Antimanic-like activity of candesartan
in mice: Possible involvement of antioxidant,
anti-in
fl
ammatory
and neurotrophic mechanisms
Júlia Ariana de Souza Gomes
a, Greicy Coelho de Souza
a,
Michael Berk
b,c,d, Lígia Menezes Cavalcante
a,
Francisca Cléa F. de Sousa
a, Josiane Budni
e,
David Freitas de Lucena
a, João Quevedo
e,f,
André F. Carvalho
g, Danielle Macêdo
a,naNeuropharmacology Laboratory, Department of Physiology and Pharmacology, Federal University
of Ceara, Fortaleza, CE, Brazil
bIMPACT Strategic Research Centre, School of Medicine, Deakin University, Geelong, Vic., Australia cFlorey Institute of Neuroscience and Mental Health, Australia
dOrygen Youth Health Research Centre, University of Melbourne, Parkville, Vic., Australia
eLaboratory of Neurosciences, Graduate Program in Health Sciences, Health Sciences Unit, University
of Southern Santa Catarina, Criciúma, SC, Brazil
fCenter for Experimental Models in Psychiatry, Department of Psychiatry and Behavioral Sciences,
The University of Texas Medical School at Houston, Houston, TX, USA
gTranslational Psychiatry Research Group, Department of Clinical Medicine, Faculty of Medicine, Federal
University of Ceara, Fortaleza, CE, Brazil
Received 7 April 2014; received in revised form 13 April 2015; accepted 7 August 2015
KEYWORDS
Candesartan; Mania; Antioxidant; Anti-inflammatory;
BDNF; GSK3beta
Abstract
Activation of the brain angiotensin II type 1 receptor (AT1R) triggers oxidant and pro-inflammatory mechanisms which are involved in the neurobiology of bipolar disorder (BD).
Candesartan (CDS) is an AT1 receptor antagonist with potential neuroprotective properties. Herein we investigated CDS effects against oxidative, neurotrophic inflammatory and cognitive
effects of amphetamine (AMPH)-induced mania. In the reversal protocol adult mice were given AMPH 2 mg/kg i.p. or saline and between days 8 and 14 received CDS 0.1, 0.3 or 1 mg/kg orally, lithium (Li) 47.5 mg/kg i.p., or saline. In the prevention treatment, mice were pretreated with
www.elsevier.com/locate/euroneuro
http://dx.doi.org/10.1016/j.euroneuro.2015.08.005
0924-977X/&2015 Elsevier B.V. and ECNP. All rights reserved.
n
Corresponding author.
Tel.:+55 85 3366 8337; fax:+55 85 3366 8333.
CDS, Li or saline prior to AMPH. Locomotor activity and working memory performance were assessed. Glutathione (GSH), thiobarbituric acid-reactive substance (TBARS) and TNF-αlevels were evaluated in the hippocampus (HC) and cerebellar vermis (CV). Brain-derived neuro-trophic factor (BDNF) and glycogen synthase kinase 3-beta (GSK-3beta) levels were measured in the HC. CDS and Li prevented and reversed the AMPH-induced increases in locomotor activity. Only CDS prevented and reversed AMPH-induced working memory deficits. CDS prevented
AMPH-induced alterations in GSH (HC and CV), TBARS (HC and CV), TNF-α(HC and CV) and BDNF (HC) levels. Li prevented alterations in BDNF and phospho-Ser9-GSK3beta. CDS reversed AMPH-induced alterations in GSH (HC and CV), TBARS (HC), TNF-α(CV) and BDNF levels. Li reversed AMPH-induced alterations in TNF-α (HC and CV) and BDNF (HC) levels. CDS is effective in reversing and preventing AMPH-induced behavioral and biochemical alterations, providing a rationale for the design of clinical trials investigating CDS's possible therapeutic effects.
&2015 Elsevier B.V. and ECNP. All rights reserved.
1.
Introduction
Although advances have been made, understanding of the pathophysiology of BD remains elusive. Animal models have been important tools for investigating BD neurobiology and novel treatment approaches (Young et al., 2011). The phar-macological animal model of mania induced by D
-ampheta-mine (AMPH) is associated with behavioral and biochemical manifestations including oxidative imbalance, mitochondrial damage and alterations in neuroplasticity (Frey et al., 2006b) which resemble BD pathophysiology (Berk et al., 2011).
Postmortem brain and clinical studies have demonstrated peripheral as well as central nervous system alterations in diverse parameters of oxidative stress and antioxidant enzymes (Andreazza et al., 2008), including decreased levels of glutathione (GSH) and increased thiobarbituric acid reactive substance (TBARS), a marker of lipid peroxidation. Deficits in GSH result in oxidative imbalance and subsequent oxidation of phospholipids, proteins, and DNA (Kulak et al., 2013).
Involvement of inflammatory mechanisms and neuroim-mune alterations is seen in the pathophysiology of BD (Berk et al., 2011). Immune disturbances have been related to the severity and recurrence of mood episodes, illness progres-sion, cognitive dysfunction and higher rates of comorbidities (Berk et al., 2011). Accordingly, increased peripheral levels of pro-inflammatory cytokines have been reported at differ-ing stages of the illness, such as IL-6 and TNF-α, during depressive episodes as well as IL-2, IL-4, IL-6 and TNF-α in mania when compared with euthymic patients and healthy subjects (Kim et al., 2007). To date, as far as we know no studies have evaluated alterations in TNF-αlevels in animals submitted to the AMPH-induced model of mania.
Neurotrophic signaling pathways are also compromised in BD and, in this regard, brain-derived neurotrophic factor (BDNF) and glycogen synthase kinase 3-beta (GSK-3beta) are two important targets related to this pathway (Picchini et al., 2004). Serum BDNF is reduced in BD during manic and depressive episodes when compared and euthymic patients and healthy controls, even in drug-naive patients (de Oliveira et al., 2009). This neurotrophin is also decreased in discrete brain areas of AMPH induced animal models of mania (Frey et al., 2006a; Macêdo et al., 2012). Regarding GSK-3beta, a growing body of evidence indicates a link between this kinase
and the therapeutic effects of mood stabilizers (Gould and Manji, 2005). Lithium inhibits GSK-3beta both directly and indirectly, and these mechanisms may be related to the neuroprotective effects of this mood stabilizer (Picchini et al., 2004).
There are substantial unmet needs in the pharmacotherapy of BD. For example, the chronic use of the classical mood stabilizer lithium is related to insufficient response, unwanted side effects, i.e. renal and thyroid dysfunction, subtle emo-tional and cognitive impairment and weight gain (Licht, 2012). There are few available treatments for the management of cognitive dysfunction in BD (Dean et al., 2012). This reinforces the need for genuinely novel pharmacotherapies for BD.
The last decade had witnessed an increased interest in the study of the brain renin–angiotensin system (RAS) (McKinley et al., 2003). This system is related to pro-inflammatory and pro-oxidant effects in brain areas related to mood regulation, such as the hippocampus (Bild et al., 2013). Angiotensin II (AngII) receptor blockers (ARBs) are pleiotropic agents with anxiolytic (Saavedra et al., 2006) and neuroprotective activities (Yagi et al., 2013). Addition-ally these substances present antioxidant action (Bild et al., 2013) as well as up-regulate BDNF levels (Kishi et al., 2012). Based on the reported psychopharmacological effects of ARBs, we recently hypothesized that these agents may provide a novel treatment opportunity for the prevention and/or treatment of BD (de Gois Queiroz et al., 2013). Noteworthy, there is an excess burden of cardiovascular risk factors in BD patients compared to the general population (Kilbourne et al., 2004). Furthermore, in many forms of experimental hyperten-sion, the neuropeptide AngII as well as components of the intrinsic brain RAS presents with an increased expression and activity (Veerasingham and Raizada, 2003). There is limited data to suggest a higher rate of hypertension in BD than the general population (Kilbourne et al., 2004). In keeping with this view the RAS system may play a role in the shared biology linking BD to cardiovascular morbidity.
BDNF and phospho-GSK-3beta) alterations induced by AMPH in mice, an acknowledged animal model of mania. The neurochemical evaluations were conducted in brain areas related to BD pathophysiology namely hippocampus (Frey et al., 2006b) and cerebellar vermis (Singh et al., 2011).
2. Experimental procedures
2.1. Animals
Two hundred seventy four treatment-naive adult male mice,
weighing 20–25 g, were obtained from the animal house facility of
Federal University of Ceara and were group-housed, 6–8 per cage, in
standard polycarbonate cages (4220.520 cm) with standard
environmental conditions (22711C, humidity 6075% and 12 h
light–dark cycle) with ad libitum access to food and water. The
experimental procedures were performed from 8:00 a.m. to 2:00 p. m. in accordance to the Guide for the Care and Use of Laboratory Animals, from the US Department of Health and Human Services. The experimental protocol was approved by the Federal University of Ceara Animal Care and Use Committee (Protocol number 065/ 2013). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Drugs
D-amphetamine (AMPH) and lithium carbonate were obtained from
Sigma (St. Louis, MO, USA) and were directly dissolved in saline solution (NaCl 0.9%, w/v). Candesartan (CDS) was purchased from AstraZeneca Laboratories (Cotia, SP, Brazil) and suspended in 0.5% carboxyl methyl cellulose (CMC). The drugs were made up freshly
within 1–2 h of dosing and were administered in a volume of 0.1 ml/
10 g body weight. The doses of CDS and lithium used here were
based on previous reports (Lu et al., 2005;Macêdo et al., 2012).
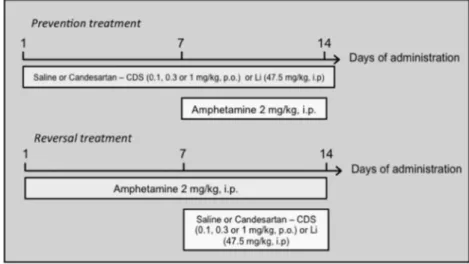
2.3. Study design
Two treatment protocols (prevention and reversal) were used and are
schematically shown inFig. 1. Each group consisted of 6–10 randomly
allocated animals. In the reversal model, we mimicked the treat-ment of acute mania in accordance to a previously proposed protocol (Frey et al., 2006b). For this, mice received one daily intraperitoneal injection (i.p.) of AMPH 2 mg/kg or saline (SAL), for 14 days. Between the 8th and 14th day, AMPH and saline-treated animals additionally received CDS [0.1, 0.3 or 1 mg/kg orally (p.o.)], lithium
(47.5 mg/kg i.p.) or SAL daily. In the prevention model (Fig. 1), we
simulated the maintenance phase of BD treatment (Frey et al.,
2006b). Animals were treated daily with lithium (47.5 mg/kg i.p.), CDS (0.1, 0.3 or 1 mg/kg p.o.) or SAL (p.o.) for 14 days. On the 8th treatment day, animals were additionally treated with either AMPH (2 mg/kg; i.p.) or SAL once a day. The time interval between drugs administration in all situations was 30 min. Since the administration of lithium or CDS alone caused no behavioral or oxidative alterations, we decided to remove these groups from the enzyme immunoassay
(ELISA) determinations. Again, due to the absence of a dose–response
effect by CDS we decided to exclude the group administered CDS 0.1 mg/kg from these measurements. This decision was based on the rational use of laboratory animals (and rules from the ethical committee of our institution).
Behavioral determinations of locomotor activity were performed
by means of the openfield test and spatial working memory was
evaluated through the Y-maze task, which were registered at the 14th day of treatment, 2 h after the last drug administration. A minimum of six animals was kept per group. Distinct animals were
used for each behavioral determination, i.e., 6–10 animals per
group were allocated to openfield test while 7 animals per group
were allocated to the Y maze task. Following behavioral
determi-nations, mice were sacrificed by decapitation and the hippocampus
and cerebellar vermis were dissected, rapidly frozen, and stored at
801C until neurochemical determinations were performed. For
neurochemical assays, i.e. ELISA or oxidative stress determinations, the groups were formed by random samples from animals submitted to each one of the behavioral tests in order to avoid bias in the neurochemical results. Furthermore, ELISA and oxidative stress determinations were performed in different brain samples, since these methods require different preparation of sample homogenate
as described inSection 2.6.
2.4. Serum lithium measurement
The serum of lithium-treated animals was separated by centrifugation.
Assays were performed with a digitalflame photometer (Labnova
AP-1500 series, São Paulo, Brazil). The serum levels of lithium in each animal ranged from 0.8 to 1.1 mEq/L, as recommended for the routine
treatment of BD (Hopkins and Gelenberg, 2000).
2.5. Behavioral determinations
2.5.1. Open-field test (Archer, 1973)
The open-field area was made of acrylic (with transparent walls and
a black floor, 30 cm30 cm15 cm) divided into nine squares of
equal areas. The apparatus was placed in a red light room. The animals were gently placed on the left corner and allowed to freely explore the arena for 5 min. The number of squares crossed was used to determine horizontal activity (crossing) and rearing beha-vior was used to determine vertical activity. This observational time has been shown to discriminate putative antimanic agents in the
AMPH-induced animal model of mania (Frey et al., 2006b;Macêdo
et al., 2013).
2.5.2. Spontaneous alternation in the Y-maze (Conrad et al., 1996)
Each mouse was placed at the end of one arm of the maze and allowed to freely move through the apparatus during 8 min. The apparatus consisted of a Y-shaped maze with three white, opaque
plastic arms (length 35, width 5, wall height 10) at a 1201angle
from each other arms. A correct alternation was defined as entries
in all three arms on consecutive occasions. The percentage of correct alternations was calculated as follows: total of alterna-tions/(total arm entries 2), as described in detail elsewhere (Dall'Igna et al., 2007).
2.6. Biochemical determinations
2.6.1. Tissue preparation
For oxidative stress analysis, the hippocampus and cerebellar vermis were homogenized ten times [weight/volume (w/v)] with ice-cold 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 10,000 rpm for 15 min, for collection of the supernatant.
For ELISA determinations, tissue samples were homogenized
eight times (w/v) in the case of TNF-αdetermination, and 20 times
(w/v) in the case of phospho-Ser9-GSK-3beta and BDNF with cold phosphate-buffered saline (PBS, pH 7.4). A protease inhibitor cocktail (Sigma-Aldrich) was added to the buffer and the homo-genate was centrifuged at 14,000 rpm for 30 min.
2.6.2. Neurochemical assays
GSH, TBARS and TNF-αwere determined in the hippocampus and
cerebellar vermis and BDNF and phospho-Ser9-GSK-3beta only in the hippocampus.
2.6.3. Assay for GSH (Sedlak and Lindsay, 1968)
The method was based on Ellman's reagent (DTNB) reaction with
free thiol groups. Briefly, the supernatants were mixed with 0.4 M
Tris–HCl buffer, pH 8.9 and 0.01 M DTNB. Reduced glutathione levels
were determined by the absorbance at 412 nm and were expressed as ng of GSH/g wet tissue.
2.6.4. Measurements of lipid peroxidation
Lipid peroxides formation was analyzed by measuring thiobarbituric acid reacting substances (TBARS) levels according to a method
described in detail elsewhere (Ohkawa et al., 1979). The lipid
peroxidation was determined by the absorbance at 535 nm and was
expressed asmmol of malondialdehyde (MDA)/mg of protein.
2.6.5. Determination of TNF-α, BDNF and phospho-Ser9-GSK-3beta levels
The levels ofTNF-αand BDNF (R&D systems, Minneapolis, MN, USA)
as well as phospho-Ser9-GSK-3beta [Human/Mouse/Rat Phospho-GSK-3beta (S9) ELISA; Millipore, USA] were determined in each
sample by enzyme immunoassays according to each specific
man-ufacturers' directions. Results are expressed as pg/g tissue.
2.7. Statistical analysis
Behavioral and biochemical data (GSH and lipid peroxidation levels)
were statistically analyzed using a 22 factorial ANOVA. Two
between-subjects factors were used: lithium (0 and 47.5 mg/kg) or CDS (0, 0.1, 0.3 and 1 mg/kg). AMPH treatment (0 and 2 mg/kg)
SAL AMPH 0
20 40 60 80 100
Number of Crossings
(5 min)
Prevention
*
# # # #
SAL AMPH 0
5 10 15 20 25
Number of rearing
(5 min)
Prevention
*
# # # #
SAL AMPH 0
50 100 150
Number of Crossings
(5 min)
Reversal
SAL Li CDS 0.1 CDS 0.3 CDS 1 *
# # # #
SAL AMPH 0
10 20 30
Number of rearing
(5 min)
Reversal
*
# # # #
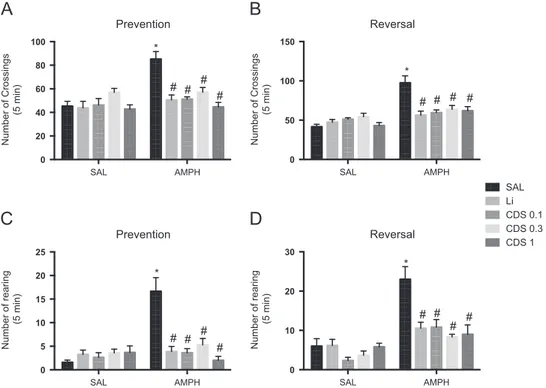
Fig. 2 Number of crossings in the prevention (A) and reversal (B) protocols. Number of rearings in the prevention (C) and reversal (D) protocols. Prevention protocol n=6–10 animals per group and reversal protocol n=6–8 animals per group. Bars represent
mean7SEM of the number of crossings or rearings. CDS=candesartan; AMPH=amphetamine; Li=lithium; SAL=saline. *Po0.05
was the within-subjects factor. In the case of a significant interac-tion between factors Tukey's post-hoc tests were used.
In the analysis of BDNF and phospho-GSK-3beta levels a one-way ANOVA was used. When a main effect of treatment was detected
(Po0.05), Tukey's post-hoc test was used to determine the level of
significance for each drug treatment (lithium and 0.1, 0.3 or 1 mg/
kg CDS). Nonparametric analyses with Kruskal–Wallis test followed
by Dunn's multiple comparisons test were performed for TNF-α
results. Before ANOVA, D'Agostino–Pearson omnibus test was
con-ducted to verify the normal distribution of the data. All results are
expressed as mean7standard error of the mean (SEM). For all
analyses, the significance level was set atPr0.05. Data analyses
were performed using GraphPad Prism software, version 6.0f for Mac (San Diego California, USA).
3.
Results
3.1. Candesartan prevented and reversed hyperlocomotion induced by AMPH
As illustrated in Fig. 2A, the analysis of the number of crossings in the prevention protocol revealed significant interaction between lithium [F (1, 31)=10.75, P=0.0026] and CDS [F (3, 56)=10.61, Po0.0001]AMPH. Ampheta-mine administration significantly increased the number of crossings when compared to SAL-treated mice (Po0.0001). When lithium [F(1, 31)=12.66,Po0.0012] or CDS [F(3, 56) =9.964, Po0.0001] were used as factors significant main effects of these treatments were observed. The adminis-tration of lithium significantly prevented the hyperlocomo-tion induced by SAL+AMPH (Po0.001). Candesartan 0.1, 0.3 or 1 mg/kg also significantly prevented the alterations
induced by SAL+AMPH (Po0.0001). In the reversal protocol (Fig. 2B) a significant interaction between lithium [F(1, 23) =16.59,P=0.0005] and CDS [F(3, 45)=10.71,Po0.0001] AMPH was observed, with significant main effects of both lithium and CDS (lithium: [F(1, 23)=9.416, 0.0054]; CDS: [F (3, 45)=4.765, P=0.0057]). In this protocol SAL+AMPH significantly increased the number of crossings when com-pared to SAL-treated animals (Po0.0001), while lithium (Po0.0001) or CDS 0.1 (Po0.0001), 0.3 (Po0.001) or 1 (Po0.0001) mg/kg significantly reversed this increase.
The evaluation of rearing in the prevention (Fig. 2C) and reversal (Fig. 2D) protocols revealed a significant interac-tion between lithiumAMPH and CDSAMPH (prevention protocol: lithiumAMPH [F (1, 31)=24.28, Po0.0001], CDSAMPH [F (3, 56)=16.27, Po0.0001]; reversal proto-col: lithiumAMPH [F (1, 23)=8.998, P=0.0066], CDSAMPH [F(3, 45)=5.934,P=0.0019]), with significant main effects of lithium (prevention protocol: [F (1, 31) =14.33, P=0.0009], reversal protocol [F (1, 23)=8.528, P=0.0079]) and CDS treatments (prevention protocol: [F(3, 56)=9.881,Po0.0001], reversal protocol [F(3, 45)=9.742, Po0.0001]). In both prevention and reversal protocols SAL +AMPH significantly increased the number of rearings when compared to SAL-treated mice (Po0.0001) while lithium (Po0.0001) or CDS 0.1 (Po0.0001), 0.3 (Po0.0001) or 1 (Po0.0001) mg/kg significantly reversed this increase.
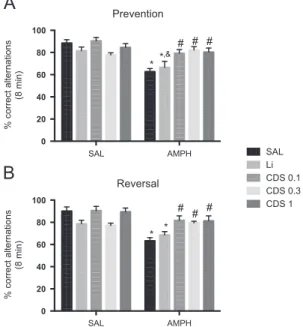
3.2. Candesartan, but not lithium, prevented and reversed AMPH-induced deficits in working memory
Bipolar disorder patients during mania present substantial deficits in working memory compared to healthy controls (Martínez-Arán et al., 2004), based on this evidence, we tested the occurrence of working memory deficits in mice submitted to the AMPH-induced model of mania as well as the effects of CDS or lithium in this behavioral paradigm using the Y-maze task (Fig. 3). The two-way ANOVA analysis of Y maze results in the prevention protocol (Fig. 3A) revealed no significant interaction between lithiumAMPH [F (1, 24)=1.959, P=0.1744], although a main effect of AMPH treatment was observed [F (1, 24)=28.29, Po0.0001]. When CDS was used as factor a significant CDSAMPH interaction was observed [F (3, 48)=8.614, P=0.0001] with the significant main effect of CDS treat-ment [F (3, 48)=3.365, P=0.0261]. Tukey's post-hoc test revealed that the administration of SAL+AMPH increased working memory deficits when compared to SAL-treated animals (Po0.0001). The administration of lithium before AMPH also significantly increased working memory deficits when compared to SAL or SAL+lithium-treated mice. On the contrary, CDS 0.1 (Po0.001), 0.3 (Po0.001) or 1 (Po0.001) mg/kg significantly prevented this alteration. In the reversal protocol (Fig. 3B) there was a significant lithiumAMPH interaction [F (1, 24)=6.492, P=0.0177]. In this protocol the administration of SAL+AMPH or lithium+AMPH increased working memory deficits when compared to SAL-treated animals. When CDS was used as factor a significant interaction between CDSAMPH was observed [F (3, 48) =6.423,P=0.0010] with significant main effect of CDS [F(3, 48)=4.095,P=0.0115]. Post-hoc test showed that CDS 0.1 SAL AMPH
0 20 40 60 80 100
% correct alternations
(8 min)
Prevention
* *
,&
# # #
SAL AMPH 0
20 40 60 80 100
% correct alternations
(8 min)
Reversal
SAL Li CDS 0.1 CDS 0.3 CDS 1
* * #
# #
Fig. 3 Percentage of correct alternations in the Y maze of animals submitted to the prevention and reversaltreatments (n=7 animals per group). Bars represent mean7SEM of the % of correct alternations. CDS=candesartan; AMPH=amphetamine;
Li=lithium; SAL=saline. *Po0.05 versus SAL; &Po0.05 versus
SAL+lithium;Po0.05 versus SAL+AMPH according to two-way
(Po0.001), 0.3 (Po0.001) or 1 (Po0.001) mg/kg signifi -cantly reversed the increase in working memory deficits induced by SAL+AMPH.
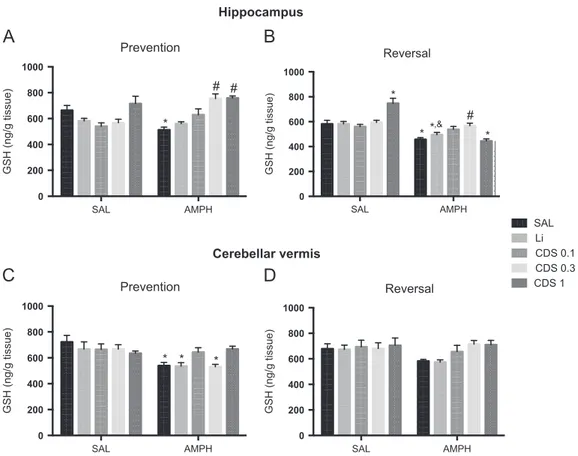
3.3. Candesartan and lithium prevent and reverse AMPH-induced alterations in oxidative stress parameters
Since pro-oxidative alterations are observed in BD patients (Andreazza et al., 2008) and also in animal models of mania (Macêdo et al., 2013), we evaluated the levels of the main endogenous antioxidant, GSH and lipid peroxidation in the hippocampus and cerebellar vermis. In the evaluation of hippocampal levels of GSH in the prevention protocol (Fig. 4A) we observed a significant interaction between lithiumAMPH [F(1, 25)=5.954,P=0.0221] and CDSAMPH [F (3, 52)=8.693, Po0.0001], in this latter case with the significant main effect of CDS treatment [F (3, 52)=8.559, P=0.0001]. Tukey's test showed a decrease in GSH levels in SAL+AMPH-treated mice when compared to SAL-treated ones (Po0.001). Candesartan 0.3 (Po0.001) or 1 (Po0.0001) mg/ kg significantly prevented this decrease. In the reversal protocol (Fig. 4B) no significant interaction between lithiumAMPH was observed. On the other hand, a significant CDSAMPH interaction [F (3, 50)=15.22, Po0.0001] was detected with the significant main effect of CDS treatment [F (3, 50)=4.153, P=0.0105]. Post-hoc analysis revealed a
significant decrease in GSH levels in animals administered SAL +AMPH (Po0.01) or CDS 1+AMPH (Po0.01) when compared to SAL-treated mice. Lithium+AMPH-treated animals also presented decreased levels of GSH when compared to SAL (Po0.05) or lithium+SAL-treated (Po0.05) ones. A significant increase in GSH levels was observed only in mice treated with CDS 0.3+AMPH when compared to SAL+AMPH (Po0.05). The administration of CDS 1+SAL increased GSH levels when compared to SAL (Po0.05).
In the cerebellar vermis, prevention protocol (Fig. 4C), no significant interaction between lithiumAMPH was observed. When CDS was used as factor there was a significant CDSAMPH interaction [F(3, 46)=4.108,P=0.0115] without the main effect of CDS. In this brain area we observed significant decreased levels of GSH in animals administered SAL+AMPH (Po0.05), lithium+AMPH (Po0.05) or CDS 0.3 +AMPH (Po0.05) when compared to SAL-treated. In the reversal protocol (Fig. 4D) no significant interaction between lithiumAMPH or CDSAMPH was detected.
The analysis of hippocampal lipid peroxidation of animals submitted to the prevention protocol (Fig. 5A) revealed a significant interaction between lithiumAMPH [F (1, 24) =13.15, P=0.0013] and CDSAMPH [F (3, 45)=13.81, Po0.0001] with significant main effects of lithium [F(1, 24) =6.508, P=0.0175] and CDS [F (3, 45)=8.395, P=0.0002]. Post-hoc test showed an increase in lipid peroxidation in SAL +AMPH-treated mice when compared to SAL-treated (Po0.001) ones that was prevented by lithium (Po0.001),
SAL AMPH
0 200 400 600 800 1000
GSH (ng/g tissue)
Prevention
*
# #
SAL AMPH
0 200 400 600 800 1000
GSH (ng/g tissue)
Prevention
* * *
SAL AMPH
0 200 400 600 800 1000
GSH (ng/g tissue)
Reversal
* *
#
* *,&
SAL AMPH
0 200 400 600 800 1000
GSH (ng/g tissue)
Reversal
SAL Li CDS 0.1 CDS 0.3 CDS 1
Hippocampus
Cerebellar vermis
Fig. 4 Reduced glutathione (GSH) levels in the hippocampus (top panel) and cerebellar vermis (bottom panel) afterpreventionand
reversaltreatments (n=6–8 animals per group). Bars represent mean7SEM. CDS=candesartan; AMPH=amphetamine; Li=lithium;
SAL=saline. *Po0.05 versus SAL;&Po0.05 versus SAL+lithium;Po0.05 versus SAL+AMPH according to two-way ANOVA followed by
Tukey's post-hoc test.
or CDS in the three doses studied (Po0.01). The animals administered CDS 0.3+AMPH presented decreased levels of lipid peroxidation also when compared to SAL-treated mice (Po0.05). In the reversal protocol (Fig. 5B) there was no significant lithiumAMPH interaction. When CDS was used as factor a significant CDSAMPH interaction was observed [F (3, 47)=5.252, P=0.0033] with the main effect of CDS treatment [F (3, 47)=4.824, P=0.0052]. According to
post-hoc analysis CDS 0.1 (Po0.01), 0.3 (Po0.01) or 1 (Po0.01) mg/kg significantly decreased the levels of lipid peroxidation when compared to SAL+AMPH-treated animals, although these levels remained increased when compared to saline-treated mice and drug-matched controls (Po0.01).
Considering lipid peroxidation levels in the cerebellar vermis, in the prevention protocol (Fig. 5C) significant lithiumAMPH [F(1, 23)=10.30,P=0.0039] and CDSAMPH
SAL AMPH
0 50 100 150 200 250
MDA equivalents (µ
mol/ g tissue)
Prevention
*
#
*,#
# #
SAL AMPH
0 50 100 150 200
MDA equivalents (µ
mol/ g tissue)
Prevention
* *,&
*,&,#
*,&*,&
SAL AMPH
0 100 200 300 400
MDA equivalents (µ
mol/ g tissue)
Reversal
*
*,& *,&,#
SAL AMPH
0 50 100 150 200
MDA equivalents (µ
mol/ g tissue)
Reversal
SAL Li CDS 0.1 CDS 0.3 CDS 1
* *
*,&,#
*,&
*,&,#
Hippocampus
Cerebellar vermis
Fig. 5 Malondialdehyde (MDA) levels, a marker of lipid peroxidation, in the hippocampus (top panel) and cerebellar vermis (bottom panel) after prevention and reversal treatment (n=6–8 animals per group). Bars represent mean7SEM. CDS=candesartan;
AMPH=amphetamine; Li=lithium; SAL=saline. *Po0.05 versus SAL;&Po0.05 versus each respective drug-matched control;Po0.05
versus SAL+AMPH according to two-way ANOVA followed by Tukey's post-hoc test.
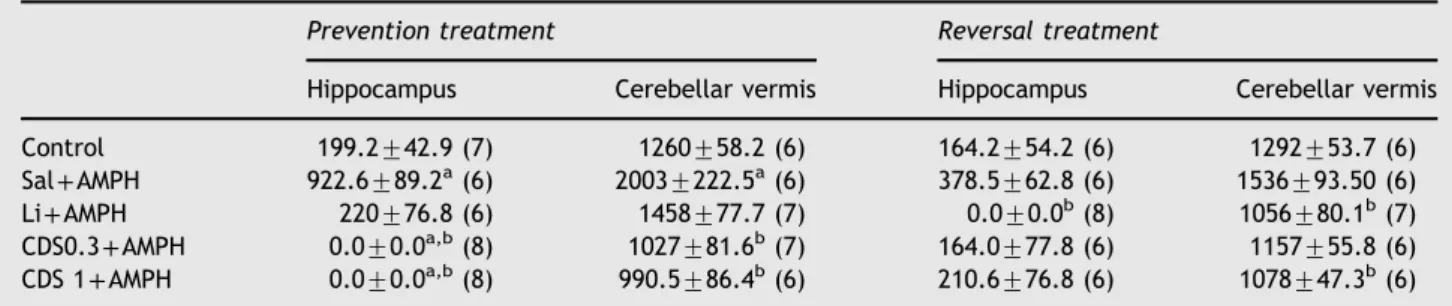
Table 1 TNF-α levels in the hippocampus and cerebellar vermis of animals submitted to the prevention and reversal
treatments.
Prevention treatment Reversal treatment
Hippocampus Cerebellar vermis Hippocampus Cerebellar vermis
Control 199.2742.9 (7) 1260758.2 (6) 164.2754.2 (6) 1292753.7 (6) Sal+AMPH 922.6789.2a(6) 20037222.5a(6) 378.5762.8 (6) 1536793.50 (6) Li+AMPH 220776.8 (6) 1458777.7 (7) 0.070.0b(8) 1056780.1b(7)
CDS0.3+AMPH 0.070.0a,b(8) 1027781.6b(7) 164.0777.8 (6) 1157755.8 (6) CDS 1+AMPH 0.070.0a,b(8) 990.5786.4b(6) 210.6776.8 (6) 1078747.3b(6)
Abbreviations: CDS 0.3=candesartan 0.3 mg/kg; CDS 1=candesartan 1 mg/kg; AMPH=amphetamine; Li=lithium; Sal=saline. The
number of animals in each group is in parenthesis. a
Po0.05 versus control.
b
[F(3, 46)=8.099,P=0.0002] interactions were observed with the significant main effect of CDS [F (3, 46)=2.826, P=0.0489]. Tukey's test revealed increased levels of lipid peroxidation in SAL+AMPH-treated mice when compared to SAL (Po0.0001). The levels of lipid peroxidation were sig-nificantly decreased in mice administered CDS 1+AMPH when compared to SAL+AMPH (Po0.001). The AMPH-treated ani-mals pretreated with lithium, CDS 0.1, 0.3 or 1 mg/kg presented higher levels of lipid peroxidation when compared to SAL and each respective drug-matched control. In the reversal protocol (Fig. 5D) we also observed significant inter-actions between lithiumAMPH [F(1, 22)=11.11,P=0.0030] and CDSAMPH [F (3, 42)=10.24, Po0.0001] with the significant main effect of CDS [F(3, 42)=10.18, Po0.0001]. In this brain area the animals treated with SAL+AMPH (Po0.001) or lithium+AMPH (Po0.001) presented higher levels of lipid peroxidation when compared to SAL. On the other hand, the administration of CDS 0.1, 0.3 or 1 +AMPH increased the levels of lipid peroxidation when compared to SAL or each respective drug-matched control (Po0.0001).
3.4. Candesartan and lithium administration decreases TNF-αlevels in mice submitted to the prevention and reversal protocols
Increased levels of the pro-inflammatory cytokine TNF-α have been reported during manic and depressive episodes of BD (Kim et al., 2007). Accordingly, in the prevention protocol significant increased levels of TNF-αwere detected in the animals administered SAL+AMPH when compared to controls in both hippocampus (Po0.001) and cerebellar vermis (Po0.01) as depicted inTable 1. In the hippocampus pre-treatment with CDS 0.3 (Po0.0001) or 1 mg/kg (Po0.0001) completely abolished the increase in TNF-α levels induced by SAL+AMPH. In the cerebellar vermis in
the same way as hippocampus CDS 0.3 (Po0.01) or 1 mg/kg (Po0.01) normalized the levels of TNF-αincreased by SAL +AMPH. In the reversal procedure, SAL+AMPH caused no significant alteration in TNF-α levels either in the hippo-campus or in the cerebellar vermis. Post-treatment with lithium significantly decreased the levels of TNF-α in both brain areas when compared to SAL+AMPH-treated animals (Po0.05). The same was observed with the post-administration of CDS 1 mg/kg (Po0.01).
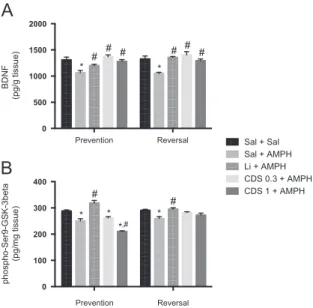
3.5. The administration of candesartan and lithium alters BDNF and phospho-GSK-3beta levels in the brain areas of mice submitted to AMPH-induced mania
Because the mechanism of action of lithium involves altera-tions in neurotrophins and GSK-3beta (Gould and Manji, 2005), we decided to evaluate the effect of CDS in these parameters in the hippocampus. As illustrated inFig. 6, in the prevention protocol, the administration of SAL+AMPH significantly decreased the levels of both parameters when compared to control animals (BDNF – [F(4,32)=6.426, Po0.01]; phospho-Ser9-GSK-3beta–[F(4,37)=30.10,Po0.001]). The administra-tion of lithium (Po0.001), CDS 0.3 (Po0.001) or 1 (Po0.001) mg/kg significantly prevented the alterations in BDNF levels induced by AMPH (Fig. 6A). In relation to phospho-Ser9-GSK-3beta, only lithium (Po0.01) significantly prevented AMPH-induced alterations in this parameter (Fig. 6B). In the reversal protocol, the administration of SAL+AMPH decreased BDNF levels, which was significantly reversed by lithium or CDS at both doses [F(4,29)=4.882, Po0.001] (Fig. 6A). Similarly to the observed in the prevention protocol decreased levels of phospho-Ser9-GSK-3beta were observed in SAL+AMPH-treated animals (Po0.01) when compared to SAL-treated ones whereas only lithium (Po0.001) significantly reversed this alteration.
4.
Discussion
To our knowledge this is thefirst study to demonstrate that CDS, an ARB, prevented and reversed AMPH-induced hyper-locomotion in mice, acknowledged as a pharmacological animal model of mania (Young et al., 2011). Furthermore, CDS prevented and reversed working memory deficits and the majority of AMPH-induced neurochemical abnormalities akin to the effects of lithium, with the exception of working memory deficits that were not ameliorated by lithium administration. Additionally, as far as we know, this is the first study to demonstrate, in an animal model of mania induced by AMPH: i) the presence of important alterations in the cerebellar vermis, a brain area altered in youth at risk for BD (Singh et al., 2011) and ii) increments in TNF-α levels in the hippocampus and cerebellar vermis, a cytokine suggested as a possible biological target for BD (Brietzke and Kapczinski, 2008).
Regarding behavioral alterations during manic episodes, psychomotor agitation is a hallmark manifestation (Young et al., 2011) and is assessed in animal models of mania by hyperlocomotion in the openfield test (Frey et al., 2006b). This is concordant with the hypothesis of dopamine dysre-gulation as a core component of the pathophysiology of BD, Prevention Reversal
0 500 1000 1500 2000
BDNF
(pg/g tissue)
Sal + Sal Sal + AMPH Li + AMPH CDS 0.3 + AMPH CDS 1 + AMPH
* *
# # # #
# #
Prevention Reversal 0
100 200 300 400
phospho-Ser9-GSK-3beta
(pg/mg tissue)
* * *
#
#
*,#
Fig. 6 Levels of BDNF (A) and phospho-Ser9-GSK-3beta (B) in the hippocampus of mice submitted to the prevention and reversal protocols (n=6–8 animals per group). Bars represent mean7SEM. CDS=candesartan; AMPH=amphetamine; Li=lithium; SAL=
sa-line. *Po0.05 versus SAL;Po0.05 versus SAL+AMPH according
to one-way ANOVA followed by Tukey's post-hoc test.
and the role of AMPH as a dopamine agonist in this paradigm (Berk et al., 2007). In keeping with this view, in the present study, AMPH administration substantially increased locomo-tion in the open field test, which was prevented and reversed by lithium, thereby reproducing the predictive validity of the model (Macêdo et al., 2013). Likewise, CDS at the three doses studied prevented and reversed AMPH-induced hyperlocomotion, which suggests it may possess antimanic-like activity.
In recent years the validity of animal models of mania has been a queried (Young et al., 2011). In this regard, some researchers have emphasized the need in these models to address more behavioral parameters than just levels of activity (Einat, 2006). A clinically relevant phenotype would be to measure cognition (Young et al., 2011) based on the well documented cognitive impairments observed in BD patients (Martínez-Arán et al., 2004). In this context, impairments in executive function and verbal memory in BD are frequently observed alterations (Martínez-Arán et al., 2004). In our experimental conditions, AMPH was able to induce working memory deficits which were prevented and reversed by CDS, but not lithium. This result obtained with CDS seems to be of interest, because lithium, a well-known mood stabilizer used to treat BD for over 50 years (Licht, 2012), has been associated with some reports of cognitive blurring and specific memory deficits in BD patients (Senturk et al., 2007). However, there are some reports that individuals who are excellent lithium responders may have preserved cognition (Rybakowski et al., 2009). In hypertensive rats (Kishi et al., 2012) ARBs prevented cognitive deficits, suggesting that this system may have an important pathophysiological role in cognitive impairment (Yagi et al., 2013).
Overall, the dysregulation of brain RAS leads to an excessive activation of AT1 receptors by AngII which is associated with an increase in sympathetic drive along with hormonal responses to stress, activation of pro-inflammatory pathways as well as changes in glutamate release and ROS formation (Jung et al., 2007). The mechanisms underlying AngII neuro-toxic effects include: i) induction of ROS production by activation of the reduced nicotinamide adenine dinucleotide phosphate NADPH-oxidase complex (NOX) (Seshiah et al., 2002), ii) regulation of the activity of several transcription factors, leading to an increase in the synthesis of pro-inflammatory mediators, e.g. EPAC1 (exchange protein directly activated by cAMP) activation (Xie et al., 2014) and iii) activation of GSK-3beta (Agarwal et al., 2013). In keeping with this view, the AT1 receptor blockade has been recently proposed by our research group as a potential target for the pharmacotherapy for BD (de Gois Queiroz et al., 2013).
The repeated administration of AMPH has been associated with oxidative imbalance, inflammatory activation, mitochon-drial damage and alterations in neuroplasticity (Macêdo et al., 2013;Valvassori et al., 2010) which mirror relevant pathways involved in BD pathophysiology (Berk et al., 2011). Therefore, we decided to determine the possible involvement of these pathways in the antimanic effects of CDS, based on the previously demonstrated neuroprotective and neurotrophic properties of this drug (Lu et al., 2005).
Importantly, our study sought abnormalities in the cerebellar vermis in an animal model of mania. This brain area is referred to as “limbic cerebellum” based on its involvement in the integration of affective and cognitive behaviors (Schmahmann
et al., 2007). The involvement of the cerebellar vermis in the pathophysiology of BD has been suggested by previous clinical studies (Singh et al., 2011), although it has not been examined in preclinical models of BD.
One of the novelties of the present study is the demon-stration of oxidative imbalance and increased TNF-α levels in the cerebellar vermis of mice submitted to AMPH-induced mania, which directs attention to a closer evaluation of this brain area in future studies addressing mania.
Candesartan prevented and reversed the oxidative imbal-ance caused by AMPH, although, regarding the evaluation of lipid peroxidation in the cerebellar vermis only CDS 1 mg/kg prevented AMPH-induced increase in this parameter. In our experimental protocol, lithium was not able to significantly prevent or reverse the alteration in GSH and in some cases lipid peroxidation levels, being capable to prevent the increase in lipid peroxidation induced by AMPH in the hippocampus. A previous study in which lithium was admi-nistered twice daily had shown that depending on the brain area and protocol (i.e., prevention or reversal) studied, co-administration of lithium and AMPH increased lipid perox-idation when compared to control animals (Frey et al., 2006b). A tentative explanation for the discrepancy between these previous findings (Frey et al., 2006b) and ours is that we used a single-dose schedule for lithium administration. On the other hand, in the present study CDS prevented and reversed the majority of AMPH-induced oxidative changes. Hence, ARBs present antioxidant proper-ties in brain tissues (Lu et al., 2005) and especially CDS was able to restore GSH levels and decrease lipid peroxidation in the hippocampus of rats submitted to chronic cerebral hypoperfusion (Ozacmak et al., 2007). It is worth to mention that in the present study, alterations caused by CDS did not display a dose–response relationship. Hence previous pre-clinical studies using similar doses of CDS (i.e., 0.1, 0.3 and 1 mg/kg) in other experimental designs have not dose-dependent effects for CDS (Kozak et al., 2008).
While clinical studies have shown immunological abnorm-alities in the pathophysiology of BD (e.g., increased periph-eral levels of pro-inflammatory cytokines) (Brietzke and Kapczinski, 2008;Kim et al., 2007), our preclinical approach is the first to show, alterations in TNF-α levels in the hippocampus and cerebellar vermis of mice submitted to AMPH-induced mania.
TNF-α has an important role in neuroplasticity, cell resi-lience and neuronal survival (Brietzke and Kapczinski, 2008). Previous report observed increased levels of this cytokine during manic and depressive episodes of BD (Kim et al., 2007) calling, thus, the attention for TNF-αas an interesting novel target for BD treatment (Brietzke and Kapczinski, 2008).
lithium response was observed (Guloksuz et al., 2012). Regarding CDS, this ARB reduces the expression of pro-inflammatory cytokines and their receptors in brain areas involved in the regulation of emotion, cognition, and memory in animals submitted to lipopolysaccharide-induced neuron-flammation (Benicky et al., 2011).
Herein, the increase in TNF-αlevels induced by AMPH was accompanied by a decrease in BDNF and phospho-GSK-3beta (Ser-9) in the hippocampus. Interestingly, both lithium and CDS reduced TNF-αand restored BDNF levels, but only lithium increased phospho-GSK-3beta (Ser9) levels in this brain area. BDNF binds to the tyrosine receptor kinase B (TrkB) receptor resulting in PI3K activation and consequently the regulation of GSK-3beta (Bondy and Cheng, 2004). GSK-3 is a pluripotent kinase which is at the convergence of a wide array of signaling pathways regulated by lithium and diverse mood stabilizer drugs (Gould and Manji, 2005). Hence, the neuroprotective effect of lithium involves GSK-3 inhibition (Li et al., 2010). GSK-3 is constitutively activated and its phosphorylation at Ser-9 is a step whereby multiple protective signaling pathways converge (Gould and Manji, 2005).
Levels of phospho-Ser9-GSK-3beta in BD patients treated with lithium are higher than in healthy controls (Li et al., 2010). In the present study, lithium prevented AMPH-induced alterations in phospho-GSK-3beta, whereas CDS lacked such effects. Conversely, CDS decreased phospho-GSK-3beta (Ser-9) levels. As mentioned above, phospho-GSK-3beta is inhibited by N-terminal phosphorylation at Ser-9 and is activated by Tyrosine phosphorylation at Tyr-216. Agarwal et al. (2013)reported a slight but significant increase in the phosphorylation of GSK-3beta (Ser-9) and an up-regulation in the phosphorylation of GSK-3beta (Tyr-216) in neuronal cells exposed to AngII, suggesting that this peptide is related to GSK-3beta activation (Bijur and Jope, 2003). Therefore, in the present study CDS may have caused a reduced activation, and not an increase in inactivation of GSK-3beta. Thus, future studies addressing the ratio of p-GSK-3beta (Tyr-216)/p-p-GSK-3beta (Ser-9) expression are necessary to indicate the level of activation of GSK-3beta in animals submitted to CDS administration.
The present study has some limitations that need to be addressed. First, the AMPH-induced animal model of mania does not precisely mimic all the symptoms of the related human disorder and also does not address the developmen-tal component of BD. Furthermore, animal models of mania do not have pathological validity outside the dopamine dysregulation hypothesis. Second, the ratio of protein expression of p-GSK-3beta (Tyr-216)/p-GSK-3beta (Ser-9) should be determined in future studies to better account for CDS effects in this neurochemical parameter.
In conclusion, the present study suggests that CDS may have antimanic effects. The possible mechanisms involved in this action may include antioxidant, anti-inflammatory and neurotrophic effect in brain areas related to BD pathophysiology, namely the cerebellar vermis and hippo-campus. Ourfindings strengthens the hypothesis that block-age of AT1 receptors may be a novel target for the management of BD. Our study opens perspectives for the design of proof-of-concept trials of AT1 antagonists either as an adjunctive treatment for bipolar disorder. Furthermore, this novel target may act as a cognitive enhancer for BD.
Role of funding source
This study was partially supported by the Brazilian Institutions CNPq and CAPES.
Contributors
Authors JASG, GCS, DFL and LMC contributed to the design of the study and performed the behavioral experiments. Authors DM and JQ designed the study and wrote the paper while MB and AFC analyzed the data and wrote the paper. Authors FCFS and JB performed the biochemical experiments.
Con
fl
ict of interests
The authors declare no conflict of interests.
Acknowledgment
The authors thank Mrs Vilani Bastos for technical support.
References
Agarwal, D., Dange, R.B., Raizada, M.K., Francis, J., 2013. Angio-tensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3beta in neuronal culture. Br. J. Pharmacol. 169, 860–874.
Andreazza, A.C., Kauer-Sant'Anna, M., Frey, B.N., Bond, D.J., Kapczinski, F., Young, L.T., Yatham, L.N., 2008. Oxidative stress markers in bipolar disorder: a meta-analysis. J. Affect. Disord. 111, 135–144.
Archer, J., 1973. Tests for emotionality in rats and mice: a review. Anim. Behav. 21, 205–235.
Benicky, J., Sánchez-Lemus, E., Honda, M., Pang, T., Orecna, M., Wang, J., Leng, Y., Chuang, D.-M., Saavedra, J.M., 2011. Angiotensin II AT1 receptor blockade ameliorates brain infl am-mation. Neuropsychopharmacology 36, 857–870.
Berk, M., Dodd, S., Kauer-Sant'anna, M., Malhi, G.S., Bourin, M., Kapczinski, F., Norman, T., 2007. Dopamine dysregulation syn-drome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. Suppl. 434, 41–49.
Berk, M., Kapczinski, F., Andreazza, A., Dean, O., Giorlando, F., Maes, M., Yücel, M., Gama, C., Dodd, S., Dean, B., 2011. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 35, 804–817.
Bijur, G.N., Jope, R.S., 2003. Glycogen synthase kinase-3 [beta] is highly activated in nuclei and mitochondria. Neuroreport 14, 2415–2419.
Bild, W., Hritcu, L., Stefanescu, C., Ciobica, A., 2013. Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Prog. Neuropsycho-pharmacol. Biol. Psychiatry 43, 79–88.
Bondy, C.A., Cheng, C.M., 2004. Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 490, 25–31.
Brietzke, E., Kapczinski, F., 2008. TNF-alpha as a molecular target in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychia-try 32, 1355–1361.
Conrad, C.D., Galea, L.A.M., Kuroda, Y., McEwen, B.S., 1996. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine treatment. Behav. Neurosci. 110, 1321–1334.
Dall'Igna, O.P., Fett, P., Gomes, M.W., Souza, D.O., Cunha, R.A., Lara, D.R., 2007. Caffeine and adenosine A(2a) receptor
antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 203, 241–245.
de Gois Queiroz, A.I., Medeiros, C.D., Ribeiro, B.M., de Lucena, D. F., Macedo, D.S., 2013. Angiotensin receptor blockers for bipolar disorder. Med. Hypotheses 80, 259–263.
de Oliveira, G.S., Cereser, K.M., Fernandes, B.S., Kauer-Sant'Anna, M., Fries, G.R., Stertz, L., Aguiar, B., Pfaffenseller, B., Kapc-zinski, F., 2009. Decreased brain-derived neurotrophic factor in medicated and drug-free bipolar patients. J. Psychiatr. Res. 43, 1171–1174.
Dean, O.M., Bush, A.I., Copolov, D.L., Kohlmann, K., Jeavons, S., Schapkaitz, I., Anderson-Hunt, M., Berk, M., 2012. Effects of N-acetyl cysteine on cognitive function in bipolar disorder. Psychiatry Clin. Neurosci. 66, 514–517.
Einat, H., 2006. Modelling facets of mania—new directions related to the notion of endophenotypes. J. Psychopharmacol. 20, 714–722.
Frey, B.N., Andreazza, A.C., Ceresér, K.M., Martins, M.R., Valvas-sori, S.S., Réus, G.Z., Quevedo, J., Kapczinski, F., 2006a. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 79, 281–286.
Frey, B.N., Valvassori, S.S., Reus, G.Z., Martins, M.R., Petronilho, F. C., Bardini, K., Dal-Pizzol, F., Kapczinski, F., Quevedo, J., 2006b. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychiatry Neurosci. 31, 326–332.
Gould, T.D., Manji, H.K., 2005. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsy-chopharmacology 30, 1223–1237.
Guloksuz, S., Altinbas, K., Aktas Cetin, E., Kenis, G., Bilgic Gazioglu, S., Deniz, G., Oral, E.T., van Os, J., 2012. Evidence for an association between tumor necrosis factor-alpha levels and lithium response. J. Affect. Disord. 143, 148–152.
Hopkins, H.S., Gelenberg, A.J., 2000. Serum lithium levels and the outcome of maintenance therapy of bipolar disorder. Bipolar Disord. 2, 174–179.
Jung, K.-H., Chu, K., Lee, S.-T., Kim, S.-J., Song, E.-C., Kim, E.-H., Park, D.-K., Sinn, D.-I., Kim, J.-M., Kim, M., 2007. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J. Pharmacol. Exp. Ther. 322, 1051–1058.
Kilbourne, A.M., Cornelius, J.R., Han, X., Pincus, H.A., Shad, M., Salloum, I., Conigliaro, J., Haas, G.L., 2004. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 6, 368–373.
Kim, Y.-K., Jung, H.-G., Myint, A.-M., Kim, H., Park, S.-H., 2007. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 104, 91–95. Kishi, T., Hirooka, Y., Sunagawa, K., 2012. Telmisartan protects
against cognitive decline via up-regulation of brain-derived neurotrophic factor/tropomyosin-related kinase B in hippocam-pus of hypertensive rats. J. Cardiol. 60, 489–494.
Kozak, W., Kozak, A., Johnson, M.H., Elewa, H.F., Fagan, S.C., 2008. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose–response study. J. Pharmacol. Exp. Ther. 326, 773–782.
Kulak, A., Steullet, P., Cabungcal, J.-H., Werge, T., Ingason, A., Cuenod, M., Do, K.Q., 2013. Redox dysregulation in the patho-physiology of schizophrenia and bipolar disorder: insights from animal models. Antioxid. Redox Signal. 18, 1428–1443. Li, X., Liu, M., Cai, Z., Wang, G., 2010. Regulation of glycogen
synthase kinase-3 during bipolar mania treatment. Bipolar Disord. 12, 741–752.
Licht, R.W., 2012. Lithium: still a major option in the management of bipolar disorder. CNS Neurosci. Ther. 18, 219–226.
Lu, Q., Zhu, Y.-Z., Wong, P.T.-H., 2005. Neuroprotective effects of candesartan against cerebral ischemia in spontaneously hyper-tensive rats. Neuroreport 16, 1963–1967.
Macêdo, D.S., de Lucena, D.F., Queiroz, A.I.G., Cordeiro, R.C., Araújo, M.M., Sousa, F.C., Vasconcelos, S.M., Hyphantis, T.N., Quevedo, J., McIntyre, R.S., 2013. Effects of lithium on oxida-tive stress and behavioral alterations induced by lisdexamfeta-mine dimesylate: relevance as an animal model of mania. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 43, 230–237.
Macêdo, D.S., Medeiros, C.D., Cordeiro, R.C., Sousa, F.C., Santos, J.V., Morais, T.A., Hyphantis, T.N., McIntyre, R.S., Quevedo, J., Carvalho, A.F., 2012. Effects of alpha-lipoic acid in an animal model of mania induced byD-amphetamine. Bipolar Disord. 14,
707–718.
Martínez-Arán, A., Vieta, E., Reinares, M., Colom, F., Torrent, C., Sánchez-Moreno, J., Benabarre, A., Goikolea, J.M., Comes, M., Salamero, M., 2004. Cognitive function across manic or hypo-manic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry 161, 262–270.
McKinley, M., Albiston, A., Allen, A., Mathai, M., May, C., McAllen, R., Oldfield, B., Mendelsohn, F., Chai, S., 2003. The brain renin–
angiotensin system: location and physiological roles. Int. J. Biochem. Cell Biol. 35, 901–918.
Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358.
Ozacmak, V., Sayan, H., Cetin, A., Akyıldız-Igdem, A., 2007. AT1
receptor blocker candesartan-induced attenuation of brain injury of rats subjected to chronic cerebral hypoperfusion. Neurochem. Res. 32, 1314–1321.
Padmos, R.C., Hillegers, M.H., Knijff, E.M., Vonk, R., Bouvy, A., Staal, F.J., de Ridder, D., Kupka, R.W., Nolen, W.A., Drexhage, H.A., 2008. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of infl amma-tory genes in monocytes. Arch. Gen. Psychiatry 65, 395–407. Picchini, A.M., Manji, H.K., Gould, T.D., 2004. GSK-3 and
neuro-trophic signaling: novel targets underlying the pathophysiology and treatment of mood disorders? Drug Discov. Today: Dis. Mech. 1, 419–428.
Rybakowski, J.K., Permoda-Osip, A., Borkowska, A., 2009. Response to prophylactic lithium in bipolar disorder may be associated with a preservation of executive cognitive functions. Eur. Neuropsychopharmacol. 19, 791–795.
Saavedra, J.M., Armando, I., Bregonzio, C., Juorio, A., Macova, M., Pavel, J., Sanchez-Lemus, E., 2006. A centrally acting, anxioly-tic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzo-diazepine binding. Neuropsychopharmacology 31, 1123–1134. Schmahmann, J.D., Weilburg, J.B., Sherman, J.C., 2007. The
neuropsychiatry of the cerebellum – insights from the clinic. Cerebellum 6, 254–267.
Sedlak, J., Lindsay, R.H., 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25, 192–205.
Senturk, V., Goker, C., Bilgic, A., Olmez, S., Tugcu, H., Oncu, B., Cem Atbasoglu, E., 2007. Impaired verbal memory and other-wise spared cognition in remitted bipolar patients on mono-therapy with lithium or valproate. Bipolar Disord. 9, 136–144. Seshiah, P.N., Weber, D.S., Rocic, P., Valppu, L., Taniyama, Y.,
Griendling, K.K., 2002. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res. 91, 406–413. Singh, M.K., Spielman, D., Libby, A., Adams, E., Acquaye, T., Howe,
M., Kelley, R., Reiss, A., Chang, K.D., 2011. Neurochemical deficits in the cerebellar vermis in child offspring of parents with bipolar disorder. Bipolar Disord. 13, 189–197.
Veerasingham, S.J., Raizada, M.K., 2003. Brain renin–angiotensin system dysfunction in hypertension: recent advances and per-spectives. Br. J. Pharmacol. 139, 191–202.
Wang, H.-M., Zhang, T., Li, Q., Huang, J.-K., Chen, R.-F., Sun, X.-J., 2013. Inhibition of glycogen synthase kinase-3β by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem. Int. 63, 345–353.
Xie, P., Joladarashi, D., Dudeja, P.K., Sun, L., Kanwar, Y.S., 2014. Modulation of angiotensin II-induced inflammatory cytokines by Epac1–Rap1a–Nhe3 pathway: implications in renal tubular patho-biology. Am. J. Physiol.: Renal Physiol. 19, 19.
Yagi, S., Akaike, M., Ise, T., Ueda, Y., Iwase, T., Sata, M., 2013. Renin–angiotensin–aldosterone system has a pivotal role in cognitive impairment. Hypertens. Res. 36, 753–758.
Young, J.W., Henry, B.L., Geyer, M.A., 2011. Predictive animal models of mania: hits, misses and future directions. Br. J. Pharmacol. 164, 1263–1284.