ContentslistsavailableatScienceDirect
Carbohydrate
Polymers
jo u r n al h om ep age :w w w . e l s e v i e r . c o m / l o c a t e / c a r b p o l
Structural
characteristics
are
crucial
to
the
benefits
of
guar
gum
in
experimental
osteoarthritis
Rondinelle
R.
Castro
a,
Christine
Maria
M.
Silva
b,
Rodolfo
M.
Nunes
b,
Pablyana
L.R.
Cunha
c,
Regina
Celia
M.
de
Paula
c,
Judith
P.A.
Feitosa
c,
Virgínia
C.C.
Girão
d,
Margarida
M.L.
Pompeu
e,
José
Alberto
D.
Leite
f,
Francisco
A.C.
Rocha
b,∗aSuperiorInstituteofBiomedicalSciences,StateUniversityofCeará,Fortaleza60714-903,Brazil bDepartmentofInternalMedicine,FederalUniversityofCeará,Fortaleza60430-170,Brazil
cDepartmentofOrganicandInorganicChemistry,FederalUniversityofCeará,Fortaleza60451-970,Brazil dDepartmentofMorphology,FacultyofMedicine,FederalUniversityofCeará,Fortaleza60430-170,Brazil eDepartmentofPathology,FacultyofMedicine,FederalUniversityofCeará,Fortaleza60441-750,Brazil fDepartmentofSurgery,FederalUniversityofCeará,Fortaleza60430-170,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2February2016
Receivedinrevisedform1May2016 Accepted11May2016
Availableonline13May2016
Chemicalcompoundsstudiedinthisarticle:
Galactomannan(PUBCHEMCID:439336) Chonsurid(PUBCHEMCID:24766)
Keywords:
Guargum Osteoarthritis Oxidation Pain Sulfation
a
b
s
t
r
a
c
t
Protein-freeguargum(DGG)wasoxidized(DGGOX)orsulfated(DGGSU)byinsertionofnewgroupsin C-6(manose)andC-6(galactose),forDGGOXandDGGSU,respectively.Ratsweresubjectedtoanterior cruciateligamenttransection(ACLT)oftheknee,jointpainrecordedusingthearticularincapacitation test,andtheanalgesiceffectofintraarticular100gDGG,DGGOXorDGGSUsolutionsatdays4–7was evaluated.OthergroupsreceivedDGGorsalineweekly,fromdays7to70andjointdamageassessed usinghistologyandbiochemistryasthechondroitinsulfate(CS)contentofcartilage.Themolarmassof CSsampleswasobtainedbycomparingtheirrelativeelectrophoreticmobilitytostandardCS.DGGbut notDGGOXorDGGSUsignificantlyinhibitedjointpain.DGGsignificantlyreversedtheincreaseinCS, itsreducedelectrophoreticmobility,andhistologicalchangesfollowingACLT,ascomparedtovehicle. StructuralintegrityaccountsforDGGbenefitsinexperimentalosteoarthritis.
©2016ElsevierLtd.Allrightsreserved.
1. Introduction
Osteoarthritis(OA)isaleadingcauseofdisabilityandchronic painwithincreasingprevalenceandcoststohealthcaresystems. Therecognitionthatinflammatorycellsplay amajorroleinOA pathogenesishasledtotheconceptofanimportant immunome-diatedinflammatorycomponentinOApathogenesis(Berenbaum, 2013).OAtreatmentstillreliesonattemptstoprovidesymptom improvement whereas chondroprotective compounds, meaning treatmentstoalterOAprogressionand/oroutcome,areanunmet need(McAlindonetal.,2014).
Viscosupplementationhas beenproposedas a termto indi-catethe recoveryof theviscoelastic properties of thesynovial
∗Correspondingauthor.
E-mailaddress:arocha@ufc.br(F.A.C.Rocha).
fluidaftertheadministrationofhighmolarmasshyaluronicacid solutions or its analogues (Hunter, 2015). Despite a persistent debateontheclinicalefficacyofviscosupplementation,currentOA treatmentguidelinesrecommendthisstrategy,particularlytotreat patientswithkneeOA(Bruyèreetal.,2014;Hochbergetal.,2012; McAlindonetal.,2014).Systematicreviewsandmeta-analysisof dataonviscosupplementationefficacyarecontroversialprobably becauseofdifferentselectionofarticlesandalackof standardiza-tionofpatientselectionandevaluation(Hunter,2015).
Themechanismsresponsiblefortheviscosupplementation effi-cacy are yet to be demonstrated. Though some claim that the higher the molar mass of the agent, coupled to the gel state, thebettertheresults,there arenodefinitivedatatoprovethis assumption(Bannuruetal.,2015).Indeed,highmolarmasshylans (around 106g/mol) had similar efficacy,as compared to lower
masscompounds (5–7.5×105g/mol)in providing pain reliefin
OA(PasqualiRonchettietal.,2001).Thesustainedclinicalrelief
http://dx.doi.org/10.1016/j.carbpol.2016.05.031
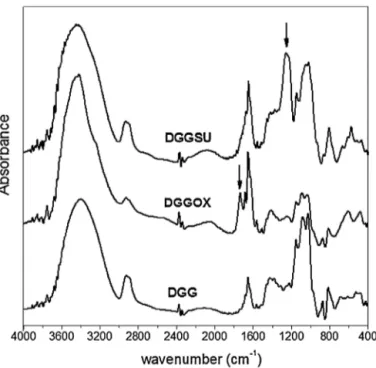
Fig1. FTIRspectrumofdeproteinizedguargum(DGG),oxidized(DGGOX),and sulfated(DGGSU)derivatives.
thatmaybeachievedafterasetof3–5injectionsorevena sin-gleinjectionofthesecompoundsmaylastforupto8–12months (Kirwan, 2001) argues against a local rheological effect to explainthetherapeuticmechanism.Consideringthatendogenous hyaluronicacidclearancefromthejointfluidisestimatedat20h andin OAjointthisclearance timeis evenless (Hunter, 2015), thepossibility thatexogenously administeredhyaluronates last lessthan1dayinthejointisverylikely.Furthermore,theissueof whetherviscosupplementationagentsdisplayachondroprotective effectinOAisalsoyettobedefined.
Guargumisahighlyviscouspolysaccharidederivedfromthe seedendospermoftheplantCyamopsistetragonolobus.Itis pre-dominantlycomposedofagalactomannanwithalongcentralchain ofmanoseresidues,joinedbytype-(1→4)glycosidiclinksand residuesofgalactosejoinedbytype␣-(1→6)linksattachedtothe sidesofthemannancentralcore.Thisgumwaspurifiedbyfour dif-ferentmethodsinordertoreducetheimpurities,especiallyprotein residues(Cunha,Maciel,Sierakowski,dePaula,&Feitosa,2007).
UsinganexperimentalOAmodelinrats,wedemonstratedthat theintraarticular(i.a.)injectionofthepurifiedDGG, meaninga protein-free guargum derivative,provided analgesia similarto thatofHylanG-F20,acommerciallyavailable viscosupplementa-tionagent(Castroetal.,2006).Additionally,thegalactomannan providedanalgesiawhenitwasgivenasaviscousorsaline solu-tion.Thatviscouspreparationpresentsaviscositysimilartothat ofHylanG-F20,110and120Pas,respectively,buttheviscosityof thegalactomannansolutionwasonly3.6Pas,atthesameshearrate andtemperature(Cunha,Castro,Rocha,dePaula,&Feitosa,2005). Thus, weproposedthat viscosupplementationdoesnotdepend onlyontheviscoelasticpropertiesofthecompound.Rather,itcould derivefromalocalpharmacologicaleffectyettobedescribed.
Inthepresentstudy,throughderivatizationoftheDGG (oxida-tionorsulfation)weprovidefurtherevidencethatthebiochemical structure,ratherthantheviscosity,accountstoexplaintheclinical effectsofthispolysaccharideinexperimentalOA.Additionally,the datashowthati.a.administrationofaDGGsolutionpreventsjoint damageinanOAmodel.
Male Wistarrats(180–200g) fromourown animalfacilities were used throughout the experiments. Animals were housed in cages (6/cage) in temperature-controlled rooms with a 12h light/darkcyclewithfreeaccesstowaterandfood.Atthestartof anyexperiments,ratswere2.5monthsofage.Alleffortsweremade tominimizeanimalsufferingandthenumberofanimalsused.The experimentalprotocolwasapprovedbyourlocalethics commit-tee(protocolnumber113/07)thatfollowedtheguidelinesofthe BrazilianCollegeofAnimalExperimentation(COBEA).
2.2. Methods
2.2.1. Sulfationofguargum
Theprotein-freeDGGwassulfatedusingmethodologyearlier reported by Moura Neto, Maciel, Cunha, de Paula, and Feitosa (2011)andrecentlyusedforsulfationofa galactomannanfrom Dimorphandragardneriana(MouraNetoetal.,2014).Some mod-ificationswereperformed,asfollows:amassof3gofDGGwas dissolvedin225mLofformamideovernight,followedbythe addi-tionof60mLpyridine.Thesolutionwascooledto4◦Cand18mLof chlorosulfonicacid(CSA)wasdroppedoveraperiodof3h.The pro-portionSR/SU(molarratioofsulfationreagenttosugarunit)was maintainedin14.6.After12hat13◦C,thesolutionwas neutral-izedwithNaHCO3,dialyzedagainstwater,thesolidprecipitated,
washedmanytimeswithethanol,andfiltered.Thedryproductwas denotedasDGGSU.
2.2.2. Oxidationofguargum
The protein-free DGG was oxidized following the method reportedbySierakowski,Milas,Desbrièes,andRinaudo(2000)and Cunhaetal.(2007),asfollows:amassofDGG(2g)wasdissolved in1Ldistilledwater understirringovernight.Thesolutionwas cooledinanicebath,sodiumhypochlorite(9mL)wasadded,and pHadjustedto9.2.TEMPOreagent(18.4mg)andNaBr(160mg) wereadded.TheoxidationproceededatconstantpH,adjustedwith NaOH.Borohydride(50mL)wasincludedtostopthereaction.The pHwasdecreasedto7,thegumprecipitatedwithEtOH,andafter 12hinrefrigerator,filtered,andwashedwithEtOH.DGGOXisthe designationoftheproduct.
2.2.3. Characterizationofguargumderivatives
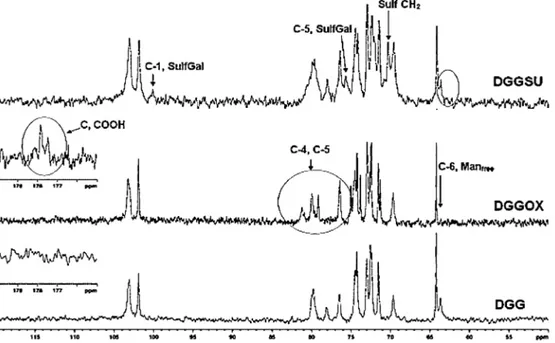
Fig.2.13CNMRspectrumofdeproteinizedguargum(DGG),oxidized(DGGOX),andsulfated(DGGSU)derivatives.
ShimadzumodelIRPrestige21spectrophotometerbetween400 and4000cm−1.Thepositionoftheintroducedgroupswasdefined
usingNuclear MagneticResonanceanalysis,withuni(RMN 1H,
RMN13CandDEPT135)orbidimensionaltechniques
(Heteronu-clearMultipleQuantumCoherence,1H–13CHMQC).Thegumswere
dissolvedindeuteratedwater for12handanalyzedinaBruker modelAvance-DMX-500spectrometer.
Thestaticlight scattering measurementswereperformed in a multi angle photometer fromMalls Dawn Wyatt Technology Corporation,workingwithaHe-Nelaser(632.8nm).Thegums con-centrationsvariedfrom3.0×10−4to1.15
×10−3g/mLinNaNO 3
0.1mol/L,usedafterfiltrationthrough0.2and0.45mMillipore membranes.
Theapparentviscositywasdeterminedin 1%(w/v)aqueous solutionsatshearrate200s−1,andtemperature25◦C,bytheuse ofaRheometerBrookfieldcone-platemodelLV-DVIII.The intrin-sicviscosity([])wascalculatedfromflowtimeofsolutionsina capillaryUbbelohdeviscometerfromCannonInstrumentsmodel 1I-71.Thesolventusedwas0.1mol/LNaCl,andthetemperature wascontrolledat25◦C.Hugginsplotwasappliedforthe[] deter-mination.
Themolarmassdistributionwasestimatedbygelpermeation chromatographyin aShimadzuequipmentand refractiveindex detector(RID-6A).Anultrahydrogellinearcolumn(7.8×300mm, Exclusionlimit7.0×106gmol−1,WatersTMCorporation),0.1mol/L
NaNO3 assolvent,flowat0.5mL/min,andambienttemperature
weretheexperimentalconditionsforGPCanalysis.
2.2.4. Analysisofglycosaminoglycansfromthearticularcartilage Thecartilageofthedistalfemoralextremitieswasexcisedwith asurgicalbladeimmediatelyaftersacrificeofanimalsandweighed afterovernightdrying(80◦C).Thematerialwassubjectedto pro-teolysisusing PROLAV 750TM (Prozyn, SP, Brazil) suspended in
Tris–HCl/NaCl50mmol/L/150mmol/Lbuffer(pH8.0),andfurther precipitatedinabsoluteethanol,followedbydilutionindistilled water. Thismaterial wasseparated ona 0.6% w/vagarose gel-electrophoresisindiaminopropaneacetatebuffer(50mmol/L,pH 9.0).Gelswerestainedwith0.1%w/vtoluidine-blue(Bezerraetal., 2004).Forcomparison,standardC4S,C6S,andheparansulfatewere subjectedtothesameprotocol.Quantificationwasmadeby densit-ometry(525nm).DatawereexpressedasgCS/mgdriedcartilage.
2.2.5. Anteriorcruciateligamenttransection(ACLT)model
Rats were anesthetized with i.m. ketamine (50mg/kg) and xylazine(10mg/kg).After preparingfor localaseptic surgery, a parapatellarincisionwasmade,followedbylateraldisplacementof thepatella,therebyprovidingaccesstothejointspace.Theanterior cruciateligamentistheneasilyvisibleandwassurgicallyexcised tryingtoavoiddamagetotheunderlyingcartilageandmeniscus. Theincreaseinanteriordisplacementofthetibiainrelationtothe femurwasusedtoassurethattheligamentwastransected.The jointcapsuleandskinweresuturedwithVycril(6-0)and monony-lon(4-0)threads,respectively.Ashamgroupwassubjectedtoskin incision,patelladisplacement,andexposureofthejointwithout damagetotheligaments,followedbywoundclosure.Anaivegroup receivednomanipulation.
2.2.6. Measurementofjointpain
Thearticularincapacitationmethod,asdescribedearlier,was usedwithmodifications(Castroet al.,2006).Theanimalswere puttowalkonasteelrotarydrum (30cmwide×50cm diame-ter),whichrotatesat3rpm.Speciallydesignedmetalgaiterswere wrappedaroundbothhindpaws.Afterplacementofthegaiters,the animalswereallowedtowalkfreelyforhabituation.Therightpaw wasthenconnectedviaasimplecircuittoamicrocomputerdata input/outputport.Thepawelevationtime(PET),asrecordedinthe presentstudy,isthetimeinsecondsthatduringa10-minperiod thehindpawisnotincontactwiththecylinder.Thearticular inca-pacitationmeasuredisinhibitedbyclassicalanalgesiccompounds leadingtotheassumptionthatitreflectsjointpain.Forcomparison betweentreatedgroups,resultsarereportedasthemeanofthePET obtaineddaily,startingatday4,untilday7.
2.2.7. Histopathology
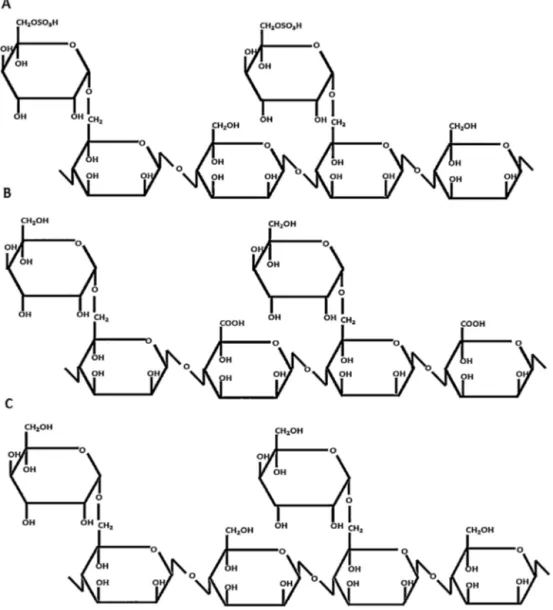
Fig.3. Chemicalstructuresofdeproteinizedguargum(DGG),oxidized(DGGOX),andsulfated(DGGSU)derivatives.
forboth condylesandexpressedasone resultforeach sample. Semi-quantitativehistopathologicalgradingwasperformedbytwo independentpathologists(VCCG,MMLP)blindedtogroup alloca-tionaccordingtotheOsteoarthritisResearchSocietyInternational (OARSI)histopathologygradingandstagingsystem(Pritzkeretal., 2006).Themaximalpossiblefinalscore(meanofmeasuresmadeby thetwopathologists)was24.Resultsareexpressedasthemedian (interquartilerange-IQR)valuesforeachtreatmentgroup.
2.2.8. Pharmacologicalmanipulation
Inordertoinvestigatewhetherthebiochemicalstructureis rele-vanttotheanalgesiceffectprovidedbythegalactomannan,groups ofratssubjectedtoACLTreceivedintra-articular(i.a.)injectionsof 100gin50LsalinesolutionsofeitherDGG,DGGSUorDGGOX derivatives,atday4followingACLT.Thearticularincapacitation, asreflectingjointpain,wasmeasureddaily,untilday7.Inanother setofexperiments,in anattempttoevaluatea potential chon-droprotectiveeffect ofthe galactomannan,meaninga potential jointstructuralbenefit,groupsofratsreceivedeitherDGGsolution (100g/50Li.a.)orsalineonceaweek,startingatday7,during 10weeks.
2.2.9. Statistics
Resultsarepresentedasthemeans+95%CIforpain measure-mentsand medians(IQR)for histologymadeonsix animalsin
eachgroup.Assessmentofnormalityofthepainbehaviourdata wasdoneusingtheD’Agostino-PearsonOmnibustest.Differences betweenmeanswerecomparedusingone-wayanalysisofvariance (ANOVA)followedbyTukey’stest;P<0.05wasconsideredas sig-nificant.Forcomparisonofmedians,theKruskal–Wallistestwas used,followedbyDunn’stest;P<0.05wasconsideredsignificant.
3. Resultsanddiscussion
3.1. Characterizationofproteinfreeguargumandderivatives
TheFT-IRspectra(Fig.1)ofderivativesareverydifferentfrom thatoftheproteinfreeDGG.IntheDGGSUanewandintenseband appearsat1262cm−1,assignedtoasymmetricalstretchingofS O
vibration.SpectraldifferencecanbeseeninDGGOXincomparison withunmodifiedDGG.AbandattributedtoC Ostretching vibra-tionisnowpresentinDGGOXspectrumduetotheinsertionof COOHgroup.
Fig.4.Gelpermeationchromatographycurvesofdeproteinizedguargum(DGG), oxidized(DGGOX),andsulfated(DGGSU)derivativessolution.
groupsper6carbons.FromdataontheSandCcontent,andusing Eq.(1)(Melo,Feitosa,Freitas,&dePaula,2002),theDSvalueof 0.60wascalculated.ThedegreeofsubstitutionfortheDGGOXwas 0.36,closetothevalueobtainedfortheoxidationof galactoman-nanfromLeucaemaleucocephalainsimilarcondition(Sierakowski etal.,2000).
DS=
( S%
atomicmassofS)
( C%
atomicmassofCx6)
=2.25(S%
C%) (1)
ThederivativeswereanalyzedbyNMRinordertoverifythe prefer-entialpositionofsulfationandoxidation.Comparisonbetweenthe
13CNMRspectrumofDGGOXandDGG(Fig.2)revealedtwomain
differences:(a)theabsenceofthesignalat63.7ppmpresentinthe unmodifiedDGG,and(b)presenceoftwonewsignalsatthe177.5 and177.8ppm.TheabsenceofthesignalfromC-6offreemannose indicatesthatthismonosaccharidewastotallysubstitutedinthe oxidationreaction.ThenewsignalsareduetothepresenceofC O groupfromCOOH.
The1H–13CHSQCspectra(SupplementaryinformationFig.S1)
exhibittheshifttoalower␦valueoftheprotonH-1frommannose residueinDGGOX,whichconfirmsthesubstitutionatC-6from mannose.Newsignalsat70.3,75.6,and100.5ppmwereobserved in13CNMRspectrumofsulfatedgum(Fig.2).Thefirstoneisdueto
theinsertionofsulfategroupintheprimarycarbon(C-6).Theproof thatthisnewsignalisattributedtosubstitutedCH2wasobtained
fromDEPT135(SupplementaryinformationFig.S2).Thesignals fromCH2appearinoppositeamplitudetothoseofCHandCH3.
Fig.5.Sulfationoroxidationofdeproteinizedguargum(DGG)abrogatesanalgesia inanosteoarthritismodel.RatsweresubjectedtoACLT.Jointpainwasassessed dailyastheincreaseinthepawelevationtime(PET),usingthearticular incapac-itationtest.AshamgroupwassubjectedtothesurgicalprocedurewithoutACLT andreceivedsalinebygavage.Thenon-treated(NT)groupreceivedsaline.Groups received100g/50Li.aofeitherDGG,sulfatedDGG(DGGSU)oroxidizedDGG (DGGOX),starting4daysafterACLT.ResultsarereportedasthemeanofthePET (s/10min)obtaineddaily,startingatday4,untilday7;n=6animals/group;*P<0.05 comparedtoNTusingone-wayANOVA,followedbyTukey’stest.
Thesignalsat75.6and 100.5ppmareattributedtoC-5andC-1 fromsulfatedgalactose,shiftedfromoriginal␦.
Thesulfationoccurredpreferentiallyinthecarbon6ofthe galac-toseresidueoftheoriginalgalactomannanwhereastheoxidation occurredpreferentiallyinthecarbon6ofthemannoseresidue,as showninFig.3.Theguargumderivativeswerealsocharacterized byviscosityand molarmassdetermination.Thegelpermeation curves (Fig. 4)indicate chain degradation in oxidized and sul-fated samples.The guar gumshows a monomodal distribution withelutionvolume7.6mL.Theoxidizedderivativealsopresents amonomodaldistribution,butwiththeelutionvolumeshiftedto highervolume(7.8mL).AlowermolarmassisobtainedforDGGOX. ThecurveprofileforDGGSUisdifferent.Amainpeakoccursat 7.8mL,buttwoshoulderscanbevisualized,at7.4and9.4mL.The distributionofmolarmassismoreheterogeneous,withlow frac-tionofunsulfatedmaterialandotherfractionwithintensechain degradation(9.4mL).Duetotheionicchargeofthederivativesand theunavailabilityofproperstandardsthemolarmasscannotbe quantifiedbythiskindofGPC/detector.Theweightaveragemolar mass(Mw)wascalculatedbylightscatteringexperiments,basedon
Zimmplots.Allderivativesshowlowermolarmassthanthe orig-inalguargum.TheDGGpresentsMw3.9×106g/mol,andDGGOX
andDGGSUshow2.6×106and2.8×106g/mol,respectively.The
slightlyhigherMw valueofDGGSU,incomparisonwithDGGOX
maybeduetothepresenceofthefractionoflowelutionvolume, andtheinfluenceofhighdimensionchainovertheMw.
Table1
Maincharacteristicsofunmodifiedandmodifiedguargum.
Characteristics Guargum
Non-modified sulfated oxidized
Insertedgroup – OSO3Na COOH
Substitutiondegree 0.00 0.60 0.36
Insertposition – C-6-galactose C-6-mannose
Intrinsicviscosity(dL/g)a 6.2 4.9 2.2
Weightaveragemolarmass(g/mol) 3.9×106 2.8
×106 2.6
×106
Apparentviscosity(mPas)b 58 39 9
aSolvent0.1mol/LNaClat25◦C.
themeans±SDofCS(g/mgofdriedcartilage)ingroupsof6animals.*P<0.05comparedtosham;#P<0.05,comparedtoNT(one-wayANOVA,followedbyTukey’stest).
(B)Cartilagesampleswereranona6%polyacrylamidegelelectrophoresis.Therelativemobilitywasobtainedbytheratiobetweenthepeakpositionofthesampleandthe peakpositionofstandardchondroitin-4-sulfate(C4S).TheMwoftheCSfromthevariousgroupsampleswasassessedbydeterminingitsrelativemobilitytostandardC4S
raninparallelona6%polyacrylamide-gelelectrophoresis.ValuesrepresenttheratioofthepeakpositionofthedigitalizedgelimagecurvesandthatofC4S,arbitrarilyset at1.0;n=6animals/group.#P<0.05comparedtoNT(one-wayANOVA,followedbyTukey’stest).
Theintrinsicviscosityandtheapparentviscositywere deter-minedand thevalues showedinTable1.TheDGGOXpresents thelowestintrinsic(2.2dL/g)andapparentviscosity(9.0mPas). The unmodified DGG exhibits the highest values ([]=6.2dL/g andapp=58mPas).IntermediatevaluesareobtainedforDGGSU
([]=4.9dL/gandapp=39mPas).
3.2. Structuremodificationsoftheguargumabrogatejoint analgesia
As we have reported previously, a single administration of a DGG solution(100g/50L)at day4 following ACLT signifi-cantlyreducedjointpain,ascomparedtoanimalsthatreceived saline(Fig.5).AdministrationofeithertheDGGOXortheDGGSU preparationssignificantlypreventedjointanalgesiapromotedby the original DGG solution (Fig.5), meaning that the structural integrityofthepolysaccharideiscrucialtoitsanalgesiceffect.It shouldberemarkedthatthemolarmassofthecompounds,aswell astheirintrinsicviscosity,werenotsignificantlymodifiedafter oxi-dationorsulfationofthegalactomannan.Coupledtoourprevious reportshowingthatthegalactomannaninhibitionofjointpainin thisOAmodeloccursregardlessofusingagelorsalineformulation (Castroetal.,2006),thisfindingaddssupporttoourproposalthat pharmacologicratherthanrheologicalmechanismsaccountforthe analgesia.
We are not aware of similarmodifications in thestructural ofcommerciallyavailableviscosupplementationagents. Rheolog-ical properties of hyaluronic acid preparations are claimed to be responsible for the beneficial effect of those compounds in patientswithOA.Apparently,high-molarmassHApreparations improveviscoelasticitythusamelioratingjointlubrication. How-ever,invitrostudiesareusedtosupportthisassumption,whichis questionedforinstancebytheobservationthatasustainedreliefis experiencedbypatientsexposedtoviscosupplementation.In keep-ingwiththisskepticism,arecentinvivostudyinrabbitsshowed that theviscoelasticityofthe synovialfluidwassimilar in ani-mals that received saline, as well as low or high molar mass HA preparations, but only the high molecular weight HA was chondroprotective(Elmorsyet al.,2014).Thefactthat ourguar gumpreparationsaresaline-solubleand theirrheologic proper-tiesdifferfromthoseHApreparations,whichareviscous(Elmorsy etal.,2014),arguesthatmolecularstructure ratherthan intrin-sicviscosityaccountforthebiologicalactivityofintraarticularly administered polysaccharidesin OA. Thus, ifwe have toadmit
Fig.7.DGGsolutionpreventsjointdamageseenathistologyintheACLTmodel.Rats weresubjectedtoACLTandkilledafter70days.GroupsreceivedGG(100g/50L i.a)orsaline(50Li.a)(NT),weekly,startingatday7untilsacrificeatday70 afterACLT.Femoralextremitieswereexcisedandprocessedforhematoxylin–eosin andtoluidine-bluestaining;evaluationusedtheOARSIgradingandstaging sys-tem.Resultsaremedians(range)ofn=6animals/group.*P<0.05comparedtoNT; #P<0.05,comparedtoSham(Kruskal–Wallis,followedbyDunn’stest).
Fig.8.DGGsolutionpreventshistologicalalterationsintheACLTmodel.RatsweresubjectedtoShamoperation(AandD)orACLT(BandE).Thedistalfemoralextremities wereexcised70daysafterACLTandprocessedforhematoxylin–eosin(leftpanel)ortoluidine-blue(rightpanel)staining.Groupsreceivedsaline(BandE)or100g/50L DGG(CandF)i.art.,weekly,startingatday7untilsacrificeatday70afterACLT.Thisrepresentativeillustrationshowspreservationofthearticularcartilageliningand stainingintensityinshamgroup(AandD),ascomparedtothesalinegroup(BandE)thatshowsfissure,erosion(arrow)andareaofdenudation(arrow-head)thatarenot noticedinthegroupthatreceivedDGG(EandF)(originalmagnification×40).
as1.0.Themolarmass(MM)canthenbeestimatedasinversely relatedtotherelative mobility ofthesample. Asa comparison C6S(MM=5.88×104g/mol),underthesameconditions,presents
arelativemobilityof0.708.
3.3. Galactomannanpreventsstructuraljointdamagein experimentalOA
Aswe showedpreviously (Silva et al., 2009), cartilage sam-plesfromratssubjectedtoACLTdisplayanincreaseintheGAG contentaswellasareducedelectrophoreticmobilityoftheGAG, as compared to sham-operated animals. In the present study, administrationoftheoriginallypurifiedDGGsolutionsignificantly reversedboththeincreaseinGAGcontentandthealtered elec-trophoreticmobilityoftheextractedGAG,ascomparedtoanimals thatreceivedsaline(Fig.6a–b),thusindicatingachondroprotective effectofthegum.
TheincreaseintheGAGcontentofthecartilagesamplesfrom theOAgroupwerereportedpreviouslyinourhands(Silvaetal., 2009)aswellasinotherstudies(Hosseininia,Lindberg,&Dahlberg, 2013).Ithasbeenproposedthatthisincreaserepresentsanearly
anabolicresponsetotheinjurythatsubsides,leadingplacetoa catabolicstageandprogressivedestructionofthejointcartilage. Byinterferingwiththeongoingprocess,theadministrationofDGG apparentlypreventedjointdamagetoanimalssubjectedtoACLT. Thoseeffects wereassociatedtoapronouncedinhibitionofthe structuraldamageathistopathology.Hence,inadditiontoprovide analgesia,theDGGsolutionhadajointprotectiveeffect.
3.4. Galactomannanadministrationpreventsjointdamageat histopathologyinexperimentalOA
Fig.7showsthehistologicalscoresatboththefemoraland tib-ialextremitiesofratssubjectedtoACLTwhich,asexpected,were clearlymoresevereandextensive,ascomparedtosham-operated animals. Administration of the purified DGG, using the same concentration(100g)thatwasanalgesic,significantlyprevented the severity and the extension of the joint damage seen at histopathology,ascomparedtoanimalsthatreceivedthevehicle.
ingredient in all commercially available viscosupplementation agents, wespeculate that structural integrity is crucialto their clinicalefficacy.
4. Conclusions
Tothebestofourknowledge,webelievethisisthefirststudy toshowthatstructuralmodificationsapartfromthemolarmass ofthecompoundarecrucialtodefineanalgesiaassociatedto vis-cosupplementation.SincewehavepreviouslyshownthatDGGis effectiveregardlessofbeingaviscousorsaline-solublepreparation, ourpresentdatafurtherindicatethatpolysaccharidesprovide anal-gesiaandchondroprotectioninOAthroughpharmacologicalrather thanrheologicalmechanisms.
Acknowledgements
This work was supported by grants 302218/2014-9 and 459334/2014-0 from CNPq (Conselho Nacional de Desenvolvi-mentoeTecnológico-Brasil).TheauthorsaregratefultoDr.Maria RitaSierakowski(UFPr)forhersupportwiththemodificationof thepolysaccharide.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.carbpol.2016.05. 031.
References
Azero,E.G.,&Andrade,C.T.(2002).Testingproceduresforgalactomannan purification.PolymerTesting,21,551–556.
Bannuru,R.R.,Schmid,C.H.,Kent,D.M.,Vaysbrot,E.E.,Wong,J.B.,&McAlindon, T.E.(2015).Comparativeeffectivenessofpharmacologicinterventionsfor kneeosteoarthritis:asystematicreviewandnetworkmeta-analysis.Annalsof InternalMedicine,162(1),46–54.
heparinandotheracidificmucopolysaccharidesfrommammaliantissues.
AnalyticalBiochemistry,46,209–218.
Elmorsy,S.,Funakoshi,T.,Sasazawa,F.,Todano,M.,Tadano,S.,&Iwasaki,N.(2014).
Chondroprotectiveeffectsofhigh-molecular-weightcross-linkedhyaluronic acidinarabbitkneeosteoarthritismodel.OsteoarthritisandCartilage,22(1), 121–127.
Hochberg,M.C.,Altman,R.D.,April,K.T.,Benkhalti,M.,Guyatt,G.,McGowan,J., etal.(2012).AmericanCollegeofRheumatology2012recommendationsfor theuseofnonpharmacologicandpharmacologictherapiesinosteoarthritisof thehand,hipandknee.ArthritisCareResearch,64,465–474.
Hosseininia,S.,Lindberg,L.R.,&Dahlberg,L.E.(2013).Cartilagecollagendamage inhiposteoarthritissimilartothatseeninkneeosteoarthritis;acase-control studyofrelationshipbetweencollagen,glycosaminoglycanandcartilage swelling.BMCMusculoskeletalDisorders,9,14–18.
Hunter,D.J.(2015).Viscosupplementationforosteoarthritisoftheknee.TheNew EnglandJournalofMedicine,372,1040–1047.
Kirwan,J.(2001).Isthereaplaceforintra-articularhyaluronateinosteoarthritisof theknee?Knee,8,93–101.
McAlindon,T.E.,Bannuru,R.R.,Sullivan,M.C.,Arden,N.K.,Berenbaum,F., Bierma-Zeinstra,S.M.,etal.(2014).OARSIguidelinesforthenon-surgical managementofkneeosteoarthritis.OsteoarthritisandCartilage,22,363–388.
Melo,M.R.S.,Feitosa,J.P.A.,Freitas,A.L.P.,&dePaula,R.C.M.(2002).Isolation andcharacterizationofsolublesulfatedpolysaccharidefromtheredseaweed Gracilariacornea.CarbohydratePolymers,49,491–498.
MouraNeto,E.,Maciel,J.S.,Cunha,P.L.R.,dePaula,R.C.M.,&Feitosa,J.P.A. (2011).Preparationandcharacterizationofachemicallysulfatedcashewgum polysaccharide.JournaloftheBrazilianChemicalSociety,22(10),1953–1960.
MouraNeto,E.,Sombra,V.G.,Richter,A.R.,Abreu,C.M.W.S.,Maciel,J.S.,Cunha, P.L.R.,etal.(2014).ChemicallysulfatedgalactomannanfromDimorphandra gardnerianaseed:characterizationandtoxicityevaluation.Carbohydrate Polymers,101,1013–1017.
PasqualiRonchetti,I.,Guerra,D.,Taparelli,F.,Boraldi,F.,Bergamini,G.,Zizzi,F., etal.(2001).Morphologycalanalysisofkneesynovialmembranebiopsiesfrom arandomizedcontrolledclinicalstudycomparingtheeffectsofsodium hyaluronate(Hyalgan)andmethylprednisoloneacetate(Depomedrol)in osteoarthritis.Rheumatology,40,158–169.
Pritzker,K.P.,Gay,S.,Jimenez,S.A.,Ostergaard,K.,Pelletier,J.P.,Revell,P.A.,etal. (2006).Osteoarthritiscartilagehistopathology:gradingandstaging.
OsteoarthritisandCartilage,14,13–29.
Sierakowski,M.R.,Milas,M.,Desbrièes,J.,&Rinaudo,M.(2000).Specific modificationsofgalactomannans.CarbohydratePolymers,42,51–57.