PUBLIC HEALTH
Ectoparasite Occurrence Associated with Males and Females of Wild
Rodents
Oligoryzomys flavescens
(Waterhouse) and
Akodon azarae
(Fischer)
(Rodentia: Cricetidae: Sigmodontinae) in the Punta Lara Wetlands, Argentina
M
arcelal
areschiCentro de Estudios Parasitológicos y de Vectores, CCT La Plata, CONICET-UNLP, Calle 2 n° 584, 1900, La Plata, Argentina; mlareschi@cepave.edu.ar
Edited by Eunice Galati – FSP/UP
Neotropical Entomology 39(5):818-822 (2010)
ABSTRACT - The objective of this research was to study infestation parameters and indexes of ectoparasites associated with each sex of the wild rodents Oligoryzomys flavescens (Waterhouse) andAkodon azarae (Fischer)in the Punta Lara wetlands, Argentina. A trend towards higher mean
abundance (MA) and ectoparasite specific richness was observed in males of O. flavescens whereas those values were similar for both A. azarae sexes. The prevalence of the following ectoparasites was
significantly higher on males (P < 0.05): Mysolaelaps microspinosus Fonseca (65.2%) and Hoplopleura travassosi Werneck(73.9%) on O. flavescens, and Ixodes loricatus Neumann (71.4%) on A. azarae. Only H. travassosi mean abundance was significantly higher on males (MA = 44.1). Since I. loricatus and Hoplopleura spp. are involved in the transmission of pathogens that cause diseases in animals and humans, and whose reservoirs are rodent hosts, these results are epidemiologically important.
KEY WORDS: Chigger, flea, mite, parasite, sucking-lice, tick
Sigmodontine rodents are hosts of numerous ectoparasite species. Many of these ectoparasite species are epidemiologically important because they are involved in the transmission of pathogens that cause diseases in humans and domestic and wild animals, and whose reservoirs are rodent hosts. So, arthropods might also play an important role in epizootic diseases and their perpetuation among those rodents (Morand et al 2006), such as is Polygenis spp. (Linardi & Guimarães 2000), Ornythonyssus bacoti (Hirst) and Androlaelaps spp. (Lareschi & Mauri 1998).
Host-parasite associations are the result of evolutionary and ecological processes (Kim 1985). Individuals in a rodent population vary in ways that may affect their interactions with their parasites. For example, specimens of the same age but different sexes usually differ in their physiology, morphology, ethology and ecology, all of which may
influence their ectoparasite populations (Marshall 1981).
Studies on the relationship between host sex and ectoparasite presence typically show either male or female bias of parasitism in relation to the biology and ecology of rodent species (Marshall 1981). In Argentina, the sex of the sigmodontine rodent Scapteromys aquaticus has been shown to affect its ectoparasite burden and richness (Lareschi 2004). In Oxymycterus rufus, sex influences the prevalence and mean abundance of ectoparasites, with the exception of those accidentally associated, which are similar in male and female rodents (Lareschi 2006). The ectoparasites associated with the rodents
Oligoryzomys flavescens and Akodon azarae have been studied previously (Autino & Lareschi 1998, Castro & Cicchino 1998, Lareschi & Mauri 1998). The relationship between rodent sex and ectoparasite infestation has been considered for the egg distribution of lice (Phthiraptera) among different areas of host bodies (Lareschi & Liljesthröm 2000).
Previous studies have reported morphological, biological and ecological differences between the sexes of O. flavescens and A. azarae (Massoia 1961). These sex differences may affect parasitological parameters and indexes of ectoparasites associated with each host sex.
Ectoparasite abundance, prevalence, preference and specific
richness associated with male and female hosts are analyzed on the basis of this hypothesis.
Materials and Methods
Rodents. Samples of rodents were obtained in accordance with the regulations and policies of the Dirección de Administración y Difusión Conservacionista del Ministerio de Asuntos Agrarios de la Provincia de Buenos Aires. Rodents were captured from March 1990 to December 1991 in 22 trapping sessions. Captures were conducted with 7.5 cm x 15 cm x 8 cm live-trap cages, arranged in a 3 x 10 grid (30 traps total) with an inter-trap distance of 3 m. Traps were baited with oiled bread and set for one night in each trapping session. Rodents were killed with sulfuric ether and frozen in individual plastic bags in order to avoid contamination with ectoparasites from other rodents. Ulyses F. J. Pardiñas (Centro Nacional Patagónico, Puerto Madryn, Chubut, Argentina) and Carlos Galliari (Centro de Estudios Parasitológicos y de Vectores, La Plata, Argentina)
identified the rodents.
Ectoparasites. The fur of the hosts was searched for ectoparasites with a magnifying lens for 30 min, and with the use of combs, tooth brushes and forceps. The ectoparasites were preserved in 70% ethanol. For taxonomic identification, mites, chiggers and ticks were cleared in lactophenol and
individually mounted in Hoyer´s medium; fleas and lice were
cleared by using KOH and mounted in Canadian balsam. Acari
were identified in accordance with the keys, drawings and
descriptions given by Strandmann & Wharton (1958), Furman (1972), Krantz (1978) and Marques et al (2004); fleas, in accordance with Smit (1987) and Linardi & Guimarães (2000); and lice, with Johnson (1972). Ectoparasites were differentiated as adults (males and females), larvae, nymphs and eggs (only those containing an embryo were considered, in accordance with Liljesthröm & Lareschi 2000). Representative specimens of rodents and ectoparasites were deposited at the Colección del Departamento de Entomología and the Colección del Departamento de Vertebrados, Museo de La Plata, Argentina, respectively, and are available upon request.
Data analysis. Species richness (S), mean abundance (MA), prevalence (P) (Bush et al 1997), specificity index (SI) (Marshall 1981) and the Sorensen Similarity index (Css) (Morales & Pino 1987) were calculated. The significance of differences in mean abundance and prevalence between host sexes was analyzed with a t-test (Moroney 1968) and normal deviation (N) (Snedecor & Cochran 1979), respectively. These methods match those used for other species of sigmodontine rodents (Lareschi 2004, 2006).
Results
The number of specimens of each ectoparasite species collected from hosts of different sexes and species is shown in Table 1. A total of 15 ectoparasite species were collected on both hosts. The between-gender similarity in species composition of ectoparasites was higher for O. flavescens (Css= 90.00%) than for A. azarae (Css = 70.59%).
Thirty-nine individuals of O. flavescens (males = 23, females = 16) were captured. Hoplopleura travassosi showed the highest mean abundance on both sexes, being
significantly higher on males (t = 4.40; P < 0.05). Mysolaelaps
microspinosus Fonseca and H. travassosi prevalence was also
significantly higher on males (N = 1.97 and 2.25 respectively, P
< 0.05). Only P. atopus and A. rotundus preferred females and, except for L. paulistanensis and G. wolffsohni, which did not show strong preference for the host sex, all the other species showed a tendency towards male hosts (Table 2).
Twenty-two individuals of A. azarae (males = 14, females = 8) were captured. The mean abundance did not differ
significantly between host sexes (P < 0.05), and H. aitkeni was the most abundant species. Only I. loricatus prevalence was
significantly different between sexes, being higher on males (N
= 3.51; P < 0.05). Regarding host sex preference, P. atopus, H. aitkeni and O. bacoti selected females, while A. fahrenholzi, A. rotundus and I. loricatus preferred males (Table 2).
Discussion
Results show that total prevalence and total mean
abundance of ectoparasites were not significantly different
between sexes of both host species. However, total mean
abundance as well as the ectoparasite specific richness was
higher on O. flavescens males, in agreement with previous studies on S. aquaticus in Punta Lara (Lareschi 2006), and on other rodent species in Brazil (Linardi et al 1984). In contrast, males and females of A. azarae showed similar
mean abundance and ectoparasite specific richness (eight for
males and nine for females) in accordance with studies on O. rufus from Punta Lara (Lareschi 2004). These results agree
with previous findings showing that the host sex influence on
ectoparasites depends on the characteristics of each rodent species (Marshall 1981).
Previous studies on ectoparasites associated with O. flavescens and A. azarae consisted of species lists and collection localities (Autino & Lareschi 1998, Castro & Cicchino 1998, Lareschi & Mauri 1998), and all ectoparasite– host associations recorded in this study have already been mentioned in the literature (Lareschi 1996, 2000, Lareschi &
Iori 1998). Host sex influence on ectoparasites was considered
only when the spatial distribution of the eggs of H. travassosi and H. aitkeni on the host‘s body (O. flavescens and A. azarae, respectively) was studied. The similarity between males and females was observed although the proportion
of eggs laid at each site was significantly different between
rodent sexes (Lareschi & Liljeström 2000).
In the present study three significant differences in
prevalence were noticed between host sexes (M. microspinosus and H. travassosi on O. flavescens; I. loricatus on A. azarae), and only one in mean abundance (H. travassosi on O. flavescens). Studies on ectoparasite infestation parameters
and indexes corroborated the finding that M. microspinosus and H. travassosi were associated with O. flavescens, whereas I. loricatus was associated with A. azarae (Lareschi 1996, 2000, Beldoménico et al 2005). These differences observed in the prevalence and/or mean abundance of ectoparasite species agree with previous findings on sigmodontines (Lareschi 2004). These results also suggest that susceptibility to colonization by a particular ectoparasite species, expressed
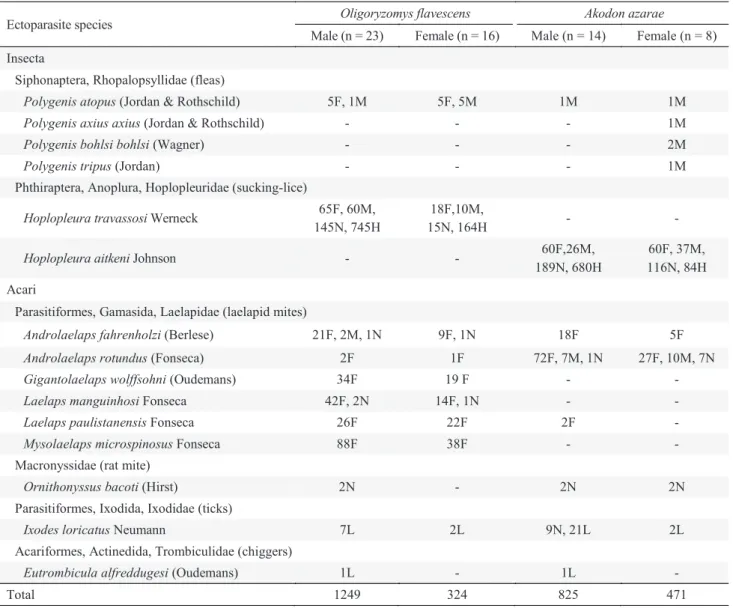
Table 1 Number (n) of specimens of every ectoparasite species collected from males and females of Oligoryzomys
flavescens and Akodon azarae. Punta Lara, Argentina (March 1990 - December 1991). F = adult females; M = adult males; L = larvae; N = nymphs; H = eggs containing an embryo.
Ectoparasite species Oligoryzomys flavescens Akodon azarae
Male (n = 23) Female (n = 16) Male (n = 14) Female (n = 8) Insecta
Siphonaptera, Rhopalopsyllidae (fleas)
Polygenis atopus (Jordan & Rothschild) 5F, 1M 5F, 5M 1M 1M
Polygenis axius axius (Jordan & Rothschild) - - - 1M
Polygenis bohlsi bohlsi (Wagner) - - - 2M
Polygenis tripus (Jordan) - - - 1M
Phthiraptera, Anoplura, Hoplopleuridae(sucking-lice)
Hoplopleura travassosi Werneck 65F, 60M, 145N, 745H
18F,10M,
15N, 164H - -
Hoplopleura aitkeni Johnson - - 60F,26M,
189N, 680H
60F, 37M, 116N, 84H Acari
Parasitiformes, Gamasida, Laelapidae (laelapid mites)
Androlaelaps fahrenholzi (Berlese) 21F, 2M, 1N 9F, 1N 18F 5F
Androlaelaps rotundus (Fonseca) 2F 1F 72F, 7M, 1N 27F, 10M, 7N
Gigantolaelaps wolffsohni (Oudemans) 34F 19 F - -
Laelaps manguinhosi Fonseca 42F, 2N 14F, 1N - -
Laelaps paulistanensis Fonseca 26F 22F 2F -
Mysolaelaps microspinosus Fonseca 88F 38F - -
Macronyssidae (rat mite)
Ornithonyssus bacoti (Hirst) 2N - 2N 2N
Parasitiformes, Ixodida, Ixodidae (ticks)
Ixodes loricatus Neumann 7L 2L 9N, 21L 2L
Acariformes, Actinedida, Trombiculidae (chiggers)
Eutrombicula alfreddugesi (Oudemans) 1L - 1L -
Total 1249 324 825 471
than by its abundance which would be related to an individual density-dependent response of the host to a certain ectoparasite burden (Liljesthröm & Lareschi 2001). In addition, all the significant differences in prevalence mentioned above showed higher values for males, in agreement with other similar studies (Linardi et al 1984, Lareschi 2006).
This result suggests that particular characteristics and
strategies of this rodent sex would influence its ectoparasite
population. Studies on the home range size and overlapping of wild rodents show that the average distance traveled by males in the breeding season is greater than that traveled by females (Bonaventura et al 1990). Furthermore, interspecific competition limits the use of territory by females during the reproductive season (Dalby 1975). Therefore, males would have better chances of a greater number of contacts with potential mates, which may affect the ectoparasite uptake.
Of all the ectoparasite species identified, O. bacoti and E.
alfreddugesi infested only males of O. flavescens, whereas only females of A. azarae were parasitized with E. alfreddugesi, L. paulistanenesi and fleas (except for P. atopus, which was also found on males).The occurrence of all of these species with only one host sex as well as their low prevalence and mean abundance values suggest accidental associations.
Oligoryzomys flavescens and A. azarae, which are
important reservoirs of significant human viruses (Gómez
Villafañe et al 2005, Pardiñas et al 2006), are representative sigmodontine species of the Buenos Aires Province (Massoia 1961, Pardiñas et al 2004, 2006, Udrizar Sauthier 2005). The conservation of biodiversity as well as population control
of rodents might be difficult unless the factors governing
Acknowledgments
This study is part of the author’s doctoral dissertation at the Universidad Nacional de La Plata (Argentina). I thank G Liljesthröm (Centro de Estudios Parasitológicos y de Vectores, Argentina) for his help with the statistical analyses, P M Linardi (Universidade Federal de Minas Gerais, Belo Horizonte, Brazil), A Cicchino (Universidad de Mar del Plata, Argentina) and U F J Pardiñas for comments on an earlier version of my doctoral dissertation. My thanks are also due
to U F J Pardiñas and C Galliari for rodent identification and
to Lucas Garbin (CEPAVE) for the English revision. The
taxonomic identification of ticks was verified by S Nava and A
Guglielmone (Instituto Nacional de Tecnología Agropecuaria, Estación Experimental Agropecuaria Rafaela, Argentina), that of rat mites and chiggers by R Mauri (CEPAVE), and of lice by D Castro (Museo de la Plata) and A Cicchino. I also thank the Department of Areas Protegidas y Difusión Conservacionista del Ministerio de Asuntos Agrarios de la Provincia de Buenos Aires for its assistance and permission for the undertaking of this research. The study was funded by Universidad Nacional de La Plata, Argentina (projects N520
and N541), and Agencia Nacional de Promoción Científica y
Tecnológica, Ministerio de Educación de la Nación Argentina (projects PICT 21546 and 33019).
References
Autino A, Lareschi M (1998) Siphonaptera, p.279-290. In Morrone J
J, Coscarón S (dir) Biodiversidad de artrópodos argentinos. Una perspectiva biotaxonómica. La Plata, Ediciones Sur, 599p. Beldoménico P M, Lareschi M, Nava S, Mangold A J, Guglielmone
A A (2005) The parasitism of immature stages of Ixodes loricatus Neumann, 1899 (Acari: Ixodidae) on wild rodents. Associates and host preference in riparian localities of Argentina. Exp Appl Acarol 36: 139-148.
Bonaventura S M, Piantanida M J, Gurini L, Sánchez López M I (1990) Habitat selection in population of cricetinae rodents in the region Delta (Argentina). Mamm 55: 339-354.
Bush A O, Lafferty K D, Lotz J M, Shostak A W (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol83: 575-583.
Castro D, Cicchino A (1998) Anoplura, p.125-139.In Morrone J J, Coscarón S (dir) Biodiversidad de artrópodos argentinos. Una perspectiva biotaxonómica. La Plata, Ediciones Sur, 599p. Dalby P (1975) Biology of Pampa rodents, Balcarce área, Argentina.
Publ Mus Mich St Univ Biol Ser 5: 149-272.
Dascanio L M, Barrera M, Frangi J (1994) Biomass structure and dry matter dynamics of subtropical alluvial and exotic Ligustrum forest at the Río de la Plata, Argentina. Vegetatio 115: 61-76. Furman D P (1972) Laelapid mites (Laelapidae: Laelapinae) of
Venezuela. Brigh Young Univ Scie, Bull Biol Ser 27: 1-58. Gomez Villafañe I E, Miño M, Cavia R, Hodara K, Courtalón P,
Sauárez O, Bush M (2005) Roedores. Guía de la Provincia de Buenos Aires. L.O.LA., Buenos Aires, 100p.
Ectoparasite
Oligoryzomys flavescens Akodon azarae
Male Female Male Female
MA SD (%) SI MA SD (%) SI MA SD (%) SI MA SD (%) SI
A. fahrenholzi 1.0 1.9 39.1 119.5 0.6 1.1 37.5 71.3 1.3 1.8 42.9 121.9 0.6 0.7 50.0 58.5
A. rotundus 0.1 0.4 4.3 100.0 0.1 0.3 6.3 75.0 5.7 5.2 78.6 101.2 5.5 7.7 75.0 97.5
E. alfreddugesi 0.1 0.2 4.3 - - - 0.1 0.3 7.14 - - - - -
G. wolffsohni 1.5 1.6 65.2 108.1 1.2 1.9 50.0 86.8 - - - -
H. aitkeni - - - 49.1 50.3 64.3 98.2 51.6 92.8 37.5 103.2
H. travassosi 44.11 65.3 73.91 140.8 12.9 33.1 43.8 41.3 - - - -
I. loricatus 0.3 0.5 26.1 130.4 0.1 0.3 12.5 52.2 2.4 3.2 71.41 147.8 0.3 0.7 12.5 15.7
L. manguinhosi 1.9 3.0 52.2 126.5 0.9 2.7 25.0 61.6 - - - -
L. paulistanensis 1.1 1.6 43.5 91.8 1.4 1.8 50.0 111.4 0.1 0.5 7.1 - - - - -
M. microspinosus 3.8 5.6 65.21 117.1 2.4 6.3 31.3 72.5 - - - -
O. bacoti 0.1 0.4 4.3 - - - 0.1 0.5 7.1 77. 8 0.3 0.7 12.5 138.9
P. atopus 0.3 0.5 21.7 73.2 0.6 1.10 37.5 146.3 0.1 0.30 7.1 78.9 0.1 0.4 12.5 277.8
P. a. axius - - - 0.1 0.4 12.5 -
P. tripus - - - 0.1 0.4 12.5 -
P. b. bohlsi - - - 0.3 0.7 12.5 - Total 54.3 74.6 91.3 20.3 45.1 93.8 58.9 51.3 92.9 58.9 98.8 87.5
Table 2 Mean abundance (MA), standard deviation (SD), prevalence (%) and preference (SI) of ectoparasites collected from Oligoryzomys flavescens and Akodon azarae males and females. Punta Lara, Argentina (March 1990 - December 1991).
Johnson P T (1972) Sucking lice of Venezuelan rodents, with remarks on related species (Anoplura). Brigh Young Univ Scie, Bull Biol Ser 17: 1-62.
Kim K Ch (1985) Coevolution of parasitic arthropods and mammals. London University Press, London, 682p.
Krantz G W (1978). A manual of acarology. Oregon State University Book Stores, Corvallis, 509p.
Lareschi M (1996) Nuevas citas de ácaros parásitos de roedores para la provincia de Buenos Aires. Rev Soc Entomol Argent 55: 66. Lareschi M (2004) Ectoparásitos asociados a los machos y a las
hembras de Oxymycterus rufus (Rodentia: Muridae). Estudio comparativo en la selva marginal del río de La Plata, Argentina. Rev Soc Entomol Argent 63: 39-44.
Lareschi M (2006) Interrelationship between the sex of the water rat Scapteromys aquaticus and its infestation with ectoparasites in La Plata river marshland, Argentina. Rev Biol Trop 54: 673-679.
Lareschi M, Iori A (1998). Nuevas citas de Siphonaptera (Rhopalopsyllidae; Hystrichopsyllidae) parásitos de roedores de la provincia de Buenos Aires, Argentina. Revta Bras Ent 41: 165-168.
Lareschi. M, Liljesthröm G (2000) Distribución espacial de los huevos de tres especies del género Hoplopleura Enderlein (Phthiraptera: Hoplopleuridae) ectoparásitas de roedores (Rodentia: Muridae: Sigmodontinae). Rev Soc Entomol Argent 59: 16-18.
Lareschi M, Mauri R (1998) Dermanyssoidea, p.581-590. In Morrone J J, Coscarón S (dir) Biodiversidad de artrópodos argentinos. Una perspectiva biotaxonómica. La Plata, Editorial Sur, 599p.
Liljesthröm G, Lareschi M (2001) Fecundity of Hoplopleura
scapteromydis (Phthiraptera, Hoplopleuridae) ectoparasite of
Scapteromys aquaticus (Rodentia: Muridae) in Río de la Plata marshland, Argentina. J Parasitol 87: 951-956.
Linardi P M, Botelho J R, Cunha H C, Moreira N S (1984) Ectoparasitos de roedores da região urbana de Belo Horizonte, MG. I. Interação entre ectoparasitos e hospedeiros. Mem Inst Oswaldo Cruz 79: 239-247.
Linardi P M, Guimarães R L (2000) Sifonápteros do Brasil. São Paulo, Ed. MZUSP, FAPESP, 291p.
Marques S, Barros-Battesti D M, Onofrio V C, Famadas K M, Faccini J L H and Keirans J E (2004) Redescription of larva, nymph and
adults of Ixodes (I.) loricatus Neumann, 1899 (Acari: Ixodidae) based on light and scanning electron microscopy. Syst Parasitol 59: 135-146.
Marshall A G (1981) The ecology of ectoparasitic insects. New York, Academic Press, 459p.
Massoia E (1961) Nota sobre Cricétidos de la Selva Marginal de Punta Lara. Publ Mus Mem Cs Nat. Mar del Plata 1: 115-134. Morales G, Pino L A (1987) Parasitología cuantitativa. Caracas,
Fundación Fondo Editorial. Acta científica venezolana, 132p.
Morand S, Krasnov B, Poulin R, Degen A A (2006) Micromammals and macroparasites: Who is who and how they interact?, p. 3-9. In Morand S, Krasnov B, Poulin R (eds) Micromammals and macroparasites. From Evolutionary Ecology to Management. Tokyo, Springer, 666p.
Moroney M J (1968) Hechos y estadisticas. Buenos Aires, Eudeba, 440p.
Pardiñas U F J, Abba A, Merino M (2004) Micromamíferos (Didelphimorphia y Rodentia) del sudoeste de la Provincia de Buenos Aires (Argentina): taxonomía y distribución. Mastozool Neotrop 11: 211-232.
Pardiñas U F J, D’Elía G, Teta P, Ortiz P, Jayat P, Cirignoli S (2006) Akodontini Vorontsov, 1959 (sensu D’Elía, 2003), p.146-166. In Barquez R M, Díaz M M, Ojeda R A (eds) Mamíferos de Argentina. Sistemática y distribución. Mendoza, Sociedad Argentina para el Estudio de los Mamíferos (SAREM), 359p. Ringuelet R (1962) Rasgos faunísticos de las reservas Naturales de
la provincia de Buenos Aires. Physis 23: 83-92.
Smit F G A M (1987) An illustrated catalogue of the Rothschild
Collection of fleas (Siphonaptera) in the British Museum (Natural
History). Vol VII. Malacopsylloidea. British Museum (Natural History), Oxford, Oxford University Press, 380p.
Snedecor G W, Cochran W G (1979) Métodos estadísticos. México, Companía Editorial Continental S.A., 703p.
Strandmann R W, Wharton G W (1958) Manual of Mesostigmatid mites. Contrib. N°4 of the Institute of Acarology, Maryland, 330p.
Udrizar-Sauthier D E, Abba A M, Pagano L G, Pardiñas U F J (2005) Ingreso de micromamíferos brasílicos en la Provincia de Buenos Aires, Argentina. Mastozool Neotrop 12: 91-95.