w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Review
article
Modern
techniques
of
magnetic
resonance
in
the
evaluation
of
primary
central
nervous
system
lymphoma:
contributions
to
the
diagnosis
and
differential
diagnosis
Antonio
José
da
Rocha
a,b,∗,
Bruno
Vasconcelos
Sobreira
Guedes
b,
Talita
Maira
Bueno
da
Silveira
da
Rocha
b,
Antonio
Carlos
Martins
Maia
Junior
a,b,
Carlos
Sérgio
Chiattone
aaFleuryMedicinaeSaúde,SãoPaulo,SP,Brazil
bSantaCasadeMisericórdiadeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received1July2014 Accepted30November2015 Availableonline31December2015
Keywords:
CNSlymphomas Lymphomatosiscerebri Neurolymphomatosis Ocularlymphomas
Magneticresonanceimaging Brainneoplasm
NonconventionalMR
a
b
s
t
r
a
c
t
In addition to findings from conventionalmagnetic resonance imaging,modern mag-neticresonanceimagingtechniqueshave providedimportantinformationabout tumor metabolism,invivometaboliteformation,watermoleculediffusion,microvasculardensity, andblood-brainbarrierpermeability,allofwhichhaveimprovedtheinvivodiagnostic accu-racyofthismethodintheevaluationofprimarycentralnervoussystemlymphoma.These nonconventionalmagneticresonancetechniquesareusefulintheclinicalpracticebecause theyenhanceconventionalmagneticresonanceimagingbyreinforcingthepossibilityofa diagnosisandbyallowingtheearlydetectionofdiseaserecurrence.
Thisreportisareviewofthemostrelevantcontributionsofnonconventionalmagnetic res-onancetechniquestotheimagingdiagnosisofprimarycentralnervoussystemlymphoma, thedifferentialdiagnosisofthisdisease,andtheprognosisofpatients.Thispaperaimsto describeawiderangeofpresentationsofprimarycentralnervoussystemlymphoma,their appearanceinimaging,andthedifferentialdiagnosesofthisdisease.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
Introduction
Primary central nervous system lymphomas (PCNSL) have a predictable imaging appearance in their classical
∗ Correspondingauthorat:SantaCasadeMisericórdiadeSãoPaulo,RuaCesárioMottaJunior,112,VilaBuarque,01221-020SãoPaulo,SP,
Brazil.
E-mailaddress:a.rocha@uol.com.br(A.J.daRocha).
presentation. Conventional magnetic resonance imaging (MRI) features can be suggestive of PCNSL but are not specific. Because of the form of presentation, the list of differentialdiagnosesislong,andmainlyincludes inflamma-tory/infectious diseases particularly of the central nervous
http://dx.doi.org/10.1016/j.bjhh.2015.12.001
system(CNS),toxoplasmosisinAIDSpatients,andprimary andsecondarymalignancies.1–3
Moderntechniquesofmagneticresonancehaveincreased the clinical applicability of this imaging method because theyprovideimportantinformationabouttumormetabolism,
in vivo metabolite formation, water molecule diffusion, microvascular density, and blood–brain barrier (BBB) per-meability. Although histology and an investigation of the cerebrospinal fluidfor meningeal disease are stillthe gold standardinthediagnosisofCNSlymphoma,theseadvanced techniquescan improve the diagnostic accuracyof PCNSL, particularlywhenthebrainparenchymaisaffected.1,4–9
Thisreportisareviewofthemostrelevantcontributionsof advancedMRItechniquestotheimagingdiagnosisofPCNSL, thedifferentialdiagnosisofthisdisease,andtheprognosisof patients.
Contribution
of
nonconventional
magnetic
resonance
techniques
to
the
diagnosis
of
primary
central
nervous
system
lymphoma
and
the
prognosis
of
patients
Thevariousimagingmethodsthatarecurrentlyavailableno longer exclusively provide descriptive anatomical informa-tion.Nowadvancedimagingtechniquesallowanassessment offunctional parameters,therebymaximizingthepotential ofthesetechniquesforanaccuratediagnosisandtreatment assessment.
Despite treatment advances, survival among PCNSL patientsintheUnitedStateshasremainedpoor.However, sur-vivalhasimprovedovertimeinthesubsetofPCNSLpatients whoarehumanimmunodeficiencyvirus(HIV)-negative.10
NonconventionalMRI techniquesare useful in the clin-ical practice because they enhance conventional MRI by reinforcingthe possibility ofa diagnosisofPCNSL,by con-tributingtotheprognosisofpatients,andbyallowingtheearly detectionofdiseaserecurrence.1,8,11
Diffusion-weighted
imaging
Diffusion-weightedimaging(DWI)reflectsthe macromolecu-larmotionofintra-andextracellularwaterandishelpfulin distinguishingbetweenPCNSLandothertumorsand tumor-mimickinglesions.DuetothehypercellularityofPCNSL,water diffusionisoftenrestricted,whichmakesthesetumorsappear hyperintense on DWI and hypointense on apparent diffu-sioncoefficient(ADC)maps.AdecreasedADCvalueindicates increasedcellularity.4,8,12Thetechniquethatisgenerallyused
todeterminetheADCvalueinvolvescalculatingtheADCratio ofthesolidcomponentofthetumorandcomparingthisratio tothatofthenormal-appearingwhitematter.Restricted diffu-sionwithanADCthresholdvalue≤1.1×10−3mm2/shasbeen
recommended to differentiate PCNSL from other intracra-nialfocallesions.7AnADCthresholdvalue>1.6×10−3mm2/s
increasestheprobabilityofadiagnosisoftoxoplasmosisover PCNSLinimmunocompromisedpatients.However,therehas beensignificantoverlappinginthesevaluesinnecrotic ring-enhancinglesions.4,13
A recent report revealed that low ADC values in pre-therapeutic tumorswere predictive ofshorter progression-free survival and overall survival. Furthermore, increasing ADC values during treatment suggest a positive response to therapy, whereas decreasing ADC values suggest tumor progression.12
Diffusiontensorimaging(DTI)takesadvantageofhighly ordered white matter fibers, which have a directionally ordered structuredue topreferentialwaterdiffusion along thelongitudinalaxisofaxonsandmyelinsheaths.This tech-niquehasrecentlyemergedasasensitivetoolforthedetection ofalterationsinthestructureofwhitematter.Additionally, differentdegreesofcellularityandcellularorganizationmay affectthefractionalanisotropy(FA)value.TheFAvaluesfor PCNSL are significantly lower than those forglioblastoma; therefore,thesevaluescanhelpinthedifferentiationofthese tumors.14
Furtherstudiesthatcombinehistologicalcorrelation tech-niqueswith imagingfindings are neededtodetermine the impactofthemeasurementofADConpatientprognosisand treatmentplanning.However,werecommendperforminga careful ADC analysis ofdifferent solid parts of the tumor beforetreatmentandaftertreatmentasanadditional non-invasivetooltoestimatelocalinvivoresponses.
Perfusion-weighted
imaging
Perfusion MRI can beused toestimate the nutritive deliv-ery ofarterialblood tothecapillarybedinbiologicaltissue usingsignalintensity–timecurvestodeterminethecapillary densityindynamicsusceptibilitycontrast(DSC)T2-weighted echoplanarsequences.Thepost-processingofacquireddata allowsforasemiquantitativeanalysisofphysiological param-eters,particularlyoftherelativecerebralbloodvolume(rCBV), relative cerebralblood flow (rCBF), and time-to-peak(TTP). AnotherperfusionMRItechniquethatcanbeusedtoevaluate vascularpermeabilityinadynamiccontrast-enhanced(DCE) T1-weighted sequence is to simultaneously administer an intravenousinjectionofaparamagneticagent(gadolinium).
PCNSLareassociatedwithmildlyincreasedoroccasionally decreasedperfusionthatisusuallyhigherthanthatinmost focal braininflammatory,demyelinatingand infectious dis-easesbutlowerthanthatinhigh-gradegliomas(rCBV>1.75).7
PCNSLlesionsareusuallystronglyenhancedduetothe dis-ruption of the BBB and the fast leakage across the BBB; however,theselesionsshowalowrCBV,whichisinagreement withhistologicaldatathatdemonstratealackof neoangio-genesisinlymphomas,mainlywhentheyarehighlycellular orthereisconsiderableperivascularorintravasculartumor infiltration.5
Figure1–DiffuselargeB-celllymphomainthecorpuscallosum.AnaxialT1imageafterintravenousgadolinium
administration(A)showsahomogeneousenhancedmassinthespleniumofthecorpuscallosum,whichispredominantly intherighthemisphere(arrow).Anaxialapparentdiffusioncoefficientmap(B)confirmedaverylowsignalintensityinthe solidlesion.Notethehyperintensityoftheperilesionalvasogenicedema.Protonmagneticresonancespectroscopy(C) showsdecreasedN-acetylaspartatelevelsandhighcholine/N-acetylaspartateandcholine/creatinineratios.Notethe increasedlipidandlactatepeaks(0.9–1.3ppm).Amagneticresonanceperfusionsequence(dynamicsusceptibilitycontrast magneticresonanceimageT2*)(D)confirmedtheabsenceofneoangiogenesis(lowrelativecerebralbloodvolume).Notethe highpercentageofsignalintensityrecovery(theascendingpartofthecurveabovethebaselineasindicatedbythevertical arrowheads).
that can beused todifferentiate between malignant brain tumors.6
Perfusion computed tomography (CT) is available but involves ionizing radiation and a morelimited area ofthe brain. In contrast to MRI, only a few studies have been publishedonperfusionCTinbraintumors.However,the diag-nosticvalueofperfusionCTinbraintumorsmayincreasein thefutureduetoitsadvantagesrelatedtospatialresolution andthelinearcorrelationbetweenthecontrastconcentration andtheattenuationofthetissue.15
Magnetic
resonance
spectroscopy
Proton magnetic resonance spectroscopy (MRS) allows for thesemiquantitativeevaluationofinvivotissuetomeasure
metabolites,suchasN-acetylaspartate(NAA),choline(Cho), creatinine (Cr), lactate (Lac), and lipids (Lip). In PCNSL, MRS has demonstrated elevated Lip and Lac peaks, high Cho/Crratios,decreasedNAAlevelsandhighCho/NAAratios (Figure 1).4,11,16,17 The presence of Lac or Lip at baseline
hasbeen associatedwithpoorprogression-freeand overall survival.11
PCNSLgrowrapidlyandbehavesimilartohigh-gradebrain tumorswithevidenceofhighcellmembraneturnoveronMRS (ahighChopeak),neuronaldamage(decreasedNAAlevels), andanaerobiosis(highlactatelevels).4,11,16,17 Thesefindings
Table1–Findingsobtainedusingnonconventionalmagneticresonancetechniquesthatareusefulfordifferentiating PCNSLfrommimiclesions.
Primarycentralnervoussystemlymphoma
Diffusion-weightedimaging Restrictedwatermoleculemovement(typicallyhomogeneousin immunocompetentpatients)
Apparentdiffusioncoefficientvalue ≤1.1×10−3mm2/s
Protonmagneticresonancespectroscopy Increasedlipidandlactatelevels(0.9–1.3ppm)
IncreasedcholinelevelsanddecreasedN-acetylaspartatelevels Dynamiccontrast-enhancedmagneticresonanceimaging Highpermeabilityoftheblood-brainbarriermaybeobserved Dynamicsusceptibilitycontrastmagneticresonanceimaging Lowerrelativecerebralbloodvolume(<1.75)thaninhigh-grade
gliomasormetastases
Ahighpercentageofsignal-intensityrecoveryabovethebaseline wouldfavoradiagnosisoflymphoma.
Note:Allofthesefeaturesshouldbecarefullyinterpretedinimmunocompromisedpatientsduetotheoccurrenceofnecrosisand hemor-rhageandtheconsiderableoverlappingfeaturesbetweenprimarycentralnervoussystemlymphomaandbraininfections,particularlyearly toxoplasmosis.
Differential
diagnosis
TheearlydiagnosisofPCNSLiscrucialtodefinetherapeutic procedures.WhenPCNSLaresuspectedbasedonimaging,a tissuebiopsyismandatoryandgrosstumorresectionmust be avoided.18 The nonconventional MRIfeatures of PCNSL
(Table 1)and mimicking brainlesions shouldbe known in ordertosupportthesuspicion.
The
imaging
features
of
primary
central
nervous
system
lymphoma
and
other
focal
brain
lesions
and
the
differential
diagnosis
of
these
lesions
PCNSLisassociatedwithahighernuclear-to-cytoplasmratio andarelativelylowintratumoralwatercontent,which indi-cateprominentCThyperdensityandhypointensitysignalsin T2-weightedimages.Theseimagingfeaturesareimportantfor distinguishingbetweenPCNSLandotherfocalbrainlesions thatusually demonstratea higherwatercontent (hyperin-tenseonT2-weightedimages)suchasgliomas,metastases, andtumefactivedemyelinatinglesions.14,19
Neurosarcoidosis rarely presents as a brain tumor-like lesion but exhibits hyposignal intensity on T2-weighted imagesanddisseminatesperivascularlywithvariablecontrast enhancement without necrosis. Therefore, the differential diagnosisofPCNSLiscrucial.
Metastaticdiseases related tomelanomaand renal cell, breast, and lungcarcinomas are morelikelyto haveblood productsthanPCNSL.Moreover,incontrasttoPCNSL,brain metastasesareusuallylocatedatthegray-whitematter junc-tions (hematogenouslydisseminated)and theyoftenincite markedperitumoraledema.1
Severalhigh-gradegliomasmayexhibithyposignal inten-sityonT2andoftencontainbloodproductswithinfiltrated margins and heterogeneous contrast enhancement, which makesitdifficulttodifferentiategliomasfromlymphomas. The classical ‘mirror image’ or ‘butterfly pattern’ that is due to the symmetrical involvement of the genu or the splenium of the corpus callosum has been observed in
PCNSL.14SubependymaldiseasemayoccurinbothPCNSLand
high-gradeglioma.1PCNSLlesionsoftenhavemorerestricted
diffusionandlowerADCvaluesthanhigh-gradegliomasand metastases.8
Peripheral enhancement patterns of PCNSL, including ‘ring-like’and‘openring-like’aspects,mustbedistinguished from those ofclassical brain demyelination.19 PCNSL have
solidportions withthicker andnon-uniformenhancement, which isnot restrictedto periventricular zones but affects deep gray matter as a single lesion or multiple lesions.20
Moreover,highCholevelsandChoratios(Cho/NAA>1.9)on MRS, areas of restricted watermolecule diffusionon DWI, the absence of high rCBV levels (<1.75) and a high per-centage ofsignal intensity recovery above the baseline on DSC-MRIwouldfavoraPCNSLdiagnosisoverotherdiagnostic possibilities.4,6,7,11,17
When PCNSL are suspected, corticotherapy should be avoidedordiscontinuedbecausetumorshrinkageresultsin radiographicregressioninapproximately40%ofpatients(‘the vanishingtumor’),whichissuggestiveofPCNSL(Figure2).The detectionofa‘vanishingtumor’shouldnotbeconsideredin thediagnosisofPCNSLbecausesarcoidosis,brain demyelina-tion,acuteencephalomyelitis,andothermalignancies(more rarely)canexhibitdramaticresponsestosteroids.3,18
ADTIanalysismaybeusedtodistinguishbetweenPCNSL andglioblastoma.AquantitativeanalysisofFAandADC val-uesfoundthatthesevaluesweresignificantlydecreasedwhen thesolidportionsofthetumorswerecomparedwith normal-appearingwhitematterinthecontralateralhemisphere.The FAandADCvaluesinprimarycerebrallymphomawere sig-nificantlylowerthanthoseinglioblastoma.14
TheMRSfindingsforPCNSL,high-gradegliomasand tume-factive demyelinatinglesions maybesimilar; therefore,we recommendacarefulinterpretationofthistechnique.Itis use-fultoconsideralloftheconventionaland nonconventional featurestosupporttheclinicalpresumptionofeachdisease, tomakea histologicaldiagnosis,and todefinetherapeutic procedures.1,4,6,7
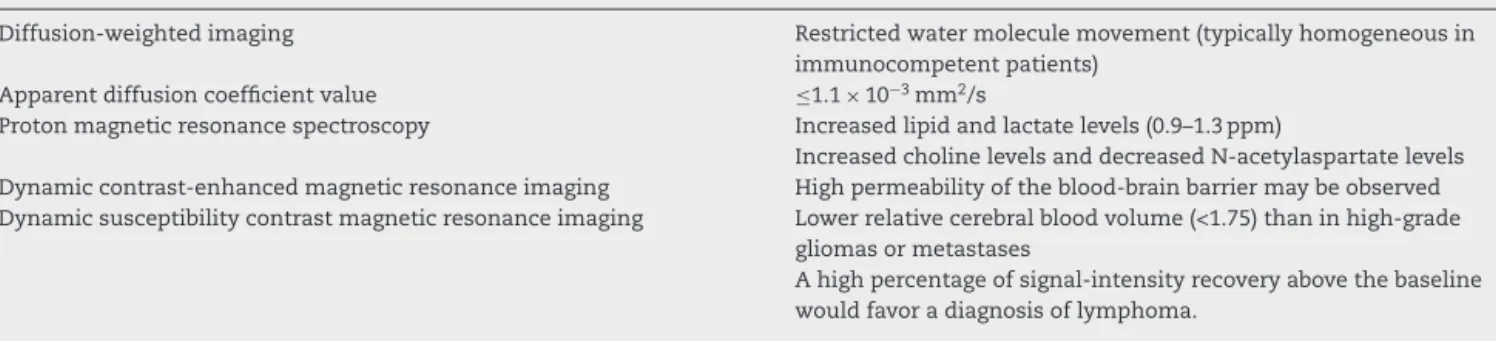
Figure2–Corticotherapyeffectsinaprimarycentralnervoussystemlymphoma.AnaxialT1imageafterintravenous gadoliniumadministration(A)showsmultifocalprimarycentralnervoussystemlymphomaasdeephomogeneous enhancedmassesinthecorpuscallosum,whichareadjacenttothethirdventricle(arrowheads).Acomparative T2-weightedimage(B)depictsnodularhypointensityinthelesionswithperilesionalvasogenicedema(arrowheads).A comparativeT1imageafterintravenousgadoliniumadministration(C)wasobtainedaftercorticotherapyanddemonstrated evidenttumorshrinkage(‘vanishingtumors’).
may be used to differentiate focal brain lesions in PCNSL from neurotoxoplasmosis, cryptococcosis, tuberculosis and intracranial abscesses.21 DWI with ADC maps is useful to
distinguishbetween toxoplasmosisand PCNSL. Early toxo-plasmosismaybeassociatedwithareasofrestricteddiffusion on DWI; however, toxoplasmosis lesions have significantly greaterdiffusionthaninPCNSL.ADCratiosthatrangedfrom 1.0to1.6havebeenreportedforbothPCNSLand toxoplas-mosis,whereasADCratiosgreaterthan1.6wereassociated solelywithtoxoplasmosis.13Inaddition,toxoplasmosismay
beindicatedbythepresenceofLipandLacandadecreasein theNAA/CrratioonMRS.Conversely,markedlyelevatedCho levelswouldfavoraPCNSLdiagnosis.4,11TheaccuracyofMRS
isinverselyproportionaltotheextensionofnecrosis(Figure3).
TheconventionalandnonconventionalMRIand patholog-icalfeaturesofCNSpost-transplantationlymphoproliferative disorder(PTLD)aresimilartothoseofPCNSLandhavebeen observedinimmunocompromisedpatients.22–24Animaging
patternthatisassociatedwithCNSPTLDincludesperipherally enhancingintraparenchymallesions,whichareusually multi-focalsupratentorialmassesthatmayextendtotheependymal surfaceleptomeningeswithnodularenhancement.Imaging playsacrucialroleindiagnosingdisease,guidingbiopsyand monitoringdiseaseresponsesfollowingtherapy.25
Figure3–AfocalbrainlesioninanAIDSpatient(toxoplasmosisversuslymphoma).AnaxialT1imageafterintravenous
gadoliniumadministration(A)showsalargeperiventricularlesionintheleftthalamuswithextensivenecrosis(asterisk) andthickperipheralenhancement.Anaxialapparentdiffusioncoefficientmap(B)confirmedaverylowsignalintensityin thesolidportionofthelesion(arrowheads)comparedwiththecentralareaofnecrosis(asterisk).Additionally,notethe hyperintensityoftheperilesionalvasogenicedema.Protonmagneticresonancespectroscopy(C)confirmedthepresenceof elevatedlipidandlactate(0.9–1.3ppm)andcholinelevelswithreducedN-acetylaspartatelevels.Amagneticresonance perfusionsequence(dynamicsusceptibilitycontrastmagneticresonanceimageT2*)(D)confirmedtheabsenceof neoangiogenesis(lowrelativecerebralbloodvolume).Thesefeaturessupportedthediagnosisoflymphoma.
Becauseof immunosuppression,transplant patientsare at riskfordevelopingseveral CNSinfectionsthatmay leadto meningitis, encephalitis, and focal abscesses. Several CNS infections share many overlapping imaging features with PTLD,particularlyinthefirstmonthsaftertransplantation. NonconventionalMRIhelpsthepromptdiagnosisofdisease, theguidanceofbiopsyandtheinitiationofappropriate treat-ment.
Peculiar
presentations
of
primary
central
nervous
system
lymphomas
Leptomeningealinfiltrationinthecourseoflymphoma, par-ticularly in systemic disease, is not rare. However, this
imaging appearance is nonspecific and may alternatively occur after CNS invasion by infection or in several other primary and systemic malignancies (meningeal carcino-matosis). Associated periventricular masses, mainly with subependymal infiltration, may corroborate a diagnosis of CNSlymphomainadifferentialdiagnosis.1Dural-based
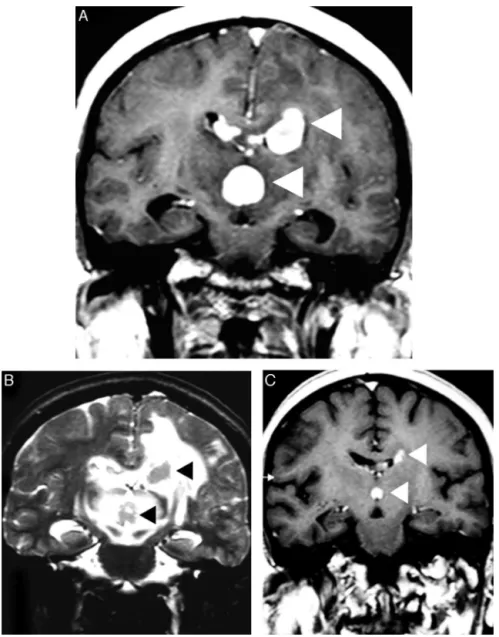
lym-phoma,particularlythemucosa-associatedlymphoidtissue (MALT) subtype, mimicsmeningiomas; therefore, a careful interpretationofimagingfindingsintheclinicalscenariois recommended.Fewreportshavedescribedthetypical presen-tation of intracranial MALT lymphoma-mimicking globular meningiomas(Figure4).1,26–28
Figure4–Mucosa-associatedlymphoidtissue(MALT) lymphomamimickingmeningioma.AsagittalT2-weighted image(A)showsasolidextra-axiallesionthatinfiltrated theduramaterintheparietalregion(arrowheads).A comparativeimageonaT1-weightedsequenceafter intravenousgadoliniumadministration(B)confirmed homogeneouscontrastenhancementthatwasassociated withanextensiveduratail,whichmimickedthe
appearanceofameningioma(arrowhead).
thepachymeninges,mainlyintheabsence ofboneerosion (Figure5).However,this pattern oflesionisnotspecific to PCNSLbecauseithasbeenobservedinsystemiclymphoma andinmanymimiclesions,includingmetastaticinfiltration from prostate carcinoma and breast cancer, osteomyelitis, meningioma,plasmacelltumors,andhistiocytosis.29
Anon-enhancingpresentationofPCNSLwasfoundin1% ofpatientsinalargeseriesofimmunocompetentpatientsand was documented in immunocompromised patients.21,30,31
‘Lymphomatosis cerebri’ is frequently misdiagnosed as diffusenonspecific leukoencephalopathy,Binswanger’s dis-ease (subcortical ischemic vascular dementia), infectious
encephalomyelitisincludingprogressivemultifocal leukoen-cephalopathy, toxic or metabolic abnormalities, unknown autoimmune diseases, or neoplasms such as gliomatosis cerebri.9,32–35DWIandMRSmaysupportapresumptive
diag-nosis of brain infiltrative neoplasia; however, an accurate diagnosisisonlypossibleafterabrainbiopsy.36MRSmayshow
increasedCho levelsand Cho ratios(Cho/NAA>1.9),which suggestinfiltrativeneoplasticdisease(Figure6).35DWIis
vari-ableandcandepictheterogeneousareaswithrestrictedwater moleculemovement.PerfusionMRItechniquesarenot help-fulbecausetherCBVisnotincreasedandthereisnoabnormal permeabilityoftheBBB.
Intravascularlymphomaisoftenmisdiagnosedas multifo-caldemyelination,septicencephalopathy,infectiouslesions, ormultifocalandrecurrentstrokesduetoembolicdiseases or CNS vasculitis (systemic or primary angiitis).34,37
Cere-bralangiographymayrevealmultivesselsegmentalstenoses anddilatations(‘bead-likepattern’)thatareconsistentwith vasculitis.38Intravascularlymphomamayfollowa
relapsing-remitting or relapsing-progressive clinical course due to fluctuating ischemic damage caused by the small vascu-lar occlusions.1,37 A correct presumptive diagnosis may be
supportedbypersistentandprogressiveDWI abnormalities thatareassociatedwithvariablegadoliniumenhancement.36
An extensive imaging investigation is not enough to con-firmintravascularlymphoma,andanearlybrainbiopsyisof paramountimportancetoachieveadefinitivediagnosisand todefinetherapeuticprocedures.37,39,40
Whenprimaryintraocularlymphomasareconsidered,the imagingfeaturesarenotspecificandarefrequently inconclu-sive.Conversely,theimagingfeaturesoforbitallymphomas oftenmimicotherdiseases.Thesediseasesincludemetastatic disease, inflammatory pseudotumors, sarcoidosis, histio-cytosis including Erdheim-Chester disease, meningiomas, cavernous hemangioma, ocular melanomas, and primary lacrimalglandlesions.1,41Incontrasttoorbitalpseudotumors,
primaryorbitallesionsarepainless.42
A DWI analysis is useful to distinguish between lym-phomasandidiopathicorbitalinflammatorypseudotumors, opticnervesheathmeningiomasandgliomas.43,44The
supe-rior lateral quadrant and the extraconal space are typical locationsoforbitallymphomas.Anintriguingpatternis char-acterized by the infiltrative behavior of the tumor, which shapesthe contouroftheeyeballand typicallycrossesthe bony limits.Osteolysismay notbe present.Effective treat-ment responseswereobservedinthefollow-up imagingof orbital lymphomas; however, severalcases oflocal relapse werefound.45
Neurolymphomatosis is extremely rare; therefore, this diagnostic possibility is often overlooked. This disease is oftenmisdiagnosedaschronicinflammatorydemyelinating polyneuropathy. The possibility of concomitant lymphoma arises when thickenedcranial nerves, cauda equinaeroots and/or peripheralnervesare demonstrated,particularly on MRI. Neurolymphomatosisshould beconsidered invarious types of neuropathy evenwhen the diagnostic criteria for chronicinflammatorydemyelinatingpolyneuropathyaremet, particularlyinpatientswhocomplainofpain.46
Figure5–Acranialvaultlymphoma.AnaxialT2-weightedimage(A)showsasmallmulticompartmentallesioninthe occipitoparietalregion(arrow).Axialdiffusion-weightedimaging(B)showsrestrictedwatermoleculediffusioninthesolid portionsofthelesion.AcomparativeT1imageafterintravenousgadoliniumadministration(C)showsanenhancedlesion thatinvolvesthescalp,theskullbone,andthepachymeningesandextendstotheleptomeninges(arrowhead).A
non-enhancedcomputedtomographyscan(D)confirmedtheabsenceofboneerosion.
orrootthickening and variable contrastenhancementthat mimics the imaging appearance of neurolymphomatosis. Additionally, hypertrophy ofthe cauda equinanerve roots isanonspecificfinding andmay beobservedinhereditary and acquired demyelinating neuropathies or in secondary neoplasias due to infiltration of the nerve roots by the neoplasm.Hypertrophyofthecaudaequinanerverootshas beendescribedasalikelyparaneoplasticphenomenoninan elderlypatientwithlymphoplasmacyticlymphoma.47In
cer-taincircumstances,theimagingpatternofneurofibromatosis may mimic the pattern of neurolymphomatosis; however, understandingthesystemicfeaturesofthisneurocutaneous syndromewillbeusefultomakeanaccuratediagnosis.
Primary lymphoma is a rare cause of myelopathies. SpinalMRIhasdemonstratedmultifocallesionswithtypical homogeneous gadolinium enhancement. Conus medullaris or cauda equina involvement is characteristic of primary intramedullaryspinalcordlymphoma;however,a patholog-icalconfirmationoftenrequiresaCNSbiopsy.48,49Lymphoma
Figure6–Lymphomatosiscerebriinanimmunocompetentpatient.Anaxialfluid-attenuatedinversionrecoveryimage(A) showsunspecificdiffusehyperintenseareasonbothbrainhemispheresthatcrossthespleniumofthecorpuscallosum withnomasseffect.Nocontrastenhancementwasobserved(notshown).Acomparativefluid-attenuatedinversion recoveryimage(B)wasobtainedaftertwomonthswithoutspecifictherapyconfirmingtheextensiveprogressionofthe disease,andexhibitingconfluenthyperintensityofbothbrainhemispheres,predominantlyoftheleft.Minimalmasseffect andnogadolinium-enhancedareaswereobserved.Aprotonmagneticresonancespectroscopystudy(C)wasusefulto supportapresumptivediagnosisanddemonstratedincreasedcholinelevelsandanincreasedcholine/N-acetylaspartate ratio(>1.9),whichwereassociatedwithreducedN-acetylaspartatelevelsandelevatedlactatepeaks.
Conclusions
PCNSLaredistinctiveandrarepresentationsofcertain sub-typesoflymphoma,generallythenon-Hodgkindiffuselarge B-celltype,andcontrast-enhancedMRIistheimaging tech-niqueofchoicetoevaluatemostpatientsandtheirvariable presentations.
Modern neuroimaging techniques may help us suspect PCNSLearlier,whichwouldresultintimelytreatmentandless CNSdamage.Radiologistsandhematologistsmustbefamiliar withtheroleofthesenewadvancedtechniques,whichmay helpinthedifferentiationofPCNSLandothermimiclesions.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.HaqueS,LawM,AbreyLE,YoungRJ.Imagingoflymphomaof thecentralnervoussystem,spine,andorbit.RadiolClin NorthAm.2008;46(2):339–61,ix.
2.BaehringJM,LongtineJ,HochbergFH.Anewapproachtothe diagnosisandtreatmentofintravascularlymphoma.J Neurooncol.2003;61(3):237–48.
3.IwamotoFM,DeAngelisLM.Anupdateonprimarycentral nervoussystemlymphoma.HematolOncolClinNorthAm. 2006;20(6):1267–85.
4.ZachariaTT,LawM,NaidichTP,LeedsNE.Centralnervous systemlymphomacharacterizationbydiffusion-weighted imagingandMRspectroscopy.JNeuroimaging.
2008;18(4):411–7.
6. ManglaR,KolarB,ZhuT,ZhongJ,AlmastJ,EkholmS. PercentagesignalrecoveryderivedfromMRdynamic susceptibilitycontrastimagingisusefultodifferentiate commonenhancingmalignantlesionsofthebrain.AJNRAm JNeuroradiol.2011;32(6):1004–10.
7. Al-OkailiRN,KrejzaJ,WooJH,WolfRL,O’RourkeDM,JudyKD. Intraaxialbrainmasses:MRimaging-baseddiagnostic strategy–initialexperience.Radiology.2007;243(2):539–50. 8. HaldorsenIS,EspelandA,LarssonEM.Centralnervous
systemlymphoma:characteristicfindingsontraditionaland advancedimaging.AJNRAmJNeuroradiol.2011;32(6): 984–92.
9. HaldorsenIS,KrakenesJ,KrossnesBK,MellaO,EspelandA. CTandMRimagingfeaturesofprimarycentralnervous systemlymphomainNorway,1989–2003.AJNRAmJ Neuroradiol.2009;30(4):744–51.
10.NordenAD,DrappatzJ,WenPY,ClausEB.Survivalamong patientswithprimarycentralnervoussystemlymphoma, 1973–2004.JNeurooncol.2011;101(3):487–93.
11.RaizerJJ,KoutcherJA,AbreyLE,PanageasKS,DeAngelisLM, LisE,etal.Protonmagneticresonancespectroscopyin immunocompetentpatientswithprimarycentralnervous systemlymphoma.JNeurooncol.2005;71(2):173–80. 12.BarajasRFJr,RubensteinJL,ChangJS,HwangJ,ChaS.
Diffusion-weightedMRimagingderivedapparentdiffusion coefficientispredictiveofclinicaloutcomeinprimarycentral nervoussystemlymphoma.AJNRAmJNeuroradiol.
2010;31(1):60–6.
13.CamachoDL,SmithJK,CastilloM.Differentiationof toxoplasmosisandlymphomainAIDSpatientsbyusing apparentdiffusioncoefficients.AJNRAmJNeuroradiol. 2003;24(4):633–7.
14.TohCH,CastilloM,WongAM,WeiKC,WongHF,NgSH,etal. Primarycerebrallymphomaandglioblastomamultiforme: differencesindiffusioncharacteristicsevaluatedwith diffusiontensorimaging.AJNRAmJNeuroradiol. 2008;29(3):471–5.
15.CianfoniA,ColosimoC,BasileM,WintermarkM,BonomoL. BrainperfusionCT:principles,techniqueandclinical applications.RadiolMed.2007;112(8):1225–43. 16.BraksE,UrbachH,PelsH,TraberF,BlockW,SchildHH.
Primarycentralnervoussystemimmunocytoma:MRIand spectroscopy.Neuroradiology.2000;42(10):738–41.
17.TaillibertS,GuillevinR,MenuelC,SansonM,Hoang-XuanK, ChirasJ,etal.Brainlymphoma:usefulnessofthemagnetic resonancespectroscopy.JNeurooncol.2008;86(2):225–9. 18.FerreriAJ.HowItreatprimaryCNSlymphoma.Blood.
2011;118(13):510–22.
19.ZalneraitisEL,VonsattelJP.Case26-1998.A15-year-oldgirl withhemiparesis,slurredspeech,andanintracraniallesion. NEnglJMed.1998;339(8):542–9.
20.ZhangD,HuLB,HenningTD,RavaraniEM,ZouLG,FengXY, etal.MRIfindingsofprimaryCNSlymphomain26 immunocompetentpatients.KoreanJRadiol. 2010;11(3):269–77.
21.ThurnherMM,RiegerA,Kleibl-PopovC,SettinekU,HenkC, HaberlerC,etal.Primarycentralnervoussystemlymphoma inAIDS:awiderspectrumofCTandMRIfindings.
Neuroradiology.2001;43(1):29–35.
22.BuellJF,GrossTG,HanawayMJ,TrofeJ,Roy-ChaudhuryP,First MR,etal.Posttransplantlymphoproliferativedisorder: significanceofcentralnervoussysteminvolvement. TransplantProc.2005;37(2):954–5.
23.CavaliereR,PetroniG,LopesMB,SchiffD,International PrimaryCentralNervousSystemLymphomaCollaborativeG. Primarycentralnervoussystempost-transplantation lymphoproliferativedisorder:anInternationalPrimary
CentralNervousSystemLymphomaCollaborativeGroup Report.Cancer.2010;116(4):863–70.
24.SenzoloM,FerronatoC,BurraP.Neurologiccomplications aftersolidorgantransplantation.TransplInt.
2009;22(3):269–78.
25.LakeW,ChangJE,KennedyT,MorganA,SalamatS,Baskaya M.Acaseseriesofprimarycentralnervoussystem post-transplantlymphoproliferativedisorder:imagingand clinicalcharacteristics.Neurosurgery.2013;72(6):960–70 [discussion970].
26.IwamotoFM,AbreyLE.Primarydurallymphomas:areview. NeurosurgFocus.2006;21(5):E5.
27.RottnekM,StrauchenJ,MooreF,MorgelloS.Primarydural mucosa-associatedlymphoidtissue-typelymphoma:case reportandreviewoftheliterature.JNeurooncol.
2004;68(1):19–23.
28.AbboudH,CarpentierA,Martin-DuverneuilN,KujasM, Hoang-XuanK.MALTlymphomapresentingasa meningioma.JNeurooncol.2005;75(2):221.
29.daRochaAJ,daRochaTM,daSilvaCJ,PaesRP,BrunieraP, ChiattoneCS.Cranialvaultlymphoma:asystematicreviewof fivepatients.JNeurooncol.2010;100(1):9–15.
30.KükerW,NägeleT,KorfelA,HecklS,ThielE,BambergM, etal.Primarycentralnervoussystemlymphomas(PCNSL): MRIfeaturesatpresentationin100patients.JNeurooncol. 2005;72(2):169–77.
31.LachenmayerML,BlasiusE,NiehusmannP,KovacsA, StuplichM,EichlerO,etal.Non-enhancingprimaryCNS lymphoma.JNeurooncol.2011;101(2):343–4.
32.BakshiR,MazziottaJC,MischelPS,JahanR,SeligsonDB, VintersHV.Lymphomatosiscerebripresentingasarapidly progressivedementia:clinical,neuroimagingandpathologic findings.DementGeriatrCognDisord.1999;10(2):152–7. 33.KanaiR,ShibuyaM,HataT,HoriM,HirabayashiK,TeradaT,
etal.Acaseof‘lymphomatosiscerebri’diagnosedinanearly phaseandtreatedbywholebrainradiation:casereportand literaturereview.JNeurooncol.2008;86(1):83–8.
34.MoussouttasM.Intravascularlymphomatosispresentingas posteriorleukoencephalopathy.ArchNeurol.2002;59(4):640–1. 35.deToledoM,López-ValdésE,FerreiroM,CerveraJL,RamosA,
CabelloA,etal.Lymphomatosiscerebriasthecauseof leukoencephalopathy.RevNeurol.2008;46(11):667–70. 36.FischerL,KochA,SchlegelU,KochHC,WenzelR,SchröderN,
etal.Non-enhancingrelapseofaprimaryCNSlymphoma withmultiplediffusion-restrictedlesions.JNeurooncol. 2011;102(1):163–6.
37.BeristainX,AzzarelliB.Theneurologicalmasqueradeof intravascularlymphomatosis.ArchNeurol.2002;59(3):439–43. 38.SongDK,BoulisNM,McKeeverPE,QuintDJ.Angiotropiclarge celllymphomawithimagingcharacteristicsofCNSvasculitis. AJNRAmJNeuroradiol.2002;23(2):239–42.
39.LozsadiDA,WieshmannU,EnevoldsonTP.Neurological presentationofintravascularlymphoma:reportoftwocases anddiscussionofdiagnosticchallenges.EurJNeurol. 2005;12(9):710–4.
40.KonikkaraJJ,PerurenaOH,WarachS,BausermanSC.A 62-year-oldmanwithfluctuatingneurologicaldeficitsand skinlesions.JAMANeurol.2013;70(1):120–4.
41.McKelviePA.Ocularadnexallymphomas:areview.AdvAnat Pathol.2010;17(4):251–61.
42.YanJ,WuZ,LiY.Thedifferentiationofidiopathic
inflammatorypseudotumorfromlymphoidtumorsoforbit: analysisof319cases.Orbit.2004;23(4):245–54.
43.FatimaZ,IchikawaT,IshigameK,MotosugiU,WaqarAB,Hori M,etal.Orbitalmasses:theusefulnessofdiffusion-weighted imaginginlesioncategorization.ClinNeuroradiol.
44.SepahdariAR,PolitiLS,AakaluVK,KimHJ,AbdelRazekAA. Diffusion-weightedimagingoforbitalmasses:
multi-institutionaldatasupporta2-ADCThresholdmodelto categorizelesionsasbenign,malignant,orindeterminate. AJNRAmJNeuroradiol.2014;35(1):170–5.
45.PriegoG,MajosC,ClimentF,MuntaneA.Orbitallymphoma: imagingfeaturesanddifferentialdiagnosis.InsightsImaging. 2012;3(4):337–44.
46.TomitaM,KoikeH,KawagashiraY,IijimaM,AdachiH, TaguchiJ,etal.Clinicopathologicalfeaturesofneuropathy associatedwithlymphoma.Brain.2013;136Pt8:2563–78.
47.KumarN,DyckPJ.Hypertrophyofthenerverootsofthe caudaequinaasaparaneoplasticmanifestationof lymphoma.ArchNeurol.2005;62(11):1776–7.
48.FlanaganEP,O’NeillBP,PorterAB,LanzinoG,HabermanTM, KeeganBM.Primaryintramedullaryspinalcordlymphoma. Neurology.2011;77(8):784–91.