T Cell Numbers Relate to Bone Involvement in Gaucher Disease
Submitted 04/09/99; revised 04/15/99
(communicated by Ernest Beutler, M.D. 04/19/99)
Lucia Lacerda
1, Fernando A. Arosa
2, Rosa Lacerda
3, Jose´ Cabeda
3,4, Grac¸a Porto
3,4, Olga Amaral
1,
Ana Fortuna
5, Rui Pinto
1, Pedro Oliveira
6, Christine E. McLaren
7, Clara Sa´ Miranda
1, Maria de Sousa
2,3ABSTRACT: The major elements of bone pathology in Gaucher disease are a failure of osteoclast and osteoblast function,
resulting in osteopenia and also osteonecrosis. T lymphocytes have recently been found to be involved in the regulation of
osteoblast/osteoclast activity in vitro. In the present report the peripheral blood T major lymphocyte subsets were
investigated in a group of genotyped type 1 Gaucher disease patients. A total of 31 patients were studied: 21
non-splenectomized (5 N370S homozygotes) and 10 splenectomized (of whom 1 was a N370S homozygote). The results
show that non-splenectomized patients present a decrease in absolute numbers of peripheral blood T lymphocytes, specially
the CD4
⫹T subset. However, when patients were analyzed with respect to the presence of bone disease, the number of
CD8
⫹T lymphocytes was found to be statistically significantly lower in patients presenting bone involvement. Furthermore,
lower numbers of CD8
⫹T lymphocytes were significantly correlated with higher levels of plasma tartrate resistant acid
phosphatase (TRAP) activity, a putative marker of osteoclast cell activity. These in vivo findings are in agreement with the
results reached in vitro by others. They provide an additional marker of disease severity in Gaucher disease. In the group of
genotyped Gaucher disease patients, the majority of the N370S homozygous patients presented a clinically milder
phenotype, including the absence of bone involvement, confirming earlier reports predicting that a number of these patients
may remain undiagnosed. Collectively the homozygosity for the N370S mutation and normal T cell numbers may provide
additional markers for the clinical heterogeneity of Gaucher disease.
r
1999 Academic PressKeywords: Gaucher’s disease, chitotriosidase, tartrate resistant acid phosphatase, CD8 positive T lymphocytes and bone pathology
INTRODUCTION
The most prevalent lysosomal storage disease, Gaucher disease, is an autosomal recessive hereditary disorder of glycosphingolipid metabolism, characterized by accumula-tion of glucosylceramide in cells due to the deficient activity of the lysosomal enzyme glucocerebrosidase (E.C.3.2.1.45). The occurrence of type 1 Gaucher disease (McKusick 230800), the non-neuronophatic form, has an estimated incidence of about 1 in 40.000–200.000 births. A higher prevalence is observed among Ashkenazi Jews in which the incidence is 1 in 625–1.500 (1). Type 1 Gaucher disease is a multi-system disease associated with striking variation in its clinical onset, severity and course.
Moreover, genetic variation in the glucocerebrosidase locus has not provided a reliable prediction of clinical phenotype or prognosis of the disease. In fact, the determination of the frequency of the prevalent N370S mutated allele in the Portu-guese population indicated that the majority of Gaucher disease patients homozygous for this mutation remained undiagnosed (2).
Although glucocerebrosidase is present in almost all cell types, the accumulation of glucosylceramide is charac-teristically observed in macrophages, as the result of their role in the turnover of red blood cells and consequently on the degradation of membrane glycolipids. The occurrence of these lipid loaded macrophages (Gaucher cells) in tissues
1Department of Genetics Neurobiology, Institute for Molecular and Cell Biology of Porto University, Porto, Portugal.
2Department of Pathology and Molecular Immunology, Institute for Molecular and Cell Biology of Porto University, Porto, Portugal. 3Department of Immunology, Abel Salazar Institute for Biomedical Sciences, University of Porto, Porto, Portugal.
4Department of Hematology, St. Anto´nio General Hospital, Porto, Porto, Portugal. 5Clinical Department, Jacinto de Magalha˜es Institute of Medical Genetics, Porto, Portugal. 6Department of Production and Systems, University of Minho, Braga, Portugal.
7Division of Epidemiology, Department of Medicine, University of California, Irvine, CA 92697-7550, USA.
Reprint request to: Prof. Maria de Sousa, Departamento de Patologia e Imunologia Molecular, Instituto de Biologia Molecular e Celular da Universidade do Porto, Rua do Campo Alegre, 823, 4150 Porto, Portugal, telephone 351 2 6074900 (ext: 367), fax 351 2 6099157, e-mail: mdesousa@ibmc.up.pt.
1079-9796/99 $30.00 Copyrightr1999 by Academic Press All rights of reproduction in any form reserved
and organs, underlies the most common signs of pancytope-nia, organomegaly and skeletal deterioration (reviewed in 3). Infiltration of normal bone marrow by lipid-laden Gaucher cells results in apparently progressive displacement of the fat-rich marrow with a consequent shift in haematopoi-etic activity from proximal to more distal sites (4). The pathophysiology is however not clearly understood. In particular, the balance between the impact of altered num-bers and increased activity of osteoclasts and the possibly suppressed osteoblast activity (5). Enzyme supplementation therapy (6) which has as its principal aim the correction and/or prevention of ongoing formation of the lipid loaded macrophages, has proven to be safe and effective in improv-ing the haematological parameters and reducimprov-ing the organo-megaly in Gaucher disease patients. The general experience is however that the skeletal response to therapy lags behind all other symptoms (7). In fact, although tissue distribution studies with tracer doses of radio-iodinated mannose-terminated enzyme show avid uptake in the marrow in proportion to the putative cellular pool of macrophages in this organ (8),direct -glucosidase measurements in bone marrow aspirates showed very little activity of the infused enzyme in the marrow macrophages (9). Recent reports of studies in vitro point to the involvement of T lymphocytes in bone homeostasis via the regulation of osteoclast differentia-tion (10, 11). In the present study, the T lymphocyte profile was studied in Gaucher disease patients in order to verify if abnormalities in the immune system could also be associated to the clinical expression of the disease. Plasma chitotriosi-dase activity, a human chitinase and a putative marker of macrophage activation (12) which may reflect the overall Gaucher cell accumulation in the body (reviewed in 14), and the tartrate resistant acid phosphatase (TRAP) activity (13), which may constitute a marker of osteoclastic activity (15), were also studied.
PATIENTS AND METHODS
Glucocerebrosidase Activity
The biochemical diagnosis of the Gaucher disease patients was done by determining the residual activity of glucocerebrosidase in peripheral blood total leukocytes (16).
Genotype Analysis
Patients were genotyped using previously described methods. Most of their genotypes have already been re-ported (17, 18).
Clinical Evaluation
The patients’ present ages range from 10 to 62 years, 11 of them being male and 20 women. In general all
non-splenectomized patients suffered from enlargement of the liver and spleen with subsequent pancytopenia and abdomi-nal pain. Ten patients had been subjected to total splenec-tomy which, with the exception of one patient, resulted in the correction of anaemia and thrombocytopenia.
The accumulation of Gaucher cells in bone marrow is associated with varying degrees of necrosis, fibrous prolifera-tion and resorpprolifera-tion of the bony trabeculae, followed by erosion of the endosteal surface of the cortex and modelling deformities. Bone pathology was evaluated by treating clinicians by several methods including X-ray, magnetic resonance imaging (MRI) and bone tomodensitometry. The lesions detected range from diffuse osteoporosis (stage 1), to medullary expansion (stage 2), to osteolysis (stage 3), to necrosis/sclerosis (stage 4), to destruction and collapse (stage 5) (19). One of the earliest clinical signs of bone involvement is the Erlenmeyer flask deformity of the distal femur and the proximal tibia (stage 2) which although not pathognomonic of the disease, is seen in most patients at presentation.
For each patient, the clinical severity was calculated on the basis of the Zimran severity score index (SSI), which takes into account the age of onset, the degree of organomegaly, liver function tests, clinical signs of liver disease, cytopenia, bone involvement and other organ involvement (20).
Patients submitted to intravenously administered en-zyme supplementation therapy started treatment with alglu-cerase, human glucocerebrosidase purified from human placenta and enzymatically deglycosylated to reveal termi-nal mannose residues, which are recognized by the macro-phages membrane mannose-specific receptors, allowing the targeting and intracellular trafficking of the enzyme (21). Presently all treated patients are receiving the recently available imiglucerase (human glucocerebrosidase ex-pressed as the recombinant product in engineered Chinese hamster ovary cells and enzymatically deglycosylated). With respect to the dosage and frequency of administration, all the presented five patients started with 60 U/kg/every 2 weeks. Treatment regimens were changed according to the patient’s clinical response, as follows: Patient 19, 33 and 35 reduced the receiving dose to 30U after respectively 3, 6 and 9 months of treatment. Patient 33 returned to the initial dose after the 12th month of treatment. Patients 19, 41, 35, 33 and 1 changed to imiglucerase at respectively the 14, 18, 18, 27th and the 33rd month of treatment.
All studies were done with the patients’ consent.
Tartrate Resistant Acid Phosphatase
(TRAP) Activity
TRAP activity was measured in plasma separated from heparinised peripheral blood (22). Acidified plasma was incubated with 5 mmol/l 4-methylumbelliferyl-phosphate substrate, prepared in 0.1 mol/l sodium citrate buffer, pH 6.0, with 0.02 mol/l tartaric acid.
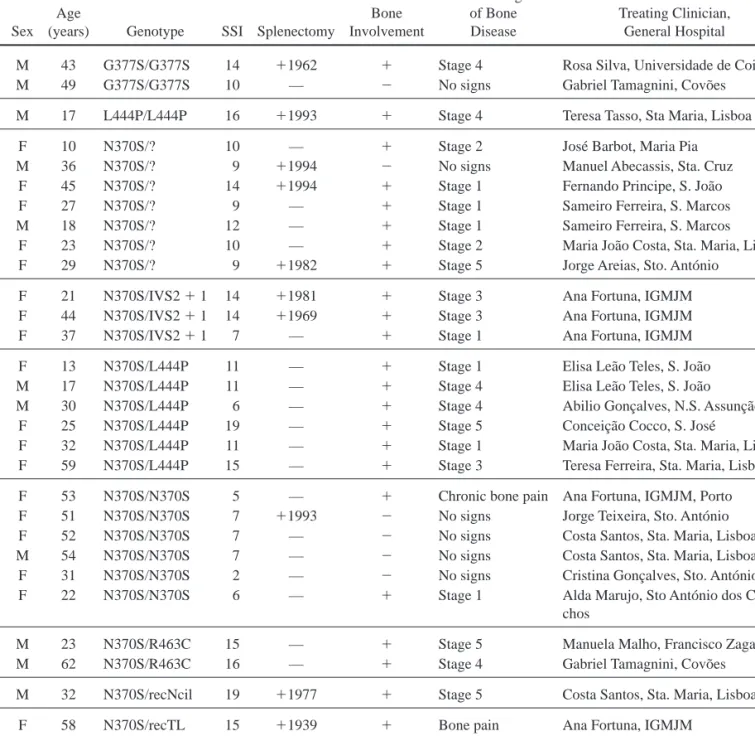
Table 1. Characteristics of GD Patients
Case Sex Age
(years) Genotype SSI Splenectomy
Bone Involvement Clinical Signs of Bone Disease Treating Clinician, General Hospital
29 M 43 G377S/G377S 14 ⫹1962 ⫹ Stage 4 Rosa Silva, Universidade de Coimbra 49 M 49 G377S/G377S 10 — ⫺ No signs Gabriel Tamagnini, Covo˜es
41 M 17 L444P/L444P 16 ⫹1993 ⫹ Stage 4 Teresa Tasso, Sta Maria, Lisboa 19 F 10 N370S/? 10 — ⫹ Stage 2 Jose´ Barbot, Maria Pia 30 M 36 N370S/? 9 ⫹1994 ⫺ No signs Manuel Abecassis, Sta. Cruz 31 F 45 N370S/? 14 ⫹1994 ⫹ Stage 1 Fernando Principe, S. Joa˜o 35 F 27 N370S/? 9 — ⫹ Stage 1 Sameiro Ferreira, S. Marcos 39 M 18 N370S/? 12 — ⫹ Stage 1 Sameiro Ferreira, S. Marcos 46 F 23 N370S/? 10 — ⫹ Stage 2 Maria Joa˜o Costa, Sta. Maria, Lisboa 47 F 29 N370S/? 9 ⫹1982 ⫹ Stage 5 Jorge Areias, Sto. Anto´nio
7 F 21 N370S/IVS2⫹ 1 14 ⫹1981 ⫹ Stage 3 Ana Fortuna, IGMJM 8 F 44 N370S/IVS2⫹ 1 14 ⫹1969 ⫹ Stage 3 Ana Fortuna, IGMJM 14 F 37 N370S/IVS2⫹ 1 7 — ⫹ Stage 1 Ana Fortuna, IGMJM 10 F 13 N370S/L444P 11 — ⫹ Stage 1 Elisa Lea˜o Teles, S. Joa˜o 11 M 17 N370S/L444P 11 — ⫹ Stage 4 Elisa Lea˜o Teles, S. Joa˜o 23 M 30 N370S/L444P 6 — ⫹ Stage 4 Abilio Gonc¸alves, N.S. Assunc¸a˜o 33 F 25 N370S/L444P 19 — ⫹ Stage 5 Conceic¸a˜o Cocco, S. Jose´
34 F 32 N370S/L444P 11 — ⫹ Stage 1 Maria Joa˜o Costa, Sta. Maria, Lisboa 56 F 59 N370S/L444P 15 — ⫹ Stage 3 Teresa Ferreira, Sta. Maria, Lisboa
5 F 53 N370S/N370S 5 — ⫹ Chronic bone pain Ana Fortuna, IGMJM, Porto 18 F 51 N370S/N370S 7 ⫹1993 ⫺ No signs Jorge Teixeira, Sto. Anto´nio 21 F 52 N370S/N370S 7 — ⫺ No signs Costa Santos, Sta. Maria, Lisboa 32 M 54 N370S/N370S 7 — ⫺ No signs Costa Santos, Sta. Maria, Lisboa 38 F 31 N370S/N370S 2 — ⫺ No signs Cristina Gonc¸alves, Sto. Anto´nio 42 F 22 N370S/N370S 6 — ⫹ Stage 1 Alda Marujo, Sto Anto´nio dos
Capu-chos
1 M 23 N370S/R463C 15 — ⫹ Stage 5 Manuela Malho, Francisco Zagalo 51 M 62 N370S/R463C 16 — ⫹ Stage 4 Gabriel Tamagnini, Covo˜es 36 M 32 N370S/recNcil 19 ⫹1977 ⫹ Stage 5 Costa Santos, Sta. Maria, Lisboa 12 F 58 N370S/recTL 15 ⫹1939 ⫹ Bone pain Ana Fortuna, IGMJM
20 F 26 N370S/X 5 — ⫹ Stage 2 Maria Joa˜o Costa, Sta. Maria, Lisboa 37 F 37 X/X 6 — ⫹ Bone pain Alberto Rosa, Sto. Espirito
Chitotriosidase Activity
Chitotriosidase activity was measured in plasma sepa-rated from heparinised blood (12). Acidified plasma was incubated with 0.0260 mmol/l 4-methylumbelliferyl- -D-N,N8,N9-triacetylchitotriose substrate prepared in 0.1mol/l citrate–phosphate buffer, pH 5.2.
Hematological Parameters
Whole blood cell counts were done in a Coulter JS automatic cell counter.
Freshly collected peripheral blood mononuclear cells were stained with CD4-FITC (or PE), CD8-FITC (or PE) and CD3-FITC monoclonal antibodies from DAKOPATTS and analyzed in a FACscan as described previously (23).
Statistical Analysis
Subjects were subdivided into three categories accord-ing to genotype. The chi-square test for independence was used to examine the association between bone involvement (present, absent) and genotype subgroup (NS370S/NS370S, NS370S/L444, and other). The nonparametric Kruskal– Wallis analysis of variance was applied to test for differences in the average Zimran severity score index in the three genotype subgroups. The Kruskal–Wallis test was also used to compare the average number of peripheral blood CD4⫹ and CD8⫹ T lymphocytes found in controls with that of non-splenectomized Gaucher disease patients subdivided according to presence or absence of bone involvement. Spearman rank correlation coefficients were calculated to test for a linear association between the number of CD4⫹ and CD8⫹ T lymphocytes and plasma TRAP enzymatic activity. All statistical tests were performed at the level of significance of 0.05. These data were analyzed using SPSS (Statistical Program for the Social Sciences).
We also performed longitudinal patient-specific analy-ses to detect changes in the mean number of T lymphocyte cells and mean chitotriosidase and TRAP activity in those patients treated with alglucerase/imiglucerase enzyme supple-mentation therapy for at least 24 months. For the analysis from a single patient, multiple regression models were formed in a hierarchical step-down fashion (24). The response variables were percent of initial activity for CD4⫹ cells, CD8⫹cells, chitotriosidase activity and TRAP activity. To model the mean change in each response variables, indicator variables represented whether a change did, or did not, occur during each specified month of therapy. All possible subsets of indicator variables (i.e. months) were considered using the method known as Leaps and Bounds (25). Thus, models with one or more change(s) in the mean response were compared to that of the null model (no change in the mean response during therapy). A minimum risk
criteria was applied for model selection (26). Specialized programs for these longitudinal analyses were written using the statistical programming language S-PLUS 4.5 (Math-Soft, Inc., Seattle, Washington).
RESULTS
Patients’ characteristics including age, sex, genotype, clinical presentation with respect to splenectomy, bone involvement, and the overall disease severity, presented as severity score (SSI) on the basis of the respective clinician assessment, are shown in Table 1. When analyzed by genotype, the association between bone involvement (pre-sent, absent) and genotype subgroup (NS370S/NS370S, NS370S/L444, and other) was statistically significant (p⫽.013). A lower proportion of N370S/N370S homozy-gous patients was found to present bone involvement (Table 1).
As can be observed in Figure 1, this is reflected in a comparatively milder overall clinical presentation of the N370S homozygotes, as assessed by the Zimran severity score index (SSI) on the basis of the respective clinician assessment (Figure 1).
Peripheral Blood CD4
⫹and CD8
⫹T Lymphocyte
Subpopulations
The study of the peripheral blood total leukocytes showed that the large majority of non-splenectomized Gaucher disease patients presented with leucopenia (mean⫾ sd; 3 995 ⫾ 1 721 cells ⫻ 106/l, n⫽ 20) whereas
the splenectomized ones presented in general a leucocytosis (13 240⫾ 4 901, n ⫽ 10, p ⬍ 0.01).
Non-splenectomized patients were analyzed separately and when compared to controls, with one exception, Gau-cher disease patients were found to present decreased numbers of the CD4⫹ T lymphocyte subset (Figure 2).
Figure 1. Clinical severity (as assessed by the Zimran’s
severity score index) of 31 genotyped Gaucher disease patients.
Although the number of Gaucher disease patients without bone involvement is low (n⫽ 4), the number of CD8⫹T cells tended to be distinct on the basis of the presence or absence of bone disease (Figure 2).
In an analysis comparing the number of T cell subsets in Gaucher disease patients with that of controls, a significant difference (p ⬍ 0.05) between the number of both CD4⫹ (483.1 ⫾ 173.4) and CD8⫹ (333.9⫾156.0) T lymphocyte subsets (cells⫻ 106/l) was observed in non-splenectomized
patients presenting bone involvement (n⫽ 16), as compared to controls (respectively 941.2⫾ 289.6 and 435.0 ⫾ 136.1, n⫽ 56) (Table 2). The available number of Gaucher disease patients without bone disease is however not sufficient to substantiate the differences in T cell numbers between patients with and without bone involvement.
Peripheral Blood T Lymphocyte CD4
⫹and CD8
⫹Subpopulations and Plasma Tartrate Resistant
Acid Phosphatase (TRAP) Activity
To study whether T lymphocyte numbers could be associated with osteoclastic activity, plasma TRAP
enzy-matic activity was measured in all the patients. As shown in Figure 3, in non-splenectomized Gaucher disease patients with bone involvement, a significant negative correlation was observed between TRAP activity and CD8⫹T lympho-cyte cell numbers (r⫽ ⫺0.508, p ⬍ 0.05). With respect to the CD4⫹T lymphocyte subset, of the regression models fit to the data (e.g. linear, cubic, exponential) none had acceptable goodness-of-fit.
Peripheral Blood CD4
⫹and CD8
⫹T Lymphocyte
Sub-Populations in Patients Submitted to the
Enzyme Supplementation Therapy
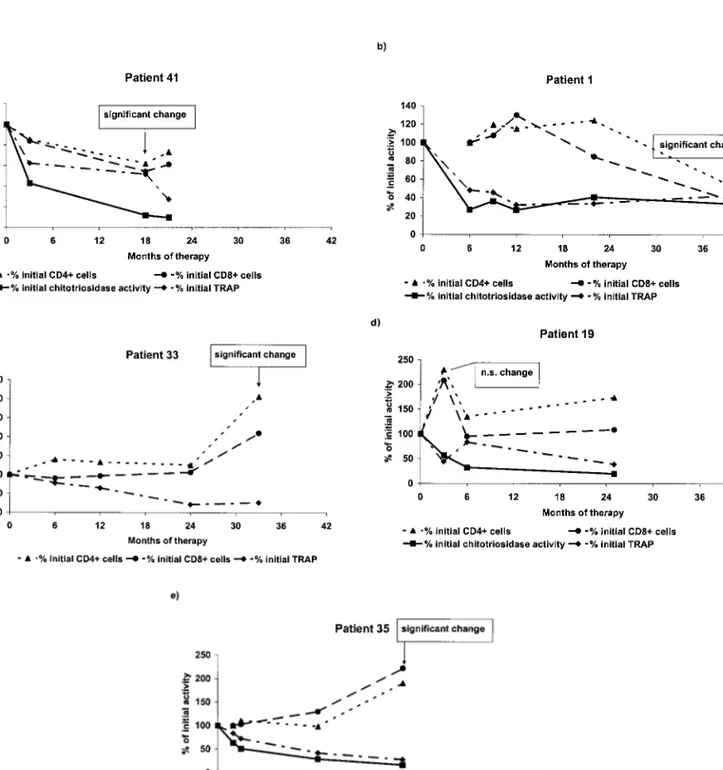
To address the question whether the abnormally low T cell numbers presented by patients with bone involvement reflected disease activity, the peripheral blood T lymphocyte subset numbers were followed in 5 patients undergoing alglucerase/imiglucerase enzyme supplementation therapy for at least 24 months. As shown in Figure 4, in four from
Figure 2. Peripheral blood CD4⫹and CD8⫹T lymphocytes (cells ⫻ 106/l) in non-splenectomized Gaucher disease
patients with and without bone involvement. a) CD4⫹ T lymphocytes; b) CD8⫹T lymphocytes.
Figure 3. Peripheral blood CD8⫹T lymphocytes (cells⫻ 106/l) and plasma tartrate resistant acid phosphatase, TRAP
activity (nmol/h/ml) of non-splenectomized Gaucher disease patients Spearman rank correlation.
Table 2. Peripheral Blood CD4⫹and CD8⫹T Lymphocyte Subsets (cells⫻106/l) in Non-splenectomized GD Patients
and Controls GD patients with bone involvements Controls CD4ⴙT 483.1⫾ 173.4 941.2⫾ 289.6 p⬍0.01 lymphocytes (16) (56) CD8ⴙ 333.9⫾ 156.0 435.0⫾ 136.1 p⬍0.05 lymphocytes (16) (56)
Values represent the mean⫾ sd of each population gated on total lymphocytes. The number of analyzed individuals is indicated between brackets. Probability (p) values below 0.05 are considered significant.
Figure 4. Peripheral blood CD4⫹and CD8⫹T lymphocyte subsets (% of initial number of cells) and plasma chitotriosidase activity (% of initial activity) in patients submitted to the enzyme supplementation therapy. Significant changes in the number of T lymphocyte were indicated by arrows. Patient 33 presents an inherited chitotriosidase deficiency.
five Gaucher disease patients, longitudinal patient-specific analysis showed that statistically significant changes in T lymphocyte cells occurred only after 12 months of therapy. Two patients showed a decrease in mean CD4⫹and CD8⫹ cells from pretreatment values. For patient 41, a significant decrease in mean CD4⫹ cells was observed by 18 months after the beginning of enzyme supplementation therapy. This decrease to 68% of initial T lymphocyte numbers (95% confidence interval: 48 to 84%) was accompanied by a corresponding decrease in CD8⫹ cells of 58% of initial number (41 to 75%). At this time the therapeutic regimen was changed to imiglucerase. Similarly, patient 1 showed an estimated mean decrease of 45% of initial numbers (13 to 47%) in CD4⫹cells and a percentage decrease in CD8⫹cells of 30% (13 to 47%) by 42 months of therapy. We note that the treatment was changed to imiglucerase at the 33rdmonth
of treatment. In contrast, two additional patients responded to therapy with an increase in mean CD4⫹and CD8⫹cells. For patient 33, an estimated increase of 306% of baseline in CD4⫹ T cells (271%, 341%) had occurred by 33 months after beginning of therapy, 6 months after beginning of therapy with imiglucerase, with a corresponding mean percentage increase in CD8⫹cells of 211% (196 to 226%). By 24 months of therapy, patient 35 showed a statistically significant mean increase of 191% from baseline (184%, 198%) in CD4⫹cells and of 222% (203%, 241%) in CD8⫹ cells, 6 months after beginning of therapy with imiglucerase. Using this longitudinal analysis, the change in T cells of patient 19, observed at the 3rd month of therapy was not significant.
The abnormally high chitotriosidase activity in Gaucher disease has been a useful marker of response to therapy, being thus included in this study. One of the patients (patient 33) presents the inherited deficiency in chitotriosidase activity, which is observed in about 6% of the population (12). With this exception, and as can be observed in Figure 4, therapy has the effect of progressively lowering chitotriosi-dase activity to a sustained level. With respect to TRAP activity, in some patients it can be observed a comparatively slower and irregular decrease (patients 41, 1 and 19), observation that agrees to the unclear evolution of bone disease in these patients. Using longitudinal patient-specific statistical analyses, we examined the chitotriosidase and TRAP activity in these five patients, to test for statistically significant changes from pretreatment mean baseline activ-ity. We found that for 4 of 5 patients one to two significant decreases occurred that preceded significant changes in CD4⫹ and CD8⫹ T cells. For example, chitotriosidase activity decreased to 33% of initial activity (95% confidence interval: 24 to 42%) by the 6th month of therapy for patient 1 and to 57% of initial activity (43 to 71%) by the 3rdmonth of
therapy for patient 41. Similarly, by the 6th and 3rd months of therapy respectively, significant decreases in TRAP activity were observed from 41% of initial activity (25%, 58%) for patient 1 and to 57% of initial activity (43 to 71%)
for patient 41. Using this longitudinal analysis, no statisti-cally significant mean changes in chitotriosidase or TRAP activity were observed for patient 19.
The coefficient of determination for models of changes in mean blood T lymphocyte subsets, chitotriosidase, or TRAP activity varied from 79 to 100% indicating a good fit of longitudinal non-linear regression models to the data.
Clinically it must be stated that after about 24 months of treatment, patient 33 showed imagiological signs of improve-ment (e.g. closed pathological fracture) whereas patient 1 started to present vertebral compression. With respect to the patients submitted to less period of treatment, no improve-ment was observed in the bone lesions.
DISCUSSION
Bone involvement is one of the least understood aspects of the clinical heterogeneity of Gaucher disease. In this and in an earlier study of Portuguese Gaucher disease patients genotyped for the most frequent glucocerebrosidase muta-tions (17), it was found that patients homozygous for the N370S mutated allele have a milder presentation of Gaucher disease. In the present study, 4 of the 6 N370S homozygous Gaucher disease patients were classified as having no bone involvement. Although this indicates that genotype is impli-cated in the outcome of clinical phenotype, it is an insuffi-cient explanation for the mechanism(s) involved in the pathophysiology of Gaucher disease. The study of periph-eral blood T lymphocytes in Gaucher disease patients showed that the number of CD8⫹ T lymphocytes was statistically significantly lower in patients presenting bone involvement. Decreased CD8⫹T lymphocyte numbers have also been reported in other diseases associated with bone resorption (27, 28). Studies of the influence of T lympho-cytes in co-cultures with bone marrow cell populations containing osteoclast precursors, demonstrated that deple-tion of T lymphocytes from the cultures resulted in the expansion of the number of osteoclasts in vitro (9). More recently (10), a mechanism was proposed for the osteoclas-togenesis inhibition, in which IL-18 (produced by stromal cells or other cells of the bone microenvironment) act on T cells via an unidentified receptor, to increase GM-CSF production, which ultimately inhibits osteoclastogenesis. These models are useful in dissecting the components of a particular effect but their exact relevance must be tested in vivo. The finding of a statistically significant correlation between the number of CD8⫹T lymphocytes and increased TRAP activity (a putative marker of osteoclastic activity) is indeed in keeping with the observations reported in vitro. The finding of low CD4⫹T lymphocytes is also of particular interest in view of earlier work where an increase in GM3 ganglioside was reported in tissues of Gaucher disease patients (29) and more recent studies showing that ganglio-sides interaction with CD4 favors its internalization and degradation (30, 31).
The present study of serial samples obtained during enzyme supplementation therapy has the additional interest of permitting to do a longitudinal analysis of lymphocyte changes with correction of the enzymatic defect. The progressive decrease of chitotriosidase is an indicator of diminishing glucocerebrosidase loaded macrophages (Gau-cher cells) activation. With respect to the patients submitted to the larger period of therapy, in spite of the initial reduction of chitotriosidase activity in patient 1, the spleen remained highly fibrotic and a significant decrease in numbers of lymphocytes was observed. Interestingly, this significant decrease in T lymphocytes coincided with worsening of the bone involvement. In patient 33 the significant decrease of splenomegaly and increase in numbers of T lymphocytes coincided with an improvement in bone involvement. Inde-pendently of the reported low delivery of the enzyme to the marrow macrophages (9), in those GD patients in whom the CD8⫹ T lymphocyte decreased numbers are due to the spleen sequestration, the reduction of splenomegaly due to the enzyme supplementation could be expected to result in an improvement of bone pathology.
The present results albeit preliminary constitute an additional illustration of the growing importance of the T lymphocyte system in the putative regulation of other systems as already demonstrated in other diseases. They point also to the potential usefulness of the determination of subset lymphocyte numbers as an indicator of severity of disease and a predictive marker of response to treatment.
ACKNOWLEDGMENTS
This work received financial support from JNICT projects PRAXIS 2/2.1/SAU/1323/95, PECS/TTSAU/ 88/95 and BD 5547/95. Support was also received from the National Institutes of Health, USA, by Research Grants R15 HL48349 and F06 TW02117 (CEM). The authors are grateful to Prof. J. Aerts, whose laboratory performed the chitotriosidase genotype analysis of Gau-cher disease patients.
REFERENCES
1. Beutler E, Grabowski G. Gaucher disease. In: Scriver C, Beaudet A, Sly W, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease on CD-ROM. New York: McGraw-Hill, Inc. 1997.
2. Lacerda L, Amaral O, Pinto R, Oliveira P, Aerts J, Sa´ Miranda MC. Gaucher disease: N370S glucocerebrosi-dase gene frequency in the Portuguese population. Clin Genet 45:298–300, 1994.
3. Cox T, Schofield J. Gaucher’s disease: clinical features and natural history. Baillie`res Clin Haematol 10:657– 689, 1997.
4. Rosenthal D. Quantitative imaging of the skeleton in patients with Gaucher disease, Gaucher Clinical Per-spectives 3:4–8, 1995.
5. Siffert R, Platt G. Gaucher disease: Orthopedic consid-erations. In: Desnick R, Gatt S, Grabowski G, eds. Gaucher disease. A Century of Delineation and Re-search. New York: Alan R Liss, pp. 617–626, 1982. 6. Barton N, Brady R, Dambrosia et al. Replacement
therapy for inherited enzyme deficiency: macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med 324:1464–1470, 1991.
7. Elstein D, Hadas-Halpern I, Itzchaki M, Lahad A, Abrahamov A, Zimran A. Effect of low-dose enzyme replacement therapy on bones in Gaucher disease patients with severe skeletal involvement. Blood Cells Mol Dis 22:104–111, 1996.
8. Mistry P, Wraight E, Cox T. Therapeutic delivery of proteins to macrophages: implications for treatment of Gaucher’s disease. Lancet 348:1555–1559, 1996. 9. Beutler E, Kuhl W, Vaughan LM. Failure of
alglucer-ase infused into Gaucher disealglucer-ase patients to localize in marrow macrophages. Mol Med 1:320–324.
10. John V, Hock J, Short L, Glasebrook A, Sells, Galvin R. A role for CD8⫹ T lymphocytes in osteoclast differentiation in vitro. Endocrinology 137:2457– 2463, 1996.
11. Horwood N, Udagawa N, Elliott J et al. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest 101:595–603, 1998.
12. Hollak C., van Weely S, van Oers M, Aerts J. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest 93:1288– 1292, 1994.
13. Aerts J, Hollak C. Plasma and metabolic abnormalities in Gaucher’s disease. Baillie`res Clin Haematol 10:691– 709, 1997.
14. Ek-Rylander B, Bill P, Norgard M, Nilsson S, Anders-son G. Cloning, sequence, and developmental expres-sion of type 5, tartrate-resistant, acid phosphatase of rat bone. J. Biol Chem 266:24684–24689, 1991. 15. Ek-Rylander B, Flores M, Wendel M, Heinegard D,
Andersson G. Dephosphorylation of osteopondin and bone sialoprotein by osteoclastic tartrate-resistant acid-phosphatase. Modulation of osteoclast adhesion in vitro. J Biol Chem 269:14853–14856, 1994.
16. Sa´ Miranda MC, Aerts JM, Pinto R et al 1990. Activity of glucocerebrosidase in extracts of different cell types from type 1 Gaucher disease patients. Clin Genet 38:218–227, 1990.
17. Amaral O, Lacerda L, Santos R, Pinto R, Aerts J, Sa´ Miranda MC. Type I Gaucher disease: molecular, biochemical and clinical characterization of patients from Northern Portugal. Biochem Med Metab Biol 49:97–107, 1993.
18. Amaral O, Pinto E, Fortuna M, Lacerda L, Sa´ Miranda MC. Type 1 Gaucher disease: identification of N396T and prevalence of glucocerebrosidase mutations in the Portuguese. Hum Mutat 8:280–281, 1996.
19. Hermann G, Goldblatt J, Levy R, Goldsmith SJ, Desnick RJ, Grabowski GA. Gaucher’s disease type 1: assessment of bone involvement by CT and scintigra-phy. Am J Rad 147:943–948, 1986.
20. Zimran A, Gross E, West C, Sorge J, Kubitz M, Beutler E. Prediction of severity of Gaucher’s disease by identification of mutations at DNA level. Lancet 2:349–352, 1989.
21. Furbish F, Steer C, Krett N, Barranger J. Uptake and distribution of placental glucocerebrosidase in rat hepatic cells and effects of sequential deglycosylation. Biochem. Biophys. Acta 673:425–434, 1981.
22. Magalha˜es J, Pinto R, Lemos M, Sa´ Miranda M, Poenaru L. Age dependency of serum acid phospha-tase in controls and Gaucher patients. Enzyme 32:95– 99, 1984.
23. Arosa F, da Silva A, Godinho et al. 1994. Decreased CD8-p56lck activity in peripheral blood T-lympho-cytes from patients with hereditary haemochromatosis. Scand J Immunol 39:426–432, 1994.
24. McLaren CE, Kambour EL, McLachlan GJ, Lukaski HC, Li X, Brittenham GM, McLaren GD. Multiple linear regression and finite mixture distribution
model-ing for sequential analysis of hematological data. Stat Med, in press, 1999.
25. Furnival GM, Wilson RW, Jr. Regression by leaps and bounds. Technometrics 16:599–511, 1974.
26. Eubanks RL. Spline Smoothing and Nonparametric Regression, New York: Marcel Deker, Inc., pp. 438, 1988.
27. Consolini R, Cini P, Cei B, Botton E. Thymic dysfunc-tioning histiocytosis X. Am J Pediatr Hematol/Oncol 9:146–148, 1987.
28. Nagasawa T, Nitta H, Watanable H, Ishikawa I. Reduced CD8⫹ peripheral blood T lymphocytes in rapidly progressive periodontitis. Arch Oral Biol 40: 605–608, 1995.
29. Philipart N, Menkes J. Characterization of the main splenic glycolipids in Gaucher’s disease: Evidence for the site of metabolic block. Biochem Biophys Res Commun 15:551–555, 1964.
30. Grassi F, Lopalco L, Lanza P et al. Chemical residues of ganglioside molecules involved in interactions with lymphocyte surface targets leading to CD4 masking and inhibition of mitogenic proliferation. Eur J Immu-nol 20:145–150, 1990.
31. Saggioro D, Sorio C, Calderazzo et al. Mechanism of action of the monosialoganglioside GM1 as a modula-tor of CD4 expression. J Biol Chem 268:1368–1375, 1993.