere
infections/100 person-years
(95%CI)
Infectionsites,n
(%)
Deaths,n
Nuki,200216 Anakinra 309 4(4) 1.3(0.4–3.3) – – 0c
Listing,200522 Anakinra 70 1(2) 2.9(0.4–9.9) 3.2(0.4–11.5) Arthritis:1
Acute
osteomyelitis:1
0
Brassard,200621 Anakinra 1414 19(19) 1.3(0.8–2.1) – – 0
Fleischmann,a
200617
Anakinra 1103 105(122) 11.1(9.3–13.1) 5.4(4.5–6.4) Pneumonia:29
Cellulitis:18
Sepsis:5
Other:70
3(sepsis)
Keystone,200718 Rituximab 1039 –(59) 5.7(4.4–7.3) 5.1(4.0–6.6) UTI:–
Bronchitis:–
Sepsis:–
UTI:–
2
(bronchopneu-moniaand
sepsis)
Genovese,200919 Rituximab 185 12(13) 7.0(3.8–11.7) 7.0(4.1–12.0) Gastroenteritis:
4
Pneumonia:2
Diverticulitis:2
Bronchitis:1
UTI:2
Cellulitis:1
Septicarthritis:
1
0
Schiff,200920 Abatacept 1046 25(25) 2.4(1.6–3.5) – Pneumonia:6
Bronchitis:3
0
CI95%,confidenceintervalof95%;URTI,upperrespiratorytractinfection;UTI,urinarytractinfection.
a Patientswhoparticipatedinthefirstsixmonthsoftheplacebo-controlledclinicaltrialwereincluded.
b TheWinPepiprogramwasusedforcalculations,with95%CI,Fisher’sexacttest.
c Theauthorofthisarticlereportedtheoccurrenceoftwodeathsnotattributedtoanakinra.
twodeaths,theydidnotconsideranakinraasacausal fac-tor.Listing etal.22 reportedapatientwhodevelopedseptic arthritis,whichprogressedtoosteomyelitis.
Threestudies17,21,22wereclassifiedasofintermediate qual-ity,andafourthstudyreceivedalow-qualification16(Table4).
Rituximab
Rituximabwas administered in 1224 patients evaluated in twoopen-labelclinicaltrialswithdurationsfromsix18to1219 months(Table1).Heretoofemalegenderprevailed, witha meanage of51.8 years,withhigh RAactivity,according to DAS28(>5.1),andwithameandiseasedurationrangingfrom 11to12years(Table2).
Table3liststhetwostudiesrelatedtorituximabinwhich 72 episodesofsevere infection (5.9%)were reported inthe totalnumberofpatientstreated,andthehighestincidence wasnotedinthestudybyGenoveseetal.19Theseinfections occurredinvarioussites,particularlyupperrespiratorytract (URT),urinarytract,andgastrointestinaltract.
AlthoughKeystone et al.18 havenot specifiedall severe infectionsites,theseauthorsreportedtwodeathsattributed totheuseofrituximab:oneofthemdueto bronchopneumo-niaandtheotherduetoneutropenicsepsisaftertheuseof trimethoprim.
Both studies18,19 were classified as high-quality trials (Table4).
Abataceptandtocilizumab
Onlyanopen-labelclinicaltriallastingsixmonthsevaluated abatacept.20Theauthorsincluded1046patientswithsimilar characteristicsastogender,age,andRAdurationand activ-ityreportedinthestudiesrelatedtoanakinraandrituximab. Pneumoniaandbronchitiswerethemaintypesofinfection observed(Tables2and 3).Thequalityofthe Schiffetal.20 studywasconsideredintermediate(Table4).Intheperiodof thereview,noobservationalstudyevaluatedinfectionsinRA patientstakingtocilizumab.
Discussion
Table4–Qualityassessmentofselectedstudiesontheriskofsevereinfectionsinpatientswithrheumatoidarthritis
associatedwiththeuseofnon-anti-TNF-␣biologicalDMARDsusinganinstrumentbasedontheNewcastle-OttawaScale.
Assessmentofeachitem(selectionofparticipants,losses,outcome,andselectionofvariables)andofthetotalscore.
Firstauthor,year Selectionofparticipantsa Lossesb Outcomec Totalscore
Nuki,200216 1 0 0 1
Listing,200522 1 0 1 2
Brassard,200621 1 0 1 2
Fleischmann,200617 1 0 1 2
Keystone,200718 1 1 1 3
Genovese,200919 1 1 1 3
Schiff,200920 1 1 0 2
DMARDs,DiseaseModifyingAntirheumaticDrugs.
a Selectionofparticipants–1pointfordescribedsamplerepresentativeofthepopulation(complete,randomorsystematic).
b Losses–1pointforproportionofdescribedlossesunder20.0%.
c Outcomeevaluation–1pointforavailableordescribedoutcomemeasurementinstrument.
Thestrengthsofthisreviewconsistofthewidesearch con-ducted,ofthe broaddefinitionofsevere infections,and of itsoriginality,takinginto accountthat, althoughother SRs fromobservationalstudieshavebeenpublished,such stud-iesdonotseparatelyevaluatethesesamedrugs.Wedetected only one review23 of observational studies that, although assessingthesafetyofthetherapeuticdrugsevaluatedinthis study,presentedtheirresultscollectively,alsoincludingTNF-␣
antagonists.
Curtisetal.24describedacohort(1998–2011)inwhichthe ratesofsevere bacterialinfections associatedwiththe use ofrituximabwere4.4(95%CI3.1–6.4)and,forabatacept,2.8 (95%CI1.7–4.7),per100patient-years.Ourresultsweresimilar sincethefrequencyofinfectionwasalsohigherforrituximab versusabatacept.
Ameta-analysispublishedbySinghetal.,25which evalu-atedsevere infectioninpatientswithRAtreatedwithnine biologicdrugs, includedthesethreemedications studiedin ourSR,buttheauthorsdidnotincludeobservationalstudies andpresentedcollectiveresultsfortheninedrugs,showing anincreaseinsevereinfectionswiththeuseofbiologicalsin conventionaldoses(OR=1.90;95%CI1.50–2.39).
Theincidenceofinfectionsrelatedtotheuseofabatacept inthisstudy(2.4%),possiblyduetotheshortfollow-upperiod (sixmonths)ofthesingleidentifiedtrial,20wassimilartothe incidencefoundbySalliotetal.11(2.5%)inarandomizedtrial lasting12months.26
InthisSR,severeinfectionratesforanakinrawere hetero-geneous,rangingfrom1.3%inthesmallerfollow-upstudies (Nukietal.16 –12months,andListingetal.22–18months) to11.1%inthestudywiththelargestsample(1103patients) andwitha36-monthfollow-up.17InanSRthatincludedfour clinicaltrialswithasix-monthduration,Salliotetal.11found only1.4%ofsevereinfections.
In the studies included in our SR which evaluated rituximab,18,19thefollow-uptimes,ofsixto12months,were similartothoseofthethreetrialsincludedbySalliotetal.11 Thus,thehighestfrequencyofsevereinfectionsattributedby ustorituximabcouldbepartiallycreditedtotheinclusion cri-terionofKeystoneetal.,17whoadmittedpatientsdiagnosed withdiabetesmellitus,unlikethecaseinSalliotetal.’sSR.11
InthecomparisonofourSRversusSRspublishedbySalliot etal.11 andStorageetal.,4weobservedthattherespiratory
tractisthemainsiteofinfectionwithrespecttoanakinraand abatacept. However,withtheuse ofrituximab,urinary and gastrointestinaltracts(inadditiontotherespiratorytract)are alsomainsitesofsevereinfection,bothinourSRasinSalliot etal.11
Thenumberofpublishedobservationalstudiesonadverse events with the use of non-TNF-␣ antagonist DMARDs is
scarce.Thisisalimitationthataffectsthepresentreviewand alsootherpublishedreviewsonthistopic.11,27
Weemphasizethatinadditiontothefactthatsevere infec-tionshaveoccurredwithafrequencyconsideredascommon, theseinfectionshaveresultedindeaths(threein125severe infectionswithanakinra,twoin72withrituximab,andzero withabatacept).Ifwetakeintoaccountthatthemaximum follow-uptimeinmostofthestudiesanalyzedwasonlyabout threeyears,notallowingtheobservationofagreaternumber offatalcases,weemphasizetheimportanceofSRstothe clin-icalpracticeofrheumatology.Consideringtheriskofsevere infectionsbroughtaboutbythesedrugs,patientsshouldhave theirrespiratoryandurinarytractsregularlymonitoredwith anactivesurveillanceforinfections,particularlythosemore severeones,inordertopreventconditionssuchas bronchop-neumoniaorsepsis.
Funding
InstitutoNacionaldeInfectologiaEvandroChagas,Fundac¸ão OswaldoCruz.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.AlamanosY,VoulgariPV,DrososAA.Incidenceand prevalenceofrheumatoidarthritis,basedonthe1987 AmericanCollegeofRheumatologycriteria:asystematic review.SeminArthritisRheum.2006;36:182–8.
550
rev bras reumatol.2016;56(6):543–550DiagnósticoeTratamentodaArtriteReumatoide.RevBras Reumatol.2007;47:151–9.
3. ScottDL,WolfeF,HuizingaTWJ.Rheumatoidarthritis.Lancet. 2010;376:1094–108.
4. StorageSS,AgrawalH,FurstDE.Descriptionoftheefficacy andsafetyofthreenewbiologicsinthetreatmentof rheumatoidarthritis.KoreanJInternMed.2010;25:1–17. 5. CohenSB,EmeryP,GreenwaldMW,DougadosM,FurieRA,
GenoveseMC,etal.Rituximabforrheumatoidarthritis refractorytoanti-tumornecrosisfactortherapy.Arthritis Rheum.2006;9:2793–806.
6. WeinblattMW,CombeB,CovucciA,ArandaR,BeckerJC, KeystoneE.Safetyoftheselectivecostimulationmodulator abataceptinrheumatoidarthritispatientsreceiving backgroundbiologicandnonbiologicdisease-modifying antirheumaticdrugs.Aone-yearrandomized,
placebo-controlledstudy.ArthritisRheum.2006;54:2807–16. 7. EmeryP,FleischmannR,Filipowicz-SosnowskaA,
SchechtmanJ,SzczepanskiL,KavanaughA,etal.Theefficacy andsafetyofrituximabinpatientswithactiverheumatoid arthritisdespitemethotrexatetreatmentresultsofaphase IIbrandomized,double-blind,placebo-controlled,
dose-rangingtrial.ArthritisRheum.2006;54:1390–400. 8. KremerJM,DougadosM,EmeryP,DurezP,SibiliaJ,ShergyWJ,
etal.Treatmentofrheumatoidarthritiswiththeselective costimulationmodulatorabatacepttwelve-monthresultsofa phaseIIb,double-blind,randomizedplacebo-controlledtrial. ArthritisRheum.2005;52:2263–71.
9. CohenBS,MorelandLW,CushJJ,GreenwaldMW,BlockS, ShergyWJ,etal.Amulticentre,doubleblind,randomised, placebocontrolledtrialofanakinra(Kineret),arecombinant interleukin1receptorantagonist,inpatientswith
rheumatoidarthritistreatedwithbackgroundmethotrexate. AnnRheumDis.2004;63:1062–8.
10.GartlehnerG,HansenRA,JonasBL,ThiedaP,LohrKN.The comparativeefficacyandsafetyofbiologicsforthetreatment ofrheumatoidarthritis:asystematicreviewand
metaanalysis.JRheumatol.2006;33:2398–408.
11.SalliotC,DougadosM,GossecL.Riskofseriousinfections duringrituximab,abataceptandanakinratreatmentsfor rheumatoidarthritis:meta-analysesofrandomised placebo-controlledtrials.AnnRheumDis.2009;68:25–32. 12.BernatskyS,HabelY,RahmeE.Observationalstudiesof
infectionsinrheumatoidarthritis:ametaanalysisoftumor necrosisfactorantagonists.JRheumatol.2010;37:928–31. 13.MoherD,LiberatiA,TetzlaffJ,AltmanDG.Preferredreporting
itemsforsystematicreviewsandmeta-analyses:thePRISMA Statement.PLoSMed.2009;6:e1000097.
14.MeyboomRHB,EgbertsAC,GribnauFWJ,HeksterYA. Comparingtherapeuticbenefitandrisk.Pharmacovigilance inperspective.DrugSaf.1999;21:429–47.
15.WellsG,SheaB,O’ConnellD,PetersonJ,WelchV,LososM, etal.TheNewcastle-OttawaScale(NOS)forassessingthe qualityifnonrandomizedstudiesinmeta-analyses.Ontario; [s.d.].Availableat:http://www.ohri.ca/programs/
clinical epidemiology/oxford.htm[accessed01.02.15].
16.NukiG,BresnihanB,BearMB,McCabeD.Long-termsafety andmaintenanceofclinicalimprovementfollowing treatmentwithanakinra(recombinanthumaninterleukin-1 receptorantagonist)inpatientswithrheumatoidarthritis: extensionphaseofarandomized,double-blind,
placebo-controlledtrial.ArthritisRheum.2002;46:2838–46. 17.FleischmannRM,TesserJ,SchiffMH,SchechtmanJ,
BurmesterG-R,BennettR,etal.Safetyofextendedtreatment withanakinrainpatientswithrheumatoidarthritis.Ann RheumDis.2006;65:1006–12.
18.KeystoneE,FleischmannR,EmeryP,FurstDE,van VollenhovenR,BathonJ,etal.Safetyandefficacyof additionalcoursesofrituximabinpatientswithactive rheumatoidarthritis:anopen-labelextensionanalysis. ArthritisRheum.2007;56:3896–908.
19.GenoveseMC,BreedveldFC,EmeryP,CohenS,KeystoneE, MattesonEL,etal.Safetyofbiologicaltherapiesfollowing rituximabtreatmentinrheumatoidarthritispatients.Ann RheumDis.2009;68:1894–7.
20.SchiffM,PritchardC,HuffstutterJE,Rodriguez-ValverdeV, DurezP,ZhouX,etal.The6-monthsafetyandefficacyof abataceptinpatientswithrheumatoidarthritiswho underwentawashoutafteranti-tumournecrosisfactor therapyorweredirectlyswitchedtoabatacept:theARRIVE trial.AnnRheumDis.2009;68:1708–14.
21.BrassardP,KezouhA,SuissaS.Antirheumaticdrugsandthe riskoftuberculosis.ClinInfectDis.2006;43:717–22.
22.ListingJ,StrangfeldA,KaryS,RauR,vonHinueberU, Stoyanova-ScholzM,etal.Infectionsinpatientswith rheumatoidarthritistreatedwithbiologicagents.Arthritis Rheum.2005;52:3403–12.
23.RamiroS,Gaujoux-VialaC,NamJL,SmolenJS,BuchM, GossecL,etal.SafetyofsyntheticandbiologicalDMARDs:a systematicliteraturereviewinformingthe2013updateofthe
EULARrecommendationsformanagementofrheumatoid
arthritis.AnnRheumDis.2014;73:529–35.
24.CurtisJR,YangS,PatkarNM,ChenL,SinghJA,CannonGW. Riskofhospitalizedbacterialinfectionsassociatedwith biologictreatmentamongUSveteranswithrheumatoid arthritis.ArthritisCareRes.2014;66:990–7.
25.SinghJA,CameronC,NoorbaloochiS,CullisT,TuckerM, ChristensenR,etal.Riskofseriousinfectioninbiological treatmentofpatientswithrheumatoidarthritis:asystematic reviewandmeta-analysis.Lancet.2015;386:258–65.
26.MorelandLW,AltenR,VandenBoschF,AppelboomT,LeonM, EmeryP,etal.Costimulatoryblockadeinpatientswith rheumatoidarthritis:apilot,dose-finding,double-blind, placebo-controlledclinicaltrialevaluatingCTLA-4Igand LEA29Yeightyfivedaysafterthefirstinfusion.Arthritis Rheum.2002;46:1470–9.