_____________________________ *)
Corresponding author: marina.jovanovic@famm.unze.ba
UDK 549.613.4; 622.785
Preparation of Mullite Ceramics from
Bayer Electrofilter Fines
and Low Kaolinite Clay
Erna Tuti
ć
, Marina Jovanovi
ć
*
), Adnan Mujkanovi
ć
Faculty of Metallurgy and Materials Science University of Zenica, Bosnia and
Herzegovina
Abstract:
In this paper are investigated microstructural, phase, physical and mechanical properties of mullite ceramics obtained from clay and Bayer electrofilter fines. Clay and alumina were mixed in such ratio that provides the amount of Al O2 3 and SiO2 corresponding composition of mullite. One mixture was manually mixed and other was milled three hours in ceramic mill. After isostatic molding and heat treating at 1580°C and 1635°C XRD analysis revealed approximately 60% of mullite and 40% of corundum in first mixture and in second mixture 70% of mullite and 30% of corundum. Two types of mullite were observed in SEM micrographs beside corundum grains and glassy phase. The results of investigation indicate that preparation of mullite ceramics from these raw materials is possible, if clay and alumina are well homogenized and milled before molding.
Keywords: Primary mullite, Secondary mullite, Clay, Bayer electrofilter fines, Sintering
1. Introduction
mullite is formed by dissolution of alumina to silica rich glass and precipitation of secondary mullite [3,8]. The rate of secondary mullite formation is very slow at temperatures below 1555°C, but extremely fast at temperatures above 1600°C due to strong effect of the eutectic liquid formation at 1595°C [5]. Temperature and the level forming secondary mullite depend on the nature and dispersity of starting materials and on the amount and composition of glassy phase [9,10,11]. Secondary mullite crystals are smaller because they are nucleated from the transient liquid phase and primary mullite crystals are larger and with a higher aspect ratio. Primary mullite is arisen from kaolinite and has grown in the firing [4,5,6,12,13].
There are many papers concerning mullite preparing from kaolin and alumina [2,5,6,8,12-15], but there is a little investigation on using cheaper raw materials [21]. The objective of this research is to examine the possibility of preparing mullite ceramics from domestic raw materials and to determine the effect of simultaneous milling on properties of material obtained by sintering of clay and Bayer electrofilter fines.
2. Materials and experimental procedure
Raw materials used in this investigation are clay “Klokoti” [20] and electrofilter fines, by-products of the Bayer process for the production of alumina from bauxite. The deposit of clay “Klokoti“ is situated in the Central Bosnia and Bayer electrofilter fines are from alumina factory “Alumina” d. o. o. Zvornik, B&H. The chemical and mineralogical composition and particle size distribution were investigated. Chemical composition was determined by atomic absorption spectrometry. Particle size analysis was carried out using laser analyzer Malvern Mastersizer 2000. XRD was performed on a Shimadzu diffractometer XRD-6000 with Cu Kα radiation, with accelerating voltages of 40 kV and current 30 mA, in the range of angles 2-80° 2θ with a step 0.02° 2θ and a dwell time of 0.6 seconds.
According to chemical composition of clay and Bayer electrofilter fines, in order to get a mixture which has approximate composition of mullite (72 % Al2O3 and 28 % SiO2), two mixtures were made:
- Specimens which are in this paper marked as A contain 39 % of clay sieved through mesh openings 0.5 mm and 61 % of Bayer electrofilter fines, mixed manually,
- Specimens marked as B contain 38.5 % of the clay and 61.5 % of Bayer electrofilter fines, milled together in ceramic mill for 3 hours.
Both types of specimens, without any additions, were isostatic pressed at 100 MPa into cylindrical cartridge case and treated at 1580°C and 1635°C in the tunnel furnace within the cycle of 36 hours, with two hours keeping on the highest temperature.
3. Results and discussion
The results of chemical analysis of unsieved clay “Klokoti” and clay sieved through mesh openings 0.5 mm and Bayer electofilter fines are given in tab. 1. It shows that the percentage of impurity in clay is high, especially the content of K2O, that indicates that clay has illite and muscovite. Low loss of ignition indicates low content of kaolinite [9]. Sieving was slightly changed the chemical composition of clay.
Tab. I Chemical composition of clay “Klokoti” and Bayer electrofilter fines. Chemical composition [%]
Clay “Klokoti” Component
Unsieved Sieved Alumina
SiO2 69.1 67.6 0.021
CaO < 0.01 < 0.01 0.027
MgO 0.25 0.25 -
Fe2O3 1.36 1.46 0.017
Al2O3 18.4 18.9 98.14
TiO2 0.82 0.87 -
Na2O 0.39 0.41 0.74
K2O 4.96 5.57 -
LOI 4.96 5.03 1.02
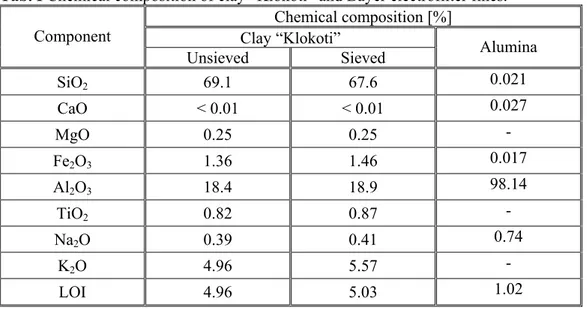
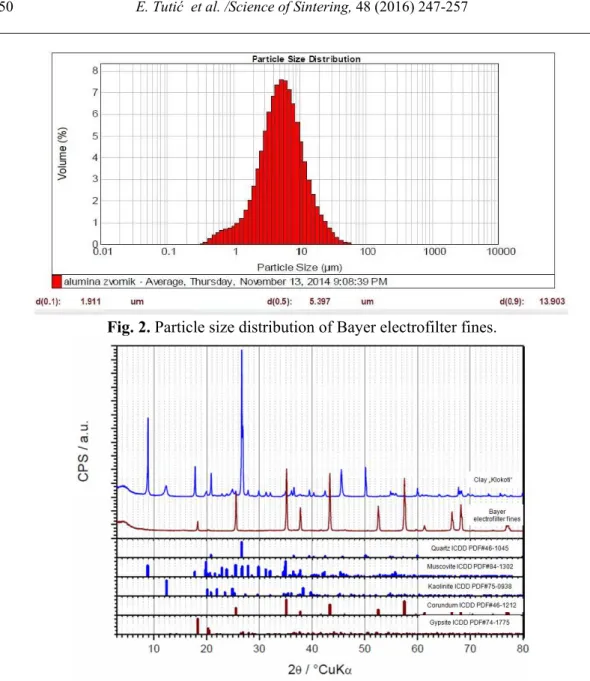
The particle size distribution of the clay “Klokoti” and Bayer electrofilter fines is shown in figs. 1 and 2, respectively. The mean particle size (d50) of the clay “Klokoti” is 18.102 µm. Bayer electrofilter fines have finer particle size distribution with mean particle size of 5 µm (fig. 2). These grain distributions are coarser in comparing with other investigations [2,5,6,8,12-15]. The XRD patterns of the clay and Bayer electrofilter fines are shown in fig. 3. The main phases in clay are kaolinite, muscovite and quartz. Muscovite and illite have very similar XRD patterns [10], so that is very difficult to differentiate them in this way. Bayer electrofilter fines consist mainly of α-Al2O3 and some gibbsite.
Fig. 2. Particle size distribution of Bayer electrofilter fines.
Fig. 3. XRD patterns of clay and Bayer electrofilter fines.
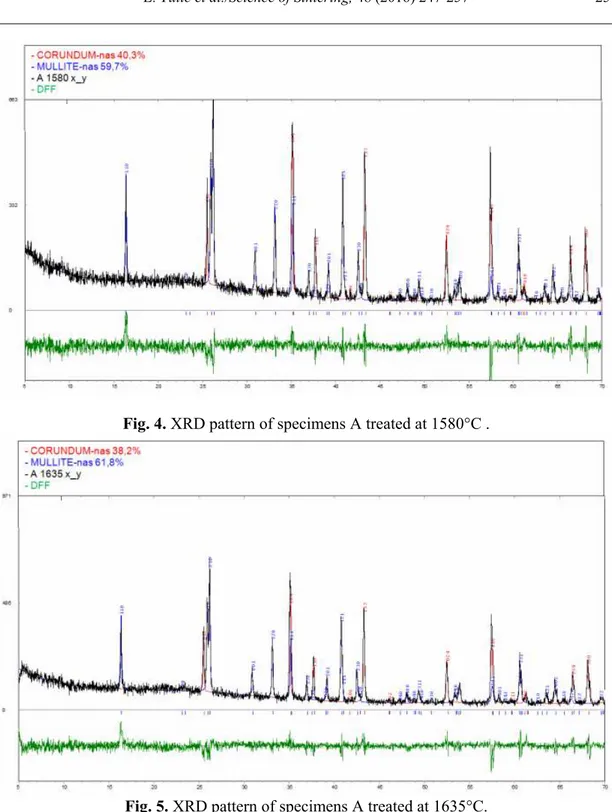
XRD patterns of mullite specimens are shown on figs. 4, 5, 6 and 7. Phase composition of crystal phase of mullite specimens is given in tab. 2. XRD analysis show that specimens consist of mullite and corundum and glassy phase. Specimens B have about 15% greater amount of mullite than specimens A because of finer particles in specimens B. Namely, grain size has a significant effect on mullitization, because activity of components present in the system largely depends on the particle size [7,11,14]. Specimens treated at higher temperature have only about 3.5% greater amount of mullite than specimens treated at lower temperature, because the temperature difference is only 55°C.
Tab. II Phase composition of crystal phase of specimens A and B.
Type of specimens
A B
Phase composition [mass%]
1580°C 1635°C 1580°C 1635°C
Mullite 59.7 61.8 68.6 71.2
Fig. 4. XRD pattern of specimens A treated at 1580°C .
Fig. 5. XRD pattern of specimens A treated at 1635°C.
Fig. 6. XRD pattern of specimens B treated at 1580°C.
Fig. 7. XRD pattern of specimens B treated at 1635°C.
Fig. 9. SEM micrographs of fracture surface of specimens.
Tab. III Physical properties of specimens A and B.
Type of specimens Characteristics
A B Temperature of treatment [°C] 1580 1635 1580 1635
Volume shrinkage [%] 26.75 24.44 31.91 26.99
Loss of mass [%] 3.88 3.60 3.56 3.62
Bulk density [g/cm3] 2.47 2.39 2.50 2.47
True density [g/cm3] 3.11 3.08 3.09 3.07
Compressive strength [MPa] 117 90 132 230
True porosity [%] 20.60 22.62 19.16 19.42
Apparent porosity [%] 5.25 6.96 0.93 0.93
Closed porosity [%] 15.35 15.66 18.23 18.49
Fig. 10. Pores morphology on fracture surface of specimens.
The physical properties of specimens A and B are given in tab. III. According to the results shown in tab. III it can be concluded that volume shrinkage of specimens B is greater than volume shrinkage of specimens A because of finer particles in specimens B [18,19]. The volume shrinkage of both specimens is greater at the lower than at higher temperature.
Creation of secondary mullite is accompanied by an increase of volume and as a result there is a loosening of structure in specimens at higher temperature [16].
Density of specimens B is also greater than density of specimens A because of higher volume shrinkage, but for both specimens it is lower at higher temperature due to increase in volume when creating a secondary mullite which leads to a loosening of structure. True porosity has the opposite behavior in comparison to density.
Specimens B have low apparent porosity and water absorption, but higher closed porosity. Increase in closed porosity may be due to gas production from decomposing of thermally unstab. materials in glassy phase at high sintering temperature [15]. Because of low apparent porosity these specimens have higher compressive strength. Specimens with the highest apparent porosity (specimens A treated at 1635°C) have the lowest compressive strength.
All specimens have refractoriness above 1780°C, but specimen A treated at 1635°C was the most deformed.
phenomenon occurs at 1635°C. Oversintering is stage of thermal treating which can lead to expansion of material and reducing the density [16].
4. Conclusion
Unlike most studies which dealt with the pure synthetic aluminas and clays with high kaolinite content, this paper present investigation of mullite producing from low-cost raw materials: non-treated natural fireclay and electrofilter fines, by-products of the Bayer process.
In addition to kaolinite, clay used for sample preparation contains quartz, illite, muscovite and other impurities. These impurities enhanced liquid phase forming upon heating which results in substantial reducing the amount of mullite in the final product.
XRD analysis of mullite showed the presence of relatively large quantities of corundum although the starting mixtures were designed to mullite (3:2) composition. Granulation of the starting material is rather rough, so their reactivity is lower. Therefore, larger particles of alumina did not have enough time to dissolve in the glassy phase and to react by creating mullite.
SEM analysis showed the presence of two type of mullite: high aspect ratio mullite corresponding to primary mullite formed from clay and smaller equiaxed secondary mullite formed in reaction of alumina and released SiO from clay. The formation of secondary2 mullite is accompanied by an increase in volume and decrease in density.
Increasing the temperature leads to an increase in grain size of mullite and increasing porosity.
Simultaneous grinding of the starting materials provides the material with more uniform structure, increased amounts of mullite, increased strength and density, and reduced porosity.
Generally, it can be concluded that preparation of mullite ceramics from low cost raw materials is possible, but clay and alumina have to be milled and homogenized before molding.
Acknowledgements
This research has been financed by the Ministry of Education and Science of Federation of Bosnia and Herzegovina as a project “The possibility of producing mullite from domestic raw materials”.
5. References
1.
J. Anggono, Mullite Ceramics: Its Properties, Structure, and Synthesis, Jurnal Teknik Mesin Vol. 7, No. 1, April 2005: 1-102.
V. Viswabaskaran, F. D. Gnanam, M. Balasubramanian, Mullitisation behavior of calcined clay – alumina mixtures, Ceramics International 29 (2003) 561 – 5713.
N. S. Raut, P. Biswas, T. K. Bhattacharya, K. Das, Effect of bauxite addition on densification and mullitization behavior of West Bengal clay, Bull. Mater. Sci., Vol. 31, No. 7, December 2008, pp. 995 – 9995.
M. A. Sainz, F. J. Serrano, J. M. Amigo, J. Bastida, A. Cabalero, XRD micrstructural analysis of mullites obtained from kaolinite – alumina mixtures, Journal of the European Ceramic Society 20 (2000) 403 – 4126.
C. Y. Chen, G. S. Lan, W. H. Tuan, Preparation of mullite by the reaction sintering of kaolinite and alumina, Journal of European Ceramic Society 20 (2000) 2519-25257.
J. Aguilar-Santillan, H. Balmori-Ramirez, R. C. Bradt, Dense mullite from attritionmilled kainite and α–alumina, Journal of Ceramic Processing Research. Vol. 8, No. 1, pp. 1 - 11 (2007)
8.
V. Viswabaskaran, F. D. Gnanam, M. Balasubramanian, Mullitisation behavior of south Indian clays, Ceramics International 28 (2002) 557 – 5649.
M. Jovanović, Strukturne i fazne promjene gline u toku procesa sinterovanja, Doctoral thesis, University of Zenica, Zenica, 2009.10.
R. E. Grim, Clay mineralogy, McGraw-Hill Book Company Inc., New York 1953.11.
S. J. Beneslavskij, Mineralogija boksitov, Nerda, Moskva 1974.12.
K. G. Liu, G. Thomas, Time - temperature transformation curves for kaolinite – α -alumina, J. Am. Ceram. Soc. 77 (1994) 1545-155213.
H. R. Rezaie, W. M. Rainforth, W. e. Lee, Mullite evolution in ceramics derived from kailonite, kaolinite with added α-alumina, and sol-gel precursors, Trans, Br. Ceram. Soc. 96 (1997) 181-18714.
D. Prodanović i dr., Osvajanje tehnologije proizvodnje mulitno-korundnog sintetskog šamota sa 70/75% i 80/85% Al2O3 na bazi domaćih sirovina tehničke glinice, gline i po potrebi mineralizatora, Izvještaj, ITNMS, Beograd 1983.15.
Y. Chen et al., Sintering Behavior and Pore Structure Development of Kaolin-Alumina Ceramics, Journal of Ceramic Society of Japan, Supplement 112-1, (2004) pp. 1265-127116.
S. Drljević, Teoretske i tehnološke osnove proizvodnje vatrostalnog materijala, University of Sarajevo, Faculty of metallurgy and materials science, Zenica, 1999.17.
W. Yan, N. Li, B. Han, Effect of sintering temperature on pore characterization andstrength of porous corundum-mullite ceramics, Journal of Ceramic Processing Research, Vol. 11, No. 3, pp. 388-391 (2010)
18.
M. Jovanović, A. Beroš, Clay and alumina grain size effect on highalumina refrectories, Metalurgija – Journal of Metalurgy, No.4, Vol.13, (2007), p. 317-324 (ISSN-0354-6306)19.
M. U. Taskiran, N. Demirkol, A. Capoglu, Influence of mixing/milling on sintering and technological properties of anorthite based porcelainised stoneware, Ceramics International 32 (2006) 325-33020.
M. Jovanović, T. Volkov-Husović, Influence of Sintering Temperature on Raw and Beneficiated Clay „Klokoti“, Science of Sintering, 44 (2012) 161-16821.
T. Yu-Ming et al., The effect of Sintering Temperature on the Structure and Properties of Corundum/mullite Ceramics, Science of Sintering, 47 (2015) 273-278Са р а: У a , ,
ј ј o e e .
a ( a) e ј ђ ј l2 3
Si 2 ј a. Ј ј a, a a ј
a o . c o o o e
e 1580°C 1635°C A a ј 60% a 40%
oј , a ј 70% a 30% . a
, e e
o o .
К учн р чи: , , , ј o a a