Mimon koepckeae Gardner & Patton, 1972 is an endemic Peruvian bat whose distribution used to be restricted to the type locality in Ayacucho Department, Peru, and surround-ings (SIMMONS 2005, WILLIAMS & GENOWAYS 2008, VELAZCO & AGUIRRE 2008). This species is known from three specimens (two females and one male), which were captured in the localities of Estera Ruana and Huanhuachayo, between 1,600 and 1,900 m (GARDNER & PATTON 1972). Natural history information about M. koepckeae is scarce, and the description of its habitat offers few details, for instance a reference to cloud forests of the east-ern slope of the Peruvian Andes (GARDNER & O’NEILL 1971, GARDNER & CARTER 1972, GARDNER & PATTON 1972).
Mimon koepckeae has a controversial taxonomic history. GARDNER & PATTON (1972) described the species based on the absence of a dorsal stripe, fewer crenulations and scarce hairs on the nose-leaf basis, narrow auditory bullae, and a well de-fined cleft between protocone and hypocone in the first and second upper molars. However, KOOPMAN (1976, 1978) consid-ered M. koepckeae as a junior synonym of Mimon crenulatum (Geoffroy St.-Hilaire, 1803), based on a morphological com-parison of skins and skulls of a single specimen of M.koepckeae, collected by Terborgh in 1972 from Estera Ruana (one locality of the type series), and a series of 10 specimens of M. crenulatum. Koopman (1978) considered the characters listed by GARDNER & PATTON (1972) for M. crenulatum as corresponding to intraspe-cific variation. Since that revision, KOOPMAN (1993, 1994) kept M. koepckeae as a highland Peruvian sub-species of M. crenulatum within the sub-genus Anthorhina. Later, SIMMONS & VOSS (1998)
and SIMMONS (2005) recognized the validity of M. koepckeae, without supporting their conclusions.
Because notorious changes in habitats due to anthropo-centric activities, M. koepckeae is listed as Critically Endangered by the Peruvian legislation (MINISTERIO DE AGRICULTURA 2014), contrasting with its classification as Data Deficient by the IUCN (VELAZCO & AGUIRRE 2008, IUCN 2012a).
Herein, we report the rediscovery of M. koepckeae 40 years after its description, and present a complete morphological char-acterization of it, including new diagnostic characters support-ing its status of valid species. Based on our results, the distribution of the species is significantly broadened. In addition, the habi-tat of the species, based on analysis of the new collecting local-ity, is characterized. Finally, we also discuss the nomenclatural status of the sub-genera of Mimon, based on comparisons with other species of the genus (sensu SIMMONS 2005).
MATERIAL AND METHODS
Bat inventories were conducted in the locality of Podocarpus, Santuario Nacional Pampa Hermosa, Chanchamayo District, Chanchamayo Province, in Junin Department of Peru (Fig. 1, 10°59’49.2"S, 75°25’57.1"W, 1890 m). Ten mist nests (12 x 2.5 m each one) were set up for seven consecutive nights, with a total sampling of 70 mist nests per hour.
The morphological characterization of Mimon koepckeae presented here is based on the specimen recorded herein (Ap-pendix 1) and one topotype (AMNH 23222), which were
con-Redescription of
Mimon koepckeae
(Chiroptera: Phyllostomidae)
Natalí Hurtado
1,2,4, Edith Arias
1& Víctor Pacheco
1,31 Departamento de Mastozoología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos.
Av. Arenales 1256, Lima 14, Lima, Peru.
2 Doctorado en Cs. Mención Ecología y Evolución, Facultad de Ciencias, Universidad Austral de Chile.
3 Instituto de Ciencias Biológicas “Antonio Raimondi”, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos.
4 Corresponding author. E-mail: natalihm@gmail.com
ABSTRACT. Mimon koepckeae Gardner & Patton, 1972 is a poorly-known bat species, with only three known specimens, including the holotype. Its distribution is restricted to the type locality in Ayacucho Department, Peru, and surround-ings. This species has been synonymized with M. crenulatum by some authors. Based on a new specimen of M. koepckeae
collected from Santuario Nacional Pampa Hermosa, Junin Department, Peru, we provide an extensive morphological comparison with M. crenulatum (Geoffroy St.-Hilaire, 1803), Mimom bennettii (Gray, 1838), and Mimon cozumelae
Goldman, 1914, concluding that M. koepckeae is a valid species. As a result the distribution range of the species is extended 160 km north of the type locality. In addition, we characterize the habitat of the species, provide current data on feeding behavior, and suggest that M. koepckeae should be categorized as endangered species.
378 N. Hurtado et al.
ZOOLOGIA 31 (4): 377–388, August, 2014
trasted with photos of the type specimen (LSUMZ 15676), and those in the original description (GARDNER & PATTON 1972). The age class was defined following PACHECO & PATTERSON (1992). For morphological characters we followed LEGENDRE (1984), PACHECO & PATTERSON (1992), VELAZCO (2005), WETTERERet al. (2000), PACHECOet al. (2004), GIANNINI & SIMMONS (2007), and FRACASSOet al. (2011). Colors were defined following RIDGWAY (1912). We also followed VELAZCO (2005: 20) for morphometrics. Mimon koepckeae was compared with the other three species of Mimon (sensu SIMMONS 2005): M. crenulatum (É. Geoffroy St.-Hilaire, 1803) (n = 181), M. cozumelae Goldman, 1914 (n = 31), and M. bennettii (Gray, 1838) (n = 5). For diet analysis, we placed a graph paper under a petri dish and analyzed stomach con-tents and feces in five randomly selected areas (5 x 5 mm) us-ing a stereomicroscope with 20x magnification.
TAXONOMY
An adult female of Mimon koepckeae was collected from the locality of Podocarpus in the Santuario Nacional Pampa Hermosa, Province of Chanchamayo, Department of Junin, Peru (Fig. 1). The specimen was captured by Edith Arias (EA 216) on October 6th, 2011. Its external measurements are: total length 78 mm, tail length 23 mm, hindfoot length 11 mm, ear length 22 mm, tragus length 9 mm, forearm length 48 mm, and weight 14.5 g. This specimen was preserved as skin, with the skull re-moved, and was deposited in the mammal collection of the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, under catalogue number MUSM 41327 (Figs 2-6). This specimen was identified as Mimon koepckeae, based on the origi-nal species description (GARDNER & PATTON 1972).
Mimon koepckeae Gardner & Patton, 1972
Type locality: Huanhuachayo (12°44’00"S, 73°47’00"W), elevation 1,660 m, Ayacucho Department, Peru.
Revised diagnosis. Medium-sized bats; pelage color from reddish brown to golden brown; dorsal stripe absent; noseleaf slender, crenulated along proximal margin, and sparsely fringed with short fine hairs; skull small, with narrow rostrum; audi-tory bullae narrow; first and second upper molars with narrow, well-defined vertical cleft separating protocone and hypocone. Redescription. Size. Medium-sized bats. Forearm length between 46.9-50.2 mm; weight 14 g. Pelage. Dorsal fur at rump level short (6 mm); bicolored banding pattern is Pale Smoke Gray at base and Chestnut at tips. Ventral fur at belly level slightly shorter (5 mm); tricolored banding pattern with small Pale Smoke Gray at base, large Hair Brown on central portion, and Olive-Buff tips that turn whitish from central abdomen toward flanks. Pale Smoke Gray basal portion on head larger dorsal and ventrally. Hairs of auricular patch exhibit larger whitish portion and Chestnut tips. Dorsal stripe absent. Head. Comparatively short rostrum, about one third head length or less, mandibular pragmatism not present. Noseleaf
developed and sparse protuberances on internal surface. Tra-gus long, about one third ear length. TraTra-gus rib thick with scarce long whitish hairs. Body. Single odor gland placed at center of throat. Forearm slim, fragile, naked; length average 47.7 mm. Fifth phalange larger than third, and fourth larger than fifth. Plagiopatagium inserted at metatarsal level on external border of fifth digit. Dactilopatagium major exhibits V shaped slot. Medium and minus dactilopatagium exhibit complete pigmen-tation. Dactilopatagium brevis inserted at middle of digit II. Uropatagium wide and long, projected beyond toenail edge. External edge of uropatagium with fringe of scarce and short
hairs. Hind feet completely pigmented with dense, long black hairs on dorsal surface. Calcars longer than tibia. Tip of tail free. Skull. Rostrum short, length average 21.11 mm. In dorsal view, incisor roots form V shaped groove. Anterior edge of nostril V shaped. Maxillary roots of zygoma swollen. Interor-bital region narrower than width between-canines. Braincase edge V shaped. In lateral view, a short terrace on the pre-max-illa exhibited at incisors level. Forehead plain without medial depression. Zygomatic arches flat and thick, with hard and broad roots. Jugal bone narrower. Sagittal crest wide at frontal level and tapering towards posterior side. In ventral view, pal-Figures 2-3. External appearance of Mimon koepckeae (MUSM 41327): (2) dorsal view; (3) ventral view. Scale bar: 3 cm.
Figures 4-6. (4) Dorsal, (5) ventral, and (6) lateral views of the skull of Mimon koepckeae (MUSM 41327). Scale bar: 1 cm.
2 3
4 5
380 N. Hurtado et al.
ZOOLOGIA 31 (4): 377–388, August, 2014
ate short and wide, with concave floor. Pre-maxilla triangular, placed below maxillary level; posterior edge exhibits small rounded projection towards maxillary. Lateral posterior edges of palate wide, extending towards middle of M2 talonid. Post-palatal process well-marked. Hard palate edge V shaped; ante-rior border extended beyond middle of interpterygoid bone. Medial basisphenoid sharped septum forms basisphenoidal pits. Basioccipital flat and narrow. Basioccipital-basisphenoid suture perpendicular to basisphenoid septum. Anterior border of fo-ramen magnum shallow. Jugular fofo-ramen the same size as ju-gal breath. Occipital condyles short and narrow. Para-occipital processes developed, but shorter than occipital condyles. Au-ditory bullae laterally compressed, wider than diameter of fo-ramen magnum, with few swollen optic rings. Dental
morphology. Dental formula I 2/1, C 1/1, P 2/2, M 3/3. Up-per. Inner incisors rhomboid shaped, half canine length. Tips and almost all inner borders free. Outer incisors flat and shorter than inner ones. Canines pyramidal shaped; well-defined me-dial notch on lingual cingulum. Second premolar (P2) reduced, conical, labially placed and one sixth canine length; in ventral view, labial and lingual cingula subequal. Fourth premolar (P4) developed and conical, average size between canines and mo-lars; P4 exhibits well developed main cusp; labial cingulum narrow, extended towards talon; talon basin wide and deep; secondary cusp absent; on posterior side, metastyle below parastyle of first molar. Parastyle high of first molar (M1), high, but not reaching paracone level; mesostyle shorter than parastyle; metacone higher than paracone; preparacrista, Figures 7-14. Dorsal and ventral views of the skull of four species of Mimon: (7-8) M. koepckeae (MUSM 41327); (9-10) M. crenulatum
(MUSM 24723); (11-12) M. cozumelae (FMNH 58151); (13-14) M. bennettii (NMNH 391027). Scale bar: 1 cm.
7 8 9 10
postparacrista, premetacrista and postmetacrista with concave edges; stylar shelves triangular; anterior ectoflexus convex; posterior ectoflexus triangular; protofossa sub equal to ante-rior stylar shelf; protocone developed and pyramidal; hypo-cone less developed; vertical sulcus between protohypo-cone and hypocone is present; labial cingulum well developed; metacingulum poorly developed and not in contact with M2; metastyle not in contact with parastyle of M2. Second molar (M2) similar to M1, but paracone and metaestyle of the third molar (M3) below level of parastyle; ectoflexus less developed; stylar shelf flat; protocone less developed than in M1 and M2. Lower. Single pair of incisors (i1) present, flat and bilobed, with diagonal lateral surface wear. Canines developed and coni-cal; labial cingulum narrow; mesial cingulum ends in small conule; parastyle and distal cingulum well developed. Second premolar (p2) short, about half size of canine, with single prin-cipal cone, asymmetrically divided. Fourth premolar (p4) larger than p2, with single principal pyramidal cone, labial cingu-lum restricted to first half. First molar (m1) longer than wider; size average between canines and p4; paraconid, protoconid and metaconid linked by vertical crests with concave edges,
forming deep triangle basin; paraconid below p2 level; proto-conid stands out as the largest cuspid; and paraproto-conid and en-toconid the smallest; hypoconulid placed posteriorly to entoconulid with a small peak; on posterior side, hypoconulid does not overlap m2. Second molar (m2) similar to m1, but oblique crest exhibits convex edge; labial cingulum exhibits deep cleft between trigonid and talonid; hypoconulid larger than in m1. Paraconid, metaconid and protoconid of third molar (m3) subequal, connected by vertical crests with con-cave edges shallower than m1 and m2; talonid less developed and shorter than hypoconulid; labial cingulum as m2; labial cingulum developed at trigonid level, but fewer at talonid level. Mandible. In dorsal view, condylar process breadth is larger than the coronoid process height; coronoid processes sloped laterally; angular processes shorter than condylar processes. In lateral view, chin straight with well develop sub-mental pro-cess; posterior edge diagonal with angle less than 30°; toothrow short; coronoid process short; condylar process thick and twice larger than angular process, placed over diastema, diastema between toothrow and coronoid process; notch between an-gular and condylar processes trapezoidal.
Figures 15-18. Lateral view of skull and mandible of four species of Mimon: (15) M. koepckeae (MUSM 41327); (16) M. crenulatum
(MUSM 24723); (17) M. cozumelae (FMNH 58151); (18) M. bennettii (NMNH 391027). Scale bar: 1 cm. 15
17
16
382 N. Hurtado et al.
ZOOLOGIA 31 (4): 377–388, August, 2014
Distribution. Mimon koepckeae is known to occur only in the type locality (Huanhuachayo) and vicinities of Estera Ruana; in Ayacucho Department, and low cloud forest of Junin Department, elevation between 1660 and 1890 m.
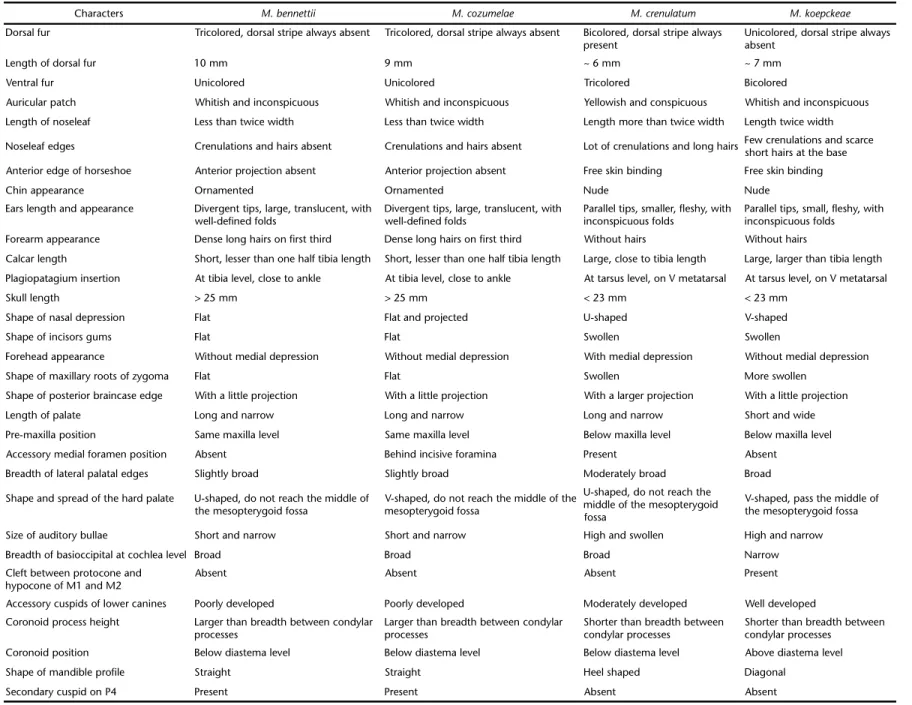
Remarks. Mimon koepckeae was compared with specimens representing the three recognized species in the genus sensu SIMMONS (2005): M. crenulatum, M. cozumelae, and M. bennettii. Compared with Mimon koepckeae, M. crenulatum is slightly larger, average body length is 84.09 mm, dorsal fur is unicolored Deep Mouse Gray and shorter, the dorsal stripe is always present, ventral fur is tricolored, auricular patch is bright and conspicuous, noseleaf crenulations and hairs are evenly dis-tributed, ears are shorter, calcar is slightly shorter, skull length is slightly larger, nasal depression is U shaped, incisors gum crests are less swollen, medial depression is present, maxillary root of zygoma is less swollen, interorbital region is wider, posterior braincase projection is more developed, palate is large and narrow, an anterior accessory medial foramen is present in some populations, lateral palatal edges are narrower, poste-rior border of the hard palate is U-shaped and does not reach the middle of mesopterygoid fossae, auditory bullae are wider, basioccipital is wider at cochlea level, cleft between protocone and hypocone is absent in M1 and M2, accessory cuspids of lower canines are less developed, coronoid process is over di-astema level, posterior edge of mandible is heel shaped.
Mimon bennettii is larger, body length is 92 mm, dorsal fur is tricolored and longer, ventral fur is unicolored, noseleaf is short and wide, noseleaf crenulations and hairs are absent, a free skin binding of the horseshow is absent, chin exhibits dense and long hairs, ears are longer, translucent with marked folds, ear tips are divergent; forearm is furred, calcar is shorter, plagiopatagium is inserted at tibia level. Skull length is larger, anterior nasal edges are flat, incisors gum crests are flat, maxil-lary root of zygoma is flat, interorbital region is comparatively narrower, palate is large and narrow, premaxillary and maxil-lary are at same level on the palate, lateral palatal edges are narrower, posterior border of the hard palate is U-shaped and does not reach the middle of mesopterygoid fossae, auditory bullae are shorter and narrower, basioccipital is wider at co-chlea level, cleft between protocone and hypocone is absent in M1 and M2, accessory cuspids of lower canine are flatted, coronoid process is over diastema level, posterior edge of man-dible is flat, secondary cuspid of P4 is present.
Mimon cozumelae is larger, average body length is 94.5 mm, dorsal fur is tricolored and longer, ventral fur is unicolored, noseleaf is short and wide, noseleaf crenulations and hairs are absent, a free skin binding of the horseshow is absent, chin exhibits dense and long hairs; ears are longer, translucent with marked folds, ear tips are divergent; forearm is furred, calcar is shorter, plagiopatagium is inserted at tibia level, skull length is larger, anterior nasal edges are flat and slightly projected, incisors gum crests are flat, maxillary root of zygoma is flat, interorbital region is comparatively narrow, palate is large and
narrow, premaxillary and maxillary are at same level on the palate, a accessory medial foramen is exhibited behind the in-cisive foramina, lateral palatal edges are narrower, posterior border of the hard palate does not reach the middle of mesopterygoid fossae, auditory bullae are shorter and narrower, basioccipital is wider at cochlea level, a cleft between proto-cone and hypoproto-cone is absent in M1 and M2, accessory cuspids of lower canine are absent, coronoid process is over diastema level, posterior edge of mandible is flat, secondary cuspid of P4 is present.
Table I provides external and cranial measurements for each species. In addition, Table II provides a summary of ex-ternal and skull-dental characters comparisons among species. Natural History. Podocarpus is located 160.1 km north-ward from the type locality, Huanhuachayo. The habitat in Podocarpus is dominated by tree species of Podocarpaceae and Clusiaceae, which are approximately of 15 m high (Fig. 19). The understory is sparse and covered with Rubiaceae and Araceae shrubs (Fig. 19, LA TORRE-CUADROSet al. 2007). Accord-ing to TOSI (1960) and HOLDRIDGE (1967), this ecosystem is cat-egorized as Very Humid low Montane forest, also known as Yungas (TOVAR-NARVÁEZet al. 2010); it is a steep landscape form-ing an altitudinal belt from 1,600 to 2,800 m elevation.
Figure 19. Cloud forest habitat of Podocarpus where Mimon koepckeae was collected in this work. Photo by Edith Arias.
Mimon koepckeae was collected in a natural canopy open-ing duropen-ing the early rainy season, with Anoura aequatoris (Lönnberg, 1921), Carollia brevicauda (Schinz, 1821), Micronyc-teris megalotis (Gray, 1842), Myotis keaysi J.A. Allen, 1914, Myotis riparius Handley, 1960, Sturnira magna de la Torre, 1966, and Vampyressa melissa Thomas, 1926. Details for this bat assem-blage will be reported elsewhere.
sexual activity. Stomach content was scarce and unidentifiable, but fecal analysis revealed remains (i.e., elytra, wings, legs, and antennas) of Elateridae (30%) and Scarabaeidae (70%) beetles.
DISCUSSION
We recognize Mimon koepckeae as a valid species, based on distinctive morphological attributes presented in this work, and thus refuting KOOPMAN’s (1976, 1978) arguments. The di-agnostic characters of this species are independent, and are not contained within the range of morphological variability of M. crenulatum (i.e. nasal shape, dorsal stripe absent). This species is distributed in low Montane Forests from Oriental slope of Central Andes in Peru, but is not sympatric with M. crenulatum (GARDNER & PATTON 1972, this work), which ranges at lower elevations (300 to 900 m).
This new record of Mimon koepckeae is a rediscovery, be-cause this report is more than 40 years after the type series
description (see criteria of SCHEFFERSet al. 2011). It is also a dis-tribution range extension of 160 km northward from the type locality and surroundings. The scarcity of records of M. koepckeae reflects the lack of intense fieldwork in the area or not enough sampling, which are largely due to difficulties ac-cessing that zone.
Mimon koepckeae and M. crenulatum were placed in the subgenus Anthorhina (GARDNER & PATTON 1972, SIMMONS & VOSS 1998). However, the taxonomic status of Anthorhina has been discussed, since HANDLEY (1960) synonymized it with Mimon. Although HUSSON (1962) considered Anthorhina as a genus, based on differences in the relative size of the upper premolars, height of bullae, and shape and nose-leaf pubescence, CABRERA (1958) and GOODWIN & GREENHALL (1961) considered Anthorhina only as a subgenus of Mimon. The characters of M. koepckeae and M. crenulatum, described herediffer from the morphology of M. bennettii and M. cozumleae, and also from the generic descrip-tion of Mimon provided by GRAY (1847), supporting recent
phy-Table I. External and cranial measurements ( in millimeters) of Mimonbennettii, M. cozumelae, M. crenulatum, and M. koepckeae. Standard deviation is in parenthesis, following by sample number.
Characters M. bennettii M. cozumelae M. crenulatum M. koepckeae
Total length 92.00(± 0.00)2 94.50(± 2.60)9 84.09(± 3.80)95 79.00(± 1.41)2 Tail length 20.50(± 0.71)2 20.61(± 2.76)9 23.26(± 2.54)96 19.50(± 4.95)2 Hindfoot length 15.00(± 0.00)2 16.83(± 0.90)9 11.57(± 1.30)96 10.00(± 1.41)2
Tragus length * * 10.22(± 1.00)96 9.50(± 0.71)2
384
N. Hurtado
et al.
ZOOLOGIA 31 (4): 377–388, August, 2014
Table II. External and cranial characters of Mimonbennettii, M. cozumelae, M. crenulatum, and M. koepckeae.
Characters M. bennettii M. cozumelae M. crenulatum M. koepckeae
Dorsal fur Tricolored, dorsal stripe always absent Tricolored, dorsal stripe always absent Bicolored, dorsal stripe always present
Unicolored, dorsal stripe always absent
Length of dorsal fur 10 mm 9 mm ~ 6 mm ~ 7 mm
Ventral fur Unicolored Unicolored Tricolored Bicolored
Auricular patch Whitish and inconspicuous Whitish and inconspicuous Yellowish and conspicuous Whitish and inconspicuous Length of noseleaf Less than twice width Less than twice width Length more than twice width Length twice width
Noseleaf edges Crenulations and hairs absent Crenulations and hairs absent Lot of crenulations and long hairs Few crenulations and scarce short hairs at the base Anterior edge of horseshoe Anterior projection absent Anterior projection absent Free skin binding Free skin binding
Chin appearance Ornamented Ornamented Nude Nude
Ears length and appearance Divergent tips, large, translucent, with well-defined folds
Divergent tips, large, translucent, with well-defined folds
Parallel tips, smaller, fleshy, with inconspicuous folds
Parallel tips, small, fleshy, with inconspicuous folds Forearm appearance Dense long hairs on first third Dense long hairs on first third Without hairs Without hairs
Calcar length Short, lesser than one half tibia length Short, lesser than one half tibia length Large, close to tibia length Large, larger than tibia length Plagiopatagium insertion At tibia level, close to ankle At tibia level, close to ankle At tarsus level, on V metatarsal At tarsus level, on V metatarsal Skull length > 25 mm > 25 mm < 23 mm < 23 mm
Shape of nasal depression Flat Flat and projected U-shaped V-shaped
Shape of incisors gums Flat Flat Swollen Swollen
Forehead appearance Without medial depression Without medial depression With medial depression Without medial depression Shape of maxillary roots of zygoma Flat Flat Swollen More swollen
Shape of posterior braincase edge With a little projection With a little projection With a larger projection With a little projection Length of palate Long and narrow Long and narrow Long and narrow Short and wide Pre-maxilla position Same maxilla level Same maxilla level Below maxilla level Below maxilla level Accessory medial foramen position Absent Behind incisive foramina Present Absent
Breadth of lateral palatal edges Slightly broad Slightly broad Moderately broad Broad
Shape and spread of the hard palate U-shaped, do not reach the middle of the mesopterygoid fossa
V-shaped, do not reach the middle of the mesopterygoid fossa
U-shaped, do not reach the middle of the mesopterygoid fossa
V-shaped, pass the middle of the mesopterygoid fossa
Size of auditory bullae Short and narrow Short and narrow High and swollen High and narrow Breadth of basioccipital at cochlea level Broad Broad Broad Narrow Cleft between protocone and
hypocone of M1 and M2
Absent Absent Absent Present
Accessory cuspids of lower canines Poorly developed Poorly developed Moderately developed Well developed Coronoid process height Larger than breadth between condylar
processes
Larger than breadth between condylar processes
Shorter than breadth between condylar processes
Shorter than breadth between condylar processes
Coronoid position Below diastema level Below diastema level Below diastema level Above diastema level Shape of mandible profile Straight Straight Heel shaped Diagonal
logenetic analyses based on morphological, molecular, and combined data, which have found Mimon to be polyphyletic (AGNARSSONet al. 2011, DÁVALOSet al. 2012); however, these analy-ses have only included M. bennettii and M. crenulatum as repre-sentatives of the genus. In order to clarify the relationships within the genus, a phylogenetic analysis including all species of Mimon needs to be conducted, because Anthorhina is a syn-onym of Tonatia (Gardner & Ferrel, 1990); then, if the clade formed by M. koepckeae and M. crenulatum is monophyletic, it would require a new generic name, as suggested by SIMMONS (2005) and WILLIAMS & GENOWAYS (2008).
Analyses of fecal samples allocate Mimon koepckeae into the insectivore guild, which agrees with the molar dilambdont pattern that predicts insectivore-feeding behavior in bats (FREE -MAN 1988). Mimon koepckeae feeds on Elateridae and Scara-baeoidae beetles, insects that inhabit upper leaves (BROWNE & SCHOLTZ 1995, COSTA & ROSA 2011). Similarly, M. crenulatum also mainly eats beetles (MELLO & POL 2006), and occasionally some moths (GIANNINI & KALKO 2005), foraging at the understory level (SIMMONS & VOSS 1998). Based on diet items, we assume that M. koepckeae uses the same foraging stratum of M. crenulatum, but our conjecture is based on the analysis of only one specimen. On the other hand, M. bennettii forages both at understory and canopy levels (SIMMONS & VOSS 1998) and its diet includes a wider spectrum of items such as beetles (Scarabaeidae, Elateridae, Passalidae, Lamperidae, Chrysonelidae), lepidopter-ans (Saturniridae), cicadas (Hemiptera), and some scorpions and spiders (CARVALHOet al. 2007). Finally, M cozumelae feeds on a variety of insects, lizards and fruits (ORTEGA & ARITA 1997). It appears that Mimon also differs in diet and feeding-foraging strategies, because members of an unnamed subgenus (formerly called Anthorhina) eat understory insects, while members of the sub-genus Mimon eat a variety of items from both under-story and canopy.
Unfortunately, the region where the habitat of M. koepckeae is locatedis under great threat from the substantial loss of habitat due to demographic expansion, and intensive agriculture. Several roads bisect the Montane forest habitats at high altitudes, and along them there are several settlements (TOSI 1960). This could promote changes in landscape use by deforestation and subsequent lost of the habitat of M. koepckeae. Likewise, the Podocarpus locality is a touristic and recreational zone within the Santuario Nacional Pampa Hermosa area (SERNANP 2012). We suggest that the area of the Podocarpus should be preserved, in order to protect one of the few areas where M. koepckeae has been recorded.
According to conservation criteria of the International Union for Conservation for Nature – IUCN (2012a), Mimon koepckeae is listed as a Data Deficient species (VELAZCO & AGUIRRE 2008). Considering the endemic condition of M. koepckeae in Peru (PACHECOet al. 2009), the potential loss of its habitat (TOSI 1960, SERNANP 2012), rarity of records (GARDNER & PATTON 1972, PACHECOet al. 2007, and this study), and its categorization as
Critically Endangered by the Peruvian legislation (MINISTERIODE AGRICULTURA 2014) and the B2 criterion (subsections a, bi, bv) of the IUCN (2012b), we suggest revision of the conservation sta-tus of M. koepckeae and categorize it as Endangered species.
ACKNOWLEDGEMENTS
We especially thank to Adela Aguilar, José Alvarez, An-thony Almeyda, Jaime Pacheco, and Alexis Larico for their sup-port in the field expeditions; Anamelba Zambrano Y., Jefe del Santuario Nacional Pampa Hermosa, for granting permit for this research and field facilities; Bruce D. Patterson for his aca-demic support and Mark Hafner for providing photos of the type specimen. Also to Edgardo Rengifo and André Ampuero for revisions; and Amelia Corso and Ann-Lloyd Hufstader for English review of an earlier version of this manuscript. We wish to thank the partial support of the FMNH Scholarship Com-mittee to NH. This work was partially supported by grants 111001031 and 121001061 of CSI-UNMSM to VP.
LITERATURE CITED
AGNARSSON, I.; C.M. ZAMBRANA-TORRELIO; N.P. FLORES-SALDANA & L.J. MAY-COLLADO. 2011. A time-calibrated species-level phylogeny of bats (Chiroptera, Mammalia). PLOS Currents Tree of Life 3 (RRN1212): 1-29. doi: 10.1371/currents.RRN1212. BROWNE, D.J. & C.H. SCHOLTZ. 1995. Scarabaeiformia.
Scaraba-eoidea. Scarabs, stag beetles, dung beetles, rain beetles, etc. The Tree of Life Web Project Version 01 January 1995 (under construction). Available online at: http://tolweb.org/ Scarabaeoidea/9077/1995.01.01 [Accessed: 14/II/2013]. CABRERA, A. 1958. Catálogo de los mamíferos de America del
Sur. Revista del Museo Argentino de Ciencias Naturales “Berbardino Rivadavia” e Instituto Nacional de Investigación de la Ciencia Naturales, Buenos Aires 4 (1): IV+307p.
CARVALHO, F.; A. DA CRUZ-NETO & J.J. ZOCCHE. 2007. Notas sobre distribução e dieta de Mimon bennettii (Gray, 1838) no Sul de santa Catarina, Brasil (Chiroptera; Phyllostomidae). In: Anais do VIII Congresso de Ecologia do Brasil. Caxambu, Sociedade de Ecologia do Brasil, p. 1-2.
COSTA, C. & S.P. ROSA. 2011. Elateridae. Click Beetles, Wireworms. The Tree of Life Web Project Version 26 February 2011. Available online at: http://tolweb.org/ Elateridae/9190/2011.02.26 [Accessed: 14/II/2013]. DÁVALOS, L.M.; A.L. CIRRANELLO; J.H. GEISLER & N.B. SIMMONS. 2012.
Understanding phylogenetic incongruence: lessons from phyllostomid bats. Biological Reviews 87: 991-1024. doi: 10.1111/j.1469-185X.2012.00240.x
386 N. Hurtado et al.
ZOOLOGIA 31 (4): 377–388, August, 2014
FREEMAN, P.W. 1988. Frugivorous and animalivorous bats (Micro-chiroptera): dental and cranial adaptations. Biological Journal of the Linnean Society 33 (3): 249-272. doi: 10.1111/j.1095-8312.1988.tb00811.x
GARDNER, A.L. & J.P. O’NEILL. 1971. A new species of Sturnira (Chiroptera: Phyllosotomidae) from Peru. Occasional Papers of The Museum of Zoology of Louisiana State University 42: 1-7.
GARDNER, A.L. & D.C. CARTER. 1972. A new Stenodermine bat (Phyllostomidae) from Perú. Occasional Papers of The Museum of Texas Tech University 2: 1-4.
GARDNER, A.L. & J.L. PATTON. 1972. New species of Philander (Marsu-pialia: Didelphidae) and Mimon (Chiroptera: Phyllostomidae) from Peru. Occasional Papers of The Museum of Zoology of Louisiana State University 43: 1-12.
GARDNER A. L. & C. S. FERREL. 1990. Comments on the nomenclature of some Neotropical bats (Mammalia: Chiroptera). Proceedings of the Biological Society of Washington103: 501-508.
GIANNINI, N.P. & E.K.V. KALKO. 2005. The guild structure of animalivorous leaf-nosed bats of Barro Colorado Island, Panama, revisited. Acta Chiropterologica7 (1): 131-146. doi: 10.3161/1733-5329(2005)7[131:TGSOAL]2.0.CO;2 GIANNINI, N.P. & N.B. SIMMONS. 2007. The chiropteran premaxilla:
A reanalysis of morphological variation and its phylogenetic interpretation. American Museum Novitates 3585: 1-44. GOODWIN, G.G. & A.M. GREENHALL. 1961. A review of the bats of Trinidad and Tobago. Descriptions, rabies infection, and ecology. Bulletin of the American Museum of Natural History 122: 187-302.
GRAY, J.E. 1847. Characters of six new genera of bats not hitherto distinguished. Proceeding of the Zoological Society of London 1847: 14-16.
HANDLEY, C.O. 1960. Descriptions of new bats from Panamá. Proceedings of the United States National Museum 112 (3442): 459-479.
HOLDRIDGE, L. R. 1967. Life zone ecology. San Jose, Tropical Science Center, 216p.
HUSSON, A.M. 1962. The bats of Suriname. Zoologische Verhande-lingen, Rijksmuseum van Natuurlijke Historie 58: 1-282. IUCN. 2012a. IUCN Red List of Threatened Species. Version
2012.2. Available online at: www.iucnredlist.org. [Accessed: 23/II/2013].
IUCN. 2012b. IUCN Red List Categories and Criteria. Gland, UICN, version 3.1, 2nd ed., 34p.
KOOPMAN, K.F. 1976. Zoogeography, p. 39-48. In R.J. BAKER; J.K. JONES & D.C. CARTER (Eds). Biology of the New World Family Phyllostomatidae. Lubbock, Texas Tech Press, Part I, 219p. KOOPMAN, K.F. 1978. Zoogeography of Peruvian bats with special emphasis on the role of the Andes. American Museum Novitates2671: 1-33.
KOOPMAN, K.F. 1993. Order Chiroptera, p. 137-241. In D.E. WIL -SON & D.M. REEDER (Eds). Mammal species of the World, a taxonomic and geographic reference. Washington, DC,
Smithsonian Institution Press, 2nd ed., 1207p.
KOOPMAN, K.F. 1994. Chiropteran systematics, p. 75-76. In J. NIETHAMMER; H. SCHLIEMANN & D. STARCK (Eds). Handbuch der Zoologie. Berlin, Walter de Gruyter Press, vol. 8, part 60, 224p. LA TORRE-CUADROS, M.A.; S. HERRANDO-PÉREZ & K.R. YOUNG. 2007. Diversity and structural patterns for tropical montane and premontane forests of central Peru, with an assessment of the use of higher-taxon surrogacy. Biodiversity Conservation 16 (10): 2965-2988. doi: 10.1007/s10531-007-9155-9
LEGENDRE, S. 1984. Étude adontologique des représentants actuels du groupe Tadarida (Chiroptera, Molossidae). Implications phylogéniques, systematiques et zoogéographiques. Revue suisse of Zoology 91 (2): 299-442.
MELLO, M.A.R. & A. POL. 2006. First record of the bat Mimon crenulatum (E. Geoffroy, 1801) (Mammalia: Chiroptera) in the state of Rio de Janeiro, Southeastern Brazil. Brazilian Journal of Biology 66 (1B): 295-299.
MINISTERIODE AGRICULTURA. 2014. Decreto Supremo No. 004-2014-MINAGRI. El Peruano, p. 520497-520504.
ORTEGA, J. & H.T. ARITA. 1997. Mimon bennettii. Mammalian Species 549: 1-4.
PACHECO, V. & B.D. PATTERSON. 1992. Systematics and biogeographic analyses of four species of Sturnira (Chiroptera: Phyllosto-midae), with emphasis on Peruvian forms. Memorias del Museo de Historia Natural, Universidad Nacional Mayor de San Marcos 21: 57-81.
PACHECO, V.; S. SOLARI & P.M. VELAZCO. 2004. A new species of Carollia (Chiroptera: Phyllostomidae) from the Andes of Peru and Bolivia. Occasional Papers of the Museum of Texas Tech University 236: 1-15
PACHECO, V.; E. SALAS; L. CAIRAMPOMA; M. NOBLECILLA; H. QUINTANA; F. ORTIZ; P. PALERMO & R. LEDESMA. 2007. Contribución al conoci-miento de la diversidad y conservación de los mamíferos en la cuenca del río Apurímac, Perú. Revista Peruana de Biología 14 (2): 169-180.
PACHECO, V.; R. CADENILLAS; E. SALAS; C. TELLO & H. ZEBALLOS. 2009. Diversidad y endemismo de los mamíferos del Perú. Revis-ta Peruana de Biología 16 (1): 5-32.
RIDGWAY, R. 1912. Colour standards and nomenclature. Wa-shington, D.C., A. Hoen & Company Press, 42p.
SCHEFFERS, B.R.; D.L. YONG; J.B.C. HARRIS; X. GIAM & N.S. SODHI. 2011. The World’s Rediscovered Species: Back from the Brink? PLoS ONE 6 (7): e22531.
SERNANP. 2012. Plan Maestro del Santuario Nacional Pampa Hermosa, periodo 2012-2017. Lima, Servicio Nacional de Áreas Naturales Protegidas por el Estado – SERNANP, Resolución Presidencial N° 213-2012-SERNANP, 55p. SIMMONS, N.B. 2005. Order Chiroptera, p. 312-529. In: D.E. WIL
-SON & D.M. REEDER (Eds). Mammal species of the World: a taxonomic and geographic reference. Baltimore, Johns Hopkins University Press, 3rd ed., vol. 1, 744p.
TOSI, J.A. 1960. Zonas de vida natural en el Perú: Memoria explicativa sobre el Mapa Ecológico del Perú. Lima, Ins-tituto Interamericano de Ciencias Agrícolas de la OEA, Zona Andina, Proyecto 39, Programa de Cooperación Técnica, Boletín Técnico 3, 271p.
TOVAR-NARVÁEZ, A.; C. TOVAR-INGAR; J. SAITO-DÍAZ; A. SOTO HURTADO; F. REGAL-GASTELUMENDI; Z. CRUZ-BURGA; C. VELIZ-ROSAS; P. VÁSQUEZ RUESTA & G. RIVERA CAMPOS. 2010. Yungas Peruanas – Bos-ques montanos de la vertiente oriental de los Andes del Perú: Una perspectiva ecorregional de conservación. Lima, CDC – UNALM, 150p.
VELAZCO, P.M. 2005. Morphological phylogeny of the bat genus Platyrrhinus Saussure, 1860 (Chiroptera: Phyllostomidae)
with the description of four new species. Fieldiana, Zoology 105: 1-54.
VELAZCO, P.M. & L. AGUIRRE. 2008. Mimon koepckeae, IUCN Red List of Threatened Species. Version 2012.2. Available online at: www.iucnredlist.org [Accessed: 03/II/2013].
WETTERER, A.L.; M.V. ROCKMAN & N.B. SIMMONS. 2000. Phylogeny of Phyllostomid bats (Mammalia: Chiroptera): data from diverse morphological systems, sex chromosomes, and restriction sites. Bulletin of the American Museum of Na-tural History 248: 1-200.
WILLIAMS, S.L. & H.H. GENOWAYS. 2008. Genus Mimon, p. 281-286. In A.L. GARDNER (Ed.). Mammals of South America. Chicago, The University of Chicago Press, vol. 1, 669p.
Appendix 1.Specimens examined. Voucher of specimens are deposited in Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos (MUSM), American Museum of Natural History (AMNH), Field Museum of Natural History (FMNH) and National Mu-seum of Natural History (NMNH).
Mimon bennettii (n = 5). BRAZIL, Minas Gerais: Sete Lagoas (19°27’00"S, 44°14’00"W, NMNH 391027). São Paulo: Bairro do Matodentro, Caverna Tiraprosa (23°26’00"S, 46°39’00"W, AMNH 256294). COLOMBIA: CORDOBA: Tierralta: Socorre, upper Rio Sinu (07°51’00"N, 76°17’00"W, FMNH 69425–69427)
Mimon cozumelae (n = 31). Belize, Cayo District: 05 m West Augustine, a Cave along Río Frio Cave (16°58’00"N, 88°59’27"W, FMNH 58152–58153). Barton Creek at Western Highway (17°12’00"N, 88°57’00"W, FMNH 58155–58156). Churchyard, Glenwood Farm (17°17’00"N, 88°34’00"W, FMNH 58154). Listowel School (Baking Pot), along Belize River (17°12’11"N, 89°01’43"W, FMNH 58157). Western Highway at Barton Creek (17°12’00"N, 88°57’00"W, FMNH 108764–108766). Orange Walk District: Gallon Jug (17°33’00"N, 89°01’00"W, AMNH 274575). Lamanai (17°45’50"N, 88°39’00"W, AMNH 277692). Toledo District: Aguacate, along Creek (16°10’12"N, 89°05’24"W, FMNH 108767). Crique Negro (15°58’00"N, 89°06’00"W, NMNH 506467). Pueblo Viejo (16°12’19"N, 89°08’30"W, FMNH 58150–58151). GUATEMALA, Peten: Tikal National Park (17°15’00"N, 89°39’00"W, FMNH 58567). PANAMA: Bocas del Toro: Almirante: Changuinola (09°26’00"N, 82°31’00"W, NMNH 315220). MEXICO: Chiapas: Palenque: Palenque (17°30’33"N, 91°58’56"W, FMNH 150630-150631). Oaxaca: Itsmo: Juchitan, 20 miles north of Matias Romero (17°03’03"N, 95°01’00"W, AMNH 185862– 185872). Yucatan: Mérida, Buenavista, Xbac (21°15’00"N, 88°49’30"W, FMNH 5845)
Mimon crenulatum (n = 181). BRAZIL, Amazonas: Borba (Santo Antonio da Uayara, Madeira River, 06°42’00"S, 69°52’00"W, AMNH 92223-92226, 92387-92388). Manaus: (Igarape Cacao Pereira, Negro River, 03°09’00"S, 60°07’00"W, AMNH 79526). São Gabriel do Cachoeira, (Taua, Uaupes River, 03°36’00"N, 69°12’00"W, AMNH 78651-78658, 78832-78833). BOLIVIA, Beni: Mamore (Baures River mouth, 12°30’00"S, 64°18’00"W, AMNH 209323). ECUADOR, Manabi: Sucre (Bahia de Caraquez, 00°36’00"S, 80°26’00"W, AMNH 64537–64541). FRENCH GUIANA: Cayenne: Sinnamary (Paracou, 04°56’00"N, 52°20’00"W, AMNH 267109, 267111-267115, 267437, 267880-267881, 267883-267884, 267886-267889). GUATEMALA, Peten: Tikal National Park (17°15’00"N, 89°39’00"W, FMNH 58568-58569). PANAMA: Bocas del Toro: Guabito (Sibube, 09°36’00"N, 82°49’00"W, NMNH 335121). Cusapin, (Peninsula Valiente, 09°08’00"N, 81°55’00"W, NMNH 57846). PERU: Amazonas: Condorcanqui (El Cenepa, Condorcanqui, P.V. 22, Falso Paquisha, Cor-dillera el Condor, 04°01’01"S, 78°24’00"W, MUSM 351). Cusco: La Convención (Echarate, Camisea, 11°53’00"S, 72°39’00"W, NMNH 582790). La Convención (Camisea, Armihuari, 11°51’51"S, 72°46’46"W, MUSM 13738-13739). La Convención (Camisea, San Mar-tin, 11°47’00"S, 72°42’00"W, MUSM 13740-13741). La Convención (Camisea, Segakiato, 11°50’42"S, 72°35’59"W, MUSM 14771).
388 N. Hurtado et al.
ZOOLOGIA 31 (4): 377–388, August, 2014
carretera Iquitos-Nauta (03°59’14"S, 73°24’55"W, MUSM 28622). Cahuide km 61 carretera Iquitos-Nauta (04°15’46"S, 73°30’05"W, MUSM 32060). Camino a El Paujil, 1,8 km al W del km 35 de la carretera Iquitos-Nauta (04°01’13"S, 73°26’47"W, MUSM 28592). Caserio Cahuide Km 59 carretera Iquitos-Nauta (04°14’32"S, 73°29’31"W, MUSM 32061–32065). Caserio Cahuide Km 60.4 carretera Iquitos-Nauta, O del camino (04°14’54"S, 73°29’58"W, MUSM 32066). Cerca Allpahuayo, km 28 SO carretera a Nauta (03°59’00"S, 73°24’51"W, MUSM 32067). Cerca de la Aldea, km 18 SO carretera a Nauta (03°54’16"S, 73°22’14"W, MUSM 32068–32069). El Dorado, km 25 de la carretera Iquitos-Nauta, app. 1.5 km al E (03°58’00"S, 73°23’37"W, MUSM 28594). El Triunfo Km 48 carretera Iquitos-Nauta (04°09’02"S, 73°28’03"W, MUSM 32070). Fundo Mery Rojas Km 19.7 carretera Iquitos-Nauta, 10 min. NO (03°54’48"S, 73°22’58"W, MUSM 32072–32073). Habanillo Km 53 carretera Iquitos-Nauta (04°11’30"S, 73°28’45"W, MUSM 32074–32079). La Habana Km 52 carretera Iquitos-Nauta (04°10’52"S, 73°28’52"W, MUSM 32083–32085). Moralillo, 1.5 km E 500 m S del km 15.2 de la carretera Iquitos-Nauta (03°54’23"S, 73°20’37"W, MUSM 28595, 28597). Ninarumi, 7.4 km al W del km 6 de la carretera Iquitos-Nauta (03°50’30"S, 73°22’51"W, MUSM 28598). Ninarumi, 7.4 km al W y 1 km al SE del km 6 de la carretera Iquitos-Nauta (03°50’59"S, 73°22’26"W, MUSM 28599). Ninarumi, 7.4 km al W y 500 m al SE del km 6 de la carretera Iquitos-Nauta (03°50’44"S, 73°22’49"W, MUSM 28601). Paujil, W km 37.45 de la carretera Iquitos-Nauta (04°03’32"S, 73°26’32"W, MUSM 28603–28606). Peña Negra, 600 m al W del km 10 de la carretera Iquitos-Nauta (03°51’19"S, 73°20’42"W, MUSM 28607, 28609). Peña Negra, 800 m al E del km 11 de la carretera Iquitos-Nauta (03°52’24"S, 73°20’08"W, MUSM 28610, 28612–28616). Peña Negra, Km 10 carretera Iquitos-Nauta (03°51’14"S, 73°20’48"W, MUSM 32095–32096). Puerto Almendra Arboretum de CEIFOR (03°50’20"S, 73°22’28"W, MUSM 32097). Quistocoha Km 5 carretera Iquitos-Nauta, 1 km O del camino, Fundo Quistococha (03°49’03"S, 73°19’55"W, MUSM 32101). San Juan (Avenida de la Participación, 03°46’43"S, 73°16’44"W, MUSM 32102). San Lucas (W km 43 de la carretera Iquitos-Nauta, 04°06’15"S, 73°27’48"W, MUSM 28617-28618). Trece de febrero Km 31.5 carretera Iquitos-Nauta, Estación de campo UNAP (03°59’59"S, 73°26’31"W, MUSM 32103). Santo Tomás (6 km al W del km 1 de la carretera Iquitos-Nauta, 03°48’35"S, 73°20’17"W, MUSM 28619). Varillal (400 m W 200 m N del km 14 de la carretera Iquitos-Nauta, 03°52’57"S, 73°21’17"W, MUSM 28621). Torres Causana (Río Lagartococha, Campamento Catalino, 00°31’42"S, 75°15’35"W, MUSM 21200). Requena, (Jenaro Herrera, 04°54’17"S, 73°40’22"W, MUSM 870, 5914). Requena (C.I. Jenaro Herrera, 04°55’01"S, 73°45’00"W, MUSM 869). Ucayali, (Contamana, Sierra de Contamana, Cerros de Canchaguaya, Aguas Calientes, 07°11’20"S, 74°56’54"W, MUSM 17959).
Madre de Dios: Manu (Alto Rio Madre de Dios, Hacienda Amazonia, 12°52’38"S, 71°23’11"W, MUSM 9826). Trocha 2° Mirador-Otorongo (12°33’38"S, 70°05’50"W, MUSM 26131). Manu (Pakitza, 11°56’47"S, 71°17’00"W, MUSM 479, 6791, 6792). Pasco: Oxapampa (Palcazu, Cerro Chontiya, 5Km Oeste Shiringamazu, carretera a Iscosazin, 10°15’00"S, 75°10’59"W, MUSM 10250-10251). Cerro Jonatan (5km E Lontananza, 10°21’57"S, 75°11’23"W, MUSM 10252). Hacienda Roca-Lux (Lontananza, Rio Mucñis, afluente del Río Iscosazin, 10°15’00"S, 75°11’00"W, MUSM 10253). Iscozacin (10°11’05"S, 75°08’43"W, MUSM 720). Río Pescado (10°22’48"S, 75°14’24"W, MUSM 24198). San Juan (10°30’00"S, 74°53’00"W, NMNH 364271). San Pablo (10°27’00"S, 74°52’00"W, AMNH 230130-230131, 230133-230136). San Martin: Rioja (Yaracyacu, Aguajal Rio mayo, 05°57’00"S, 77°11’00"W, MUSM 35204-35206). Tumbes: Tumbes (Pampas de Hospital, Qda. Angostura, 03°45’33"S, 80°22’56"W, MUSM 19346-19347). Zarumilla (Matapalo, Quebrada Naranjos, 03°50’31"S, 80°12’08"W, MUSM 19190, 19348). Rio Zarumilla (Carrizalillo, 03°44’38"S, 80°11’25"W, MUSM 22172). Rio Zarumilla (Carrizalillo 2, 03°44’38"S, 80°11’32"W, MUSM 22173). Ucayali: Coronel Portillo (Yarinacocha, 08°18’00"S, 74°36’00"W, FMNH 62120). Padre Abad (Irazola, Padre Abad, B.N. Von Humboldt, 08°46’59"S, 75°07’59"W, MUSM 8473). Purus (Rio Curanja, Balta, 10°08’00"S, 71°13’00"W, MUSM 1145). SURINAME, Sipaliwini: Coeroeni (Kayser Gebergte Airstrip, East of Zuid River, 03°07’00"N, 56°27’00"W, FMNH 93208). TRINIDAD AND TOBAGO, Trinidad: Nariva County (Bush Bush Forest, Nariva Swamp, 10°23’00"N, 61°02’00"W, AMNH 119420-119421). Saint George County (Blanchisseusse, Las Cuevas, 10°47’00"N, 61°23’00"W, AMNH 256308). Port of Spain, Belmont (10°40’00"N, 61°30’00’W, AMNH 175586). Port of Spain (10°39’00"N, 61°31’00"W, AMNH 207063). VENEZUELA, Amazonas: Atabapo (Mount Duida, Middle Camp, 03°25’00"N, 65°40’00"W, AMNH 77524). Falcon: Bolivar (Tocuyo River, 10°16’00"N, 69°56’00"W, AMNH 130687-130699, 130724, 131085-131086).
Mimon koepckeae (n = 3). PERU, Ayacucho: La Mar (Huanhuachayo, 12°44’00"S, 73°47’00"W; LSUMZ 15676). Estera Ruana (12°43’00"S, 73°49’00"W; AMNH 233222). Junin: Chanchamayo (Santuario Nacional Pampa Hermosa, Podocarpus, 10°59’49.2"S, 75°25’57.1"W; MUSM 41327).