Running title: Bioactivity of enriched phenolic extracts
Bioactivity of different enriched phenolic extracts of wild fruits from
Northeastern Portugal: A comparative study
Rafaela Guimarães,1,2 Lillian Barros,1 Ricardo C. Calhelha,1,2 Ana Maria Carvalho,1 Maria João R.P. Queiroz,2 Isabel C.F.R. Ferreira1,*
1
Centro de Investigação de Montanha (CIMO), ESA, Instituto Politécnico de Bragança,
Campus de Santa Apolónia, apartado 1172, 5301-854 Bragança, Portugal
2
Centro de Química, Universidade do Minho, Campus de Gualtar 4710-057 Braga,
Portugal
* Author to whom correspondence should be addressed (e-mail: iferreira@ipb.pt
Abstract Arbutus unedo, Prunus spinosa, Rosa micrantha and Rosa canina are good sources of phenolic compounds, including anthocyanins. These compounds have potent
antioxidant properties, which have been related to anticancer activity. Herein, the in
vitro antioxidant and antitumor properties of enriched phenolic extracts
(non-anthocyanin phenolic compounds enriched extract- PE and (non-anthocyanins enriched
extract- AE) of the mentioned wild fruits were evaluated and compared. PE gave higher
bioactive properties than the corresponding AE. It was observed a high capacity of A.
unedo phenolic extract to inhibit lipid peroxidation in animal brain homogenates (EC50
= 7.21 µg/mL), as also a high antitumor potential against NCI-H460 human cell line
(non-small lung cancer; GI50 = 37.68 µg/mL), which could be related to the presence of
galloyl derivatives (exclusively found in this species). The bioactivity of the studied
wild fruits proved to be more related to the phenolic compounds profile than to the
amounts present in each extract, and could be considered in the design of new
formulations of dietary supplements or functional foods.
Keywords Wild fruits; Northeastern Portugal; Phenolic compounds; Anthocyanins; Antioxidant activity; Antitumor effects
Abbreviations
DPPH 2,2-Diphenyl-1-picrylhydrazyl
FBS Foetal bovine serum
HBSS Hank’s balanced salt solution
SRB Sulforhodamine B
TBARS Thiobarbituric acid reactive substances
Introduction
Phenolic compounds are common constituents of fruits and vegetables that are
considered an important class of antioxidant natural substances (1-3). In fact, the
interest of plant phenolic extracts derives from the evidence of their potent antioxidant
activity and their wide range of pharmacologic properties including anticancer activity
(4). However, the considerable diversity of their structures affects their biological
properties such as bioavailability, antioxidant activity, specific interactions with cell
receptors and enzymes (5).
The antioxidant properties are conferred to phenolic compounds by hydroxyl
groups attached to aromatic rings and they can act as reducing agents, hydrogen
donators, singlet oxygen quenchers, superoxide radical scavengers and even as metal
chelators (6). They also activate antioxidant enzymes, reduce α-tocopherol radicals
(tocopheroxyls), inhibit oxidases, mitigate nitrosative stress, and increase levels of uric
acid and low molecular weight compounds (6). For many years, phenolic compounds
have been intensely studied for their antitumor, proapoptotic and antiangiogenic effects
and, in recent years, the usage of these compounds has increased considerably (4).
Anthocyanins, from the flavonoids family, are found mainly in berries and have high
antioxidant activity, which plays a vital role in the prevention of neuronal and
cardiovascular illnesses, diabetes and cancer, among others (7).
As previously demonstrated by our research group, species such as Arbutus unedo
L., Prunus spinosa L., Rosa micrantha Borrer ex Sm. and Rosa canina L. are good
sources of phenolic compounds, including anthocyanins (8). The fruits of A. unedo are
used in folk medicine as antiseptics, diuretics and laxatives (9). P. spinosa fruits have
prophylactic and therapeutic activities for inflammatory disorders such as arthritis,
rheumatism, gout, colds and gastrointestinal disorders (10,11).
The antioxidant properties of extracts of A. unedo, P. spinosa, R. micrantha and R.
canina fruits were previously reported by different authors (12-15), but nothing is
known regarding different fractions of the mentioned extracts. Fujji et al. (16) studied
the effects of an aqueous extract of R. canina hips on mouse melanoma cells, and
demonstrated that proanthocyanidins contributed greatly to its melanogenesis-inhibiting
effect on those cells. Tumbas et al. (17) reported that the flavonoids fraction from R.
canina tea showed high antioxidant activity towards 2,2-diphenyl-1-picrylhydrazyl
(DPPH), as also antiproliferative activity in three human tumor cell lines (HeLa, MCF-7
and HT-29; IC50 values 80.63, 248.03 and 363.95 mg/L, respectively).
Despite the mentioned studies reporting antioxidant properties of fruits of the four
species, as far as we know, this is the first study regarding antitumor effects of A.
unedo, R. micrantha and P. spinosa. Moreover, the available reports on antioxidant
properties refer to crude and not purified/enriched extracts, and no conclusions could be
taken about the contributions of different phenolic fractions to the bioactivity of those
fruits. Therefore, in the present work, the in vitro antioxidant and antitumor properties
of enriched phenolic extracts (non-anthocyanin phenolic compounds enriched extract
and anthocyanins enriched extract) of A. unedo, P. spinosa, R. micrantha and R. canina
wild fruits were evaluated and compared in order to clarify anthocyanins contribution
for bioactivity and the advantageous of using purified/enriched instead of crude
phenolic extracts.
Materials and Methods
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Alfa Aesar (Ward Hill, MA,
USA). Foetal bovine serum (FBS), L-glutamine, Hank’s balanced salt solution (HBSS),
trypsin-EDTA (ethylenediaminetetraacetic acid), penicillin/streptomycin solution (100
U/mL and 100 mg/mL, respectively), RPMI-1640 and DMEM media were from
Hyclone (Logan, USA). Acetic acid, ellipticine, sulforhodamine B (SRB), trypan blue,
trichloroacetic acid (TCA) and Tris were from Sigma Chemical Co. (Saint Louis, USA).
Water was treated in a Milli-Q water purification system (TGI Pure Water Systems,
USA).
Samples
The fruits of Arbutus unedo L. (strawberry-tree) from Ericaceae, and the Rosaceae
species Prunus spinosa L. (blackthorn), Rosa canina sl. (dog rose) and Rosa micrantha
Borrer ex Sm. (similar to eglantine rose) were gathered in the Natural Park of
Montesinho territory, in Trás-os-Montes, Northeastern Portugal. Strawberry-tree berries
were collected fully ripened in November 2008; well matured blackthorn and dog rose
hips were gathered in late September 2008. R. micrantha overripe hips, that is fleshy
and soft dark red fruits, were collected in late autumn 2009. Morphological key
characters from the Flora Iberica (18) were used for plant identification. The fruits with
seeds were lyophilized (Ly-8-FM-ULE, Snijders, Holland) and stored in the
deep-freezer at -20ºC for subsequent analysis.
Samples preparation
Non-anthocyanin phenolic compounds enriched extract (PE): Each sample (1 g) was
extracted with 30 mL of methanol:water 80:20 (v/v) at room temperature, 150 rpm, for
re-extracted twice with additional 30 mL portions of methanol:water 80:20 (v/v). The
combined extracts were evaporated at 35 ºC (rotary evaporator Büchi R-210) to remove
methanol. For purification, the extract solution was deposited onto a C-18 SepPak® Vac
3 cc cartridge (Phenomenex), previously activated with methanol followed by water;
sugars and more polar substances were removed by passing through 10 mL of water and
phenolic compounds were further eluted with 5 mL of methanol. The methanolic extract
obtained (designated by phenolic extract) was concentrated under vacuum and stored at
4 ºC for further use.
Anthocyanins enriched extract (AE). Each sample (1 g) was extracted with 30 mL of
methanol containing 0.5% TFA, and filtered through a Whatman nº 4 paper. The residue
was then re-extracted twice with additional 30 mL portions of 0.5% TFA in methanol.
The combined extracts were evaporated at 35 ºC to remove the methanol, and
re-dissolved in water. For purification, the extract solution was deposited onto a C-18
SepPak® Vac 3 cc cartridge (Phenomenex), previously activated with methanol
followed by water; sugars and more polar substances were removed by passing through
10 mL of water and anthocyanin pigments were further eluted with 5 mL of
methanol:water (80:20, v/v) containing 0.1% TFA. The methanolic extract (designated
by anthocyanins extract) was concentrated under vacuum, lyophilized and stored at 4 ºC
for further use.
Evaluation of bioactivity
The extracts were re-dissolved in water at a final concentration 10 mg/mL and 8 mg/mL
for antioxidant and antitumor activity evaluation, respectively. The final solutions were
evaluation in vitro assays (1000-4 µg/mL and 400-25 µg/mL for antioxidant and
antitumor assays, respectively). The results were expressed in i) EC50 (extract
concentration providing 50% of antioxidant activity or 0.5 of absorbance in the reducing
power assay) values for antioxidant activity or ii) GI50 (extract concentration that
inhibited 50% of the net cell growth) values for antitumor activity. Water was used as
negative control, and trolox and ellipticine were used as positive controls in antioxidant
and antitumor activity evaluation assays, respectively.
Antioxidant activity assays. To evaluate the antioxidant activity the following assays
were used: DPPH radical-scavenging activity assay; reducing power assay; inhibition
of β-carotene bleaching assay; and lipid peroxidation inhibition by thiobarbituric acid
reactive substances (TBARS) assay (14, 19).
DPPH radical-scavenging activity was evaluated by using a ELX800 microplate Reader
(Bio-Tek Instruments, Inc; Winooski, USA), and calculated as a percentage of DPPH
discolouration using the formula: [(ADPPH-AS)/ADPPH] × 100, where AS is the absorbance
of the solution containing the extract at 515 nm, and ADPPH is the absorbance of the
DPPH solution. Reducing power was evaluated by the capacity to convert Fe3+ into
Fe2+, measuring the absorbance at 690 nm in the microplate Reader mentioned above.
Inhibition of β-carotene bleaching was evaluated though the β-carotene/linoleate assay;
the neutralization of linoleate free radicals avoids β-carotene bleaching, which is
measured by the formula: β-carotene absorbance after 2h of assay/initial absorbance) ×
100. Lipid peroxidation inhibition in porcine (Sus scrofa) brain homogenates was
evaluated by the decreasing in TBARS; the color intensity of the
inhibition ratio (%) was calculated using the following formula: [(A - B)/A] × 100%,
where A and B were the absorbance of the control and the extract solution, respectively.
Antitumor activity. Five human tumor cell lines were used: MCF-7 (breast
adenocarcinoma), NCI-H460 (non-small cell lung cancer), HCT-15 (colon carcinoma),
HeLa (cervical carcinoma) and HepG2 (hepatocellular carcinoma). Cells were routinely
maintained as adherent cell cultures in RPMI-1640 medium containing 10%
heat-inactivated FBS (MCF-7, NCI-H460 and HCT-15) and 2 mM glutamine or in DMEM
supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin and 100 mg/mL
streptomycin (HeLa and HepG2 cells), at 37 ºC, in a humidified air incubator containing
5% CO2. Each cell line was plated at an appropriate density (7.5 × 103 cells/well for
MCF-7, NCI-H460 and HCT-15 or 1.0 × 104 cells/well for HeLa and HepG2) in
96-well plates and allowed to attach for 24 h. Cells were then treated for 48 h with various
extract concentrations. Following this incubation period, the adherent cells were fixed
by adding cold 10% trichloroacetic acid (TCA, 100 µL) and incubated for 60 min at 4
ºC. Plates were then washed with deionized water and dried; sulforhodamine B solution
(0.1% in 1% acetic acid, 100 µL) was then added to each plate well and incubated for
30 min at room temperature. Unbound SRB was removed by washing with 1% acetic
acid. Plates were air-dried, the bound SRB was solubilized with 10 mM Tris (200 µL)
and the absorbance was measured at 540 nm in the microplate reader mentioned above
(20).
Hepatotoxicity. A cell culture was prepared from a freshly harvested porcine liver
obtained from a local slaughter house, and it was designed as PLP2. Briefly, the liver
100 µg/mL streptomycin and divided into 1×1 mm3 explants. Some of these explants
were placed in 25 cm2 tissue flasks in DMEM medium supplemented with 10% fetal
bovine serum, 2 mM nonessential amino acids and 100 U/mL penicillin, 100 mg/mL
streptomycin and incubated at 37 ºC with a humidified atmosphere containing 5% CO2.
The medium was changed every two days. Cultivation of the cells was continued with
direct monitoring every two to three days using a phase contrast microscope. Before
confluence was reached, cells were subcultured and plated in 96-well plates at a density
of 1.0×104 cells/well, and cultivated in DMEM medium with 10% FBS, 100 U/mL
penicillin and 100 µg/mL streptomycin (20).
Statistical analysis
All the assays were carried out in triplicate in three different extracts, and the results are
expressed as mean values±standard deviation (SD). The statistical differences
represented by letters were obtained through one-way analysis of variance (ANOVA)
followed by Tukey’s honestly significant difference post hoc test with α = 0.05. These
treatments were carried out using SPSS v. 18.0 program.
Results and Discussion
The results of antioxidant activity, determined by free radicals scavenging activity,
reducing power and inhibition of lipid peroxidation in brain cell homogenates, are
shown in Table 1. The studied extracts were chemically characterized in a previous
work of our research group (8). Herein, two different enriched phenolic extracts were
prepared, in order to evaluate and compare their bioactivity: a non-anthocyanin phenolic
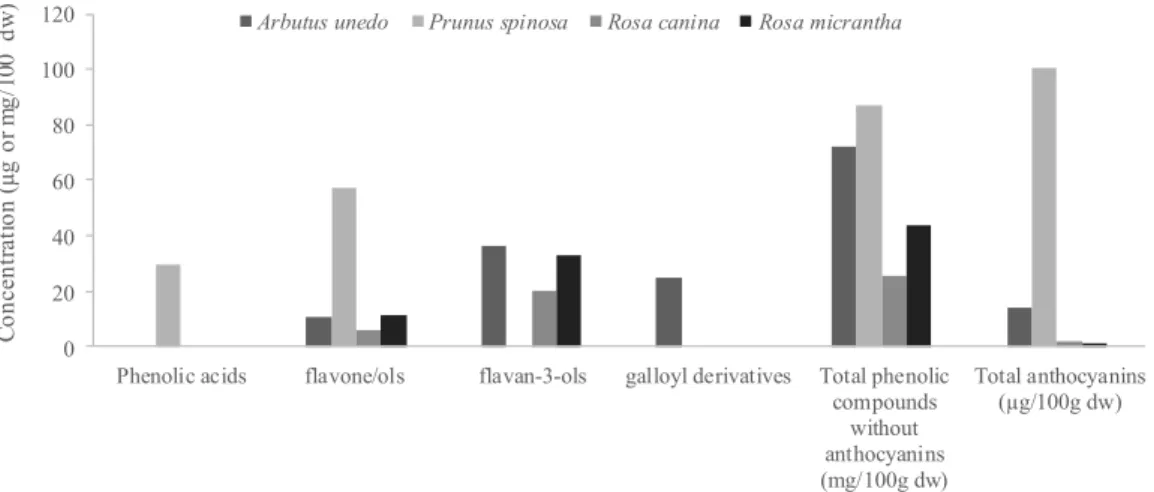
compounds enriched extract (PE; with phenolic acids, flavones/ols, flavan-3-ols and
Regarding PE of the studied wild fruits, A. unedo presented the highest antioxidant
activity in all the in vitro assays, which could be related to the presence of galloyl
derivatives (exclusively in A. unedo PE) and/or to the presence of higher levels of
flavan-3-ols. The second one with highest antioxidant effects was P. spinosa, in which
the main contributors seemed to be phenolic acids (exclusive in P. spinosa PE) and
flavones/ols, present in this PE in higher amounts. The studied Rosa species revealed
the lowest antioxidant activity, presenting similar phenolic compounds profile
(flavan-3-ols and flavones/ols); the higher levels of these compounds found in R. micrantha
comparatively to R. canina, might explain the higher antioxidant activity observed in
the first case (Table 1, Fig. 1).
Concerning AE, a pro-oxidant effect of anthocyanins seemed to occur, since the
samples with the highest amounts revealed the lowest antioxidant activity (Table 1, Fig.
1). For that reason, P. spinosa gave the lowest antioxidant activity (in β
-carotene-bleaching inhibition assay it was not possible to determine EC50 value due, in our
opinion, to pro-oxidant effects of anthocyanins), while R. canina showed the highest
antioxidant effects.
PE gave higher antioxidant properties than the corresponding AE, and according to
their chemical characterization, those properties seem to be related to galloyl
derivatives, flavan-3-ols, phenolic acids and flavones/ols. In general, PE and AE
presented higher antioxidant activity than the methanolic extracts (crude extracts) of the
same fruits previously studied by us (14,15). It seems that purified/enriched extracts
(such as the cases herein presented) are more suitable than crude extracts, in which
antagonistic effects between the compounds present could be observed, conducting to a
decrease in the antioxidant activity. The only exception was for the β-carotene
molecules rather than the ones previously mentioned are probably involved and might
bring synergistic effects.
The antitumor potential was tested in human tumor cell lines (breast, lung, colon,
cervical and hepatocellular carcinomas), and the hepatotoxicity was evaluated using a
porcine liver primary cell culture. All the extracts inhibited the growth of tumor cell
lines, except R. canina PE and AE, P. spinosa AE and R. micrantha AE for MCF-7
(breast carcinoma). A. unedo, followed by P. spinosa, PE gave the best antitumor
inhibition (Table 2), which could be correlated as mentioned above for antioxidant
activity (similar behaviour), to the phenolic groups present in each of the wild fruits
(Fig. 1), i.e., exclusive presence of galloyl derivatives and the highest levels of
flavan-3-ols for A. unedo PE, and exclusive presence of phenolic acids and the highest levels of
flavones/ols for P. spinosa PE. Regarding AE, samples with the highest amounts of
anthocyanins (P. spinosa and A. unedo) revealed the highest antitumor effects, except in
the case of MCF-7 that was not inhibited by P. spinosa AE. None of the samples
showed toxicity for non-tumor liver primary culture.
As far as we know, this is the first study regarding antitumor effects of A. unedo, R.
micrantha and P. spinosa wild fruits. In the case of R. canina, the antitumor effects of
an aqueous extract from its hips were studied in mouse melanoma cells (16), and
similarly to the herein studied PE, the higher contributors are proanthocyanidins
(flavan-3-ols). Otherwise, the flavonoids fraction from R. canina tea showed higher
antiproliferative activity in HeLa cell line (IC50 = 80.63 µg/mL; 17) than the one
observed in the present study for PE (GI50 = 253.03 µg/mL); contrarily to the observed
result (no activity up to 400 µg/mL), those authors reported effects against MCF-7 cell
Overall, the bioactivity of the studied wild fruits proved to be more related to
phenolic compounds profile than to the amounts present in each extract, being PE more
bioactive than AE. It should be highlighted the high capacity of A. unedo PE to inhibit
lipid peroxidation in animal brain homogenates (EC50 = 7.21 µg/mL), as also its
antitumor potential against NCI-H460 human cell line (non-small lung cancer; GI50 =
37.68 µg/mL). Regarding chemical characterization of the mentioned sample, the
presence of galloyl derivatives exclusively in A. unedo wild fruits could be related to its
higher bioactivity. Further studies are needed in order to confirm the specific role of
these compounds in antioxidant and antitumor effects. Due to the observed bioactive
properties, the mentioned species could be considered in the design of new formulations
of dietary supplements or functional foods.
Acknowledgements Foundation for Science and Technology (FCT, Portugal) for PEst-OE/AGR/UI0690/2011, PEst-C/QUI/UI0686/2011, BD/78307/2011 (R. Guimarães),
BPD/68344/2010 (R. Calhelha) and researcher contract of L. Barros.
The authors declare that they have no conflict of interest.
References
1. Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic
compounds. Review. Trend Plant Sci 2:152-159
2. Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, Chen SS
(2004) Flavonoids in food and their health benefits. Plant Food Hum Nutr
59:113-122
3. Szajdek A, Borowska EJ (2008) Bioactive compounds and health-promoting
4. Carocho M, Ferreira ICFR (2013) The role of phenolic compounds in the fight
against cancer- A review. Anti-Cancer Ag Med Chem 13:1236-1258.
5. Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols.
J Nutr 130:2073-2085
6. Carocho M, Ferreira ICFR (2013) A review on antioxidants, prooxidants and
related controversy: Natural and synthetic compounds, screening and analysis
methodologies and future perspectives. Food Chem Toxicol 51:15-25
7. Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (2003) Analysis and biological
activities of anthocyanins. Phytochemistry 64:923-933
8. Guimarães R, Barros L, Dueñas M, Carvalho AM, Queiroz MJRP, Santos- Buelga
C, Ferreira ICFR (2013) Characterization of phenolic compounds in wild fruits
from Northeastern Portugal. Food Chem 141:3721-3730
9. Bnouham M, Merhfour FZ, Legssyer A, Mekhfi H, Maallem S, Ziyyat A (2007)
Antihyperglycemic activity of Arbutus unedo, Ammoides pusilla and Thymelaea
hirsuta. Pharmazie 62:630-632
10. Carvalho AM (2010) Plantas y sabiduría popular del Parque Natural de
Montesinho. Un estudio etnobotánico en Portugal. Biblioteca de Ciencias 35.
Madrid: Consejo Superior de Investigaciones Científicas
11. Orhan DD, Hartevioğlu A, Küpeli E, Yesilalada E (2007) In vivo anti-inflammatory
and antinociceptive activity of the crude extract and fractions from Rosa canina L.
fruits. J Ethnopharmacol 112:394-400
12. Fortalezas S, Tavares L, Pimpão R, Tyagi M, Pontes V, Alves PM, McDougall G
Stewart D, Ferreira RB, Santos CN (2010) Antioxidant properties and
neuroprotective capacity of strawberry tree fruits (Arbutus unedo). Nutrients
13. Mendes L, Freitas V, Baptista P, Carvalho M (2011) Comparative antihemolytiic
and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and
fruit. Food Chem Toxicol 49:2285-2291
14. Barros L, Carvalho AM, Morais JS, Ferreira ICFR (2010) Strawberry-tree,
blackthorn and rose fruits: Detailed characterization in nutrients and
phytochemicals with antioxidant properties. Food Chem 120:247-254
15. Guimarães R, Barros L, Carvalho AM, Ferreira ICFR (2010) Studies on chemical
constituents and bioactivity of Rosa micrantha: an alternative antioxidants source
for food, pharmaceutical, or cosmetic applications. J Agric Food Chem
58:6277-6284
16. Fujii T, Ikeda K, Saito M (2011) Inhibitory effect of rose hip (Rosa canina L.) on
melanogenesis in mouse melanoma cells and on pigmentation in brown guinea
pigs. Biosci Biotechnol Biochem 75:489-495
17. Tumbas VT, Čanadanović-Brunet JM, Četojević-Simin DD, Ćetković GS, Dilas
SM, Gille L (2012) Effect of rosehip (Rosa canina L.) phytochemicals on stable
free radicals and human cancer cells. J Sci Food Agric 92:1273-1281
18. Castroviejo S. (coord) (2004) Flora Iberica. Plantas vasculares de la Península
Ibérica e Islas Baleares. Vol. IV. Real Jardín Botánico, Madrid: CSIC
19. Dias MI, Barros L, Sousa MJ, Ferreira ICFR (2011) Comparative study of
lipophilic and hydrophilic antioxidants from in vivo and in vitro grown Coriandrum
sativum. Plant Food Hum Nutr 66:181-186
20. Guimarães R, Barros L, Dueñas M, Calhelha RC, Carvalho AM, Santos Buelga C,
Queiroz MJRP, Ferreira ICFR (2013) Nutrients, phytochemicals and bioactivity of
wild Roman chamomile: a comparison between the herb and its preparations. Food
0 20 40 60 80 100 120
Phenolic acids flavone/ols flavan-3-ols galloyl derivatives Total phenolic compounds without anthocyanins (mg/100g dw) Total anthocyanins (µg/100g dw) Co n c e n tr a ti o n ( µ g o r m g /1 0 0 d w )
Arbutus unedo Prunus spinosa Rosa canina Rosa micrantha
Fig. 1. Concentrations of phenolic compounds present in the wild fruits, determined by HPLC-DAD-MS/ESI according to reference (8).
Table 1 Antioxidant activitya of different phenolic enriched extracts from four wild fruits (mean ± SD).
a
EC50 values (µg/mL) corresponding to the sample concentration achieving 50% of
antioxidant activity or 0.5 of absorbance in reducing power assay. n.a. It was not possible to obtain EC50 value for this extract. PE- Non-anthocyanin phenolic
compounds enriched extract; AE- Anthocyanins enriched extract. In each column, and DPPH scavenging Activity Reducing power β-carotene bleaching inhibition TBARS inhibition PE
Arbutus unedo 60.89±1.74d 36.69 ± 1.82c 432.08±19.37c 7.21 ± 0.35c Prunus spinosa 64.98 ± 6.19c 42.08 ± 0.66b 641.11 ± 80.69b 7.39 ± 0.20c Rosa canina 75.78 ± 4.10a 47.38 ± 0.93a 852.20 ± 147.67a 10.02±0.29a Rosa micrantha 69.58 ± 3.37b 47.80 ± 1.19a 755.39 ± 82.25b 8.89 ± 0.26b
AE DPPH scavenging
activity
Reducing power
β-carotene
bleaching inhibition TBARS inhibition Arbutus unedo 93.75 ± 2.26b 75.41±0.53b 950.96 ± 38.71a 23.13±3.21b Prunus spinosa 99.37 ± 2.36a 83.30 ± 0.46a n.a. 25.29 ± 0.85a Rosa canina 81.21±2.26d 72.75±2.38c 893.57±29.19b 12.39 ± 0.18c Rosa micrantha 86.33±1.69c 75.25±0.12b 904.08±55.50b 22.52±0.36b
Table 2 Antitumor activity and hepatotoxicitya of different phenolic enriched extracts from four wild fruits(mean ± SD).
a
PE MCF-7
(breast carcinoma)
NCI-H460 (non-small lung cancer)
HCT-15 (colon carcinoma)
HeLa (cervical carcinoma)
HepG2
(hepatocellular carcinoma)
PLP2 (non-tumor liver primary culture)
Arbutus unedo 153.08±10.34d 37.68±5.02d 93.36±5.98c 143.36±9.07d 128.51±7.47d >400
Prunus spinosa 270.65±9.25c 154.25±6.35c 220.44±2.89b 193.62±11.05c 169.56±6.39c >400
Rosa canina >400a 254.69±3.91a 243.67±4.65a 253.03±11.03a 281.79±5.78a >400
Rosa micrantha 374.11±8.69b 226.04±7.56b 223.25±4.23b 226.34±13.81b 255.31±9.01b >400
AE MCF-7
(breast carcinoma)
NCI-H460 (non-small lung cancer)
HCT-15 (colon carcinoma)
HeLa (cervical carcinoma)
HepG2
(hepatocellular carcinoma)
PLP2 (non-tumor liver
primary culture)
Arbutus unedo 238.11±6.74 227.43±4.09c 121.95±7.15b 149.88±8.46d 168.40±7.29c >400
Prunus spinosa >400a 282.92±4.28b 234.59±6.29a 224.58±14.09c 231.31±9.24b >400
Rosa canina >400a 305.97±4.23a 243.51±8.24a 311.16±10.18a 266.53±10.95a >400
Rosa micrantha >400a 307.68±6.05a 264.04±3.08a 252.07±11.01b 270.5±9.24a >400