Universidade de Lisboa
Faculdade de Farmácia
Hepatocellular Carcinoma: The Progress of
Treatment and Future Tendencies
Jorge Ricardo Honda
Mestrado Integrado em Ciências Farmacêuticas
Universidade de Lisboa
Faculdade de Farmácia
Hepatocellular Carcinoma: The Progress of
Treatment and Future Tendencies
Jorge Ricardo Honda
Monografia de Mestrado Integrado em Ciências Farmacêuticas apresentada à Universidade de Lisboa através da Faculdade de Farmácia
Orientador: Professora Doutor Noélia Duarte
Resumo
O cancro é uma das principais causas de morte em todo o mundo, com mais de catorze milhões de novos casos diagnosticados anualmente. Os tumores hepáticos são os segundos mais mortais entre os indivíduos do sexo masculino e o Carcinoma Hepatocelular é o responsável por mais de noventa porcento desses casos. Carcinoma Hepatocelular é o terceiro maior responsável pelas mortes decorrentes do cancro devido à falta de alternativas para o tratamento e ao diagnóstico tardio que é efetuado na maior parte dos casos. Vários fatores de risco para esta patologia foram identificados incluindo a infeção pelo vírus da Hepatite B ou C, o consumo excessivo de álcool, dieta com exposição a aflotoxinas, diabetes e obesidade. Atualmente, o sistema de estadiamento mais consensual entre as sociedades internacionais é o Barcelona Clinic Liver Cancer que divide os doentes entre cinco categorias distintas. Os doentes classificados nos três últimos estadios deparam-se com poucas alternativas terapêuticas e uma baixa probabilidade de sobrevivência. O objetivo desta revisão é compreender os métodos disponíveis para estes doentes segundo o sistema Barcelona Clinic Liver Cancer (embolia química transarterial e quimioterapia com Sorafenib), identificar as principais lacunas destas alternativas e investigar em que direção está a ser levada a terapêutica para estes doentes através dos estudos mais recentes desenvolvidos nesta área. Em suma, a terapêutica localizada é a que demonstra resultados mais favoráveis e tendências mais promissoras, devendo ser o principal foco da investigação nesta área. Esta utiliza uma característica única deste tipo de tumores, a sua abundante irrigação arterial, não-fisiológica, o que permite uma ação local eficiente sem complicações de toxicidade sistémica. No entanto, no que toca à quimioterapia, desde a aprovação do Sorafenib há cerca de dez anos, não houve grandes desenvolvimentos que levassem a uma melhoria marcada na qualidade de vida ou sobrevivência dos doentes.
Palavras-chave: Carcinoma Hepatocelular, Sorafenib, terapia localizada, TACE,
Abstract
Cancer is one of the leading causes of death worldwide, with over fourteen million new cases being diagnosed each year. Liver cancer is the second most lethal cancer among men around the globe and Hepatocellular Carcinoma in particular accounts for up to ninety percent of all those cases. Hepatocellular Carcinoma is the third most deadly cancer worldwide due to the lack of treatment alternatives and to the late stage in which most of the cases are diagnosed. Several risk factors for Hepatocellular Carcinoma have been identified including Hepatitis B and C infections, alcohol abuse, dietary exposure to aflatoxins, diabetes and obesity. Currently the most widely accepted staging system is the Barcelona Clinic Liver Cancer dividing patients between five distinct groups. The patients placed on the latter three stages have few treatment options and poor chances of survival. The aim of this review is to fully understand the procedures available for the patients (Transarterial Chemoembolization and Sorafenib systemic therapy), identifying the major flaws and analyzing the trend of research that will potentiate the enhanced treatment options of the future. In summary, the localized therapies show much more promising results and should be target of further investigation. They make use of a unique characteristic of this type of tumor and the implication of systemic toxicity is very low. Efforts should be made to overcome the major contraindications of this type of therapy making it available for a wider group of patients. Systemic therapies however have shown minor improvement since the approval of Sorafenib for HCC treatment and the trend of research does not indicate any major breakthrough for the immediate future.
Keywords: Hepatocellular Carcinoma, localized therapies, palliative treatment,
Abbreviations
AFP – Alpha fetoprotein;
BCLC – Barcelona clinic liver cancer; BHP – Bipotential hepatic progenitor;
CT – Computed tomography;
DC – Dendritic cells;
DEB-TACE – Transarterial chemoembolization with doxorubicin eluding beads;
DNA – Deoxyribonucleic Acid; HBV – Hepatitis B virus;
HCC – Hepatocellular carcinoma; HCV – Hepatitis C virus;
HKLC – Hong Kong liver cancer;
MRI – Magnetic Resonance Imaging; PDGF – Platelet-derived growth factor;
PI3K – Phosphatidylinositol 3-kynase; RFA – Radiofrequency ablation;
SHARP – Sorafenib hepatocellular carcinoma assessment randomised protocol;
TACE – Transarterial chemoembolization; TARE – Transarterial radioembolization;
US – Ultrasonography;
Index:
1 Introduction ... 7
1.1 The Burden of Cancer ... 7
1.2 Hepatocellular Carcinoma Epidemiology and Physiopathology ... 8
1.2.1 Major Risk Factors ... 9
1.2.2 Screening, Surveillance and Diagnosis ... 11
1.2.3 Staging Systems and Prognosis ... 13
1.2.4 Treatment Options ... 14
2 Objective ... 16
3 Method ... 17
4 Results ... 17
5 Discussion ... 18
5.1 The BCLC staging system recommended treatment options ... 18
5.1.1 Transarterial Chemoembolization ... 18
5.1.2 Systemic Therapies ... 21
5.2 Therapies under research ... 23
5.2.1 TACE and sorafenib Concomitant Therapy ... 23
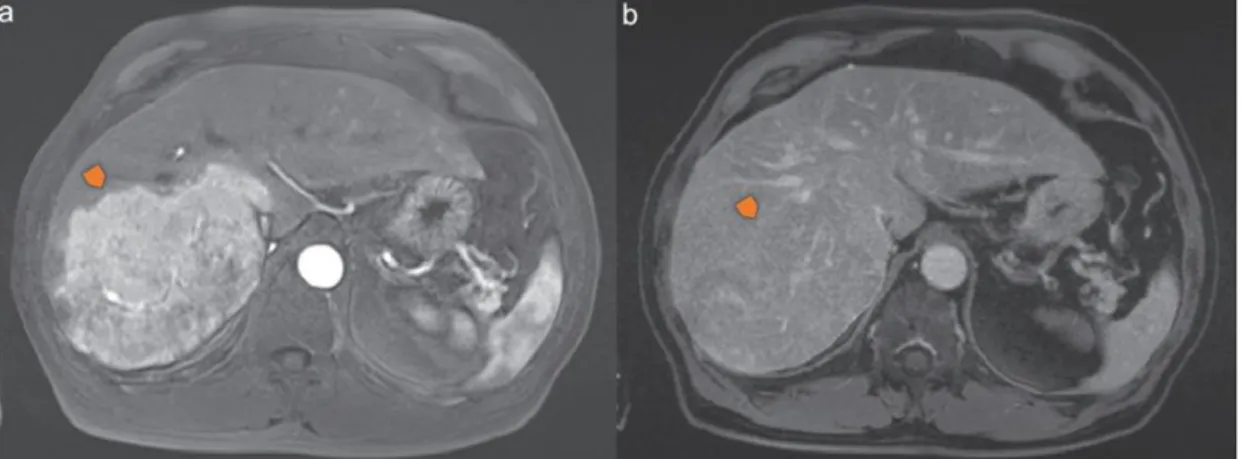
5.2.2 Immunotherapy ... 23 5.2.3 Radiotherapy ... 24 5.2.4 Chemotherapy ... 25 6 Conclusions ... 28 References ... 29 Figures Index: Figure 1 – Magnetic Resonance Imaging of the “Wash out” phenomenum in a patient with HCC (adapted from Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visc Med. 2016;32(2):116–20). Tumor location is marked with an arrow. ... 12
Figure 2 – Main aspects of the BCLC staging system (adapted from Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visc Med [Internet]. 2016;32(2):116–20). This image summarizes the main aspects of the BCLC Staging System treatment selection process. RFA: Radiofrequency Ablation; TACE: Transarterial Chemoembolization. ... 14
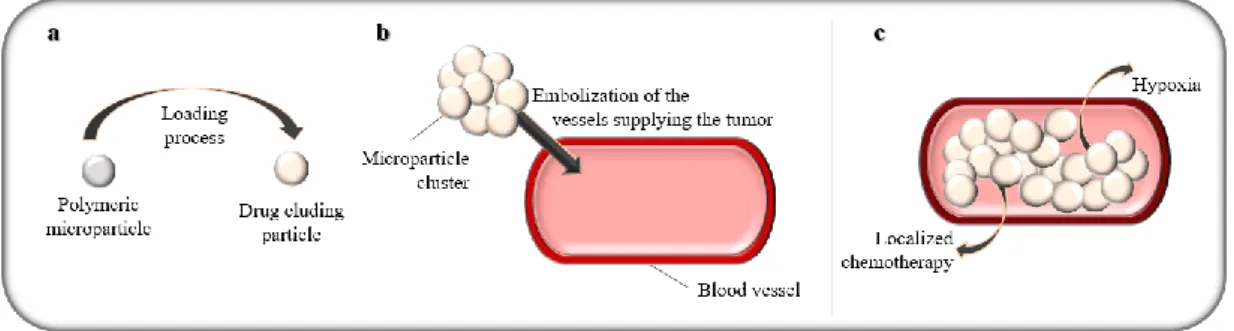
Figure 3 – Transarterial chemoembolization with polymeric microparticles. ... 20
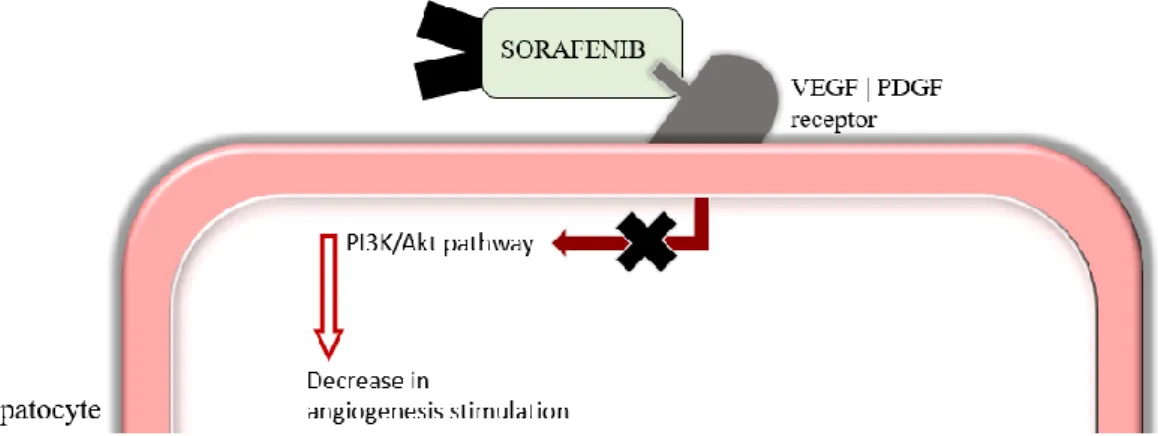
Figure 4 – Mechanism of action of sorafenib. VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; PI3K: phosphatidylinositol 3-kynase. ... 22
1 Introduction
1.1 The Burden of Cancer
Cancer is recognized as one of the leading causes of death that increases mortality and morbidity rate of countries worldwide. In 2012, over 14 million new cases of cancer were diagnosed globally, and this number is expected to grow with the increase of population, age, and adoption of changes that affect lifestyle, their society and the economy of their countries. Predictions say that by 2030 the number of newly diagnosed cancer cases will be near twenty-two million (1–3). The Low- to Mid-Income countries are expected to experience a greater increase in the cancer burden until 2030. The cost of diagnosing and treating all types of cancer tend to continue to escalate (1). As these countries meet the inevitable industrialization process that accompanies their economic growth, they will also be faced with the economic burden of diagnosing and treating the new cases of cancer. These new cases will be originated from that same industrializing process and the lifestyle changes it brings (1,2). These countries already struggle with obstacles in their healthcare providing systems like fewer diagnosis due to scarce awareness of the symptoms, lack of access to preventive vaccines and absence of resources and proper preparation of the diagnosis centers. Currently, these factors end up contributing to the failure of diagnosis methods and to the increase of the mortality and morbidity of cancer. As the number of cancer cases grow, these problems will become an even larger concern for health providing systems worldwide (4). At 2012, liver cancer is set as the second leading cause of cancer deaths among males throughout the globe (3) affecting only one third of the amount of women in comparison (5). It is also the second largest contributor for cancer mortality in the Low- to Mid-Income countries (2). Up to 90% of liver cancer diagnosed cases are categorized as Hepatocellular Carcinoma (3).
1.2 Hepatocellular Carcinoma Epidemiology and Physiopathology
Hepatocellular carcinoma (HCC) is among the five most common types of cancer (6) and is placed as the third most deadly cancer worldwide (7). The incredibly high death rate can be owed to the lack of treatment alternatives after it’s diagnosis (8). Some of the risk factors for the development of this disease are Hepatitis B and C infections, smoking, alcohol abuse, dietary exposure to aflatoxins, diabetes and obesity (3,6,9,10). Some recent studies have demonstrated that the HCC incidence varies across different races, ethnicity and geographic groups, with greater incidence in Asian males between the ages of 35 and 49, although this difference has decreased when compared to older studies (11). Despite that, HCC’s uneven distribution between developed and developing countries seem to support the thesis that a variety of environmental factors can influence the development of the disease (12).
The symptomatology can vary according to several aspects, tumor dimensions being one of them. Small tumors are usually asymptomatic, originating symptoms after it reaches 5 to 8 cm in diameter. The symptoms can be due to the hepatic decompensation like ascites and jaundice or they can be tumor related symptoms like abdominal pain, weight loss, malaise and palpable mass on examination. The most common symptom reported is a dull visceral abdominal pain (13).
Recent advances in medicine have allowed the improvement of the short-term prognosis to HCC patients, but when compared to the long-term prognosis the survival rate 10 years after the treatment is, in average, below 30% (7).
The liver is remarkably known for its capability to regenerate after an injury. Unfortunately, that same potential can be responsible for the origin of an hepatic tumor. The hepatocytes are the most copious and functionally active cells in the liver parenchyma. It has been shown that under physiological conditions or after an acute injury, mature hepatocytes are responsible for the reestablishment of the liver function through their own replication. The ductular cells are also present in the liver parenchyma and they are also referred to as the bipotential hepatic progenitor (BHP) cells. Using murine models, a recent study has shown that both types of cells contribute to tumor growth and heterogeneity (14). After chronic exposure to inflammation and oxidative DNA damage (10), BHP cells are responsible for the restoration of the homeostasis in the liver as they provide a constant supply of new hepatocytes for the
liver parenchyma (14). However, these same cells can initiate a process of excessive proliferation. That process can lead to structural aberrations in the hepatic lobules, like fibrosis and cirrhosis (10). Paracrine signaling modulation is used by mutated hepatocytes to promote the maintenance of the activation and undifferentiation of BHP cells (14).
The microenvironment created around the tumor cells is also of foremost importance when it comes to understanding and treating HCC. Studies have demonstrated that the success rate of treatments decrease when only the tumor cells are established as targets, indicating that the microenvironment around them also have the ability to influence the tumor progression. Specific extra-cellular proteins like collagens, integrins and laminins suffer alterations that lead to the creation of new signaling pathways with precise ligands and receptors. This impairer network allows further communication between tumor cells and the surrounding liver parenchyma to gather the means necessary to create an oncogenic favorable environment, through paracrine and endocrine messaging. The hepatic stellate cells are responsible for the increase in fibrogenic activity during chronic injury to the liver, as happens during the progression of HCC. These cells become activate during liver injury and start the synthesis and excretion of extra-cellular matrix proteins favoring the oncogenesis process. An increase in the matrix stiffness promotes tumor cells proliferation and creates a bigger chemotherapeutic resistance (15).
1.2.1 Major Risk Factors
One of the reasons behind the difficulty of successfully treating HCC lies on its heterogeneity. Usually, it takes a combination of several factors to achieve the oncogenesis and the response to treatment differs with the different etiologies that may have influenced tumor development, progressions and metastasis (15).
Viral infections are pivotal among the risk factor when it comes to HCC, being able to influence the oncogenesis both directly and indirectly. More than 257 million people are chronically infected by the hepatitis B virus (HBV) and around 71 million people are chronically infected with the hepatitis C virus (HCV) (16). HBV and HCV
infections are responsible for 50 and 25 per cent of all HCC cases worldwide, respectively (18).
The HBV can originate DNA mutations on the host cells and directly increase the chances of oncogenic transformation. In its life cycle, a part of the virus’ DNA is incorporated in the host’s genome and during that process there is a high probability of originating chromosomal aberrations such as deletions and copy number gains, frequently found in HCC cells. Besides that, the integration of viral DNA can also alter the protein expression in the liver cells. It can be responsible for interrupting important DNA sequences and originating truncated or wild-type proteins, as well as dysregulating the transcription pattern of other proteins due to the addition of viral promoter sites (19). Differently from the HBV, the HCV does not insert itself in the host’s genome as it is a positive-strand RNA virus that replicates itself outside the cell nucleus. Nonetheless it has been shown it influences cell proliferation through the interaction of its non-structural proteins with the cell proteins responsible for that process (20). On the other hand, the physiologic mechanism of hepatocytes proliferation during a chronic infection, where the activation of the dormant hepatocytes is done recurrently, can indirectly lead to HCC. That mechanism can be verified either in HBV and HCV infections (20,21) In the specific case of an HBV chronic infection, the immune response is mediated through the innate and the adaptive immune systems. In those settings, the CD8+ cells are the prime inducers of liver damage that occurs consequently to the attempt of extinguishing the HBV infection (21).
The alteration of genetic and epigenetic traits also seems to be directly related with the high prevalence of this type of cancer. The epigenetic changes are inherited like the genetic ones, but it concerns only the states of gene expression and chromatin organization without affecting the DNA sequence itself. One of its most studied examples is the DNA methylation process (6,17). The epigenetic alterations have been shown to modulate the self-renewal and differentiation of groups of pluripotent cells designated cancer stem cells (17). In HCC, the hypermethylation of a specific promoter is associated with the inactivation of various anti-cancer mechanisms of the cell such as cell cycle regulation, apoptosis and development of metastatic cells thus allowing the proliferation of cancerous cells (6,17).
Obesity can increase the risk of developing HCC due to the existence of excess fat and its dysfunctional accumulation in an ectopic manner. This contributes to an increase in
the oxidation of free fatty acids. The oxidation originates elevated levels of reactive oxygen species and other toxic compounds enhancing the probability of the occurrence of cancer inducing mutations. In addition to that, the production and activity of adiponectin as an antiproliferative, antiangiogenic and pro-apoptotic factor is reduced in obesity and in insulin-resistant states (18,19).
The exposure to the fungal toxin aflatoxin B1 generates N7-Guanine DNA adducts that
lead to the GC-TA mutations as early as ten weeks after exposure in murine models. These mutations are often found in HCC tumor cells and this recent study was able to connect this DNA alkylation process directly to the aflatoxin exposure (20).
1.2.2 Screening, Surveillance and Diagnosis
Both screening and surveillance are early stage diagnosis strategies and though they are different, it seems that exists a great confusion with what each of them represents. A screening program searches for the prevalence of HCC or any other pathology across the population using a screening test. A surveillance program makes use of a test in controlled intervals that is applied repeatedly to the risk groups, in its majority, and discovers the incidence of HCC. Usually the surveillance programs are preferred by the countries who invest in an early stage diagnosis strategy (21) since surveilling the population allows an increase in the practice of potential curative treatment options (22).
The first step in a surveillance program is the definition of the groups in risk. This procedure is normally applied to all patients who exhibit cirrhosis and those that are known to be infected by the HBV or the HCV (21). It should be kept in mind that not all HCC cases derive from these etiologies, but choosing these as surveillance criteria helps control the most predisposed populations (22). The surveillance method of choice in most countries is the Transabdominal Ultrasound (23) and the recommended frequency of analysis is six months for cirrhotic patients (21).
The HCC is a highly vascularized type of tumor and this aspect is typically used as a differentiative factor for its diagnosis (Figure 1) (24). During even the early stages of HCC the dysplastic nodule presents an increase in arterial flow due to the hypervascularization followed by a decrease in the portal venous flow. It is presented
(Figure 1-a) and a decrease during the venous phase (Figure 1-b). This rapid decrease in the venous flow during the late stage of the irrigation cycle is a very specific characteristic of this type of cancer, also known as the “wash out” phenomenon (23– 25). Other types of liver dysplasia also present changes in their irrigation cycle but it normally presents itself as a decrease in the arterial flow and a maintained venous flow (23).
Figure 1 – Magnetic Resonance Imaging of the “Wash out” phenomenum in a patient with HCC (adapted from Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visc Med. 2016;32(2):116–20). Tumor location is marked with an arrow.
Imaging techniques like Ultrasonography (US), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are chosen in the majority of cases. Nodules bigger than two centimeters are screened with either MRI or CT for the presence of abnormal behavior, like the “wash out” phenomenon (24,25). Nodules with sizes comprised within the 1-to-2-centimeter interval however should be evaluated using two of the above-mentioned methods for diagnosis (US, CT or MRI) (24).
Serologic markers have also been used and the most prevalently chosen is the Alpha-Fetoprotein (AFP) but its sensitivity ranges from 25 percent in tumors smaller than three centimeters to 50 percent in tumors greater than that so it is considered a weak solo method for diagnosing HCC (25) though it is recommended in association with one imaging technique (CT or MRI) in some Asian countries (22).
1.2.3 Staging Systems and Prognosis
Following an accurate staging system is crucial for the selection of the most appropriate course of action since it influences the treatment options and in that manner, the prognosis of each patient (26). Currently there are several staging systems in place for the evaluation of the degree of HCC development. Although there is not any consensus as of what system is the ideal one, the Barcelona Clinic Liver Cancer (BCLC) staging system is endorsed by the American and European societies and it shows to this date the better results (27). Its main advantage lies on the existence of a treatment plan for patients on every stage (26). In Asian countries and others with a greater prevalence of HBV related HCC there is a greater preference for other staging systems like the Hong-Kong Liver Cancer (HKLC) due to its better prognosis in cohort studies (28).
The BCLC system (Figure 2) is an algorithm that among other factors makes use of two other classification pathways. The Child-Pugh Score has as its main purpose the assessment of the severity of liver cirrhosis and the Okuda System, which establishes a score through the analysis of factors like tumor size, serum bilirubin level, severity of ascites and amount of serum albumin level. There are four major factors that define the prognosis of a certain patient. These are tumor related factors like the tumor volume and number, liver function related factors, those that can influence the efficacy of the treatment and the ones related to the overall health of the patient like the performance status. The patients are placed in one of five stages that range from 0 to D according to their performance status, tumor stage, Okuda classification and liver function study. Of these five stages, the first two provide mainly curative treatment options and the other three palliative care to improve life expectancy or general well-being. Other than that, there are factors that when present imply a negative prognosis like an elevated level of AFP, alkaline phosphatase, creatinine or bilirubin, tumor larger than five centimeters, vascular invasion and extrahepatic metastasis (29).
The fourth stage of the BCLC system also referred to as the intermediate stage (BCLC-B) is considered its major flaw. The category is reckoned as too broad engulfing patients with several different types of tumors not differentiating between uninodular, multinodular and infiltrative lesions and furthermore, associating that array of tumors with several different Performance Status scores and varying degrees of liver function (30). This flaw was compromising the prognosis of some patients who, for example, had a single nodule larger than five centimeters but without any vascular invasion, since they were being characterized as an intermediate stage patient, and their treatment options were mainly palliative(28). Recently these same patients have been included in the early stage group (BCLC-A) improving greatly the treatment options at their disposal (22).
1.2.4 Treatment Options
As mentioned above, depending on the stage of the cancer at the time of its diagnosis the treatment plan can follow one of two routes. There are curative treatment options like surgical interventions (resection or transplant), radiofrequency ablation and percutaneous ethanol injection. These techniques are applied to the patients at an earlier
Figure 2 – Main aspects of the BCLC staging system (adapted from Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visc Med [Internet]. 2016;32(2):116–20). This image summarizes the main aspects of the BCLC Staging System treatment selection process. RFA: Radiofrequency Ablation; TACE: Transarterial Chemoembolization.
stage of HCC (BCLC-0 or BCLC-A). These strategies aim to eradicate any presence of neoplasia before it has the opportunity to spread through the patient’s organism. The other option is based on palliative techniques like transarterial chemoembolization or systemic chemotherapy with sorafenib which has the main objective of increasing patient’s life expectancy and diminishing the tumor’s ability to grow and spread (31,32).
2 Objective
This work aims to perform a literature research on the progress made with new therapeutic options for the patients diagnosed with HCC at a stage equal or higher then BCLC-B. Since this type of cancer does not normally develop symptoms early on and the surveillance programs have only recently been implemented, in 25 to 70 percent of the cases (32) the diagnosis is made when the cancer has already reached a more advanced stage. These patients are usually directed towards one of the palliative treatment options which as of today, only marginally improve their life expectancy. Therefore, this is an area of much needed research regarding the Hepatocellular Carcinoma patients.
3 Method
A broad literature research was conducted mainly on the PubMed platform, rendering the articles that would fit into one of two major groups. The first group comprised the papers exposing the state of the art of what is being made according to the BCLC staging system and all the main information about TACE and sorafenib treatment procedures. This was achieved by researching combinations of the terms “Hepatocellular Carcinoma” with “intermediate stage” or “advanced stage” and “TACE” or “sorafenib”. The second group was retrieved through the combination of the terms “Hepatocellular Carcinoma” with “clinical trial”, “palliative treatment” and “stage II”. For the latter and keeping in mind that the purpose of those articles was to show the direction in which the future of treatment was headed, no articles prior to two-thousand and twelve were considered, and trials still relying on in vitro findings were excluded.
4 Results
Following the above mentioned criteria, sixty-three articles were retrieved after full reading of the papers and were separated into the two groups previously mentioned.
5 Discussion
5.1 The BCLC staging system recommended treatment options
5.1.1 Transarterial Chemoembolization
Transarterial Chemoembolization (TACE) is a palliative localized procedure suggested for inoperable HCC tumors, such as those patients with multimodal tumors or with tumors larger than five centimeters. It has been proved to provide a better prognosis to patients with unresectable tumors (33) and, as its name suggests, this approach makes use of a localized delivery of chemotherapeutic agents allied with a technique to promote the emboly of the blood vessels in the area (34).
The rationale behind the practice of TACE (Figure 3) lies on the fact that opposed to the physiologic supply of blood to the liver tissue that precedes from the portal vein in its majority, carcinogenic tissue is usually supplied with blood from the hepatic artery. In this manner, the treatment can pass by the compromise of the arterial blood supply without damaging the healthy liver tissue. This embolization process has several advantages including delivering a concentrated dosage of the chemotherapeutic agent to the liver tumor without compromising the surrounding healthy liver tissue, increasing the contact time between the drug and the tumor and preventing the spread of the chemical agent throughout the body and with that minimizing the systemic side-effects of this therapy, besides promoting the ischemia of the tumor. Other than that, it has been found that the synergy between the ischemia and the effect the chemical agent (Figure 3-c) increase the rate of necrosis of the tumor (35).
When it comes to the procedure itself, TACE has suffered some improvement since the beginning of its usage. Initially the chemical agent was delivered in a solution or emulsion form through an injection directly at the artery feeding the tumor and then the appropriate embolization agent was used to selectively cut the blood supply at the desired area, more recent techniques have appeared where the chemical agent is embedded within the material chosen to ensure the embolization process (36).
One of the embolization agents used on TACE is Lipiodol. It is an oil immiscible with water mainly composed of ethylic esters of linoleic, oleic, palmitic and stearic acids that suffered the addition of one, two or three atoms of iodine. It was initially used as a contrast medium with a higher usage percentage in lymphography procedures. This
compound does not provide the complete embolization of the blood vessel since it is a liquid oil and it can suffer washout, but it still has its place in TACE therapy since it seems to have an affinity for HCC cancerous tissue and increased time of perpetuity in those same locations due to the absence of phagocytes and Kupffer cells that contribute to its elimination at those sites (37).
The chemical agents more commonly associated with the use of Lipiodol are doxorubicin, epirubicin, aclarubicin, 5-fluorouracil mitomycin and cisplatin (38). Polymeric microparticles are another alternative for embolization agent in TACE (Figure 3). These however are more complex and can branch out into several different materials (39). They can be divided according to their degradability, the chemical agent loading procedure, and the type of interaction that lead to the release of that same agent. As of their degradability, the particles can be divided into non-biodegradable and biodegradable. The non-biodegradable particles often provide a longer period of embolization and are preferred for isolated treatments while the biodegradable ones are often used on patients who undergo several rounds of treatment, although this is not limitative of their choice for either case. The particles can be composed from ionic or non-ionic polymers which will influence the manner in which the chemical agent is released from the polymeric net. The ionic polymers undergo an ionic exchange process with the same charge ions in the surrounding medium while the non-ionic polymers allow the passage of the chemical agent through the pores in its net in an uncomplicated process of diffusion. Within the biodegradable particles there is still one other release process named bioerosion should be taken into account. As the polymer is degraded inside the organism, its erosion leads to the release of the chemical agent previously contained inside the particle. This phenomenon can be used as a strategy to ensure the release of compounds that are either insoluble on water or that have a very high molecular weight and because of that would suffer a very slow release through any other process, compromising the effectiveness of the procedure.
Regarding the loading process (Figure 3-a) the microparticles can either be preloaded or require loading prior to their application. As it would be expected the use of preloaded microparticles is preferred because the loading process itself can carry several issues. The dosage of the chemical agent present in the microparticles can be achieved through a soaking procedure where the unloaded particles are left in contact
be affected both through the concentration of the chemical agent in the liquid medium and through the time of contact between the medium and the particles. This increases the risk of achieving an incorrect final dose due to human error, depending greatly on the operator, and it has a very low reproducibility. Besides the low reproducibility, the process of loading already formed particles can lead to an uneven filling process, since the concentration of the drug may vary throughout the particle, being more concentrated in the outer layers than on the inner ones (38–40).
Figure 3 – Transarterial chemoembolization with polymeric microparticles.
The anticancer drugs most commonly loaded on these microparticles are doxorubicin, mitomycin C, cisplatin, methotrexate and paclitaxel. One other advantage with this type of embolization is the possibility of load the particles with other agents, like angiogenic agents in order to further prevent the progression of the tumor, or with anti-inflammatory agents to provide pain relief posterior to the procedure and reduce the inflammatory response that is normally originated from it. One main disadvantage is the necessity to embed the particles in some type of iodinated contrast solution to allow the visual monitorization of the process which is crucial for guidance during the administration of the particles (38).
A recent study (41) has demonstrated that there is no significative difference between opting for drug eluding microparticles or Lipiodol as the embolization agent, when it comes to mortality and morbidity rate, post-embolization syndrome or overall survival. Further information was provided by a recent paper in which both mechanisms were compared in association with Doxorubicin. In fact, both methods of release provided a good outcome as a HCC treatment alternative, but there were significant differences in Doxorubicin pharmacokinetics and systemic toxicity. Lipiodol provided a higher maximum concentration, a bigger area under the curve and consequently better bioavailability. It also presented some cases of hepatic toxicity that led to interruption
of the treatment. On the other hand, the microparticles originated a lower but more constant level of localized dosage of the drug and in doing so prevented the existence of serious adverse events of systemic toxicity (42).
Some innovation has also been applied to the chemical agent used on TACE. Fine-powdered cisplatin was used on a recent trial after reports that it presented a better response rate than other alternatives previously analyzed. The initial response rate was even more elevated than what had been predicted by the precedent studies, inferring the exactness of the protocol put to test. Unfortunately, the response rate decreased after the third month of trial indicating that the interval between administrations of TACE was probably not the better suited, being this the primal area of improvement on posterior studies. The severity of the adverse effects experienced by this therapy was comparable to what had been documented for treatment with cisplatin alone, attesting the safety of this method (43).
5.1.2 Systemic Therapies
Currently the only drug approved for the systemic treatment of HCC is sorafenib. It is an oral tyrosine kinase inhibitor that has been shown to stimulate the apoptosis of HCC cell lines and to inhibit angiogenesis on HCC tissue samples. Sorafenib was introduced as an option for HCC treatment after the SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomised Protocol) study, which was conducted in six hundred and two patients with intermediate to advanced HCC as a double-blind placebo controlled multicenter investigation and that shown a significant increase of about forty percent in median overall survival (44). The effectiveness of this therapy was later confirmed on a phase III trial conducted on the Asian-pacific population. Sorafenib was the first targeted therapy alternative for patients with advanced HCC and preserved liver function (45).
Alpha fetoprotein (AFP) has shown to react in a pattern that can be correlated with sorafenib therapy. The decrease of AFP right after the start of the treatment is correlated with a god response to sorafenib and usually indicates a good prognosis (46).
The mechanism of action of this drug is mainly based on the characteristics of the tumor itself (Figure 4). HCC can be characterized as a highly vascularized tumor and so the angiogenesis process is particularly important in this type of cancer. The Vascular
are two of the most important stimulating factors of the angiogenesis process. PDGF is responsible for the enrollment of smooth muscle cells that surround the newly formed blood vessels and VEGF has been shown to be overexpressed in HCC tissue, being associated with a poor prognosis as its expression increases. Sorafenib acts as an inhibitor on the receptors of both stimulating factors which stops the stimulation of the tyrosine kinase cascade within the cells.
Figure 4 – Mechanism of action of sorafenib. VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; PI3K: phosphatidylinositol 3-kynase.
Unfortunately, sorafenib only provided limited benefits as a therapeutic option. A concerning problem with sorafenib is the easiness with which the tumor can develop a resistance to the treatment. This is a frequent problem with all inhibitors of VEGF, where the tumor initially seems to react with stability but very quickly develops resistance. Several hypotheses have been proposed as the reasoning behind the development of resistances, many of them rely on the capability of the tumor to develop or on alternative angiogenic pathways. The upregulation of alternative angiogenic receptors is a known mechanism of resistance (47) and the phosphatidylinositol 3-kynase (PI3K)/Akt pathway is another alternative route which becomes activated and provides the maintenance of the resistance to sorafenib on tumor cells after a prolonged exposure (48). Is has also been postulated that the cancer stem cells can be responsible for the development of resistance to treatment as well as for the resurgence of a tumor after resection or remission. These cells are a minor part of the cell population within the tumor, but being able to engage in a process self-renewal and differentiation into the other cells that constitute the tumor they have shown ability to resist various types of chemotherapy, sorafenib included (49).
5.2 Therapies under research
5.2.1 TACE and sorafenib Concomitant Therapy
The combination of these two therapies has been tested in several clinical trials and they showed promising results. In these studies, the ideal value for several parameters was established including the dosage of sorafenib, the interval between the first TACE session and the beginning of administration of sorafenib and the duration of the treatment with sorafenib. The first trials were mainly conducted on small groups of patients and although they presented favorable outcomes in their conclusions when it came to inhibition of cancer progression and decrease of HCC markers blood levels, many patients presented serious adverse effects. Patients with adverse effects like abdominal pain, alopecia, fatigue and hiperbilirubinemia were considered endangered and had their treatment suspended leading to their exclusion from the trials. Fortunately, as more trials started, the problems with unexpected adverse effects were extinguished. Improvement in survival rates was also achieved when compared to sorafenib alone (50).
A recent phase three multicenter, randomized, double-blind, placebo-controlled study was carried out on a total of three-hundred and thirteen patients in the United Kingdom using TACE with Doxorubicin Eluding Beads (DEB-TACE) and a daily dose of eight-hundred milligram of sorafenib divided in two takes. This trial concluded however that there was no significant difference in overall survival time when compared to DEB-TACE alone, putting this combination no longer as a potential candidate for a future revolutionary treatment (51).
5.2.2 Immunotherapy
A study conducted on thirty patients was able to demonstrate the possible benefits from using Dendritic Cells (DC) vaccination as a method of treatment for HCC. The test group was submitted to treatment with Dendritic Cells vaccines, a novel immunologic approach to cancer therapy. The DC can be pulsed with tumor-specific antigens and they work by processing and presenting those same antigens to CD8+ T lymphocytes, activating the cellular immune response against the tumor and increasing the host’s resistance to it. The patients included in this trial were not suitable for any curative treatment strategy or even TACE, being in a very precarious position. The test group
who was being maintained on their life-supportive palliative treatment. Other than that, the tolerability to the vaccine was excellent originating only mild flu-like symptoms on the first days after administration. This alternative could be the best choice for patients with advanced stage HCC who are not suitable for any of the guideline-endorsed therapies, or even as an adjuvant to TACE in intermediate stage HCC patients (52). A different approach was researched by Bian et al. (53) when they saw potential in associating the antibody metuximab with Radiofrequency Ablation (RFA), a technique used as a resource for early-stage HCC who are not eligible for resection or transplant or for recurrent HCC after hepatectomy. RFA is a minimally invasive method that induces cell death by heat-induced coagulative necrosis and by the activation of the innate immune response. However, the high rate of recurrence of HCC and the effects on patient’s survival is problematic with this technique when used on its own. In that manner, the association with an iodine metuximab injection possessed a lot of potential. This variation of metuximab was created through the labeling of a CD174 targeted metuxibab with iodine-131 and it allowed the association of two benefits for this therapy: the activation of targeted cell-death mechanisms and the monitoring through imaging techniques due to its iodine labeling. This trial revealed that this association led to a better overall survival time, improvement of the one and two-year recurrence rates and in the median time to overall tumor recurrence, which used to be the great trouble with the use of RFA. All of this was achieved without major adverse events of systemic toxicity and without alteration of the thyroid function in all patients.
5.2.3 Radiotherapy
Systemic radiotherapy was tested on a clinical trial comprised by forty-one patients suffering from intense tumor-related symptoms like abdominal pain and discomfort, out of which twenty-one had been diagnosed with advanced stage HCC and the remaining with liver metastasis. The results showed an improvement in the quality of life of a little over one quarter of the patients, with reduction of the symptoms that led them to the trial initially. The treatment was well tolerated with the main complaints being mild fatigue and nausea and should be considered for further studies with a wider test population as a substitute for sorafenib in palliative care of BCLC-C and D patients (54).
Localized radiotherapy is also an option when it comes to treating HCC. Transarterial Radioembolization (TARE) is a microembolic technique that only partially occludes the arteries in the liver as it releases small amounts of radiation locally. This procedure is considered experimental by the HCC guidelines, so it is not recommended for any specific stage of HCC, however the truth is this technique has shown favorable results and it is the only other alternative for patients with portal vein thrombosis that cannot undergo other procedures, being restricted to sorafenib and symptomatic treatment. One major disadvantage with this technique is the necessity of a multidisciplinary team for providing the procedure and the use of a sophisticated therapy that requires specialized centers which greatly increases the cost associated with this alternative (55). The use of Yttrium-90 microspheres is a novel type of TARE. It relies on biocompatible microspheres to deliver high-energy beta radiation to the tumor through the hepatic artery in a manner that is proportional to the concentration of microspheres in each area. This technique differs from systemic radiotherapy because it preserves most of the healthy parenchyma that constitutes the liver since it delivers focalized energy to sites previously identified (56). A different study concluded that the combination of Yttrium-90 TARE and sorafenib administration could be an alternative treatment in the future. Further data related to efficacy is yet to be released but it has been proven that the association is safe and does not imply a worse adverse effects range than the treatment with sorafenib alone, even when the dosage of sorafenib used in each case was equal (57).
5.2.4 Chemotherapy
A trial assessing the efficacy and safety profile of a systemic chemotherapy association was performed on 45 patients with advanced HCC. The experimental treatment consisted of the association of sorafenib with gemcitabine, a common chemotherapeutic agent with only modest activity against HCC. Gemcitabine does however possess the capability to improve the anti-cancer activity of other chemotherapy drugs, and since the adverse effect profiles of gemcitabine and sorafenib are not coincident, they have exciting potential of providing a better treatment alternative. This combination proved to be better tolerated than other associations like doxorubicin with sorafenib, originating neutropenia to a lesser degree, although in terms of effectiveness it proved to be similar to the other association (58).
Another association was proposed by a recent study that postulated the potential benefits of associating sorafenib to capecitabine, another systemic chemotherapy drug well tolerated in patients with cirrhosis and effective against hepatobiliary tumors. The test population was small, only 15 patients met the criteria established by the investigators and so parameters like overall survival time were not estimated, but this association had a better response rate than sorafenib alone without increasing the rate of serious adverse effects (59).
For the cases where the treatment with sorafenib was discontinued due to intolerability or inability of preventing cancer progression during treatment, the axitinib alternative treatment was tested. Axitinib is a potent and selective inhibitor of the VEGF receptors one to three which has already been approved for usage against advanced liver cell carcinoma. On this trial 134 patients underwent treatment with axitinib and the results were slightly less uplifting than the investigators hoped for. The overall survival time did not suffer any significant changes on the overall population although the time to tumor progression, the clinical benefit rate and the patient-reported outcome all showed significant improvement. When further investigated, the population of patients who discontinued other antiangiogenic therapies due to incapability of enduring the treatment did actually benefit from this alternative, even in the overall survival time after treatment, showing this drug could be a second line of treatment for locally advanced or metastatic HCC (60).
As an alternative to sorafenib treatment some studies have emerged relying on the combination of other chemical agents. These new assortments are composed of Oxaliplatin in combination with other potentially therapeutic agents including gemcitabine, 5-fluorouracil, capecitabine or leucovorin. Oxaliplatin is a platinum-based drug that interacts directly with the synthesis of DNA as it binds to guanines and prevents the progression of the normal cell cycle and replication, leading to its demise. The studies concluded that these combined therapies were better tolerated than other previous combinations though they only marginally improved the median and overall survival time. When compared to sorafenib this combination did not present much benefit, but it would have a place in treatment guidelines for those patients who cannot receive sorafenib chemotherapy or for those who it is economically unfeasible. One interesting remark made is the clear difference in response to the same treatment presented by the Asian and the Western population. Regardless of treatment with
oxaliplatin-based agents or sorafenib, the Western population always showed a better overall and median survival time, differing in three or four months in most of the cases, which only validates the heterogeneity of this type of cancer, and the effect that different etiologies can have on the course of the disease (61,62).
Targeted therapies show potential on becoming a future first-line treatment, yet another alternative has emerged. It combines the anti-angiogenic agent Bevacizumab and mammalian target of rapamycin inhibitor temsirolimus which had already been tested for safety purposes. The trial was conducted on 27 patients and showed a surprisingly high overall survival of 14 months. The biggest setbacks encountered were the frequency of intravenous administrations that reduced the adhesion to the treatment and the systemic toxicity that required reduction of dosage in forty percent of the cases and led to interruption of treatment in twelve percent of the participants (63).
6 Conclusions
Hepatocellular carcinoma is a very heterogeneous disease when it comes to its etiology and that same variety originates a great barrier to an effective universal treatment method since the response to the various kinds of treatment have been documented to mutate according to the etiology most likely to have propelled the tumor. When the tumor is detected on an early stage the prognosis for the patient is often favorable. But given the lack of symptoms on the early stages, diagnosis is currently reached when the patients are already only suitable for palliative treatment options. Efforts are being made to increase the surveillance in order to initiate the therapeutic interventions early-on, but the results of these efforts will only be felt in a space of ten to twenty years. Given this, there is much and necessary growth required on the palliative treatment options available for the patients suffering from the more advanced stages of this disease.
Currently the staging systems tend to be very strict and it is very hard to adapt every case to one of the classes available. This leaves many patients with a poorer prognosis than what could be achieved if they could benefit from combinations of the treatments recommended for the different stages or even alternatives that are not comprised for being considered too experimental.
The unique irrigation pattern of HCC allows for the use of localized drug delivery and hypoxia that should be further explored in order to contour the situations that prevent the use of this therapy. The lack of systemic adverse events is a big advantage with this method and as it has already been demonstrated it is possible to overcome problems like portal vein thrombosis which is one of the major contraindications of this procedure. Systemic chemotherapy however has yet to prove itself as a significant improvement on the care of HCC patients. Sorafenib approved drug was introduced about ten years ago and at its best it prolongs the life of the patients in ten to twelve months. Combinations of sorafenib with other alternatives have shown marginal improvement and other drugs that target the same pathways have yet to be constituted as safe and efficient alternatives. Moving forward, more targeted alternatives like immunotherapy will probably be explored as the knowledge about the disease, its physiopathology, biomarkers and pathways involved in it becomes greater.
References
1. Bray F. The evolving scale and profile of cancer worldwide: Much ado about everything. Cancer Epidemiol Biomarkers Prev. 2016;25(1):3–5.
2. Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: A review. Scand J Public Health. 2017 Jul;(February):1–10. 3. Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and
Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.
4. Olver I. Cancer control—A global perspective. Eur J Cancer Care (Engl). 2017;26(1):1–6.
5. Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: Burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444–57. 6. Wahid B, Ali A, Rafique S, Idrees M. New Insights into the Epigenetics of
Hepatocellular Carcinoma. Biomed Res Int. 2017;2017.
7. MALKI A, EL-SHARKAWY A, MOHAMED M, BERGMEIER S. Antitumor
Activities of the Novel Isosteviol Derivative 10C Against Liver Cancer. Anticancer Res. 2017;37(4):1591–601.
8. Bie B, Sun J, Li J, Guo Y, Jiang W, Huang C, et al. Baicalein, a Natural Anti-Cancer Compound, Alters MicroRNA Expression Profiles in Bel-7402 Human Hepatocellular Carcinoma Cells. Cell Physiol Biochem. 2017;41(4):1519–31. 9. Kalra S, Rini B, Jonasch E. Annals of Oncology Advance Access published
January 26, 2015 1. Ann Oncol. 2015;1–18.
10. Sun B, Karin M. Inflammation and liver tumorigenesis. Front Med. 2013;7(2):242–54.
11. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing Hepatocellular Carcinoma Incidence and Liver Cancer Mortality Rates in the United States. Am J Gastroenterol. 2014;109(4):542–53.
Carcinoma: A Systematic Review and Meta-Analysis. Dis Markers. 2017;2017:1–10.
13. Salgia R, Singal AG. Hepatocellular carcinoma and other liver lesions. Med Clin North Am. 2014;98(1):103–18.
14. Tummala KS, Brandt M, Teijeiro A, Graña O, Schwabe RF, Perna C, et al. Hepatocellular Carcinomas Originate Predominantly from Hepatocytes and Benign Lesions from Hepatic Progenitor Cells. Cell Rep. 2017;19(3):584–600. 15. Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: More complicated than ever. Liver Int. 2014;34(6):834–43.
16. World Health Organization. Global hepatitis report, 2017. Geneva; 2017. 17. Raggi C, Invernizzi P. Methylation and liver cancer. Clin Res Hepatol
Gastroenterol. 2013;37(6):564–71.
18. Aleksandrova K, Stelmach-Mardas M, Schlesinger S. Obesity and liver cancer. Recent Results Cancer Res. 2016;208(1):177–98.
19. Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med. 2016;67(1):103–17.
20. Fedeles BI, Chawanthayatham S, Croy RG, Wogan GN, Essigmann JM. Early detection of the aflatoxin B 1 mutational fingerprint: A diagnostic tool for liver cancer. Mol Cell Oncol. 2017 Jul 4;4(4):1–3.
21. Cucchetti A, Cescon M, Erroi V, Pinna AD. Cost-effectiveness of liver cancer screening. Best Pract Res Clin Gastroenterol. 2013;27(6):961–72.
22. Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology. 2016;150(4):836–53.
23. Schütte K, Schulz C, Malfertheiner P. Mini-Review Hepatocellular Carcinoma: Current Concepts in Diagnosis, Staging and Treatment. Gastrointest Tumors. 2014;1:84–92.
24. Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visc Med.
2016;32(2):116–20.
25. Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23(29):5282.
26. Chang L, Wang Y, Zhang J, Guo T, Chang L, Wang Y, et al. The best strategy for HCC patients at each BCLC stage: a network meta-analysis of observational studies. Oncotarget. 2015;5(0):1–10.
27. Choi W-M, Yu SJ, Ahn H, Cho H, Cho YY, Lee M, et al. A model to estimate survival in ambulatory patients with hepatocellular carcinoma: can it predict the natural course of hepatocellular carcinoma? Dig Liver Dis. 2017;
28. Adhoute X, Pénaranda G, Raoul JL, Edeline J, Blanc J-F, Pol B, et al. Barcelona clinic liver cancer nomogram and others staging/scoring systems in a French hepatocellular carcinoma cohort. World J Gastroenterol. 2017;23(14):2545. 29. Selçuk H. Prognostic Factors and Staging Systems in Hepatocellular Carcinoma.
Exp Clin Transplant. 2017;15(Suppl 2):45–9.
30. Jun CH, Yoon JH, Cho E, Shin SS, Cho SB, Kim HJ, et al. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: A novel approach to subclassification and treatment. Medicine (Baltimore). 2017;96(17):e6745.
31. Lee Y-S, Seo YS, Kim JH, Lee J, Kim HR, Yoo YJ, et al. Can More Aggressive Treatment Improve Prognosis in Patients with Hepatocellular Carcinoma? A Direct Comparison of the Hong Kong Liver Cancer and Barcelona Clinic Liver Cancer Algorithms. Gut Liver. 2017 Sep 7;
32. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015;35(9):2155–66.
33. Wang P, Sheng L, Wang G, Wang H, Huang X, Yan X, et al. Association of transarterial chemoembolization with survival in patients with unresectable hepatocellular carcinoma. Mol Clin Oncol. 2014;2(2):203–6.
35. Salem R, Thurston KG. Radioembolization with 90Yttrium Microspheres: A State-of-the-Art Brachytherapy Treatment for Primary and Secondary Liver Malignancies. J Vasc Interv Radiol. 2006;17(9):1425–39.
36. Liu K, Zhang X, Xu W, Chen J, Yu J, Gamble JR, et al. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin Transl Gastroenterol. 2017 Jun 15;8(6):e98.
37. Ogata K, Nakakuma K, Tashiro S, Hiraoka T, Ootsuka K. Hepatocellular Metastatic. Radiology. 1985;154:15–7.
38. Giunchedi P, Maestri M, Gavini E, Dionigi P, Rassu G. Transarterial chemoembolization of hepatocellular carcinoma. Agents and drugs: An overview. Part 1. Expert Opin Drug Deliv. 2013;10(5):679–90.
39. Lewis AL, Dreher MR. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J Control Release. 2012;161(2):338–50. 40. Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials.
1996;17(2):103–14.
41. Kucukay F, Badem S, Karan A, Ozdemir M, Okten RS, Ozbulbul NI, et al. A single-center retrospective comparison of doxorubicin-loaded hepasphere transarterial chemoembolization with conventional transarterial chemoembolization for patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2015;26(11):1622–9.
42. Lilienberg E, Dubbelboer IR, Karalli A, Axelsson R, Brismar TB, Barbier CE, et al. In vivo drug delivery performance of lipiodol-based emulsion or drug-eluting beads in patients with hepatocellular carcinoma. Mol Pharm. 2017;14(2):448–58.
43. Takaki H, Yamakado K, Tsurusaki M, Yasumoto T, Baba Y, Narimatsu Y, et al. Hepatic arterial infusion chemotherapy with fine-powder cisplatin and iodized-oil suspension in patients with intermediate-stage and advanced-stage (Barcelona Clinic Liver Cancer stage-B or stage-C) hepatocellular carcinoma: multicenter phase-II clinical. Int J Clin Oncol. 2015 Aug 29;20(4):745–54. 44. Llovet J, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J, et al. Sorafenib in
45. Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
46. Miyahara K, Nouso K, Yamamoto K. Chemotherapy for advanced
hepatocellular carcinoma in the sorafenib age. World J Gastroenterol. 2014;20(15):4151–9.
47. Sampat KR, O’Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18(4):430–8.
48. Chen K-F, Chen H-L, Tai W-T, Feng W-C, Hsu C-H, Chen P-J, et al. Activation of Phosphatidylinositol 3-Kinase / Akt Signaling Pathway Mediates Acquired Resistance to Sorafenib in Hepatocellular Carcinoma Cells. J Pharmacol Exp Ther. 2011;337(1):155–61.
49. Chen J, Jin R, Zhao J, Liu J, Ying H, Yan H, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367(1):1–11.
50. Cabibbo G, Tremosini S, Galati G, Mazza G, Gadaleta-Caldarola G, Lombardi G, et al. Transarterial chemoembolization and sorafenib in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2014;14(7):831–45.
51. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–75.
52. El Ansary M, Mogawer S, Elhamid SA, Alwakil S, Aboelkasem F, Sabaawy H El, et al. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J Cancer Res Clin Oncol. 2013;139(1):39–48.
53. Bian H, Zheng J-S, Nan G, Li R, Chen C, Hu C-X, et al. Randomized Trial of [131I] Metuximab in Treatment of Hepatocellular Carcinoma After Percutaneous Radiofrequency Ablation. J Natl Cancer Inst. 2014;106(9):1–5. 54. Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell R, et al. Phase II trial
Clin Oncol. 2013;31(31):3980–6.
55. Rognoni C, Ciani O, Sommariva S, Tarricone R. Real-World Data for the Evaluation of Transarterial Radioembolization versus Sorafenib in Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Value Heal. 2017;20(3):336–44.
56. Om A, Elsayed Z. Yttrium-90 microsphere radioembolisation for unresectable hepatocellular carcinoma ( Review ) SUMMARY OF FINDINGS FOR THE MAIN COMPARISON. Cochrane Database Syst Rev. 2016;(2).
57. Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: Analysis of the European multicentre trial SORAMIC. Liver Int. 2015;35(2):620–6.
58. Srimuninnimit V, Sriuranpong V, Suwanvecho S. Efficacy and safety of sorafenib in combination with gemcitabine in patients with advanced hepatocellular carcinoma: A multicenter, open-label, single-arm phase II study. Asia Pac J Clin Oncol. 2014;10(3):255–60.
59. Patt Y, Rojas-Hernandez C, Fekrazad HM, Bansal P, Lee FC. Phase II Trial of Sorafenib in Combination with Capecitabine in Patients with Hepatocellular Carcinoma: INST 08-20. Oncologist. 2017;1–7.
60. Kang YK, Yau T, Park JW, Lim HY, Lee TY, Obi S, et al. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol. 2015;26(12):2457– 63.
61. Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. Oxaliplatin-based Chemotherapy: A New Option in Advanced Hepatocellular Carcinoma. A Systematic Review and Pooled Analysis. Clin Oncol. 2014;26(8):488–96.
62. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–8.
63. Knox JJ, Qin R, Strosberg JR, Tan B, Kaubisch A, El-Khoueiry AB, et al. A phase II trial of bevacizumab plus temsirolimus in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33(1):241–6.