w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Evaluation
of
limonoid
production
in
suspension
cell
culture
of
Citrus
sinensis
Elisângela
Fumagali
Gerolino
a,
Talita
Perez
Cantuaria
Chierrito
a,
Arquimedes
Santana
Filho
b,
Eliezer
Rodrigues
Souto
c,
Regina
Aparecida
Correia
Gonc¸
alves
a,
Arildo
José
Braz
de
Oliveira
a,∗aProgramadePós-graduac¸ãoemCiênciasFarmaceuticas,DepartamentodeFarmacia,UniversidadeEstadualdeMaringá,Maringá,PR,Brazil bLaboratoriodeQuímicadeCarboidratos,DepartamentodeBioquímica,UniversidadeFederaldoParaná,Curitiba,PR,Brazil
cDepartamentodeAgronomia,UniversidadeEstadualdeMaringá,Maringá,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5November2014 Accepted22May2015 Availableonline23June2015
Keywords: Citrussinensis Biotechnology Limonoidaglycones Limonin
Plantcellculture
a
b
s
t
r
a
c
t
Theuseofcellandplanttissueculturetechniquestoproduceeconomicallyimportantactivemetabolites
hasbeengrowing.Amongthesesubstancesaretotallimonoidaglycones,whichareproducedby“pera”
orange(Citrussinensis(L.)Osbeck,Rutaceae)andhavereceivedconsiderableattentionbecauseoftheir
anticanceractions.Themainobjectiveofthepresentstudywastoanalyzeandcomparethelevelsof
limonoidaglyconesinseeds,calluscultures(originatingfromseeds),calluscultures(originatingfrom
hypocotyls),cellsuspensionsfromhypocotylscells,andcellsuspensionsfromcotyledons.Thecell
cul-turesorC.sinensiswereobtainedbyinoculatingtwostrainsofcallusinMSmediumsupplementedwith
2.0M2,4-dichlorophenoxyaceticacid,7.0Mbenzylaminopurine,and3%(w/v)sucroseinthedark.
Thehighestconcentrationsoflimonoidaglyconethatwereobtainedwereobservedincotyledoncell
lines(240mg/100gdryweight)thatwereproducedonday21ofcultureandhypocotylcelllinesonday
7(210mg/100gdryweight).Explantsofdifferentoriginsunderthesamecultureconditionshaddifferent
limonoidaglyconecontent.Thepresentresultsmaysuggeststrategiesforenhancingtheproductivityof
biologicallyimportantlimonoidaglyconesandinvestigatingthecomplexpathwaysofthesesecondary
metabolitesinplanttissuecultures.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Biotechnologyusestechniquesandprocessesthatinvolve liv-ingorganismstoobtainspecificproductsand/ormodificationsthat increasetheproductionofchemicalsubstancesofinterestinless timeandlesscapitalinvestment(DaviesandDeroles,2014). Sec-ondarymetabolitesthatarefoundinplantsaregenerallyproduced inlowconcentrationscomparedwithprimarymetabolites. There-fore,differentstrategies,includinginvitroculturesystems,have beenextensivelystudiedtoincreasetheproductionofsecondary metabolitesinplants(Smetanska,2008;MuranakaandSaito,2010; Gilletal.,2013).Invitrocellculturesrepresentaninteresting alter-nativebecausesecondarymetabolitesofinterestareobtainedin acontrolledenvironmentthatisnotinfluencedbychangesin cli-mateorsoilconditions(Gonc¸alvesandRomano,2013;Collin,2001;
Fumagalietal.,2008).Plantsthataregrownintheirnaturalhabitat
generallyhavevaryingconcentrationsofcompoundsofinterest,
∗ Correspondingauthor.
E-mail:ajboliveira@uem.br(A.J.B.deOliveira).
depending onthe particular cropseason(Salmore and Hunter,
2001;Puricellietal.,2002;RalphsandGardner,2001).Moreover,
theirexploitationintheirnaturalenvironmentcancausegradual geneticerosion(SidhuandBel,1996).
Citrusplantsaregrownworldwide.Therearebasicallyfour com-mercialspeciesofCitrus,amongwhichCitrussinensis(L.)Osbeck, Rutaceae(sweetorange)isnotable.Variousmetaboliteshavebeen isolatedfromspeciesoftheCitrusgenus.Amongthemajor second-arymetabolitesareflavonoidsandlimonoids,andpectinsarethe primarymetabolites(Khaliletal.,2002;Berhowetal.,2000;Okwu,
2008).
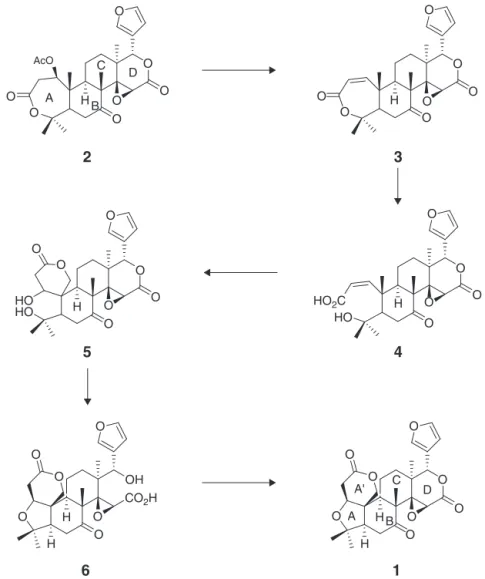
Limonoidsarehighlyoxygenatedmodifiedtriterpenesthatare derivedfromaprecursorwitha4,4,8-trimethyl-17-furanylsteroid skeletonthatisbiosynthesizedbytheacetate–mevalonate path-way in Citrus (Hasegawa and Hoagland, 1977). All naturally occurringCitruslimonoidscontainafuranringthatisattachedto ad-ringatC-17andoxygen-containingfunctionalgroupsatC-3, C-4,C-7,C-16,andC-17(Fig.1).Oxidativedegradationonthe C-17sidechainofeitheroftheseskeletonsresultsinthelossoffour carbonatomsand theformationof -substitutedfuran.Further oxidationandskeletalrearrangementsinoneormoreofthefour
http://dx.doi.org/10.1016/j.bjp.2015.05.008
O
O
O
O O
O O
O O
O O
O O
H O
A B
C D
O
AcO
O O
O O O
H O O
2
3
5
4
6
1
O O
O O O
H HO HO2C O
O
O O O
H O O
HO HO
OH
O O O
CO2H
H
H H
H A
A' C
B D O
O
O
Fig.1.LimoninbiosynthesispathwayproposedbyBreskaetal.(2007).
rings(designatedA,B,C,andD;Fig.1)giverisetodifferent skele-tonsofCitruslimonoids.Fig.1showsthebiosyntheticpathwayof limonin(1),themostabundantaglyconeinCitrusspecies.
Limonoidshavepropertiesthatinhibittheformationof chem-ically induced tumors in the mouth, stomach,small intestines, colon,lungs,andskininexperimentalanimals(Berhowetal.,2000). Anotherinterestingpropertythathasbeendescribedforlimonoid aglyconeisitsantimalarialactivity(Khaliletal.,2002).
Callusculturesandcellsinsuspensionhavebeenusedtostudy thebiosynthesisofeconomicallyimportantsecondarymetabolites
(Gonc¸alvesetal.,2010),enablingthepropagationofcelllineages
thatcontainalterationsinbiosyntheticcapabilities.
Theproductionofdifferentcompoundsinplantsisgenerally mediatedbyenvironmentalfactorsthatvaryaccordingto physio-logicalconditionsandseasonalvariations(Gilletal.,2013).Thus, cellculturesensurecontrolledconditionsthatcircumvent environ-mentalchangesandallowustoguide,inacontrolledmanner,the synthesisofthesecompounds.
Onlyafewstudieshaveutilizedcallusand/orcellculturesofC. sinensistoobtainsecondarymetabolites.Forexample,Niedzetal.
(1987)soughttoproducevolatilecompoundsfromcalluscultures,
andEndoetal.(2002)usedacellculturethatwasobtainedfrom
embryogeniccallustoproduceCitruslimonoids.
Thepurposeofthepresentstudywastoevaluatetheinvitro productionoflimonoidaglycones usingnon-embryogeniccallus culturesandcellsinsuspensionthatoriginatedfromcotyledons andhypocotylsofBrazilian“laranja-pêra”oranges(Citrussinensis).
Materialandmethods
Instrumentationandgeneralprocedures
Absorption spectra in the infrared regionwere obtained on aKBrtabletinaFouriertransforminfraredspectrometer (FTIR-Bomen, model MB-100C26). The analysis was performed on a diskcomposedof1mgofsteepedsampleswith250mgKBr.The one-dimensional(1D)/two-dimensional(2D)1H and13Cnuclear
magneticresonance(NMR)spectrawereobtainedusingaVarian MercuryPlusspectrometerat300MHzwithCDCl3asthesolvent
andTMSastheinternalstandard.Thespectraofhigh-resolution masseswereobtainedusingBrukerDaltonicsequipment(model microTOF-QII-ESI-TOF).Theplatesthatwereusedforanalytical thin-layerchromatography(TLC)were60F254aluminumsilicagel
sheets(Merck).Thechromatoplatesweredevelopedusing ultravi-oletlight(254and 365nm)andasulfuricvanillin solution.The solvents (hexane, acetone,and acetonitrile) that were used for thepreparationoftheextractsandchromatographicfractionation wereofanalyticalgrade.
Plantmaterial,calluscultures,andcells
inoculatedinbottledMSculture(MurashigeandSkoog,1962) sup-plementedwith30gsucrose,2.0M2,4-dichlorophenoxyacetic acid(2,4-D), 7.0Mkinetin,and 0.8%(w/v)agarandincubated at28◦Cinthedarktoinducecallusformation.Aftergermination
andseedlingdevelopment,thehypocotylsandsterilecotyledons wereusedasexplants.TheyweretransferredtoMSmedium sup-plementedwith 2.0M2,4-dichlorophenoxyacetic acid(2,4-D), 7.0Mkinetin,and0.8%(w/v)agarandthenincubatedat28◦C
inthedarktoinducecallusformation.Afterward,totallimonoid aglyconesweremeasuredinthethird-generationsubculture,and thegrowthprofilewasstudiedinaliquidcellculturesuspension.
Establishmentofcellculturesinsuspension
Freshcallus(0.6g)weretransferredtoaflaskthatcontained 50mlofMSliquidmediumsupplementedwith2.0M2,4-Dand 7.0Mkinetinandthencultivatedat28◦Cinthedarkonanorbital
shakerat120rotationsperminuteforfourweeks.Thesuspended cellswereremovedeverysevendays(intriplicate),weighed(fresh weight),andfrozenforsubsequentlyophilizationandverification ofthedryweightatintervalsof7,14,21,28,and35daysofculture. Thisprocedurewasperformedforthetwotypesofcallusthat wereinducedunderlaboratoryconditions:callusthatarosefrom hypocotylsandcallusthatarosefromseeds.
Obtaininglimonoidaglycones
Theisolationoflimonoidaglyconesoccurredafterextracting seedsfromthe“laranja-pêra”orange(C.sinensis,287.72g),which weredried,crushedbyturbolysis,andextracted,firstunderreflux inhexane(toeliminateoils)andtheninacetonefor4htoobtain limonoidaglycones.Theacetoneextractoftheseeds(AES;1.31g) was concentrated and resuspended in acetonitrileand filtered. Afterevaporationofthesolvent,weobservedcrystalformation. Thesamplewaspurifiedusingpreparativethin-layer chromatog-raphy(PTLC)usingCH2Cl2:acetone(9:1,v/v)asthemobilephase
insilicagel60,whichallowedisolationofthelimonoidaglycone limonin(1).Thestructureofthislimonoidwasdeterminedby1D and2D1H and13CNMR,mass spectrometry,infrared
spectros-copy, and comparisonswith theliterature(Khalil et al.,2002): limonin(1):IVmax(KBr)3488,2986,1758(lactone),1708(C O),
1391cm−1.The1Hand13CNMRdatawereinagreementwiththe
literature(Khaliletal.,2002;EM-IES493,2071[M+Na+],C 26H30O8
(calculatedmass,470.19).
O O
O O
O
O O
O
H H
1
A A'
B C
D 21
20 22 23
1 2 3
10
4
25
26 6
5 7 8 9 24 11
12 18
1317 16
15 14
Extractionoftotallimonoidaglyconesfromseedsandcallusand suspensionculturesofC.sinensis
CallusandsuspensionculturesofC.sinensiswerelyophilized andthensubjectedtoextractionbyrefluxinacetonefor4h(Ohta etal.,1993).Theacetonicextractswerefiltered,concentrated,and storedat−20◦Cuntilanalysis.
Theseedshadpreviouslyundergoneextractionbyrefluxin hex-anetoremovetheoilandsubsequentlysubjectedtoextractionby refluxinacetonetoobtainlimonoidaglyconesextract(Ohtaetal., 1993).Afterevaporationofthesolvent,themasswasdetermined, andthesampleswerestoredat−20◦Cuntilanalysis.
Quantificationoftotallimonoidaglycones
Total limonoid aglycones were quantified in a spectropho-tometer(VarianCary100UV-VIS).Afterdilutionoftheextracts inacetonitrileandreactionwithfreshlypreparedErlichreagent (0.1g4-dimethyl-amino-benzaldehyde,2.4mlperchloricacid,and 3.0mlaceticacid),thesampleswerehomogenizedandleft undis-turbedwithoutstirringfor30min.Readingswerethenperformed
at503nm(BreskaandIbarra,2007).
Limonin(1)thatwasisolatedfromtheseedswasusedasthe standard to constructtheanalytical (calibration)curve.From a 500g/mlstocksolutioninacetonitrile,10–100g/mlofthe solu-tionswasprepared,andtheanalyticalcurvewasconstructedby linearregression.
Theanalysesoftotallimonoidaglyconeswereperformedwith the following samples: seeds, callus cultures (originating from seeds),calluscultures(originatingfromhypocotyls),cell suspen-sionfromhypocotylscells,andcellsuspensionfromcotyledons, whichwereobtainedondays7,14,21,28,and35ofcultureafter lyophilizationtoobtainthedryweight.
Statisticalanalysis
Thedatawereanalyzedbyanalysisofvariance,andmeanswere comparedusingDuncan’stestandOriginMicrocal9.0software. Valuesofp<0.05wereconsideredstatisticallysignificant.Forthe determinationof totallimonoids,theresultswerebasedonthe averageofthreeindependenttestswiththreereplicationseach. Theresultsareexpressedasthemean±standarddeviation.
Results
Fromtheacetoneextractoftheseeds,weusedPTLCtoisolate andsubsequentlyidentifylimonoidaglycone(1),aprocedurethat wasmonitoredbyananalyticalcharge-coupleddevice.The extrac-tionmethodthatwasproposedbyOhtaetal.(1993)allowsoneto obtainanextractthatisrichinlimonoidaglyconesandonlytraces oflimonoidglucosides.
The mass spectra of compound 1 showed a molecular ion [M+Na]+ atm/z=493.2171,equivalenttothemolecularformula
C26H30O8,and12degrees ofunsaturation.Thespectraldatafor
compound1indicatedthepresenceoftwolactones,oneketone, fourmethyls,andonefuranring.Thephysicalandspectraldata (infraredspectroscopy,1Hand13CNMR,DEPT,and2Dcorrelation
techniques)showninattachmentthatwereconsistentwiththose reportedforlimonincompound1inKhaliletal.(2002).
Thedeterminationoftotallimonoidaglyconeswasperformed usingthemethodthatwasdevelopedbyBreskaandIbarra(2007), whichestimatestotallimonoidaglyconeconcentrationsinsamples ofCitrusinlimonin(1)equivalents.
Underthepresentconditions,theformationofacolorimetric complexwaslinearwithincreasingconcentrationsoflimonin,even withconcentrationsthatexceeded100g/ml.Thecorrelation coef-ficient(R2)fortheanalyticalcurvewas0.9984(Fig.2).
0.50 0.45 0.40 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0 20 40 60 80 100
[18/4/2008 14:41 "/graph1" (2454574)] Linear regression for data1_B: Y = A+B * X
Error Value Parameter
---1.01045 –12.53021 A
3.25581 236.42961 B
---
-P N SD R
---<0.0001 10 1.24984 0.99924
---Lim
o
ni
n
co
nce
ntr
a
tion
(
μ
g/m
l)
Absorbance
Fig.2.Analyticalcurveforthequantificationoftotallimonoidaglyconesusing limonin(1)asthestandard.Thestandardoflimonin(10–100g/ml)wasdissolved inacetonitrileandreactedwithacolorimetricreagent.
calluscultureswhenconsideringtheirreadyavailabilityandrate ofmultiplication(Fig.3).
Thedeterminedquantityoflimonoidaglyconesintheextractof C.sinensis(Pera-Rio)seeds(Table1)washigherthantheonefound intheseedsofothervarietiesofCitrussinensis,including Valen-ciaorange(213.6mg/100g),Rubybloodorange(219.3mg/100g), Queenorange(173.1mg/100g),andJaffaorange(224.2mg/100g;
RouseffandNagy,1982).
Consideringthegrowthrateandproductionoflimonoid agly-conesfromthetwocelllinesinsuspension,theresultingcellsfrom cotyledonshadaslowergrowthratethanthecellsfromhypocotyls butwithhigherconcentrationsoflimonoidaglycones(Fig.4).
Agreateramountoflimonoidaglycone(240mg/100gofcelldry weight)wasaccumulatedinthecallusculturesfromcotyledons, butthedifferencewassmallcomparedwithcallusculturesfrom
0 50 100 150 200 250 300 350
35 28
21 14
7 0
0 50 100 150 200 250 300
35 28
21 14
7 0
Cell growth (mg/fflask)
Cell growth (mg/fflask)
TLA (mg/100 g of dried mass)
TLA (mg/100 g of dried mass)
Culture time (days)
Culture time (days)
A
B
Fig.4.Cellgrowth(mg, )andproductionoftotallimonoidaglycones(mg/100g dry weight,---)in calluscultureinsuspension ofCitrussinensisfrom:(A) hypocotylsand(B)cotyledons.CallusweregrowninMSmedium(Murashigeand Skoog,1962)supplementedwith2.0M2,4-dichlorophenoxyaceticacid,7.0M kinetin,and3.0%(w/v)sucrose.
Table1
Contentoftotallimonoidaglyconesinseeds,callusandcellssuspensioncultureof Citrussinensis.
Samplea LTA(mg/100gdryweight)
Seeds 400±7.0
Callus(cotyledons) 10.0±0.5
Callus(hypocotyls) 200±1.0
Cellsuspension(hypocotyls)b 210 ±3.0 Cellsuspension(cotyledons)b 240
±5.0
aAnalyzeswereperformedintriplicate.
bThevaluesrefertothepeakproductionofLTAonthegrowthcurve.
hypocotyls(210mg/100gcelldryweight),eventhoughthiscell linehasagreatergrowthrate.
Discussion
Limonin(1)wasusedasthestandardforthespectrophotometric analysistodeterminethelevels oflimonoid aglyconesbecause itis themajorcomponentof Citrusspecies, themostabundant limonoidaglycone,andcommerciallyavailable(Bilaletal.,2013).
Furthermore,formostCitruslimonoids,thestructureofBCDrings (Fig.1)remainsthesame,whereasbiosyntheticreactionsare con-centratedontheconversionoftheAringofnomalin(2)totheA–A′
two-ringstructureoflimonin(1)(BreskaandIbarra,2007).Mostof theotheraglyconesthathavebeenisolatedfromCitruswere inter-mediates,withvariationsinthestructureoftheAring,including obacunone(3)andichangin(5)(RoyandSaraf,2006).
Althoughthis colorimetricmethodisnot currentlythemost widelyusedforCitruslimonoidanalyses(Tianetal.,2003;Vikram
etal.,2007;Solemainetal.,2005),wechosethismethodologyto
determinelimonoidaglyconecontentbecauseitdoesnotrequire expensiveequipment,thusincreasingthepotentialforits applica-tionintheanalysisoflimonoidaglyconesincallusculturesandcell suspensionsofCitrusspecies.
The combinationsof phytoregulators that wereused in this studyweresimilartothoseusedbyMansellandMcIntosh(1991), whousedacombinationof2,4-Dandkinetin,whichdifferfrom
Endoetal.(2002),whousedonlycytokinin.
Thelimonoidaglyconesconcentrationwasthehighestinthe C.sinensisseedextractcomparedwithextractsofcallusthatwere obtainedfromhypocotylandcotyledonexplants(Table1).
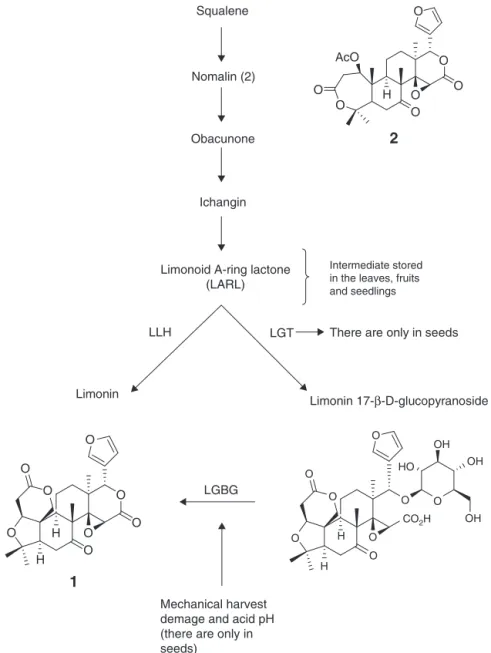
Squalene
Nomalin (2)
Ichangin
Limonoid A-ring lactone (LARL)
Limonin Limonin
17-β-D-glucopyranoside LGT
LLH
O O
O
O O
O O
O
H H
O O
O O O
H
2
1
O O
AcO
CO2H O
O O
O
O
O
O
O H
H
OH OH OH
HO
LGBG
Mechanical harvest demage and acid pH (there are only in seeds)
Intermediate stored in the leaves, fruits and seedlings
There are only in seeds Obacunone
Fig.5.MajorenzymesinvolvedinthekeystepsoflimonoidbiosynthesisinCitrus.LGT,limonoidglucosyltransferase;LLH,limonoidd-ringlactonehydrolase;LGBG,limonoid
Importantly, callus from both hypocotyls and cotyledons wereabletoproducelimonoidaglycones. However,callusfrom hypocotylshada greaterability tobiosynthesizelimonoid agly-cones. This increased biosynthesis of callus from hypocotyl explantscanbeexplainedbythefactthatnomalin(2)isthemost likelyinitialprecursorofallknownlimonoidsinCitrus.Nomalin(2) hasbeenshowntobebiosynthesizedbytheacetate–mevalonate pathwayinthephloemregionofthestem,whichispresentinthe hypocotylexplantsthatareutilizedforcallusinduction(Moriguchi etal.,2003).Thisprecursorthenmigratestoothertissues,suchas leaves,fruittissues,andseeds,whereotherlimonoidsare biosyn-thesizedindependently(Fig.5).Thistoocanexplainthesmaller quantitiesof limonoidaglycones in the callus from seeds that requirenomalin(2)to biosynthesizelimonoidaglycones (Endo
etal.,2002).
Plantcellsuspensionsculturesnormallyareinitiatedwithcallus culturelinesthatproducehighamountsofinterestingsecondary metabolite,suchaslimonoids(Ravaletal.,2003).Callusorcell cul-turescanbeundercatabolicrepression,whichpreventsthemfrom producinglimonoidsorcausesthemtoproducelimonoidsinvery smallamounts.Underfavorableconditions,however,theycan pro-ducelimonoidsagain(Ravaletal.,2003).Thecallusculturesfrom hypocotylsofC.sinensisexhibitedthischaracteristic(Table1).Thus, wealsoevaluatedlimonoidaglyconeproductionincalluscultures fromcotyledonsandcellsuspensions.
Asshown in Fig.4, both cultures had a lag phase of 14–21 daysafterinoculation,followedbyarapidgrowthphasethat sta-bilizedafter28daysofcultivation.Inthecallusculturethatwas obtainedfromhypocotyls,thelevelsoftotallimonoidaglycones increased,togetherwithanincreaseinbiomass.Thus,the concen-trationsofthesecompoundsremainedatconstitutivelylowlevels forthebiosynthesisofthesecompoundsundercultureconditions
(MansellandMcIntosh,1991).
The suspension culturefrom cotyledons however showed a dramatic increase in limonoid aglycone concentrations on day 7,which maybe attributableto theconversion of
limonin-17--d-glucopyranoside (7) to limonin aglycone (1) by limonoid glucoside -glucosidase (Fig. 5) that probably was induced by adaptation to suspension culture stress through a shear effect or aeration, mimicking the same conditions and damage that occur with mechanical harvest in orange juice processing or seed germination. This conversion of limonoid glucosides to limonoidaglyconesoccurredonlyintheseedscells(Berhowetal.,
2000).
Inthis first momentoccurreda conversion oflimonoid gly-cosidesstockedtolimonoidaglycones,showedbyincrementof TLAconcentrationfromcotyledonscellline(onday7),afterthe TLAconcentrationwasdrasticallyreducedbut withmeasurable concentrationsbycolorimetricassay(day14)asshowninFig.5. Howeverinthehypocotylscellssuspensionatday14,theTLA con-centrationwasreducedtoalevelbelowthedetectionlimitofthe assay(day14).
Theresultssuggest thatde-differentiatedcallustissuesfrom cotyledonshavealimitedcapacitytoproducelimonoidaglycones. However,callusfromhypocotylsexplantsappeared tobemore promisingforthispurpose.Ontheotherhandwhenworkingwith suspensioncellslinesthatareobtainedfromcotyledonscallusare themostappropriatebecausetheyhavethecapacitytoproduceTLA bydirectbiosynthesisandfromhydrolyzeoflimonoidglucosidesto TLA.Clearly,tissueculturesandcellsuspensionsarevaluabletools forstudyingthebiosynthesisoflimonoidaglyconesfromCitrus.The analysisandevaluationoftechniquesthatareusedtosuppressor enhancelimonoidaglyconesbiosynthesisinplantsarepossible.The resultsthatwereobtainedusingthesetechniques,combinedwith moleculartechniques,willaidinthedevelopmentofpracticaluses ofthesecultures.
Authors’contributions
EFG(Ph.D.student)andERScontributedtotherunningofthe plantcellculturelaboratoryworkandanalysis.EFGandAPSF con-tributedtoMS,NMRandcolorimetricdateanalysisanddiscussion. TCPC(undergraduatestudent)contributedtomaintenanceand cul-tivationofplantcellculturesandphytochemicalwork.AJBOwrote manuscript.RACGcontributedtocriticalreadingofthemanuscript. AJBOandRACGdesignedthestudy,supervisedthelaboratorywork andcontributedtothecriticalreadingofthemanuscript.Allthe authorshavereadthefinalmanuscriptandapprovedsubmission. Allauthorsreadandapprovedthefinalmanuscriptsubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
WethankCNPqandCAPESforfinancialsupport.Wealsothank Dr.EliezerRodriguesSouto,DepartmentofAgronomy,State Uni-versityofMaringá,Maringá,PR,Brazil,forallowingustousehis laboratoryfortheinductionandmaintenanceofcallusculturesof C.sinensis.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2015.05.008.
References
Bilal,H.,Akram,W.,Hassan,S.A.,Sahar,S.,Iqbal,M.M.,2013.Determinationof limoninandnomilincontentsindifferentcitruscultivarsusinghigh perfor-manceliquidchromatography.Pak.J.Sci.Ind.R.B56,36–40.
Berhow,M.A.,Hasegawa,S.,Manners,G.D.,2000.Citruslimonoids.Functional Chem-icalsinAgricultureandFood,2000.AmericanChemicalSociety,Washington,DC, pp.253–260.
Breska,A.P.,Ibarra,P.,2007.Colorimetricmethodfortheestimationoftotallimonoid aglyconesandglucosidecontentsinCitrusjuices.J.Agric.Food Chem.13, 5013–5017.
Collin,H.A.,2001.Secondary productformationinplanttissuecultures. Plant GrowthRegul.34,119–134.
Davies,K.M.,Deroles,S.C.,2014.Prospectsfortheuseofplantcellculturesinfood biotechnology.Curr.Opin.Biotechnol.26,133–140.
Endo,T.,Kita,M.,Shimada,T.,Moryguchi,T.,Hidaka,T.,Matsumoto,R.,Hasegawa, S.,Omura,M.,2002.Modificationoflimonoidmetabolisminsuspensioncell culturesofCitrus.PlantBiotechnol.19,397–403.
Fumagali,E.,Gonc¸alves,R.A.C.,Machado,M.F.P.S.,Vidoti,G.J.,Oliveira,A.J.B.,2008. Produc¸ãodemetabólitossecundáriosemculturadecélulasetecidosdeplantas: OexemplodosgênerosTabernaemontanaeAspidosperma.Rev.Bras.Farmacogn. 18,627–641.
Gill,S.S.,Anjum,N.A.,Hasanuzzaman,M.,Gil,R.,Trivedi,D.K.,Ahmad,I.,Pereira,E., Tuteja,N.,2013.Glutathioneandglutathionereductase:aboonindisguisefor plantabioticstressdefenseoperations.PlantPhysiol.Biochem.70,204–212. Gonc¸alves,S.,Romano,A.,2013.Invitrocultureoflavenders(Lavandulaspp.)and
theproductionofsecondarymetabolites.Biotechnol.Adv.31,166–174. Gonc¸alves,R.A.C., Cunha,A.C.,Oliveira, J.B.O.,Machado,M.F.P., Santos,R.A.M.,
2010. Aplicac¸ões da cultura de células e tecidos de plantas em reac¸ões de biotransformac¸ão. In: Marsaioli, A.J.,Porto, A.L.M. (Eds.), Biocatálise e Biotransformac¸ão:FundamentoseAplicac¸ões.EditoraSchoba,SãoPaulo,pp. 102–157,2010.
Hasegawa,S.,Hoagland,J.E.,1977.BiosynthesisoflimonoidsinCitrus. Phytochemi-stry16,469–471.
Khalil,A.T.,Maatooq,G.T.,ElSayed,K.A.,2002.LimonoidsfromCitrusreticulate.Z. Naturforsch.58c,165–170.
Mansell,R.L.,McIntosh, C.A.,1991. Citrusspp.VitroCulturetheProductionof NaringinLimonin.In:Bajaj,Y.P.S.(Ed.),Medicinaland AromaticPlantsIII, BiotechnologyinAgricultureandForestry,vol.15.Spring-Verlag,Berlin, Heildel-berg,pp.193–210.
Moriguchi,T.,Kita,M.,Hasegawa,S.,Omura,M.,2003.Molecularapproachtocitrus flavonoidandlimonoidbiosynthesis.J.FoodAgric.Environ.1,22–25. Murashige,T.,Skoog,F.A.,1962.Revisedmediumforrapidgrowthandbioassays
Muranaka,T.,Saito,K.,2010.Productionofpharmaceuticalsbyplanttissuecultures. In:Mander,L.,Liu,H.W.(Eds.),ComprehensiveNaturalProductsII:Chemistry andBiology,vol.3.Elsevier,Oxford,pp.615–628.
Niedz,R.P.,Moshonas,M.G.,Peterson,B.,Shapiro,J.P.,Shaw,P.E.,1987.Analysisof sweetorange(Citrussinensis(L.)Osbeck)callusculturesforvolatilecompounds bygaschromatographywithmassselectivedetector.PlantCellTissueOrg.51, 181–185.
Ohta,H.,Fong,C.H.,Berhow,M.,Hasegawa,S.,1993.Thin-layerandhigh perfor-manceliquidchromatographicanalysesoflimonoidsandlimonoidglucosides inCitrusseeds.J.Chromatogr.639,295–302.
Okwu,D.E.,2008.Citrusfruits:arichsourceofphytochemicalandtheirrolesin humanhealth.Int.J.Chem.Sci.6,451–471.
Puricelli,L.,Innocenti,G.,DelleMonache,G.,Caniato,R.,Filippini,R.,Cappelletti,E.M., 2002.InvivoandinvitroproductionofalkaloidsbyHaplophyllumpatavinum. Nat.Prod.Lett.16,95–100.
Raval,K.N.,Hellwig,S.,Prakash,G.,Ramos-Plasencia,A.,Srivastava,A.,Büchs,J.,2003. Stageprocessfortheproductionofazadirachtin-relatedlimonoidsinsuspension cultureofAzadirachtaindica.J.Biosci.Bioeng.96,16–22.
Ralphs,M.H.,Gardner,D.R.,2001.Distributionofnorditerpenealkaloidsintall lark-spurplantpartsthroughthegrowingseason.Biochem.Syst.Ecol.29,117–124.
Rouseff,R.L.,Nagy,S.,1982.DistributionoflimonoidsinCitrusseeds.Phytochemistry 21,85–90.
Roy,A.,Saraf,S.,2006.Limonoids:overviewofsignificantbioactivetriterpenes dis-tributedinplantskingdom.Biol.Pharm.Bull.29,191–201.
Salmore,A.K.,Hunter,M.D.,2001. Environmentalandgenotypicinfluenceson isoquinolinealkaloid content inSanguinaria canadensis. J. Chem. Ecol. 27, 1713–1747.
Sidhu, O.P., Bel,H.M., 1996. Seasonal variation in azaradirachtin inseeds of Azadirachtaindica.Curr.Sci.70,1084–1085.
Solemain,A.,Parvin,Z.,Esmaeil,M.,2005.QuantificationoflimonininIranianorange juiceconcentratesusinghigh-performanceliquidchromatographyand spectro-photometricmethods.Eur.FoodRes.Technol.221,202–207.
Smetanska,I.,2008.Productionofsecondarymetabolitesusingplantcellcultures. Adv.Biochem.Eng.Biotechnol.111,187–228.
Tian,Q.,Li,D.,Barbacci,D.,Schwartz,S.J., Patil,B.S.,2003.Electronionization mass spectrometry ofcitrus limonoids. RapidCommun. Mass Espectrom., 2517–2522.