Sara Daniela de Sousa Reis
CHARACTERIZATION OF THE EFFECTS OF PROTON PUMP
INHIBITORS ON BONE METABOLISM: IN VITRO STUDY IN
CO-CULTURES OF OSTEOCLASTS AND BREAST CANCER CELLS
AND IN CULTURES OF OSTEOBLASTS
Dissertação de Candidatura ao grau de Mestre em Oncologia Molecular
submetida ao Instituto de Ciências Biomédicas de Abel Salazar da Universidade
do Porto, realizada sob orientação do Professor Doutor João Miguel Silva e Costa
Rodrigues, professor auxiliar convidado, e co-orientação da Professora Doutora
Maria Helena Raposo Fernandes, professora catedrática da Faculdade de
Medicina Dentária da Universidade do Porto
2
Agradecimentos
Agradeço aos meus orientadores, Professor Doutor João Miguel da Costa Rodrigues e Professora Doutora Maria Helena Raposo Fernandes por todos os ensinamentos, toda a ajuda e disponibilidade permanente ao longo da elaboração deste trabalho.
Ao Professor Doutor Manuel Teixeira (Instituto Português de Oncologia), pela cedência das linhas celulares de cancro da mama.
À Doutora Paula Sampaio (Instituto de Biologia Molecular e Celular), pela disponibilidade na aquisição de imagens de microscopia confocal.
À Técnica Mónica Garcia (Faculdade de Medicina Dentária da Universidade do Porto), pela disponibilidade e ajuda laboratorial.
Aos meus colegas e amigos por todo o apoio e companheirismo.
3
Resumo
Os inibidores de bombas de protões são uma classe de fármacos amplamente utilizada em várias condições patológicas, como refluxo gastroesofágico, dispepsia e úlceras pépticas. Apesar de existirem diversas moléculas diferentes pertencentes a esta classe, sabe-se que estas partilham várias propriedades funcionais e estruturais, bem como algumas características farmacocinéticas. O seu mecanismo de ação baseia-se na
inibição de H+/K+ ATPases, responsáveis pela secreção de ácido gástrico. Neste
contexto, uma vez que no processo de reabsorção os osteoclastos solubilizam a fase óssea mineral através da secreção de ácido para a lacuna de reabsorção por bombas de protões do tipo vacuolar, V-ATPases, os inibidores de bombas de protões surgem como potenciais modeladores farmacológicos da atividade osteoclástica, particularmente em condições patológicas caracterizadas pela hiperactivação de osteoclastos, tal como acontece nas metástases osteolíticas associadas ao cancro da mama.
Neste trabalho avaliaram-se os efeitos celulares e moleculares de três inibidores de bombas de protões, nomeadamente, omeprazole, esomeprazole e lansoprazole, em culturas de células do cancro da mama, em co-culturas de osteoclastos e células do cancro da mama e, ainda, em culturas de osteoblastos. As culturas foram mantidas na presença de diferentes concentrações de cada inibidor durante 21 dias. As células foram caracterizadas aos dias 7, 14 e 21 através da proliferação/viabilidade celular, conteúdo proteico, atividade e coloração histoquímica da fosfatase alcalina e da fosfatase ácida resistente ao tartarato, presença de células multinucleadas com anéis de actina e expressão de recetores de vitronectina e calcitonina, apoptose e capacidade de reabsorção. Posteriormente, as culturas foram também tratadas com inibidores das vias MAPK/ERK, NFkB, PKC e JNK para ser avaliado o envolvimento destas vias de sinalização intracelulares na resposta celular. Observou-se que os inibidores de bombas de protões inibem a osteoclastogénese em co-culturas de osteoclastos e células do cancro da mama, de forma dependente de dose e fármaco testado, envolvendo alterações significativas nas diversas vias de sinalização intracelulares testadas. Verificou-se, ainda, que estes compostos têm, também, a capacidade de inibir o crescimento de culturas de células do cancro da mama e a osteoblastogénese. Os inibidores de bombas de protões surgem, assim, como potenciais modeladores do metabolismo ósseo no contexto da metastização óssea.
4
Abstract
Proton pump inhibitors are a class of drugs widely used in many pathological conditions, such as gastroesophageal reflux, dyspepsia and peptic ulcers. Although there are several different molecules belonging to this class, it is well known that they share many functional and structural properties as well as some pharmacokinetic characteristics. The mechanism of action relies in the inhibition of H+/K+ ATPases, responsible for gastric acid secretion. In this context, since in the resorption process osteoclasts solubilize the bone mineral phase through the secretion of acid to the resorption lacuna by proton pumps of the vacuolar type, V-ATPases, proton pump inhibitors appear as potentially pharmacological modulators of osteoclastic activity, particularly in pathological situations characterized by hiperactivation of osteoclasts, such as osteolytic metastases associated with breast cancer.
In this study were assessed the cellular and molecular effects of three proton pump inhibitors, namely, omeprazole, esomeprazole and lansoprazole, in cultures of breast cancer cells, in co-cultures of osteoclasts and breast cancer cells and in osteoblasts cultures. The cultures were maintained in the presence of different concentrations of each inhibitor for 21 days. Cells were characterized at day 7, 14 and 21 for cellular proliferation/viability, total protein content, alkaline phosphatase and tartarate resistant acid phosphatase activity and histochemical staining, presence of multinucleated cells with actin rings and expression of vitronectin and calcitonin receptors, apoptosis and resorbing ability. Later, the cultures were treated with inhibitors of the MAPK/ERK, NFkB, PKC and JNK pathways to assess the involvement of these intracellular signaling pathways in the cellular response. Results revealed that proton pump inhibitors inhibit osteoclastogenesis in co-cultures of osteoclasts and breast cancer cells, depending on the identity and dose of the tested molecule and involving significant changes in the several intracellular signaling pathways tested. Moreover, it was seen that these compounds also have the ability to inhibit cell growth in breast cancer cell cultures and osteoblastogenesis. Thus, proton pump inhibitors appear as potential modulators of bone metabolism in the context of bone metastasis.
5
Index
List of abbreviations……….7
1. Introduction………9
1.1 Bone tissue………..9
1.1.1 Structural and functional characteristics………...9
1.1.2 Bone cells………10
1.1.3 Osteoclastogenesis………12
1.1.4 Hormonal control………13
1.1.5 Signaling pathways………14
1.2 Breast cancer………17
1.2.1 Epidemiology and pathology………17
1.2.2 Risk factors………..18
1.2.3 Molecular types of breast cancer……….18
1.2.4 Therapies……….20
1.3 Bone metastasis in breast cancer……….24
1.3.1 Bone metastasis……….24
1.3.2 Types of bone metastases………26
1.3.3 Molecular types of breast cancer and metastatic behavior……….28
1.3.4 Treatment………29
1.4 Proton pump inhibitors……….30
1.4.1 Mechanism of action………..30
1.4.2 Adverse effects………...32
1.4.3 PPIs versus H2RAs………33
1.4.4 Secondary effects of PPI in bone metabolism………...34
2. Materials and methods………..36
2.1 Cell cultures………..36
2.1.1 Isolation of peripheral blood mononuclear cells (PBMC)……….36
2.1.2 Breast cancer cell lines cultures (T47D and SK-BR-3)………36
2.1.3 Co-cultures of SK-BR-3 and T47D and PBMC………..36
2.1.4 Human fetal osteoblastic cell line (hFOB) culture……….37
2.2 Characterization of cell cultures……….38
2.2.1 Total protein quantification………38
2.2.2 TRAP activity quantification………..38
2.2.3 Histochemical staining of TRAP………..38
2.2.4 ALP activity quantification……….39
6
2.2.6 Cellular proliferation/viability……….39
2.2.7 Immunofluorescence staining and visualization by CLSM………..39
2.2.8 Apoptosis quantification………40
2.2.9 Calcium phosphate resorbing ability………...40
2.2.10 Statistical analysis………40
3. Results……….41
3.1 Assessment of the effects of different PPI in cultures of SK-BR-3 and T47D………41
3.1.1 Characterization of SK-BR-3 cultures……….41
3.1.2 Characterization of T47D cultures ………..42
3.2 Assessment of the osteoclastogenic effects of different PPI in co-cultures of PBMC and breast cancer cell lines………..44
3.2.1 Characterization of PBMC + SK-BR-3 co-cultures………44
3.2.2 Characterization of PBMC + T47D co-cultures……….48
3.3 Assessment of the effects of different PPI in cultures of hFOB………52
3.3.1 Characterization of hFOB cultures (-DEX)……….52
3.3.2 Characterization of hFOB cultures (+DEX)………55
3.3.3 Characterization of the intracellular effects involved in cellular response…....57
4. Discussion………...62
5. Conclusion………..66
7
List of abbreviations
α-MEM - α-minimal essential medium
ALP – alkaline phosphatase
BMP – bone morphogenetic protein BP - bisphosphonate
CA2 – carbonic anhydrase II
cAMP – cyclic adenosine monophosphate CaSR – calcium-sensing receptor
CATK – cathepsin K
CLSM – confocal laser scanning microscopy CSF – colony-stimulating factor
CTR – calcitonin receptor
DMEM/F12 - Dulbecco’s Modified Eagle’s Medium/HAM’s Nutrient Mixture F12 ECM – extracellular matrix
EGF – epidermal growth factor ER – estrogen receptor
ERK - extracellular signal regulated kinase FGF - fibroblasts growth factor
GMCSF - granulocyte macrophage colony-stimulating factor HER2/erB2 - human epidermal growth factor receptor 2 hFOB – human fetal osteoblastic cell line
HR - hormonal receptor
H2RA - histamine 2 receptor antagonist IGF - insulin-like growth factor
IL - interleukin
JNK - C-jun N-terminal kinase
MAPK - mitogen activated protein kinases M-CSF – macrophage colony stimulating factor MITF - microphthalmia associated transcription factor MSC - mesenchymal stem cells
NF-kB - Nuclear factor kappa B OPG – osteoprotegerin
PBMC – pheripheral blood mononuclear cells PDGF - platelet-derived growth factor
PGE2 – prostaglandin E2 PKC – protein kinase C
8
pNPP - para-nitrophenilphosphate
PPI – proton pump inhibitor PR – progesterone receptor PTH - parathyroid hormone
PTHrP - parathyroid hormone related protein
RANK – receptor activator of nuclear factor kappa B RANKL - receptor activator of nuclear factor kB ligand TGF-β - transforming growth factor-betas
TNF – tumor necrosis factor
TRAF – tumor necrosis factor receptor associated factor TRAP - tartarate resistant acid phosphatase
VEGF - vascular endothelial growth factor VNR – vitronectin receptor
9
1. Introduction
1.1 Bone tissue
1.1.1 Structural and functional characteristics
Bone is a specialized connective tissue that is constantly remodeling and repairing itself. With a microstructure developed to enable and support a maximum strength associated with a minimal mass, bone tissue can be divided in two components: an organic component, composed essentially by bone cells and an extracellular matrix constituted by type I collagen (90% of total protein), proteoglycans and other proteins, which confers flexibility; and an inorganic, or mineralized, component, composed mainly by calcium and phosphate salts in the form of hydroxyapatite (Ca10(P04)6(OH)2),
representing about 60% of bone weight, and also small amounts of silicon, sodium and magnesium, which confers rigidity [1, 3, 18].
In addition to presenting a series of mechanical functions such as, providing form, protection and support, the bone also acts as a reservoir, storing and releasing ions in order to maintain a constant concentration in body fluids, and absorbing and accumulating toxins and metals preventing its adverse effects in other tissues [2, 20].
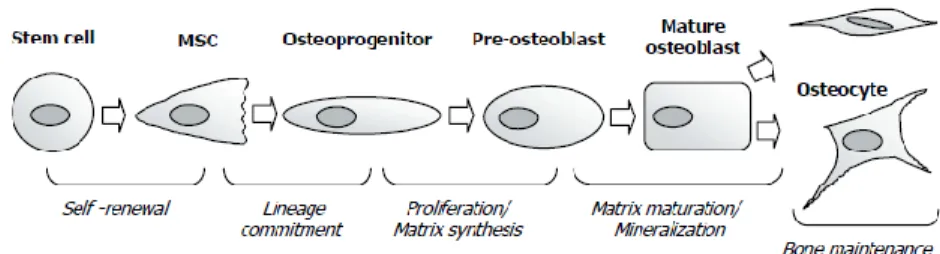
Its multicellular base comprises essentially three distinct types of cells: osteoblasts (cells responsible for the formation of new bone), osteoclasts (cells specialized in bone resorption), and osteocytes, which are osteoblast completely embedded in bone matrix that are thought to play a key role as mechanosensors (Fig. 1) [2, 20].
Figure 1. Bone remodeling process. Osteoclasts are activated and the resorption process
begins. Resorption phase takes approximately 10 days. Then, osteoblasts precursors are recruited, proliferate and differentiate. Osteoblasts produce bone mineralized matrix in a much longer process that can take several months.
10
(http://www.york.ac.uk/res/btr/Image%20Library/Bone%20remodelling.jpg)
Once formed, bone performs its remodeling process balancing these actions of bone resorption and synthesis in order to ensure the renewal of tissue with minimal temporary loss. Unbalances between these actions can result in some pathological conditions. Most adult skeletal diseases are caused by an excess of osteoclastic activity, such as osteoporosis and rheumatoid arthritis [1].
1.1.2 Bone cells
1.1.2.1 Osteoblasts
Osteoblasts arise from mesenchymal stem cells (MSC) and are the bone-forming cells. The process of differentiation of osteoblasts is less well understood than the osteoclastic one.
First, mesenchymal stem cells are committed to the osteoblast lineage. These cells, called osteoprogenitors, proliferate and differentiate into pre-osteoblasts. Pre-osteoblasts in turn start to produce extracellular matrix (ECM) and differentiate into mature osteoblasts. Mature osteoblasts ensure ECM synthesis and mineralization. However, only a fraction of the mature osteoblasts will be incorporated into newly formed bone matrix and remain as osteocytes. The remaining osteoblasts will die or become lining cells (Fig. 2) [17].
Figure 2. Stages of osteoblasts differentiation. Osteoblasts undergo a complex differentiation
process that essentially comprises: cell proliferation, matrix maturation and matrix mineralization. (Adapted from Aubin, J. E. et al.)
During the active proliferation phase, committed progenitor cells express genes that support proliferation and several genes encoding for extracellular matrix proteins, such as type I collagen and fibronectin. The accumulation of matrix proteins will contribute, in part, to the cessation of cell proliferation. During the post-proliferative phase, characterized by the high synthesis of alkaline phosphatase (ALP), the extracellular matrix progresses to
11
the mineralization phase. Here, osteoblasts synthesize several proteins that are associated with the mineralized matrix, including sialoprotein, osteopontin and osteocalcin [14, 15, 16].
It is believed that each of the differentiation steps during osteoblastogenesis is regulated by different kinds of morphogens, hormones, growth factors, cytokines and ECM proteins [12, 13].
1.1.2.2 Osteoclasts
Osteoclasts are mobile multinucleated cells that arise from precursor cells in the CD14+ monocyte-macrophage lineage [1, 23]. The osteoclast precursor cells reach the bone through the blood circulation and fuse into multinucleated cells to form mature osteoclasts in a process called osteoclastogenesis. Inactive osteoclasts adhere to bone surface through specialized adhesion structures called podosomes, forming a sealing zone sealed
by actin filaments together with αvβ3 integrin, the vitronectin receptor (VNR) [20-22].
Once formed, the resorption lacuna, or Howship’s lacuna, is acidified by the export of
hydrogen ions mediated by H+-ATPase located in osteoclasts plasma membrane. These
protons are derived from the carbonic acid formed by solubilization of CO2 by carbonic
anhydrase II (CA2). Thus, mineral phase is dissolved by H+ action, producing and
releasing into the resorption lacuna calcium, water and phosphate, according to the following equation [9, 20, 21, 75].
[Ca3(PO4)2]3Ca(OH)2 + 8 H+ → 10 Ca2+ + 6 HPO4+ 2 H2O
Hydroxyapatite (solid) (solution)
The process continues with the degradation of the organic phase, being carried out through the action of lytic enzymes such as tartarate resistant acid phosphatase (TRAP) and cathepsin K (CATK), both secreted by osteoclasts (Fig. 3) [9, 20, 21].
12
Figure 3. Functional elements of the mature osteoclast. Osteoclasts adhere to bone via
podosomes containing αvβ3 integrin. Protons generated by action of CA2 are transported into the resorption lacuna by the vacuolar-type H+-ATPase. CATK is an important enzyme for the removal of the organic components of bone in the resorption lacuna. (Adapted from Tolar, J. et al.)
1.1.3 Osteoclastogenesis
Osteoclastogenesis is a complex process regulated by different cells, such as osteoblasts and bone marrow stromal cells, responsible for the production of many modulators of the process, including the two key protein factors that shape osteoclast differentiation and activation, namely macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kB ligand (RANKL) [1, 2, 23].
M-CSF is a homodimeric glycoprotein produced by monocytes, granulocytes, endothelial cells, osteoblasts, fibroblasts, lymphocytes B and T, and some tumor cell lines. M-CSF promotes the formation of granulocytes and macrophages colonies and acts on osteoclastogenesis at an early stage in the fusion and further differentiation of monocytes [19, 24-26]. RANKL is a homotrimeric protein member of the tumor necrosis factor (TNF) family that stimulates differentiation and activation of osteoclasts by linkage to RANK receptor on the surface of precursor cells. RANKL is produced by osteoblasts but it can also be synthesized by fibroblasts, lymphocytes T and bone marrow cells.
The osteoclastogenic process may, however, be negatively regulated by osteoprotegerin (OPG) which, acting as a soluble receptor to RANKL, prevents RANKL-RANK binding, decreasing its bioavailability [1, 19, 27-30].
M-CSF and RANKL are, by themselves, enough to induce the expression of several osteoclastic features, such as the reorganization of the cytoskeleton with formation of actin rings that establish the contact with the resorption lacuna, production of TRAP, enzyme used as a marker of osteoclastic activity, synthesis of VNR, responsible for the adhesion process, and calcitonin receptor (CTR) of which the ligand is an inhibitory hormone of the osteoclastic activity, preserving bone mass (Fig.4) [1, 31].
13
Figure 4. Osteoclastogenesis. M-CSF and RANKL act in crucial phases of osteoclastogenesis,
being able to induce the expression of several osteoclastic characteristics. (Adapted from Feng, X.)
Upon resorption, bone-stored growth factors, such as bone morphogenetic proteins (BMPs), insulin-like growth factors (IGFs), and transforming growth factor-betas (TGF-β), are released and activated. These local factors, along with the systemic factors parathyroid hormone (PTH), estrogens, and prostaglandins, are constantly released in the bone marrow cavity by osteoclastic bone resorption, and recruit new osteoblasts to fill the gap created by osteoclasts [47].
1.1.4 Hormonal control
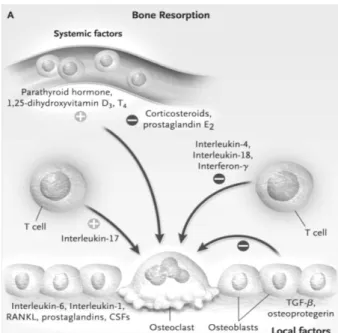
PTH (parathyroid hormone) is a peptide produced by the parathyroid glands that is important in the maintenance of normal calcium homeostasis due to its stimulatory effects on osteoclastic bone resorptive activity and renal reabsorption of calcium [32].
Parathyroid hormone related protein (PTHrP) binds PTH receptor and activates adenyl cyclase to produce cyclic adenosine monophosphate (cAMP). The effects of PTH and PTHrP on osteoclasts and precursors were studied in vivo. These studies demonstrated that neither PTH nor PTHrP had an effect on early osteoclast precursors, but increased the number of committed mononuclear osteoclast progenitors as well as mature osteoclasts [32].
However, the primary target cell for PTH appears to be the osteoblast. PTH binds to osteoblasts stimulating osteoblasts to increase their expression of RANKL and to inhibit their expression of OPG. Isolated osteoclasts seem to not resorb bone in response to PTH and only do so when osteoblasts or osteoblastic cell lines are added to the cultures. Furthermore, PTH induces RANKL expression by marrow stromal cells. On the other
14 hand, PTHrP stimulates bone formation by enhancing osteoblast differentiation and reducing osteoblast apoptosis (Fig. 5) [33].
Figure 5. Regulation of Bone Resorption. Both systemic factors and locally acting factors induce
the formation and activity of osteoclasts. Systemic hormones such as parathyroid hormone, 1,25-dihydroxyvitamin D 3, and thyroxine (T 4) stimulate the formation of osteoclasts by inducing the expression of RANKL on marrow stromal cells and osteoblasts. In addition, osteoblasts produce interleukin-6 (IL-6), interleukin-1 (IL-1), prostaglandins, and colony-stimulating factors (CSFs), which induce the formation of osteoclasts. (Adapted from Roodman, G. D.)
1.1.5 Signaling pathways
Osteoclastogenesis is an extremely complex process dependent on the action of several hormones, growth factors, and others, therefore involving multiple intracellular signaling pathways.
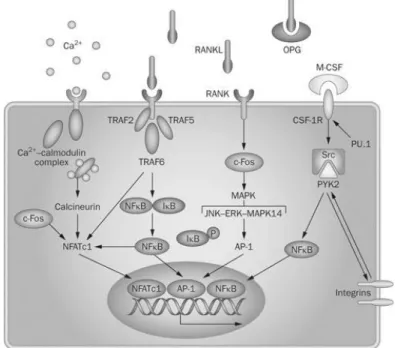
Several signaling pathways are activated by RANKL mediated protein kinase signaling. Some of them mediate directly osteoclastogenesis and others osteoclast activation. The intracellular domain of RANK contains three binding domains for TNF receptor-associated factors (TRAF). TRAF2, -5 and -6 have been shown to bind to RANK and studies revealed that TRAF6 is indeed an essential adaptor required for RANK-associated signaling (Fig. 6) [10, 34].
Among the major types of signal transduction pathways in eukaryotic cells are protein kinase cascades which culminate in the activation of a family of protein kinases known as
15 mitogen activated protein kinases, or MAP kinases. Although they vary in their substrate specificities and responses to extracellular stimuli, MAP kinases are highly conserved in their primary structure and mode of activation. All MAP kinases are serine/threonine kinases that are activated in response to their phosphorylation on invariant threonine and tyrosine residues [36]. The mitogen activated protein kinase and extracellular signal regulated kinase (MAPK/ERK) pathway triggers intracellular responses through binding of growth factors to surface receptors promoting osteoclastic proliferation and differentiation [35].
Recently, Miyazaki et al. reported that ERK is involved in survival of osteoclasts by showing that inhibition of ERK activity induced the apoptosis of osteoclast-like cells while ERK activation remarkably lengthened their survival by preventing spontaneous apoptosis. In short, results show that ERK is localized in the clear zones of osteoclasts. It seems that both the prevention of ruffled border formation and the acceleration of apoptosis in osteoclasts are associated with a decrease in phosphorylated ERK. Results suggest that ERK in osteoclasts may be involved in their survival, motility, and cell polarity [35].
Nuclear factor kappa B (NF-kB) is a family of five transcription factors that are expressed in most cell types. NF-κB dimers are normally inactive in the cytosol. Activation of this transcription factor is a consequence of phosphorylation of its inhibitory protein, IκB proteins (IκBa, IκBb, IκBe, IκBg, Bcl-3), by an upstream kinase complex termed IκB kinase (IKK), resulting in the release and translocation of NF-κB to the nuclear compartment. Once in the nucleus, they attach to κB binding sites in the promoter region of a large number of genes including IL-1, IL-6, TNFα-, and granulocyte macrophage colony-stimulating factor (GMCSF) and, after stimuli-induced IκB protein degradation in response to extracellular signals, induce their transcription [37]. Thus, NF-kB pathway regulates the expression of various genes essential to inflammatory and immune responses, by regulating the expression of a variety of pro inflammatory cytokines and other inflammatory mediators, cellular survival and proliferation. It is crucial for osteoclastogenesis in terms of differentiation of monocytic precursors into osteoclasts and further activation such as the C-jun N-terminal kinase (JNK) pathway, which acts mobilizing a series of osteoclastogenic transcription factors [5, 6, 8]. JNK is a subgroup of the MAPK family and is also known to be activated by cell stressors such UV radiation [36].
Other relevant signaling pathways are protein kinase C (PKC) and stress-activated protein kinase (p38). PKC pathway when activated induces cellular proliferation, apoptosis
16 and actin remodeling. Stimulation of the stress-activated protein kinase, p38, results in the downstream activation of the microphthalmia associated transcription factor (MITF), which controls the expression of the genes encoding TRAP and CATK, required for osteoclastic function [4, 7].
Figure 6. Signaling pathways involved on osteoclastogenic differentiation. Binding of RANKL
to its receptor RANK induces various intracellular signaling cascades, such as MAPK (JNK, ERK, MAPK 14) and NF-κB. Several transcription factors have been found crucial for osteoclast differentiation downstream of RANKL/RANK signaling. (Adapted from Edwards, J. R. and Mundy, G. R.)
17
1.2 Breast cancer
1.2.1 Epidemiology and pathology
Breast cancer is by far the most frequent cancer among women (small occurrence in men) with an estimated 1.38 million new cancer cases diagnosed in 2008 (23% of all cancers), and ranks second overall (10.9% of all cancers). It is now the most common cancer both in developed and developing regions and it is second only to lung cancer as a cause of cancer mortality.
It is estimated that in the year of 2008, the incidence rate of breast cancer in Portugal was 60 per 100,000 and the mortality rate of 13.5 per 100,000 [GLOBOCAN 2008 IARC].
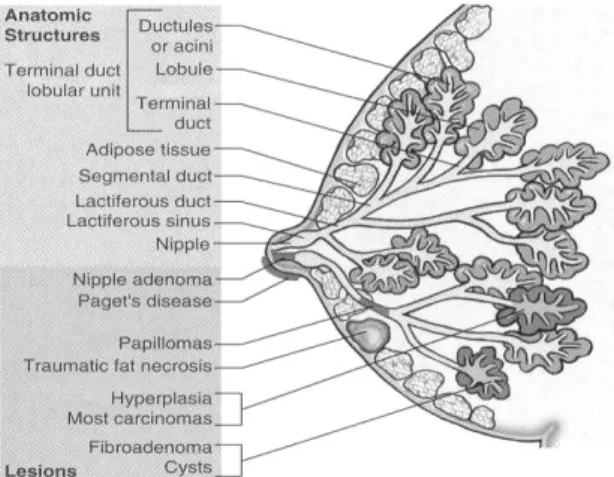
Ninety-five percent of breast cancers are carcinomas. Carcinomas can be divided into: in situ carcinoma and invasive or infiltrating carcinoma, and these may arise either in ducts or lobules. Most carcinomas show ductal differentiation (90%). Other malignant lesions are associated with mesenchymal neoplasms, such as cystosarcoma phyllodes, angiosarcoma, among others [38, 39].
Figure 7. The female breast. The anatomic structures and lesions of the female breast.
(http://medicinembbs.blogspot.pt/2010/12/breast-anatomy.html)
Benign lesions include hyperplasia, cystic changes, adenomas, papillomas and fibroadenomas, the most common benign tumor of the breast. Pathologies of the adipose tissue can also occur, called traumatic fat necrosis (Fig. 7) [38, 39].
18
1.2.2 Risk factors
There are several risk factors considered for the development of breast cancer. Obviously, the age and the occurrence of a prior breast cancer or benign lesion are two of them, but there is also a number of lifestyle, environmental and hormonal factors that are probably involved (unbalanced diet, lack of exercise, early menarche, hormone replacement therapy, nulliparity, …) [38, 39,40].
Women with a family history of breast cancer should obtain as much information as possible about those relatives, including age at onset and type of cancer. The risk of breast cancer development related to family history increases with the number of affected relatives, specific lineage and age at diagnosis [38, 39, 40].
About 5-10% of breast cancer is thought to be linked to changes in certain genes. The most common are those of the BRCA1 and BRCA2 genes. Women with mutations in these genes have a higher risk of developing breast and ovarian cancer during their lives [38, 39, 40].
Despite advances, 20-30% of patients with early breast cancers experience relapse with distant metastatic disease. Risk of recurrence is influenced by stage at initial presentation and the underlying biology of the tumor. Tumor size, nodal involvement, grade, lymphovascular invasion, and estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2/erB2) status are all independent risk factors for relapse [38, 39, 59].
1.2.3 Molecular types of breast cancer
The heterogeneity of breast cancers makes them both a fascinating and challenging solid tumor to diagnose and treat.
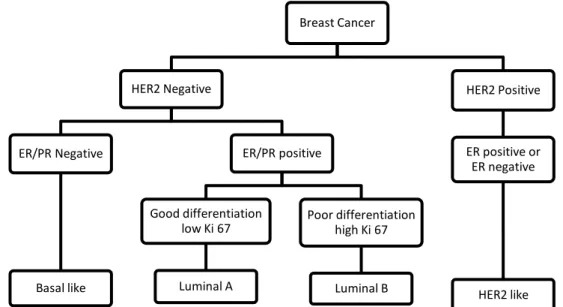
Until very recently, personalized cancer medicine in breast cancer relied on only two predictive markers, ER and HER2/erB2. Breast cancer is now recognized not as a single disease with variable morphology, but as at least four molecularly distinct neoplastic disorders: luminal-A breast cancer (low proliferative ER positive), luminal-B breast cancer (high proliferative ER positive), HER2-positive breast cancer (amplification and high expression of the ERBB2 gene and several other genes of the ERBB2 amplicon) and triple-negative or basal-like breast cancer (ER/progesterone receptor negative and HER-2-negative) (Fig. 8) [41, 42].
19 Although the immediate additional clinical value of this molecular classification is limited by its close correlation to traditional methods of testing for ER and HER2, the identification of genetic aberrations that underlie molecularly distinct subtypes of breast cancer has revealed new therapeutic targets and has reshaped breast cancer clinical trial design [41, 42].
Figure 8. Molecular types of breast cancer. To distinguish the molecular type of cancer, first
HER2 membrane receptor must be assessed and only after test for nuclear hormone receptor. Ki 67 denotes nuclear antigen ki-67, a marker of cell proliferation. (Adapted from Sotiriou, C. and Pusztai, L.)
Estrogen receptor/progesterone receptor positive (ER/PR+) tumors have a better prognosis and a better response to treatment than receptor-negative tumors. Two thirds of women with diagnosed breast cancer have ER/PR+ tumors. These tumors are highly responsive to anti-estrogen therapeutic strategies. However, despite the widespread use of hormonal adjuvant therapy, a quarter of women with ER+ disease will relapse. Since the earliest studies of the intrinsic molecular subtypes in breast cancer, the defining feature of B breast cancer has been its poor outcome compared with the luminal-A subtype. Several studies have suggested that luminal-B breast cancer is relatively insensitive to endocrine therapy compared with luminal-A [43, 49].
HER2 is over expressed in 15-20% of breast cancer, and it is associated with a highly aggressive behavior. HER family is constituted by four members, HER1, HER2, HER3 and HER4. An intracellular tyrosine kinase domain exists for HER1, HER2 and HER4 and phosphorylation of these domains by homodimerization or heterodimerization induces both cell proliferation and survival signaling [61]. HER2 is the preferred dimerization
Breast Cancer HER2 Negative ER/PR Negative Basal like ER/PR positive Good differentiation low Ki 67 Luminal A Poor differentiation high Ki 67 Luminal B HER2 Positive ER positive or ER negative HER2 like
20 partner for the other HER family members. One of the other downstream effects of these reactions is the production of vascular endothelial growth factor (VEGF) supporting angiogenesis [58].
The availability of the anti-HER2 monoclonal antibody therapy has significantly improved the prognosis of patients with HER2 positive breast cancer both in early and advanced disease stages.
Triple-negative breast cancers in particular are difficult to define. This tumor subgroup does not respond to hormonal therapies or Her2-targeted therapies due to the lack of expression of these targets. These tumors are associated with a poor prognosis.
The subtypes most in need of therapeutic advances are triple negative breast cancer and luminal-B breast cancer, where therapeutic resistance is common and where advances in molecular profiling have identified promising new therapeutic targets [43, 49].
1.2.4 Therapies
Breast cancer surgery has changed dramatically over the past 20 years. With the emergence of breast conserving therapy, many women now have the option of preserving a cosmetically acceptable breast without sacrificing survival. Conserving therapy refers to surgical removal of the tumor without removing excessive amounts of normal breast tissue. The aims of this therapy are to provide a cancer operation equivalent to mastectomy and a cosmetically acceptable breast, with a low rate of recurrence in the treated breast. The main obstacle to widespread acceptance and utilization of conserving therapy is the risk of in-breast recurrence. Depending of several factors, such as tumor size, multifocality and possibility of radiotherapy used, mastectomy may be the method of choice. Regardless of the method used, axillary lymph node dissection is always mandatory for evaluation [39, 40].
Radiation adjuvant therapy is known to substantially reduce the risk of recurrence and decrease breast cancer mortality, both when given after mastectomy and after breast-conserving surgery. Radiation therapy is generally given over a 5- or 6-week time span, with care taken to try to avoid damage to the heart or lungs. The only usual changes with breast radiation are skin erythema and possibly some transient lymphedema [39].
Adjuvant systemic therapy refers to the administration of chemotherapy, hormone therapy and/or monoclonal antibodies used following primary surgery for breast cancer. The purpose is to eliminate or delay the subsequent appearance of clinically occult
21 micrometastases. The original regimen of chemotherapy used was cyclophosphamide, methotrexate, 5-fluorouracil (CMF). Thereafter, many other regimes have been used. A major determinant of the choice of adjuvant therapy is whether an individual breast cancer expresses hormonal receptors (HR). Hormone therapy benefits patients with hormone receptor-positive breast cancer, but not those with hormone receptor negative disease [39].
Targeting the ER is the oldest molecular targeted therapy approach, and the widespread use of the selective ER modulator tamoxifen in breast cancer is responsible for major improvements in cure rates, quality of life and disease prevention for the past 25 years [39, 40].
Tamoxifen, a selective estrogen receptor modulator (SERM), inhibits the growth of breast cancer cells by competitive antagonism of estrogen at the ER, inhibiting both translocation and nuclear binding of the ER. Tamoxifen and its metabolites bind to the ER, and subsequently occurs translocation of this complex to the nucleus and binding to the response element. This binding prevents transcriptional activation of estrogen-responsive genes (Fig. 8). However, its actions are complex and it has also a partial estrogen agonist activity. Additionally, there are marked differences between the anti proliferative properties of tamoxifen and its metabolites [40, 57].
Their long-term efficacy, however, is limited by relapse of disease and development of resistance. Laboratory and clinical data have demonstrated that ER-positive breast cancers that overexpress HER2 are associated with resistance to tamoxifen and to hormonal therapy in general. Despite continuous expression of ER at relapse in either locally recurrent or secondary metastatic tumors, up to half of patients with HR-positive primary breast cancer who develop metastatic disease do not respond to first-line endocrine treatment (de novo resistance), and the remainder will eventually relapse despite an initial response (acquired resistance) [40].
Several factors may contribute to tamoxifen resistance in breast cancer, including variable expression of estrogen receptor alpha and beta isoforms, interference with binding of co-activators and co-repressors, alternatively spliced ER mRNA variants and ER modulators expression, such as epidermal growth factor (EGF) and its receptor (EGFR1, also called HER1), as well as the type 2 EGFR, also called HER2. Emerging
22 certain drug metabolizing CYP2D6 genotypes that are associated with a reduced activation of tamoxifen to its active metabolite endoxifen [40, 44, 60].
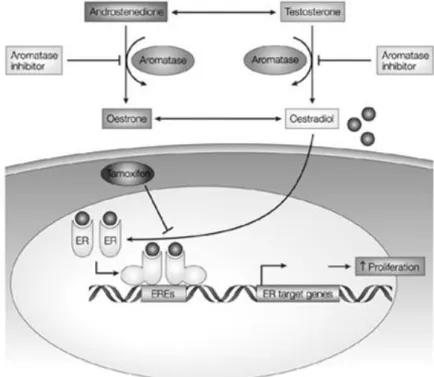
Other hormonal therapeutic agents include aromatase inhibitors that interfere with the enzyme aromatase, which plays a critical role in the production of estrogen in postmenopausal women. Aromatase is an enzyme that naturally converts estrogen from androgen. In premenopausal women, most of the estrogen is produced in the ovaries, but in postmenopausal women, most estrogen is synthesized in peripheral tissue from conversion of androgens. In contrast to tamoxifen, these compounds lack partial agonist activity (Fig. 9). Examples of this class include anastrozole, letrozole and exemestane [40, 45].
Figure 9. Comparison of the mechanisms of action of estradiol, tamoxifen and aromatase inhibitors.
Estradiol binds to ER, leading to dimerization, conformational change and binding to estrogen response elements (ERE) upstream of estrogen responsive genes including those responsible for proliferation. Tamoxifen competes with estradiol for ER binding whereas Aromatase inhibitors reduce the synthesis of estrogen from androgenic precursors. (Adapted from Johnston, S. R. D. and Dowsett, M.)
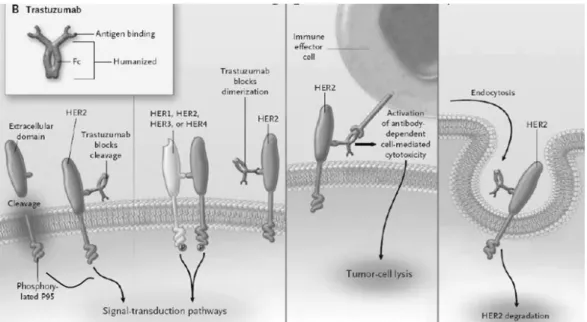
Approximately 25-30% of breast cancer tumours display amplification of the HER2 gene or overexpression of its protein product, and this is associated with an adverse prognosis. Trastuzumab is a humanized monoclonal antibody directed against the external domain of the receptor with clinical activity as a single agent in patients whose cancers overexpress HER2 (Fig. 10). Overexpression of the receptor is associated with increased disease recurrence and worse prognosis. Targeting both HER2 with
23 trastuzumab and VEGF with bevacizumab in combination with chemotherapy has become a further milestone of molecular targeted therapy. However, intrinsic and acquired resistance to endocrine and/or cytostatic treatments is still a common feature that limits the benefits of these novel therapeutic strategies. In tumors that are HER2 and ER positive, HER2 signaling is dominant. This resistance can be partially overcome by combining anti-estrogen and anti-HER2 therapies [40, 57, 61].
Figure 10. Potencial mechanisms of action of transtuzumab. Cleavage of the extracellular
domain of HER2 leaves a membrane-bound phosphorylated p95, which can activate signal-transduction pathways. Binding of trastuzumab to a juxtamembrane domain of HER2 reduces the release of the extracellular domain, thereby reducing p95. Trastuzumab may reduce HER2 signaling by physically inhibit homodimerization or heterodimerization. Trastuzumab may also recruit immune effector cells and other components of antibody-dependent cell-mediated cytotoxicity, leading to tumor-cell death. Other mechanisms such as receptor down-regulation through endocytosis have been suggested. (Adapted from Hudis, C. A.)
24
1.3 Bone metastasis in breast cancer
1.3.1 Bone metastasis
Most patients with cancer die not because of the primary tumour, but rather because it has spread to other sites. It is difficult to determine, precisely, how frequently different tumours metastasize to bone. Patients with advanced breast and prostate cancers almost always develop bone metastases. Moreover, the chances are high that, in patients who are originally diagnosed with breast or prostate cancers, the bulk of the tumour burden at the time of death will be in bone [46]. Metastasis to bone occurs in the late stages of tumor progression, in a multistep process [50, 51].
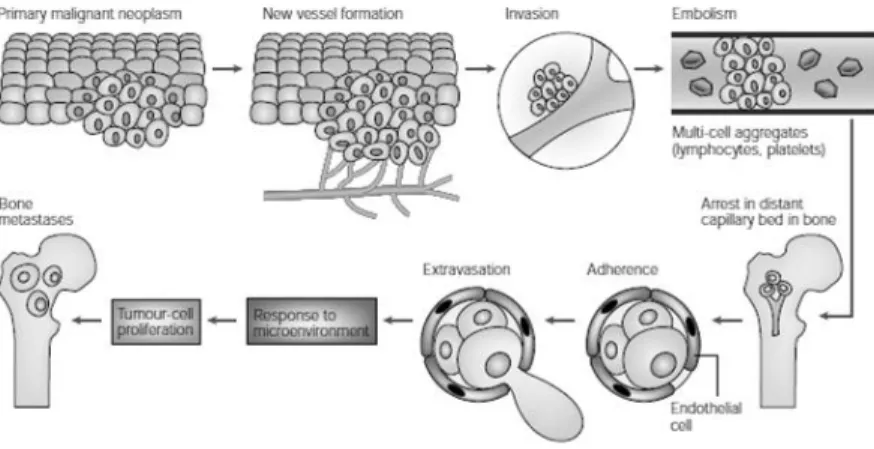
Because tumor metastasis is a complex process involving individual discrete steps, interruption of one or more of these steps can inhibit the metastatic process. Each of these steps represents cellular interactions caused by specific determinants of both the tumor and the tissue. The steps involved in the shedding of the tumor cells from the primary site involve detachment of tumor cells from adjacent cells, followed by invasion of adjacent tissue in the primary organs. The cells then enter the tumor capillaries (stimulated by specific angiogenesis factors produced by the tumor) and via these capillaries reach the general circulation. The steps involved in entering the tumor blood vessels at the primary site are similar to those involved in exiting the vasculature in the bone marrow cavity. These steps include the attachment of the tumor cells to basement membrane, the secretion of proteolytic enzymes that enable tumor cells to disrupt that membrane, and the migration of the tumor cells through it. Tumor cells that metastasize to the skeleton adhere to the endosteal surface and colonize bone (Fig. 11) [50, 51].
Figure 11. The steps involved in tumour-cell metastasis from a primary site to the skeleton.
25
carry the cancer cells to capillary beds in bone. Aggregates of tumour cells and other blood cells eventually form embolisms that arrest in distant capillaries in bone. These cancer cells can then adhere to the vascular endothelial cells to escape the blood vessels. As they enter the bone, they are exposed to factors of the microenvironment that support growth of metastases. (Adapted from Mundy, G. R.)
Although the distribution of metastases in distant organs can be predicted by the anatomic distribution of blood flow from the primary site in 30% of cases, specific properties of the tumor cell and features at the metastatic site determine where the metastasis occurs in the majority of cases [51].
The metastasis of tumor cells to specific sites in the skeleton is not a simple and random event determined solely by blood flow. Rather, it is a complex process that is dependent on specific properties of the tumor cells and on factors in the bone microenvironment that favor metastasis. Tumor cells most frequently affect the heavily vascularized areas of the skeleton, particularly the red bone marrow of the axial skeleton and the proximal ends of the long bones, the ribs, and the vertebral column [51].
Physical factors within the bone microenvironment, including low oxygen levels, acidic pH, and high extracellular calcium concentrations, may also enhance tumor growth.
Hypoxia is a major contributor to tumor metastasis, regulating secreted products that drive tumor-cell proliferation and spread. Hypoxia also contributes to resistance to radiation and chemotherapy in primary tumors. Solid tumors are particularly prone to hypoxia because they proliferate rapidly, outgrowing the malformed tumor vasculature, which is unable to meet the increasing metabolic demands of the expanding tumor. Hypoxia regulates normal marrow hematopoiesis and chondrocyte differentiation. Cancer cells capable of surviving at low oxygen levels can thrive in the hypoxic bone microenvironment and participate in the vicious cycle of bone metastasis [50].
Acidosis of the bone microenvironment also potentiates the vicious cycle of bone metastasis. Extracellular pH is tightly regulated within bone and has significant effects on osteoblast and osteoclast function with osteoclasts being maximally stimulated at pH levels of <6.9. Osteoblast mineralization and bone formation is significantly affected by acid. Tumor metastasis leads to localized regions of acidosis within the skeleton. Increased glycolysis and lactic acid production by proliferating cancer cells (due to the hypoxic conditions) and decreased buffering capacity of the interstitial fluid contribute to
26 the acidic microenvironment within primary tumors. The acid-mediated tumor invasion hypothesis states that altered glucose metabolism in cancer cells stimulates cancer cell proliferation resulting in a more invasive tumor phenotype. Acidosis alters the cellular dynamics at the interface between the tumor and normal tissue, promoting apoptosis in adjacent normal cells and facilitating extracellular matrix degradation through the release of proteolytic enzymes. Unlike normal cells, cancer cells have compensatory mechanisms to allow proliferation and metastasis even at low extracellular pH, being resistant to acid-induced apoptosis [50].
Calcium released from the mineralized bone matrix contributes to the vicious cycle of metastasis by several mechanisms. Calcium is the primary inorganic component of the bone matrix and, in the bone microenvironment, levels are maintained within a narrow physiologic range. Active osteoclastic bone resorption causes extracellular calcium levels to rise up. The effects of Ca2+ are mediated through the extracellular calcium-sensing receptor (CaSR), a G protein–coupled receptor, which, in the presence of high Ca2+,
inhibits cyclic AMP and activates phospholipase C. The CaSR is expressed in normal tissues and is overexpressed in several types of cancer, including breast and prostate cancer and it regulates the secretion of PTHrP [50].
Finally, estimates of the frequency of metastasis depend also on the sensitivity of the diagnostic technique utilized to detect them. The most commonly used techniques are bone scans, X-rays, and histologic evaluation of autopsy specimens. Both radiologic and histologic assessments are limited by the extent of the sample evaluated. Bone scans survey the whole body. However, because isotope accumulation depends on osteoblast activity, lesions with very little osteoblastic component cannot be detected [51].
1.3.2 Types of bone metastases
There are different patterns of bone effects in patients with cancer, ranging from purely or mostly destructive, designated by osteolytic metastases, to mostly bone-forming or osteoblastic metastases [46]. However, patients can have both osteolytic and osteoblastic metastasis or mixed lesions containing both elements. Both osteoblastic and osteolytic bone metastases lead to numerous skeletal complications, including bone pain, hypercalcemia, pathologic fractures, and spinal cord and nerve compression syndromes. Such complications increase morbidity and diminish the quality of life in these patients [47, 63].
27 Breast cancer is one of the most frequent tumours that originate bone metastasis, being the most common the osteolytic ones. However, 15 to 20 percent of the patients have predominantly osteoblastic lesions. Moreover, secondary formation of bone occurs in response to bone destruction. This reactive process makes it possible to detect the osteolytic lesions by scanning techniques, identifying the sites of active bone formation [47]. This increase in bone formation in patients with osteolytic lesions can be also reflected by the increase of serum ALP levels, a marker of osteoblasts activity. In contrast, the lesions in prostate cancer are predominantly osteoblastic.
In breast cancer, osteolytic bone metastasis results from a hiperactivation of osteoclasts induced directly or indirectly by several factors produced by cancer cells. Osteoblastic metastases, conversely, are believed to be caused by cancer-cell production of factors that stimulate osteoblast proliferation, differentiation and bone formation [46, 63].
1.3.2.1 Osteoblastic metastases
The mechanisms of osteoblastic metastasis and the factors involved are unknown. Endothelin-1 has been implicated in osteoblastic metastasis from breast cancer. This molecule is one of the most well studied mediators that stimulates bone formation and osteoblast proliferation in bone organ cultures. Endothelin-1 levels are increased in the circulation of patients with osteoblastic metastases and prostate cancer, being also expressed by breast cancer cell lines that cause osteoblastic metastases. Osteoblast proliferation and bone metastasis have both been shown to be inhibited in vivo by endothelin-1A-receptor antagonists. In addition to endothelin-1, platelet-derived growth factor (PDGF), a polypeptide produced by osteoblasts in the bone microenvironment, urokinase, and prostate- specific antigen may also be involved [47, 93].
1.3.2.2 Osteolytic metastases
Breast cancer cells produce factors that direct or indirectly induce osteoclasts formation. In turn, bone resorption carried out by osteoclasts release growth factors from bone matrix which stimulate tumor growth and bone destruction. This reciprocal interaction between breast-cancer cells and the bone microenvironment results in a vicious cycle that increases both bone destruction and the tumor burden [47, 64].
Bone is an abundant source of inactive growth factors which are activated during the resorption process, including TGF-β, IGFs I and II, fibroblasts growth factors (FGFs),
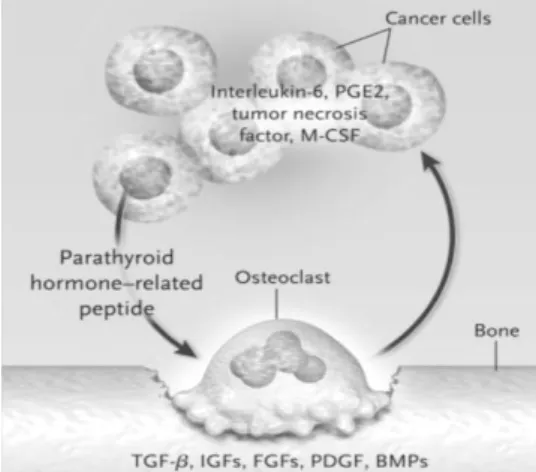
28 PDGFs, BMPs and calcium. These factors are then released providing a fertile ground in which tumour cells can grow. On the other hand, breast cancer cells and many of other solid tumours are thought to stimulate the formation of osteoclasts by the production of PTHrP. This molecule, in association with other factors produced by tumor cells, such as IL-6, prostaglandin E2 (PGE2), TNF-α (tumour necrosis factor α) and M-CSF, increase the expression of RANKL mainly by osteoblasts, which directly acts on osteoclast precursors promoting osteoclastogenesis and bone resorption (Fig. 12) [46, 47, 63, 64].
Figure 12. The Vicious Cycle of Osteolytic Metastasis. Breast-cancer cells, secrete PTHrP as
the primary stimulator of osteoclastogenesis. In addition, tumor cells produce other factors that increase the formation of osteoclasts (IL-6, PGE2, TNF-α, and M-CSF). All these factors increase the expression of RANKL. The process of bone resorption releases factors such as TGF-b,IGFs, FGFs, PDGF, and BMPs, which increase the production of PTHrP by tumor cells as well as growth factors that increase tumor growth. This symbiotic relationship between bone destruction and tumor growth further increases bone destruction and tumor growth. (Adapted from Roodman, G. D.)
Two pieces of evidence support the view that osteoclastic bone resorption is the predominant mechanism of bone destruction in bone metastasis: histological analysis and scanning electron microscopy studies invariably detect tumor cells adjacent to osteoclasts resorbing bone, and the use of bisphosphonates, potent inhibitors of osteoclastic bone resorption, in women with breast cancer metastases to bone results in reduced skeletal morbidity [51].
1.3.3 Molecular types of breast cancer and metastatic behavior
It is known that estrogen-responsive breast cancer cells have a more aggressive bone metastatic behavior than their estrogen receptor-negative counterparts which metastasize more aggressively to visceral organs (liver, lung, pleura…). There are differences in the
29 anatomic sites of relapse according to molecular subtypes. The increased incidence of brain metastases in HER2-positive and basal like breast cancer is well recognized. Luminal breast cancers appear to have a predilection for metastasis to bone and pleura. In a small study of patients with metastatic breast cancer, no differences in sites of metastasis were observed between luminal-B and luminal-A breast cancers.
1.3.4 Treatment
Bisphosphonates (BPs) are a class of pyrophosphate analogues that bind with high affinity to mineralized bone surfaces and inhibit osteoclastic bone resorption. Common examples of these drugs are pamidronate (Aredia), alendronate (Fosamax), zoledronate (Zometa), risedronate (Actonel) and clodronate (Bonefos) [46]. Bisphosphonates are used extensively to treat patients with diseases associated with bone loss, such as osteoporosis, Paget’s disease and osteolytic metastasis [54, 55].
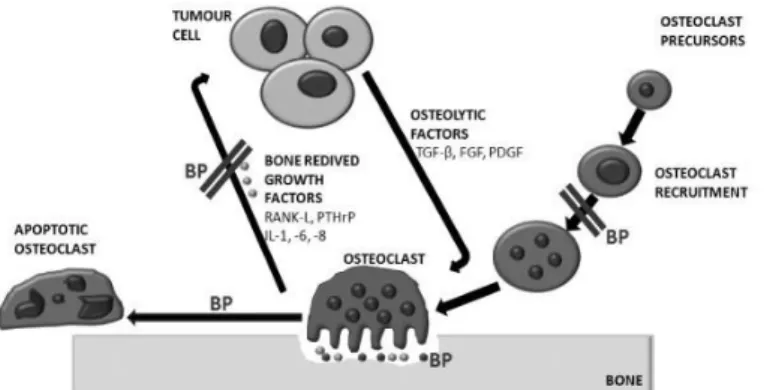
Bisphosphonates inhibit both bone lesions and tumor cell burden in bone in experimental models of breast carcinomas. They act by decreasing the recruitment, proliferation and differentiation of pre-osteoclasts, their adhesion to the mineralized matrix and, most importantly, the resorptive activity of mature osteoclasts. They also reduce the release of bone derived tumor growth factors and shorten the osteoclast lifespan by induction of programmed cell death (Fig. 13) [54, 55].
Figure 13. Inhibitory action of bisphosphonates in the vicious cycle of osteolytic metastasis.
(Adapted from Rogers, T. L.)
In addition, there is extensive evidence that these agents exert direct antitumor effects in vitro, inhibiting tumor cell adhesion, invasion, proliferation and angiogenesis, and inducing apoptosis.
30 Because BPs home to bone very quickly due to their high affinity for hydroxyapatite, they are present in the skeleton for prolonged periods.
However, BPs therapy is only for palliative treatment and does not provide a life prolonging benefit in the majority of the patients. New therapeutics targeting osteoclast activities are therefore required. They could be used alone or in combination with BPs to treat more efficiently breast cancer patients with bone (osteolytic) metastases [56].
Newer bone resorption inhibitors are currently in clinical development. These new therapeutics include OPG, anti-PTHrP antibodies, vitamin D analogues (to decrease PTHrP production) and RANK-Fc, a hybrid chimeric molecule that acts in an identical way to OPG [46, 56].
The use of CATK inhibitors has been investigated as well. CATK inhibitors are successfully used in the treatment of bone loss associated with osteoporosis. This protease is also expressed by human breast cancer cells in skeletal metastases, suggesting that CATK inhibitors could target not only osteoclasts but also tumour cells. Preclinical studies show some evidence that these inhibitors, alone or in combination with bisphosphonates, reduce breast cancer–induced osteolysis and skeletal tumor burden in animals [46, 56].
31
1.4 Proton pump inhibitors
Proton pump inhibitors (PPI) and histamine 2 receptor antagonists (H2RAs) are the most popular acid-suppressive drugs (ASDs) available, and millions of individuals currently take these medications on a continuous or long-term basis. These potent drugs are used to treat various disorders, and indications for long-term maintenance therapy with this drug class continue to expand [74]. First, the H2RAs were introduced, offering patients the first single-agent therapy that effectively reduced gastric acid secretion. Then, PPI became widely available in the early 1990s, and they generally appeared to be superior to the H2RAs in their acid-suppressing activity, symptom control and healing [69].
1.4.1 Mechanism of action
PPIs are a class of drugs widely used in many pathological conditions, such as gastroesophageal reflux, dyspepsia and peptic ulcers. Although there are several different molecules belonging to this class, it is known that they share many functional and structural properties as well as some pharmacokinetic characteristics. The mechanism of action of PPIs relies on their chemical activation in a significant acidic environment and consequent inhibition of H+/K+ATPases. Nevertheless, like any other medication, they also display several side effects [65, 67].
.
Omeprazole was the first PPI used in clinical practice in the late 1980s. Other analogues such as lansoprazole, rabeprazole and esomeprazole have been developed since that (Fig. 14) [67].
Figure 14. Structures of PPIs. Timoprazole, omeprazole, lansoprazole and rabeprazole are
benzimidazole-based PPIs. Esomeprazole is an optical isomer of omeprazole (S-omeprazole). (Adapted from Sachs, G. et al.)
32
PPIs are lipophilic and are inactive in the neutral environment of the bloodstream. After
absorption, PPIs cross the plasma membrane, enter and accumulate in the secretory canaliculi of parietal cells, where they are protonated by acid and then converted into their active form, sulfenamide. The activated sulfenamide reacts covalently with the cysteine residues on the extracellular surface of the proton pump, inhibiting gastric acid secretion (Fig. 15) [67].
Figure 15. PPIs activation. PPIs such as omeprazole concentrate in the acidic secretory
canaliculi of the parietal cell, where they are activated and generate a sulphenamide. The sulphenamide interacts covalently with the sulphydryl goups of cysteine residues in the extracellular domain of the ATPase thereby inhibiting its activity. (Adapted from Olbe, L. et al)
PPIs are very specific to the inhibition of gastric proton pumps because they are activated only in the acidic environment of the stomach, whereas they are not activated by the similar enzyme found in the colon and the kidney [67]. Differences in cysteine binding properties among the PPIs may, at least partly, underlie the differences among them in the duration of the inhibition of gastric acid secretion. Currently available benzimidazole-based PPIs have similar half-lives of 1–2 h. As these agents are usually prescribed once daily, this means that there is effectively no circulating PPI present at the end of the dosing interval. A PPI with an extended half-life might have a prolonged anti-secretory effect with consequent therapeutic advantages [65].
1.4.2 Adverse effects
As mentioned above, there are common adverse events that occur in 1-4% of patients. The most common are headache, abdominal pain, gastrointestinal alterations (nausea and diarrhea, for example), fatigue, community-acquired pneumonia, acute interstitial nephritis and allergy-related problems. However, the knowledge of the adverse events of newly marketed drugs is limited, and it is only after widespread clinical use that the side-effect profile of a drug is performed more assertively. The presence of side side-effects on
33 proton pump inhibitors is greatest for omeprazole, since this drug has been on the market for a longer period [68, 70, 73].
PPIs reduce gastric acidity, which is necessary to activate pepsinogen into pepsin, which, by its turn is important for the beginning of the digestion of dietary proteins and to release vitamin B12 from food. PPIs used for a short-term may minimally reduce the absorption of protein-bound vitamin B12. Elderly patients who already have gastric atrophy may present lower levels of vitamin B12 in serum with long term PPIs used. However, studies have shown that PPIs used for a long-term in younger patients do not reduce serum vitamin B12 concentrations. In patients long-term treated with high dose of PPIs duodenal absorption of organic and non-organic iron may be reduced. Nevertheless, this effect is small, and PPIs are not associated with an increased risk of latent iron deficiency or iron deficiency [68, 70, 73].
For the proton pump inhibitors omeprazole, lansoprazole, pantoprazole and esomeprazole, certain hepatic isoenzyme pathways, notably cytochrome P450 (CYP2C19), have an important role in the drug metabolism. In particular, among patients who are genotypically rapid, or extensive, metabolizers, proton pump inhibitors with significant CYP2C19 metabolism tend to yield lower plasma levels and, thus, have low efficacy. The contribution of the CYP2C19 pathway varies in the metabolism of the different proton pump inhibitors [69].
1.4.3 PPI’s versus H2RAs
Despite the efficiency of the action of the H2RAs, there were patients whose acid-related disorders failed to respond to or required very high doses of these drugs. Furthermore, the phenomenon of H2RA tolerance was recognized, with implications on possible failures of chronic/maintenance H2RA therapy. PPIs were subsequently developed, and, in most cases, have been found to be superior to H2RAs in their acid suppressing ability. They also relieve oesophagitis symptoms and heal erosions more effectively than H2RAs. In contrast to H2RAs or anticholinergic agents, which only partially inhibit histamine-, gastrin- or acetylcholine- stimulated acid secretion, PPIs inhibit acid secretion in response to all stimulatory agents (Fig. 16). Consequently, PPIs are exceptionally effective for the control of acid production. Moreover, PPIs elevate pH above 3-4 4 for longer time periods than do H2RAs[69].
34
Figure 16. Gastric acid secretion by parietal cell. Gastric acid secretion is a process regulated
by three types of receptors on the parietal cell (histamine, gastrin and acetylcholine). Activation of these receptors leads to activation of the gastric acid proton pump, H+K+-ATPase, which regulates acid transport and is the final common pathway to acid secretion for these three receptors. In contrast to H2RAs which only partially block these receptors, PPIs directly block the action of H+K+-ATPase, suppressing gastric acid secretion, regardless of the stimulus. (Adapted from Olbe, L. et al.)
1.4.4 Secondary effects of PPI in bone metabolism
Long-term PPI therapy and its attendant potent acid suppressive properties have generated great concern regarding to the potential effects on calcium absorption and bone metabolism. Several epidemiological studies have suggested an association between PPI use and the risk of fractures of the hip, or other sites, including spine, wrists and forearms. Some potential mechanisms by which PPI therapy may lead to fractures have been identified. First, the small intestine’s ability to absorb ingested calcium depends on pH. Without an acidic environment in the gastrointestinal tract, calcium may be retained in the food matrix preventing absorption. Second, impaired calcium absorption may lead to compensatory secondary hyperparathyroidism. Secondary hyperparathyroidism refers to the increase in circulating levels of parathyroid hormone, which, in an attempt to rectify the deficit in calcium absorption, increase the rate of osteoclastic activity [66, 72]. Finally, PPI may directly interfere with osteoclasts activity [74]. Studies have shown that omeprazole inhibits bone resorption in vitro and in vivo in humans by inhibiting osteoclastic vacuolar
H+ ATPase activity. A reduced bone resorption should lead to increases in bone density,
however, bone resorption is necessary for the normal bone development, replacing old bone and repairing microfractures. Thus, a decrease in osteoclast activity can affect bone structure and, thus, predispose patients to fractures [66, 70, 72, 74].
35 Taken together, these studies are limited by a low magnitude of association and inability to assess potential confounding factors [79]. Despite all these reservations, it seems likely that PPI may affect significantly bone metabolism, particularly in some vulnerable individuals, such as postmenopausal women, where PPI use contributes to an increased risk of fractures at various sites, in a manner related to the dose and duration of exposure to the drug [66, 70, 74, 80].
In this context, since in the resorption process osteoclasts solubilize the bone mineral phase through the secretion of acid to the resorption lacuna by proton pumps of the vacuolar type, V-ATPases, PPIs appear as potentially pharmacological modulators of osteoclastic activity, particularly in pathological situations characterized by hiperactivation of osteoclasts such as osteolytic metastases associated with breast cancer.
36
2. Materials and methods
2.1 Cell Cultures
2.1.1 Isolation of Peripheral blood mononuclear cells (PBMC)
The cells were isolated from blood of male healthy donors with ages between 25 and 35 years old. The blood was diluted with PBS (phosphatase buffer solution) supplemented
with 2 mM EDTA (1:2) and applied on top of 4ml of Ficoll-PaqueTM PREMIUM (GE
Healthcare Bioscience). After centrifugation at 400 g for 30 minutes at room temperature, PBMC were collected and washed twice with PBS supplemented with 2 mM EDTA with centrifugations at 300 g for 10 minutes at 4ºC in each. PBMC were seeded at 1.5x106cells/cm2.
2.1.2 Breast cancer cell lines culture (T47D and SK-BR-3)
Human breast cancer cell lines, estrogen-receptor positive T47D and estrogen-negative SK-BR-3, were maintained in α-Minimal Essential Medium (α-MEM) supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL penicillin, 2.5 µg/mL streptomycin, 2.5 µg/mL amphotericin B and 50 µg/mL ascorbic acid. Cultures were performed at 37ºC in a 5%
CO2 humidified atmosphere. At about 70-80% confluence, cells were detached with 0.05%
trypsin and 0.5 mM EDTA, and separately seeded at 104cells/cm2.
Cell cultures were maintained in α-MEM supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL penicillin, 2.5 µg/mL streptomycin, 2.5 µg/mL amphotericin B and 50 µg/mL ascorbic acid. Cultures were treated with different PPI (omeprazole, esomeprazole
and lansoprazole), at a concentration range of 10-9-10-3 M. Culture medium was changed
once a week and PPI were renewed at each medium change. Cell cultures were
maintained for 21 days in a 5% CO2 humidified atmosphere at 37ºC.
Cultures were assessed for cellular viability/ proliferation and apoptosis.
2.1.3 Co-culture of SK-BR-3 and T47D and PBMC
SK-BR-3 and T47D cell lines were separately seeded at 102 cells/cm2 and 104cells/cm2, respectively. Cultures were incubated for 24h at 37ºC in α-MEM supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL penicillin, 2.5 µg/mL streptomycin, 2.5 µg/mL