JCB
Report
The Dystrophin Complex Forms a Mechanically Strong Link Between the
Sarcolemma and Costameric Actin
Inna N. Rybakova, Jitandrakumar R. Patel, and James M. Ervasti
D epartment of Physiology, U niversity of Wisconsin Medical School, Madison, Wisconsin 53706
Abstract.
The absence of dystrophin complex leads to disorganization of the force-transmitting costameric cy-toskeleton and disruption of sarcolemmal membrane integrity in skeletal muscle. H owever, it has not been determined whether the dystrophin complex can form a mechanically strong bond with any costameric protein. We performed confocal immunofluorescence analysis of isolated sarcolemma that were mechanically peeled from skeletal fibers of mouse hindlimb muscle. A popu-lation of g-actin filaments was stably associated with sarcolemma isolated from normal muscle and displayed a costameric pattern that precisely overlapped with dystrophin. H owever, costameric actin was absent from all sarcolemma isolated from dystrophin-deficient m dxmouse muscle even though it was localized to cos-tameres in situ. Vinculin, a-actinin, b-dystroglycan and
utrophin were all retained on m dx sarcolemma, indicat-ing that the loss of costameric actin was not due to gen-eralized membrane instability. O ur data demonstrate that the dystrophin complex forms a mechanically strong link between the sarcolemma and the costameric cytoskeleton through interaction with g-actin filaments. D estabilization of costameric actin filaments may also be an important precursor to the costamere disarray observed in dystrophin-deficient muscle. Finally, these methods will be broadly useful in assessing the mechan-ical integrity of the membrane cytoskeleton in dystro-phic animal models lacking other costameric proteins.
Key words: dystrophin • actin • muscular dystrophy • membrane skeleton • costameres
Introduction
In skeletal muscle, dystrophin is associated with a large, oligomeric complex of proteins that includes dystroglycans, sarcoglycans, dystrobrevins, syntrophins and sarcospan (Straub and Campbell, 1997). The dystrophin complex is generally thought to link the actin-based cortical cytoskel-eton and laminin-2 in the extracellular matrix (E rvasti and Campbell, 1993). G enetic ablation of dystrophin or other components in the complex results in a variety of muscular dystrophies, which are often associated with defects in sar-colemmal membrane integrity (Petrof et al., 1993; Straub et al., 1997; D uclos et al., 1998; Cote et al., 1999). Thus, it is hypothesized that one important function of the dystro-phin complex is to mechanically stabilize the sarcolemmal membrane from shear stresses imposed during eccentric
muscle contraction (Petrof et al., 1993). A mechanically strong attachment of dystrophin with the sarcolemmal membrane has already been demonstrated (Straub et al., 1992). R egarding possible interaction with the cortical cy-toskeleton, dystrophin is located adjacent to the cytoplas-mic face of the sarcolemmal membrane in a discrete, rib-like lattice termed costameres (Porter et al., 1992; Straub et al., 1992), a cytoskeletal protein assembly that links the force-generating sarcomeric apparatus to the sarcolemmal membrane (Pardo et al., 1983a; Craig and Pardo, 1983). Costameres transmit contractile forces laterally through the sarcolemmal membrane to the basal lamina (D a-nowski et al., 1992), which may be important for maintain-ing uniform sarcomere length between active and restmaintain-ing muscle fibers of different motor units. While dystrophin is not required for the assembly of several proteins compris-ing costamere-like structures, the absence of dystrophin in humans and mice leads to an altered costameric lattice (Minetti et al., 1992, 1994; Porter et al., 1992; E hmer et al.,
A ddress correspondence to D r. James M. E rvasti, D epartment of Physiol-ogy, 127 Service Memorial Institutes, 1300 U niversity A venue, Madison, WI 53706. Tel.: (608) 265-3419. Fax: (608) 265-5512. E -mail: ervasti@ physiology.wisc.edu
on February 18, 2016
jcb.rupress.org
Downloaded from
1997; Williams and Bloch, 1999). These data suggest that dystrophin plays an important role in organizing or stabi-lizing costameres, possibly via direct binding to actin fila-ments. Biochemical studies have demonstrated that puri-fied dystrophin complex can bind actin filaments with an apparent dissociation constant in the sub-micromolar range (R ybakova et al., 1996). H owever, it has been diffi-cult to assess whether the affinity of the dystrophin/F-actin interaction measured in vitro is sufficient to support a me-chanically strong interaction in vivo.
To address this question, we isolated inside-out sar-colemmal membranes by mechanically peeling the mem-branes from single myofibers teased from normal mouse hindlimb muscles. We used confocal immunofluorescence microscopy to visualize the cytoskeletal proteins retained on the sarcolemma without interference from sarcomeric proteins (Straub et al., 1992). In brief, we have obtained the first evidence demonstrating that the dystrophin com-plex is necessary for a mechanically strong physical link-age between the sarcolemmal membrane and g-actin of the costameric cytoskeleton. Furthermore, our data point toward destabilization of g-actin filaments as a possible in-termediate between dystrophin deficiency and global dis-organization of the costameric apparatus (Minetti et al., 1992, 1994; Porter et al., 1992; E hmer et al., 1997; Williams and Bloch, 1999). Finally, our approach should be broadly applicable to assessing the contributions made by other costameric proteins toward the macromolecular organiza-tion and mechanical integrity of the membrane cytoskele-ton in skeletal muscle.
Materials and Methods
Antibodies and Fluorescent Probes
R abbit 2 polyclonal antiserum against dystrophin was obtained from an animal immunized with a recombinant protein encoding the first 246 amino acids of human dystrophin (D YS246), which was expressed and pu-rified as previously described (R ybakova et al., 1996). R abbit 56 poly-clonal antiserum against a peptide corresponding to the last 12 amino ac-ids of utrophin (O hlendieck et al., 1991) was the kind gift of D r. Kevin Campbell (U niversity of Iowa, Iowa City, IA ). The pan-actin monoclonal antibody C4 was purchased from ICN. Monoclonal antibodies specific for
a-actinin, a-sarcomeric actin, b-actin, and vinculin were purchased from Sigma-A ldrich. The preparation and characterization of g-actin polyclonal antibodies was previously described (O tey and Bulinski, 1988). The b -dys-troglycan monoclonal antibody was purchased from Vector Laborato-ries. FITC- and TR ITC-conjugates of anti-rabbit IgG and anti-mouse IgG were purchased from Jackson ImmunoR esearch Laboratories, Inc. A lexa488- and A lexa568-conjugates of phalloidin, anti-rabbit IgG and anti-mouse IgG were purchased from Molecular Probes.
Mechanical Isolation of Single Myofibers and Sarcolemma
A ge matched, or littermate control, C57BL/10ScSn-D MDmdx/J, or C57BL/ 6J-Lama2dy mice were obtained from Jackson ImmunoR esearch Labora-tories. The vastus lateralis was dissected from tendon to tendon in ice-cold R ingers solution and transferred to a 0.5-ml drop of relaxing solution (100 mM BE S, 15 mM creatine phosphate, 5 mM D TT, 4.74 mM A TP, 5.43 mM MgCl2, 7 mM E G TA , and 0.02 mM CaCl2 with the ionic strength adjusted to 180 mM with potassium proprionate, pH 7.0) surrounded by silicone oil. To isolate sarcolemma membranes, one end of a single myofi-ber was secured with a very fine forceps, the sarcolemma was hooked near the forceps with a stainless steel micro-needle (,100 mm diameter filed to a fine point) and rolled down and off the free end of the myofiber. Iso-lated sarcolemma and mechanically peeled or intact single myofibers were transferred with fine-tipped glass micropipets to a 0.02-ml drop of relaxing
solution stationed on a gelatin-coated glass slide. Isolated sarcolemma and mechanically peeled myofibers were fixed for 5 min with 4% paraformal-dehyde in PBS. To assess the location of g-actin in situ, intact myofibers from the vastus lateralis were fixed and permeabilized for 5 min with 4% paraformaldehyde and 0.1% Triton X-100 in PBS.
Immunofluorescence Analysis
Isolated myofibers and sarcolemmal membranes were blocked for 30 min at room temperature with 5% goat serum in PBS and incubated with pri-mary antibodies overnight at 48C. The samples were washed with PBS, in-cubated with the appropriate fluorescently tagged secondary antibodies for 30 min at 378C, rinsed, and sealed under coverslips in an anti-fade solu-tion. Confocal microscopy was performed with a Bio-R ad MR C1000 scan head mounted transversely to an inverted Nikon D iaphot 200 microscope in the Keck Neural Imaging Lab at the U niversity of Wisconsin. The kryp-ton/argon mixed gas air–cooled laser was set to allow only the 488- and 568-nm excitation lines, while the green and red emission signals were di-rected to separate photomultiplier tubes. Images were collected during a sequential scan to reduce the possibility of fluorescence bleed-through. To further verify that bleed-through of the green signal was not contaminat-ing the red signal, serial optical sections (z series) were collected in some experiments using both single channel and double channel modes. D igital images were cropped using A dobe Photoshop 5.0 and figure montages prepared with A dobe Illustrator 8.0.
Miscellanea
The dystrophin/utrophin-glycoprotein complexes were amplified for im-munoblot analysis from control and m dx total skeletal muscle membranes (O hlendieck and Campbell, 1991) using digitonin extraction and WG A -Sepharose chromatography (E rvasti et al., 1990). Immunofluorescence analysis of frozen cryostat sections from control and m dx muscle was per-formed as previously described (E rvasti and Campbell, 1991).
Results and Discussion
A Population of Actin Filaments Is Tightly Associated with Costameres on Isolated Sarcolemma
Costameric proteins are typically visualized by immuno-fluorescence analysis of glancing longitudinal cryosections (Craig and Pardo, 1983; Porter et al., 1992), or in perme-abilized single myofibers from mature skeletal muscle (Straub et al., 1992; E hmer et al., 1997). If used in combi-nation with widely available actin probes, analysis of dys-trophin/actin colocalization by either of these methods is greatly complicated by the intense and ubiquitous signal provided by sarcomeric actin (R ybakova, I.N., and J.M. E rvasti, unpublished results). Therefore, we adopted a method (Straub et al., 1992) that would enable us to visu-alize the costameres without interference from the sarco-meric cytoskeleton. We isolated inside-out sarcolemmal membranes by mechanical peeling of single myofibers teased from normal mouse hindlimb muscles. Sarcolem-mal membranes double stained with rabbit polyclonal antibodies to dystrophin and a fluorescent conjugate of phalloidin were examined by confocal immunofluores-cence microscopy, which revealed closely overlapping cos-tameric staining patterns consisting of alternately bright and dark transverse bands (Fig. 1 a) with an average peri-odicity of 2.8 6 0.3 mm (n5 7). H owever, only phalloidin stained the mechanically peeled myofibers (2.5 mm) while no dystrophin staining was detected (Fig. 1 b). A nalysis of sarcolemma stained with other, better-characterized anti-bodies to dystrophin yielded similar results. H owever, we found that the rabbit 2 polyclonal antiserum to dystrophin raised in our laboratory yielded the greatest
on February 18, 2016
jcb.rupress.org
Downloaded from
noise. Since the rabbit 2 antiserum was used throughout this study and had not been previously characterized, we have included evidence documenting its specificity for dys-trophin in Fig. 1 c. To confirm that phalloidin was appro-priately reporting the presence of actin, we double stained sarcolemma with rabbit 2 dystrophin antibodies and a well documented pan-actin monoclonal antibody (C4) reactive with all mammalian actin isoforms (Lessard, 1988). A gain, dystrophin and actin staining exhibited closely overlapping staining patterns suggestive of costameres (Fig. 2, a–c). A s an additional control, similar staining patterns were ob-served when sarcolemma were single-stained for dystro-phin or actin. Finally, no staining was observed when fluo-rescent secondary antibodies were incubated alone with sarcolemma, nor did the secondary antibodies exhibit any inappropriate species cross-reactivity that could poten-tially explain the closely overlapping patterns obtained for dystrophin and actin. Thus, we conclude that a population of actin filaments and dystrophin are tightly associated with
the costameric cytoskeleton of normal skeletal muscle such that both can withstand the rigors of mechanical peeling.
Costameric Actin Is Specifically Absent from Dystrophin-deficient Sarcolemma
Because dystrophin binds actin in vitro(R ybakova et al., 1996) and the costameric cytoskeleton is altered in dystro-phin-deficient muscle (Minetti et al., 1992, 1994; Porter et al., 1992; E hmer et al., 1997; Williams and Bloch, 1999), we hypothesized that costameric actin should display an ab-normal staining pattern on sarcolemma isolated from dys-trophin-deficient m dx mice. To our surprise, we observed that costameric actin was completely absent from m dx sar-colemma as reported by monoclonal antibody C4 (Fig. 2, d–f), or phalloidin (Fig. 3, a–c). The absence of actin from
m dx sarcolemma can be most directly and simply ex-plained through an actin stabilizing function normally per-formed by dystrophin. A lternatively, the loss of actin from
m dx sarcolemma might not be specifically due to the ab-sence of dystrophin but rather to some other aspect of the dystrophic pathomechanism. H owever, we have detected costameric actin in all 24 sarcolemma isolated from 8 dif-ferent control mice while costameric actin was absent from 21 different sarcolemma obtained from 7 different m dx
mice. The animals used ranged from 2 to 6 months of age with the isolated sarcolemmal diameters varying from 18– 60 mm for controls and 20–43 mm for m dx mice. Further-more, we examined costameric actin distribution in
sarco-Figure 1. D ystrophin and F-actin colocalize on mechanically
iso-lated sarcolemma in a costameric pattern. Shown is a mechani-cally isolated sarcolemma (a), or a skinned myofiber (b) both stained with A lexa488-phalloidin (green) and rabbit 2 antiserum to dystrophin (red). R ed and green channels were collected si-multaneously and areas of coincidence appear yellow. Shown on the left in (c) are immunoblots containing WG A -Sepharose elu-ates from detergent solubilized control and m dx skeletal muscle membranes stained with rabbit 2 antiserum to dystrophin (D ys), or rabbit 56 antiserum to utrophin (U tr). Shown on the right in c are transverse cryosections of control and m dx skeletal muscle stained with rabbit 2 antiserum to dystrophin. Bars: (b) 10 mm; (c) 50 mm.
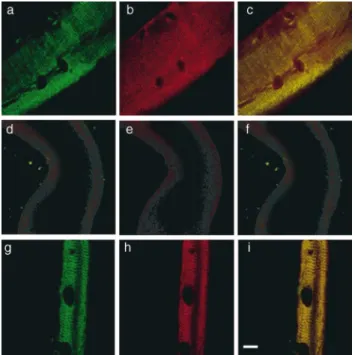
Figure 2. Costameric actin is absent from dystrophin-deficient
m dx sarcolemma. Shown are representative images of sarco-lemma isolated from control (a–c), dystrophin-deficient m dx (d– f), or laminin-2–deficient dy/dy (g–i) muscle stained with rabbit 2 antiserum to dystrophin (green) and monoclonal antibody C4 to actin (red). G reen (a, d, and g) and red (b, e, and h) channels were collected separately, or simultaneously (c, f, and i) where areas of coincidence appear yellow. D ark ovoid shaped areas are nuclear lacunae, which mark the location of peripheral myonu-clei before skinning (Straub et al., 1992). Bar, 10 mm.
on February 18, 2016
jcb.rupress.org
Downloaded from
lemma isolated from the hindlimb muscles of severely d yst r o p h ic d y /d y m ice . T h e d y /d y m o u se is d e ficie n t in la m in in -2, t h e m a jo r e xt r a ce llu la r m a t r ix liga n d fo r a-dystroglycan in the dystrophin-glycoprotein complex (Campbell, 1995). In contrast to m dx muscle, the dystro-phin-glycoprotein complex is normally expressed at the sarcolemma of dy/dy muscle (O hlendieck and Campbell, 1991) and shows little evidence of sarcolemmal membrane damage (Straub et al., 1997). Sarcolemma isolated from
dy/dy muscle retained both dystrophin and actin in an overlapping pattern (Fig. 2, g–i) similar to the costameric distribution observed in non-dystrophic control sarco-lemma (Fig. 2, a–c). These results suggest that in the ab-sence of dystrophin, costameric actin is not stably associ-ated with the sarcolemma membrane.
Because sarcolemmal integrity is compromised in dys-trophin-deficient muscle (Straub et al., 1997), the absence of costameric actin might simply reflect a nonspecific loss of peripheral sarcolemmal proteins as a consequence of the stresses imposed during mechanical peeling. H owever, we found that both vinculin (Fig. 3 a) and a-actinin (Fig. 4 e) were retained on sarcolemma isolated from m dx mus-cle. R ecently, both residual b-dystroglycan and upregu-lated utrophin in m dx muscle were shown to exhibit a cos-tameric staining pattern in situ (Williams and Bloch, 1999). Consistent with this study, we observed that b -dys-troglycan and utrophin were also retained on m dx sarco-lemma and exhibited a costameric staining pattern (Fig. 3, b–d). Importantly, these data indicate that the loss of cos-tameric actin was not due to generalized weakness of the sarcolemma membrane but is instead a specific conse-quence of dystrophin deficiency. Furthermore, our data suggest that utrophin either lacks the actin filament stabi-lizing activity of dystrophin (A mann et al., 1999), or that the amount of costameric utrophin in m dx muscle was not
sufficient to retain costameric actin on mechanically iso-lated sarcolemma.
Costameric Actin Is Appropriately Expressed and Localized in mdx Muscle In Situ
The g-actin isoform was previously shown to colocalize with vinculin at costameres in chick skeletal muscle (Craig and Pardo, 1983). H owever, the polyclonal antibody used in this study (Craig and Pardo, 1983) did not significantly stain the sarcolemma of mouse diaphragm (Pardo et al., 1983b). Thus, it remained unclear which actin isoform(s) may populate costameres of mammalian muscle. There-fore, we stained normal sarcolemma with monoclonal anti-bodies specific for a-sarcomeric actin, or b-actin, or a polyclonal antibody raised against a peptide correspond-ing to the unique amino-terminus of g-actin (O tey and Bu-linski, 1988). O nly the g-isoform antibody yielded a bright, costameric pattern of staining while a- and b-specific anti-bodies yielded only weak and diffuse background labeling (Fig. 4, a–c). A s was found using monoclonal antibody C4 (Fig. 2), or phalloidin (Fig. 3), the g-actin staining pattern evident on normal sarcolemma (Fig. 4, c and d) was absent from dystrophin-deficient m dx sarcolemma (Fig. 4 e). These data indicate that costameric actin appears to be ex-clusively comprised of the g-isoform.
It remained to be determined whether costameric actin was absent from m dx muscle before mechanical peeling,
Figure 3. R etention of vinculin, b-dystroglycan and utrophin on
m dx Sarcolemma. Shown are images of mechanically isolated sarcolemma from m dx (a–c) or control muscle (d) stained with A lexa568-phalloidin (a–d) in red, and antibodies to vinculin (a),
b-dystroglycan (b), or utrophin (c and d) in green. Bar, 10 mm. Figure 4. Costameric g-actin is appropriately expressed and lo-calized in m dx muscle in situ. Shown in a–c are images of sarco-lemma from normal mouse muscle stained with antibodies spe-cific to a-sarcomeric (a), b-nonmuscle (b), or g-actin (c). Shown in d and e are images of control (d), or m dx sarcolemma (e) dou-ble stained with monoclonal antibodies to a-actinin (green) and polyclonal antibodies to g-actin (red). A reas of coincidence be-tween the two probes appear yellow. Shown in f and g are images of fixed and permeabilized myofibers from control (f) and m dx
(g) muscle stained with antibodies specific for g-actin. Bars, 10 mm.
on February 18, 2016
jcb.rupress.org
Downloaded from
or was lost as a result of the peeling procedure. Therefore, we examined g-actin staining in single fibers from control and m dx muscle that were fixed and permeabilized, but not mechanically peeled. Both control and m dx myofibers exhibited peripheral g-actin staining patterns marked by regularly spaced transverse fluorescent bands of similar in-tensities (Fig. 4, f and g). Finally, the g-actin antibodies also labeled the periphery of Z -lines in mechanically peeled myofibers from both control and m dx muscle, how-ever, the g-actin signal appeared brighter on m dx myofi-bers compared with controls (not shown). Taken together, our results indicate that g-actin is appropriately expressed and assembled into costameres in m dx muscle, but that its stable association with the sarcolemmal membrane is se-verely compromised.
Based on its structure, protein interactions, and mem-brane defects associated with its absence or abnormality in dystrophic muscle, the dystrophin complex has long been hypothesized to mechanically stabilize the sarcolemmal membrane against the stresses imposed during muscle contraction or stretch (Petrof et al., 1993; Campbell, 1995). Whereas dystrophin is strongly anchored to the sarcolem-mal membrane (Straub et al., 1992), no study has demon-strated a mechanically strong linkage between dystrophin and any component of the costameric cytoskeleton. Thus, ours are the first data demonstrating that the dystrophin complex is necessary for a mechanically strong physical linkage between the sarcolemmal membrane and cos-tameric g-actin. Furthermore, our data point toward de-stabilization of g-actin filaments as a possible intermediate between dystrophin deficiency and global disorganization of the costameric apparatus (Porter et al., 1992; Minetti et al., 1992, 1994; E hmer et al., 1997; Williams and Bloch, 1999). Complexes comprised of ankyrin/spectrin(Williams and Bloch, 1999), vinculin/talin/a-actinin (Williams and Bloch, 1999) and intermediate filament/intermediate filament as-sociated proteins (Milner et al., 1996; A ndra et al., 1997; D alpe et al., 1999) are also localized to costameres and are capable of binding actin (Fig. 5). Therefore, it is possible that multiple, distinct costameric protein complexes act synergistically to form a strong mechanical linkage be-tween the sarcolemmal membrane and the force-generat-ing sarcomeric apparatus. In support of this possibility, targeted inactivation of desmin (Milner et al., 1996), plec-tin (A ndra et al., 1997), or BPA G n1/dystonin (D alpe et al., 1999) also cause muscular dystrophy and sarcolemmal instability. O ur methods should be valuable in further as-sessing the macromolecular organization and mechanical integrity of the membrane cytoskeleton in dystrophic ani-mal models lacking other components of costameres.
A lternatively, dystrophin may be specifically designed to mechanically stabilize costameric actin filaments. We previously demonstrated that dystrophin contains two dis-tinct and spatially separated actin binding sites located at the amino terminus and middle rod domains (R ybakova et al., 1996; A mann et al., 1998), which enables dystrophin to form an extended lateral association with F-actin and protect actin filaments from depolymerization in vitro (R ybakova et al., 1996; R ybakova and E rvasti, 1997). Studies by others suggest that the dystrophin carboxy-ter-minal domain may also participate in binding actin, either directly (H oward et al., 1998), or indirectly through a -syn-trophin (Iwata et al., 1998). A nalysis of sarcolemma from
m dx mice transgenically expressing dystrophin constructs deleted in different domains (Chamberlain et al., 1997), or mice lacking a-syntrophin (Kameya et al., 1999) will re-solve which elements in the dystrophin complex are neces-sary for strong actin filament association with the sar-colemmal membrane. Finally, the methods used in this study will be valuable in assessing whether utrophin over-expression (Tinsley et al., 1998) can rescue the mechani-cally strong linkage between costameric actin and the sar-colemmal membrane observed in normal muscle.
We thank D r. Kurt A mann for preparing the rabbit 2 antiserum to dystro-phin, D r. Kevin Campbell for providing the rabbit 56 antiserum to utro-phin and D r. J. Chloe Bulinski for providing the polyclonal antibodies specific for g-actin. We are also grateful to D r. D onata O ertel for
provid-Figure 5. Costameric actin as a common nexus for mechanical
coupling between the contractile apparatus and extracellular ma-trix of striated muscle. Shown is a schematic model depicting the molecular organization of costameres in normal (top) and dystro-phin-deficient m dx mouse muscle (bottom). Based on evidence presented in the current report, the fracture plane effected by mechanical peeling is also indicated (*……….*).
on February 18, 2016
jcb.rupress.org
Downloaded from
ing access to her stereo dissecting microscope and D r. R ichard Moss for his expert advice and encouragement.
This work was supported by grants from the National Institutes of H ealth to J.M. E rvasti (A R 42423 and A R 01985) and a D evelopment G rant from the Muscular D ystrophy A ssociation to I.N. R ybakova.
Submitted: 12 June 2000 R evised: 17 July 2000 A ccepted: 17 July 2000
References
A mann, K.J., W.X.A . G uo, and J.M. E rvasti. 1999. U trophin lacks the rod do-main actin binding activity of dystrophin. J. B iol. Chem . 274:35375–35380 A mann, K.J., B.A . R enley, and J.M. E rvasti. 1998. A cluster of basic repeats in
the dystrophin rod domain binds F-actin through an electrostatic interaction.
J. B iol. Chem . 273:28419–28423.
A ndra, K., H . Lassman, R . Bittner, S. Shorny, R . Fassler, F. Propst, and G . Wiche. 1997. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. G enes D ev. 11:3143–3156.
Campbell, K.P. 1995. Three muscular dystrophies: Loss of cytoskeleton-extra-cellular matrix linkage. Cell. 80:675–679.
Chamberlain, J.S., K. Corrado, J.A . R afael, G .A . Cox, M. H auser, and C. Lu-meng. 1997. Interactions between dystrophin and the sarcolemma mem-brane. In Cytoskeletal R egulation of Membrane Function. S.C. Froehner and V. Bennett, editors. The R ockefeller U niversity Press, New York. 19–29. Cote, P.D ., H . Moukhles, M.Lindenbaum, and S. Carbonetto. 1999. Chimaeric
mice deficient in dystroglycans develop muscular dystrophy and have dis-rupted myoneural synapses. N at. G enet. 23:338–342.
Craig, S.W., and J.V. Pardo. 1983. G amma actin, spectrin, and intermediate fil-ament proteins colocalize with vinculin at costameres, myofibril-to-sarco-lemma attachment sites. Cell M otility. 3:449–462.
D alpe, G ., M. Mathieu, A . Comtois, E . Z hu, S. Wasiak, Y. D e R epetigny, N. Leclerc, and R . Kothary. 1999. D ystonin-deficient mice exhibit an intrinsic muscle weakness and an instability of skeletal muscle cytoarchitecture. D ev. B iol. 210:367–380.
D anowski, B.A ., K. Imanaka-Yoshida, J.M. Sanger, and J.W. Sanger. 1992. Costameres are sites of force transmission to the substratum in adult rat car-diomyocytes. J. Cell B iol. 118:1411–1420.
D uclos, F., V. Straub, S.A . Moore, D .P. Venzke, R .F. H rstka, R .H . Crosbie, M. D urbeej, C.S. Lebakken, A .J. E ttinger, J. Van der Meulen, et al. 1998. Pro-gressive muscular dystrophy in a-sarcoglycan-deficient mice. J. Cell B iol.
142:1461–1471.
E hmer, S., R . H errmann, R . Bittner, and T. Voit. 1997. Spatial distribution of
b-spectrin in normal and dystrophic human skeletal muscle. A cta N euro-pathol. (B erl.) 94:240–246.
E rvasti, J.M., and K.P. Campbell. 1991. Membrane organization of the dystro-phin-glycoprotein complex. Cell. 66:1121–1131.
E rvasti, J.M., and K.P. Campbell. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell B iol.
122:809–823.
E rvasti, J.M., K. O hlendieck, S.D . Kahl, M.G . G aver, and K.P. Campbell. 1990. D eficiency of a glycoprotein component of the dystrophin complex in dys-trophic muscle. N ature 345:315–319.
H oward, P.L., H .J. Klamut, and P.N. R ay. 1998. Identification of a novel actin
binding site within the D p71 dystrophin isoform. Febs L etters. 441:337–341. Iwata, Y., Y. Pan, T. Yoshida, H . H anada, and M. Shigekawa. 1998. a
1-syntro-phin has distinct binding sites for actin and calmodulin. Febs L etters. 423: 173–177.
Kameya, S., Y. Miyagoe, I. Nonaka, T. Ikemoto, M. E ndo, K. H anaoka, Y. Na-beshima, and S. Takeda. 1999. a1-syntrophin gene disruption results in the absence of neuronal-type nitric-oxide synthase at the sarcolemma but does not induce muscle degeneration. J. B iol. Chem . 274:2193–2200.
Lessard, J.L. 1988. Two monoclonal antibodies to actin: O ne muscle selective and one generally reactive. Cell M otil. Cytosk el. 10:349–362.
Milner, D .J., G . Weitzer, D . Tran, A . Bradley, and Y. Capetanaki. 1996. D is-ruption of muscle architecture and myocardial degeneration in mice lacking desmin. J. Cell B iol. 134:1255–1270.
Minetti, C., K. Tanji, and E . Bonilla. 1992. Immunologic study of vinculin in D uchenne muscular dystrophy. N eurology. 42:1751–1754.
Minetti, C., K. Tanji, P.G . R ippa, G . Morreale, G . Cordone, and E . Bonilla. 1994. A bnormalities in the expression of b-spectrin in D uchenne muscular dystrophy. N eurology. 44:1149–1153.
O hlendieck, K., and K.P. Campbell. 1991. D ystrophin-associated proteins are greatly reduced in skeletal muscle from m dx mice. J. Cell B iol. 115:1685– 1694.
O hlendieck, K., J.M. E rvasti, K. Matsumura, S.D . Kahl, C.J. Leveille, and K.P. Campbell. 1991. D ystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. N euron. 7:499–508.
O tey, C.A ., and J.C. Bulinski. 1988. Immunolocalization of muscle and non-muscle isoforms of actin in myogenic cells and adult skeletal non-muscle. Cell M otil. Cytosk el. 9:337–348.
Pardo, J.V., J. D ’A ngelo Siliciano, and S.W. Craig. 1983a. A vinculin-containing cortical lattice in skeletal muscle: Transverse lattice elements (“cos-tameres”) mark sites of attachment between myofibrils and sarcolemma.
Proc. N atl. A cad. Sci. USA . 80:1008–1012.
Pardo, J.V., M.F. Pittenger, and S.W. Craig. 1983b. Subcellular sorting of isoac-tins: selective association of g actin with skeletal muscle mitochondria. Cell.
32:1093–1103.
Petrof, B.J., J.B. Shrager, H .H . Stedman, A .M. Kelly, and H .L. Sweeney. 1993. D ystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. N atl. A cad. Sci. USA . 90:3710–3714.
Porter, G .A ., G .M. D mytrenko, J.C. Winkelmann, and R .J. Bloch. 1992. D ys-trophin colocalizes with b-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J. Cell B iol. 117:997–1005.
R ybakova, I.N., and J.M. E rvasti. 1997. D ystrophin-glycoprotein complex is monomeric and stabilizes actin filaments in vitro through a lateral associa-tion. J. B iol. Chem . 272:28771–28778.
R ybakova, I.N., K.J. A mann, and J.M. E rvasti. 1996. A new model for the inter-action of dystrophin with F-actin. J. Cell B iol. 135:661–672.
Straub, V., and K.P. Campbell. 1997. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr. O pin. N eurobiol. 10:168–175.
Straub, V., R .E . Bittner, J.J. Léger, and T. Voit. 1992. D irect visualization of the dystrophin network on skeletal muscle fiber membrane. J. Cell B iol. 119: 1183–1191.
Straub, V., J.A . R afael, J.S. Chamberlain, and K.P. Campbell. 1997. A nimal models for muscular dystrophy show different patterns of sarcolemmal dis-ruption. J. Cell B iol. 139:375–385.
Tinsley, J., N. D econinck, R . Fisher, D . Kahn, S. Phelps, J.M. G illis, and K. D avies. 1998. E xpression of full-length utrophin prevents muscular dystro-phy in m dx mice. N ature M ed. 4:1441–1444.
Williams, M.W., and R .J. Bloch. 1999. E xtensive but coordinated reorganiza-tion of the membrane skeleton in myofibers of dystrophic (m dx) mice. J. Cell B iol. 144:1259–1270.
on February 18, 2016
jcb.rupress.org
Downloaded from