rev bras reumatol.2017;57(2):174–181
ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Biological
therapy
and
development
of
neoplastic
disease
in
patients
with
juvenile
rheumatic
disease:
a
systematic
review
Vanessa
Patricia
L.
Pereira
a,
Teresa
Cristina
Martins
Vicente
Robazzi
b,∗aUniversidadeFederaldaBahia(UFBA),FaculdadedeMedicina,Salvador,BA,Brazil bUniversidadeFederaldaBahia(UFBA),DepartamentodePediatria,Salvador,BA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6February2016 Accepted8September2016 Availableonline5December2016
Keywords:
Rheumaticdisease Children
Teenager Biologicalfactors Neoplasms
a
b
s
t
r
a
c
t
Juvenilerheumaticdiseasesaffectthemusculoskeletalsystemandbeginbeforetheageof 18.Theseconditionshavevaried,identifiableorunknownetiologies,butthoseofan autoim-muneinflammatorynaturehavebeenassociatedwithanincreasedriskofdevelopmentof cancer,regardlessoftreatment.Thisstudyaimstoassess,throughasystematicreviewofthe literatureaccordingtoPrisma(PreferredReportingItemsforSystematicReviewsand Meta-Analyses)qualitycriteria,theriskofcancerinpatientswithjuvenilerheumaticdisease, anditsassociationwithbiologicalagents.ThecriteriadescribedbytheStrengtheningthe ReportingofObservationalStudiesinEpidemiologyinitiativewereusedinordertoassess themethodologicalqualityofthoseindividualitemsselectedinthisstudy.Weanalyzed ninepublications,fromatotalof251papersinitiallyselected.Therewasanincreasein can-cerriskinthepopulationwithjuvenilerheumaticdiseaseversusthegeneralpopulation. Mostspecifiedcancerswereofalymphoproliferativenature.Sevenstudiesdidnot spec-ifythetreatmentornotdefinedanassociationbetweentreatmentandcancerrisk.Only onestudyhassuggestedthisassociation;init,theirauthorsobservedhighriskinpatients diagnosedinthelast20years,aperiodoftheadventofnewtherapies.Onestudyfound anincreasedriskinapopulationnottreatedwithbiologicalagents,suggestingadiseasein itsnaturalcourse,andnotanadverseeffectoftherapy.Studieshaveshownanincreased riskofmalignancyassociatedwithjuvenilerheumaticdisease,andthismayberelatedto diseaseactivityandnotspecificallytothetreatmentwithbiologicalagents.
©2016ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:trobazzi@gmail.com(T.C.Robazzi).
http://dx.doi.org/10.1016/j.rbre.2016.11.008
rev bras reumatol.2017;57(2):174–181
175
Uso
de
imunobiológicos
e
desenvolvimento
de
doenc¸as
neoplásicas
em
pacientes
com
doenc¸as
reumáticas
juvenis:
revisão
sistemática
Palavras-chave:
Doenc¸asreumáticas Crianc¸a
Adolescente Fatoresbiológicos Neoplasias
r
e
s
u
m
o
Asdoenc¸asreumáticasjuvenisafetamosistemamusculoesqueléticoeseiniciamantesdos 18anos.Apresentametiologiavariada,identificáveloudesconhecida,porémasdenatureza inflamatóriaautoimunetêmsidoassociadasaomaiorriscodedesenvolvimentode neo-plasias,independentementedotratamento.Esteartigopropõeavaliar,pormeioderevisão sistemáticadaliteraturadeacordocomoscritériosdequalidadePrisma(PreferredReporting ItemsforSystematicReviewsandMeta-Analyses),oriscodecâncerempacientescomdoenc¸as reumáticasjuvenisesuaassociac¸a˜ocomimunobiológicos.Oscritériosdescritospela ini-ciativaStrengtheningtheReportingofObservationalStudiesinEpidemiologyforamusadospara avaliaraqualidademetodológicaindividualdosartigosselecionadosnopresenteestudo. Foramanalisadasnovepublicac¸ões,de251incialmenteselecionadas.Houveaumentono riscodecâncernapopulac¸ãocomdoenc¸areumáticajuvenilcomparadacomapopulac¸ãoem geral.Amaioriadoscânceresespecificadosfoidenaturezalinfoproliferativa.Seteestudos nãoespecificaramaterapêuticaounãodefiniramassociac¸ãoentreelaeoriscodecâncer. Apenasumestudosugeriuessaassociac¸ãoeobservoumaiorriscoempacientes diagnostica-dosnosúltimos20anos,períododeadventodenovasterapias.Umestudoconstatoumaior riscoemumapopulac¸ãonãotratadacomimunobiológicos,sugeriutratar-sedaevoluc¸ão naturaldadoenc¸a,enãodoefeitoadversodaterapêutica.Osestudosdemonstramaumento noriscodemalignidadeassociadaadoenc¸asreumáticasjuvenisquepodeestarrelacionada àatividadedadoenc¸a,enãoespecificamenteaotratamentocomimunobiológicos.
©2016ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Thetermjuvenilerheumaticdiseaseencompassesavariety ofconditionsaffectingprimarilyorsecondarilythe muscu-loskeletalsystemandthathavetheironsetinpatientsbelow theageof18years.Theseconditionsshowvaried,identifiable orunknownetiologies,amongwhichstandoutthose result-ingfromadysregulationoftheimmunesystemandthatare associatedwithchronicinflammation.Thus,themajorityof patientswithrheumaticdiseasesaretreatedwith immuno-suppressivetherapeuticagents.1
Fromthetimethatbiologicaltherapiessuchasetanercept, adalimumabandinfliximabwereintroducedinthepediatric population(over10years), inordertoinhibittumor necro-sis factor alpha (TNF-␣), a cytokine with a wide range of
proinflammatoryactions,theseremarkabledrugshaveproven tobeextremely effectiveforthe treatment ofawide vari-etyofrheumaticandinflammatoryconditions.2,3Butin2008
theFoodandDrugAdministration(FDA),theUSgovernment agencyresponsibleforthe controlofdrugsinthatcountry, reported an increase in the malignancy rate among pedi-atricusersoftheseagents,whichoccurredafterameanof 30monthsoftreatment.1,3 Forty-eightcasesofmalignancy
(31 cases involving infliximab, two cases involving adali-mumab,and15 casesinvolvingetanercept) wereidentified. Thisresultedinaninvestigationthatrequired,since Novem-ber2009, morestern warnings,forinstance, byapplying a “blacklabel”intheboxofallTNF-␣inhibitoragents(asawayto
warnthatthesedrugscancauseseriousorevenfataladverse effects),whichraisedconcernabouttherelationshipbetween
malignancy and juvenilerheumatic disease,1,3,4 which had
beeninitiallybasedontheincreasedriskobservedinadults withrheumaticdisease.5–8
TheFDAreportwascriticizedformethodologicalreasons, and wasfollowed byseveralstudies thathaveinvestigated theassociationbetweenmalignancy,JIA,andotherjuvenile rheumaticdiseases.
The authors aimed to determine, through a systematic review,theriskofcancerinpatientswithjuvenilerheumatic diseasecomparedtothegeneralpopulationandifbiological agentsareassociatedwithmalignancyinchildrenand ado-lescentswithrheumaticdisease.
Methods
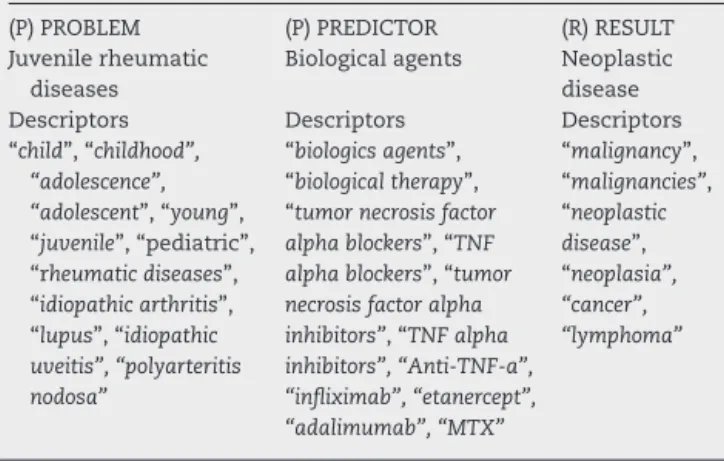
A systematicliteraturereviewwas conductedaccording to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) quality criteria. PRISMA consists of a list of 27 items that are considered essential in order to carry out a systematicreview or meta-analysis which can gather scientific evidence in a clearand reliable manner.9
The electronic databases MEDLINE/PubMed (http://www. ncbi.nlm.nih.gov/pubmed),LILACS(http://lilacs.bvsalud.org), Scielo (http://www.scielo.br) and Cochrane Library/Bireme (http://cochrane.bireme.br/portal/php/index.php) were con-sulted.
For searching the MEDLINE/PubMed database, a search of the literature guided by a question in PPR10
176
rev bras reumatol.2017;57(2):174–181Table1–DescriptorsusedforissueinaPPR (Problem/Predictor/Result)context-drivenliterature researchusingMEDLINE/PubMeddatabase.
Question:Inpatientswithjuvenilerheumaticdiseases[P],theuse
ofbiologicalagents[P]isrelatedtothedevelopmentofneoplastic disease[R]?
(P)PROBLEM (P)PREDICTOR (R)RESULT
Juvenilerheumatic diseases
Biologicalagents Neoplastic disease
Descriptors Descriptors Descriptors
“child”,“childhood”, “adolescence”, “adolescent”,“young”, “juvenile”,“pediatric”, “rheumaticdiseases”, “idiopathicarthritis”, “lupus”,“idiopathic uveitis”,“polyarteritis nodosa”
“biologicsagents”, “biologicaltherapy”, “tumornecrosisfactor alphablockers”,“TNF alphablockers”,“tumor necrosisfactoralpha inhibitors”,“TNFalpha inhibitors”,“Anti-TNF-a”, “infliximab”,“etanercept”, “adalimumab”,“MTX”
“malignancy”, “malignancies”, “neoplastic disease”, “neoplasia”, “cancer”, “lymphoma”
disease (P – problem), the use of biological agents (P –
Predictor) isrelated to the development of neoplastic dis-eases (R–Result)? Table 1sets out the descriptorsrelated toeach ofthe PPR items. For the search in Scielo,LILACS andCochraneLibrary/Biremedatabases,thesamedescriptors wereintroducedinBooleancombination.
Observationaloriginalarticles(cohortstudies, retrospec-tivestudies,and caseseries)toassess the developmentof neoplastic disease inpatientswith juvenilerheumatic dis-easeswereincluded.Therewerenorestrictionswithregard tolanguage,placeofconductionofthestudy,oryearof pub-lication.
Duplicate articles, literature reviews, case reports with fewerthanfivecases,editorials,andabstractswereexcluded. Theselectionofthestudiesfoundinthedatabaseswas performed independently by two reviewers using title and summaryevaluationandfullarticlereading whenonewas identifiedasapotentiallyeligiblepaper.Disagreementswere dealtwithafteraconference.
ThecriteriadescribedbytheStrengtheningtheReporting ofObservationalStudiesinEpidemiology(STROBE)initiative11
wereappliedinordertoassessindividuallythe methodologi-calqualityofthearticles.Thisinitiativebringsachecklistwith 22itemsofrecommendationsonwhatshouldbeincludedina morepreciseandcompletedescriptionofobservational stud-ies.Thestudiesareratedassatisfactorywhenmorethan66% ofthe explaineditemsare included;as intermediatewhen 33–65% ofthe items are included; or unsatisfactory, when less than 32% ofthe itemsare included. Theclassification ofanoriginalstudyasanintermediaryorunsatisfactoryone isrelatedtoagreaterlikelihood ofthisstudy inpresenting differentbiases.
All items included in the review were approved by the respective ethics committees of data collection sites. For thepresentstudy,andinaccordance withtheresolutionof theNationalHealthCouncil–MinistryofHealth,number196 from1996,ananalysisbytheResearchEthicsCommittee(REC) isnotrequired.
Results
Fig.1displaystheselectionprocessofpapers.
AttheendofthesearchinPubMeddatabase,fromthe65 pre-selectedpapers,61articleswereexcludedafter examina-tionoftheirtitleand/orsummary,fornothavingrelationwith theproposedtheme,andfourarticleswereincludedforafull reading.Ofthe186scientificarticlesreturnedtoourresearch inotherdatabasesthroughBooleancombinations,181articles wereexcludedafterreviewingthetitlesandabstractsbecause ofduplication(comparedwiththepapersobtainedinthe pre-vioussearch),fornotrepresentingtheoriginalarticles,orby presentingirrelevanttopicsforthepurposeofthisresearch; fivepotentiallyeligiblepaperswereincluded.
Afterreading the nine selectedpapers, secondary refer-ences cited in the articles obtained were sought, but the relevant referencesto thiswork had already been selected through database searches. Thus, this search method was irrelevanttoourresearch.
Medline/PubMed:
Selected Excluded
61 papers
65 papers
4 papers
Total
9 papers
Excluded
181 papers
Lilacs, scielo, cochrane library/bireme:
186 papers
Selected
5 papers
Reasons for exclusion:
•
Subject of study
irrelevant for this
research
•
Duplicate papers
•
Papers under
elaboration
•
Reports of less than 5
cases
r
e
v
b
r
a
s
r
e
u
m
a
t
o
l
.
2
0
1
7;
5
7(2)
:174–181
177
Table2–Generalcharacteristicsofthepapersincludedinthesystematicreview.
Authorship/year Origin Study
design/control
Numberof participants (inthestudy)
Objective Diseases Agegroup Biological agents
Rateratio (CI=95%)
Cancertype Conclusions
Simardetal., 201012
Stockholm/Sweden Retrospective analytical observational cohort/control groupstudy with comparators ofthegeneral populationfor eachcase
9027 Todeterminethe riskofcancerin patientswithJIA versusthe general population.
JIA Upto16
yearsold
– RRforcancer
inall numbers:1.1 (CI0.9–1.5); RRforMLDin JIAafter1987: 4.2(CI 1.7–10.7);RR forgeneral cancerinJIA after1987:2.3 (CI1.2–4.4).
Cancerin general,MLD
Highriskin patientswitha lessthan20 yearsJIADx;it maybe associatedwith current therapies.
Horneff, 201013
Sankt
Augustin/Germany [Germanlanguage study]
Casereport 5 Reportof5cases
documentedin theGerman registerofcancer inpatientswith JIAtreatedwith TNF-␣inhibitor
agents.
JIA Upto16
yearsold
MTX;TNF blockers (etanercept; adalimumab; infliximab)
– NHL;HL;
thyroid cancer;yolk saccancer; cervical dysplasia
Considerand reportrisksand benefitsofusing biologicalagents; long-term observationof patients.
Bernatsky etal., 201115
Montreal, Quebec/Canada
Retrospective analytical observational cohort/control groupstudy with comparators ofthegeneral populationfor eachcase
1834 Topresent preliminarydata ontheincidence ofmalignancyin JIA,compared withtheratesin thegeneral population.
JIA Meanof8.6
years (standard deviation,5.1)
– SIRforcancer
ingeneral: 0.12(CI 0.0–0.70);SIR for hematologic cancer:0.76 (CI0.02–4.21)
HL SoonafteraDx
ofJIA,theoverall riskofcanceris notincreased;it ispossiblean increasedriskof MLD.
Horneffetal., 201114
Sankt
Augustin/Germany
Observational analytical retrospective cohortstudy
1260 Toreview Germanregisters ofcancerin childrenexposed toTNFblockers andverifyif thereisahigher risk,especially forlymphoma.
JIA Upto16
yearsold
MTX;TNF blockers (etanercept; adalimumab; infliximab)
– NHL;HL;
thyroid carcinoma; yolksac carcinoma; cervical dysplasia
178
r
e
v
b
r
a
s
r
e
u
m
a
t
o
l
.
2
0
1
7;
5
7(2)
:174–181
Table2–(Continued)
Authorship/year Origin Study
design/control
Numberof participants (inthestudy)
Objective Diseases Agegroup Biological agents
Rateratio (CI=95%)
Cancertype Conclusions
Nordstrom etal., 201217
Lexington, Mas-sachusetts/USA
Retrospective analytical observational cohort/control groupstudy with20 comparators ofthegeneral populationfor eachcase
3605 Toestimatethe relativeriskof diagnosisof canceramong patientswithJIA, comparedwith patientswithout JIA.
JIA Meanof11
years
– HR:2.8(CI
0.9–8.3);SIR forJIAcohort: 4.0(CI 2.6–6.0);SIR fornon-JIA cohort:1.4(CI 0.6–2.6)
Lymphoma; softtissue cancer
Asignificantrisk ofcancer(nearly 3timesgreater) wasfoundin patientswithJIA nottreatedwith biologicalagents.
Beukelman etal., 201218
Alabama/USA Observational analytical retrospective cohort/controlled studywith twocohortsof childrenwith Dxofchronic disease withoutJIA
7812 Todeterminethe incidenceof malignancy relatedtothe treatmentof childrenwithJIA, comparedwith childrenwithout JIA.
JIA Upto16
yearsold
MTX;TNF blockers
SIRforcancer ingeneral:4.4 (CI1.8–9.0); SIRfor biological agents:6.9(CI 2.3–16);SIR forMTX:3.9 (CI0.4–14); SIRforTNF blockers:0,0 (CI0–9.7)
Braincancer; leukemia; softtissue cancer;GIT cancer
Thecancerrisk seemstobe higherin childrenwithJIA, butitisnot associatedwith theuseofTNF blockers.
Bernatsky etal., 201316
Montreal, Quebec/Canada
Retrospective analytical observational cohort/control groupstudy with comparators ofthegeneral populationfor eachcase
1020 Toevaluatethe incidenceof cancerinJSLE.
JSLE Upto18years old;meanof 12.6years (standard deviation,3.6)
– SIRfor
invasive cancer:4.7(CI 2.6–7.8);SIR for hematologic cancer:5.2(CI 1.1–15.2)
NHL/leukemia Thereisan increasedriskof cancerinJSLE thatcanarise onlyafterthe patienthas reached adulthood.
Hasijaetal., 201419
Toronto/Canada Observational analytical retrospective cohortstudy
357 Todeterminethe rateandalsorisk factorsforthe occurrenceof cancerin patientswithJIA treatedwith biologicalagents.
JIA/IU/PAN Onsetof rheumatic disease: 1.7–7.3;Dxof neoplasm; 15.3–17.9 years
MTX; infliximab; etanercept
– NPCerenal
carcinoma; MLD;PMT; sarcoma
rev bras reumatol.2017;57(2):174–181
179
T able 2 – ( Continued ) A uthorship/y ear Orig in Stud y design/contr ol Number of participants (in the stud y) Objecti v e Diseases Ag e gr oup Biolo g ical a g ents Rate ratio (CI = 95%) Cancer type Conclusions Ko k et al., 2014 20 Linkou/T aiw an Retr ospecti v e anal ytical observ ational cohort/contr ol gr oup stud y with four compar ators of the g ener al population for eac h case 2892 To in v estig ate the ma gnitude of risk associated with JIA and its tr eatment to the de v elopment of cancer in childr en in T aiw an. JIA Up to 16 y ears old MTX; TNF b loc kers HR: 3.14 (CI 1.98–4.98), RR: 2.75 (CI 1.75–4.32) and IRR: 3.21 (CI 2.01–5.05) for cancer; IRR: 7.38 for leukemia, 8.3for lymphoma, 11.07
for sar coma, 2.08 for others.
Leukemia; lymphoma; soft
tissue sar coma; other Childr en with JIA ha v e a 3-time higher cancer risk East Asia; biolo g ical a g ents do not incr ease this risk. JIA, Juv enile Idiopathic Arthritis; CI, confidence interv al; NPC, nasophar yng eal car cinoma; MLD , malignant lymphopr olifer ati v e disease; Dx, dia gnosis; HR, hazar d ratio; IRR, incidence rate ratio; JSLE Juv enile Systemic Lupus Er ythematosus; HL, Hodgkin’ s lymphoma; NHL, non-Hodgkin’ s lymphoma; MTX, methotr e xate; PA N , pol y arteritis nodosa; PMT , pilomatrixoma; RR, relati v e risk; SIR, standar dized incidence ratio; GIT , g astr ointestinal tr act; TNF-␣ , tumor necr osis factor alpha; IU , idiopathic uv eitis.
Thegeneralcharacteristics ofthepapersincludedinthe systematicreviewcanbeseeninTable2,intheirchronological orderofpublication.Therefore,thefinalproductofthis sys-tematicreviewisadescriptiveanalysisofthedatacollected. Meta-analyseswerenotperformed.
Ofthenineselectedarticles,12–20threewereconductedin
Canada,15,16,19 two inthe US,17,18 two inGermany,13,14 one
inTaiwan20andoneinSweden.12 Althoughthisreviewhas
notmaderestrictionontheyearofpublicationforthe inclu-sionofarticles, allthepaperswerewritteninthepastfive years, showingthe up-to-dateness ofthis research. Of the nine papers, eight were retrospective observational cohort studies12,14–20ratedassatisfactorybytheSTROBEInitiative,
andonewasafive-casereport.13
Thetotalnumberofpatientswithjuvenilerheumatic dis-ease evaluated inthe studies with or without progression toneoplasticdiseasewasabout27,800childrenand adoles-cents.Ofthispopulation,approximately0.5%hadaneoplastic disease(inabsolutenumberstherewasanincidenceof133 cases), which was considered as a statistically significant increasedriskforpatientsinthiscondition(comparedtothe generalpopulationreferredinthesestudies,wherethe inci-denceofmalignancywasabout0.03%).Onlyonestudydidnot supportthisresult.15
Although the search hascoveredrheumatic diseases in general, the resultsobtained onlyinvolved studies dealing with autoimmune rheumatic diseases.Most of the papers studied populationswith JIA(7 out of9articles,12–15,17,18,20
whose participantscomprisedabout 95%ofthetotal num-ber of children and adolescents with rheumatic diseases). One of these studies16 addressed a population with JSLE
(whoseparticipantscomprisedalmost3.7%ofthetotal num-ber of children and adolescents with rheumatic diseases). And anotherstudy19 addressedrheumaticdiseases in
gen-eral (whoseparticipants comprisedabout 1.3%ofthe total numberofchildrenandadolescentswithrheumaticdiseases). This last study specified that JIA was the most common diagnosis.
Approximately22%ofcancerswerespecifiedasbeingof ahematologicandlymphoproliferativenature.Moststudies didnotspecifythenatureofallneoplasmsfoundandvarious solidtumorswerecited(thyroidglandcarcinoma,yolksac car-cinoma,cervicaldysplasia,softtissuecarcinoma,braintumor, gastrointestinaltracttumor,nasopharyngealcarcinoma, kid-neycarcinoma,pilomatrixoma,amongothers).
Two15,16 ofthe ninepapersanalyzeddidnotspecify the
therapies used by the participants. One study12 found a
higher risk of developing cancer in patients with juvenile rheumaticdiseasediagnosedinthepast20years,raisingthe possibilitythatthenewtherapiesadoptedsince1999, involv-ing biological agents,would berelated. Three papers13,14,19
failedtoset anassociationbetweenthetreatment of juve-nilerheumaticdiseaseandcancerrisk.Twostudies18,20found
anon-statisticallysignificantincreasedriskofcancer devel-opmentinthesubgroupstreatedwithTNF-␣inhibitorswhen
comparedtothesubgroupnotexposedtothesedrugs.Finally, astatisticallysignificantincreasedriskofneoplasticdisease wasfoundinastudy17whichaimedtoassessthisproblemin
180
rev bras reumatol.2017;57(2):174–181Discussion
Epidemiologicalstudieshaveshownanincreaseintherateof malignancyincidenceinassociationwithjuvenilerheumatic disease.OnlythestudyofBernatskyetal.15publishedin2011
inCanadaproduceddifferentresultsversusotherstudies,for reasonsthatarenotclear.Theseauthorsinvestigateda pop-ulationdiagnosedwithjuvenileidiopathicarthritis(JIA)and concluded that in the first years after diagnosis the over-allriskofcancerwasnothigher.Butthe resultsrelatedto theriskofspecificcancerswerenotclearandonecouldnot ruleout the possibilityofanincreasedriskofhematologic malignancies.
Simardet al.12 were the first authors topublishon the
associationbetweenJIAandincidenceofmalignancy.These authors conductedaretrospective study in Swedenwith a follow-upperiodfrom1969until2007.Thiscohortwas strati-fiedintotwosubgroupsofabout20yearsoffollow-upeach.In themostrecentcohort,evidenceofanincreaseintherelative riskofcanceringeneralinJIApatientswasfound.Although dataontheuseofspecificmedicationswerenotavailablefor mostofthestudyyears,theauthorsspeculatethatthetime differenceobservedinmalignancyriskcouldbearesultofthe widespreaduseofmethotrexate(MTX)intheirmostrecent cohortofpatients.InEngland,Clearyetal.21publishedin2002
aseriesofcaseswithJIAwhoweretreatedwithMTXandwho laterdevelopedlymphoma,afindingthatgivessomesupport tothis potentialexplanation,but thisinitialreportwasnot followedbyacontrolledstudy.
Little is known about the incidence of malignancy in patientswithJSLE.TheBernatskyetal.16reportwastheonly
publicationfoundonthesubject.Althoughtheuseof medica-tionshasnotbeenevaluated,thedataobtainedsuggestthat theriskofincidenceofmalignancyinpatientswithJSLEcan beincreased,similartowhatoccursinadultpatientswithSLE. ThestudybyBeukelman et al.,18 publishedin2012and
involvingapopulationwithJIAintheUSA,evaluatedmore specifically the effects of the use of medications, but its authorsfoundstrongassociationsbetweentheuseofMTXor TNF-␣inhibitorsandtheincidenceofmalignancy.Thestudy
subsequentlyperformedbyKoketal.20in2014inTaiwan
sup-portsthisresult,butthesamplesize,thelimitednumberof outcomes,andthefollow-upperiods(inthefirststudy,5years; andinthesecondstudy,8years)wereinsufficienttoallowany definitiveconclusion.
Nordstrometal.17in2012conductedaUSstudythatfound
astatisticallysignificant increasedriskofcancerinpeople withJIAnottreatedwithbiologicalagents(uptothreetimes higherversusthegeneralpopulation).Thesedataraisedthe hypothesisthattheincidenceofcancerwouldbepartofthe naturalhistoryofthedisease,andwouldnotbeapotential adverseeffectofitstreatment.Itisunclearwhetherthedegree ofinflammatoryactivityofjuvenilerheumaticdiseasewould bethedeterminingfactorforthedevelopmentofmalignancy, butthiswouldbeconsistentwithpreviousstudiesinadults5–8
withmoreconsolidatedresultsand,inthatcase,ifitwouldbe possiblethatbiologicalagentscouldinsteadreducetherisk ofincidenceofmalignancythrougha bettercontrolofthe diseaseandlesstissuedamage.
Itiscriticaltobeawareofthepossibilityoftheplurality intheetiologyofcancerinpatientswithjuvenilerheumatic diseases, withthe additionofother influences besidesthe chronic useofmedications andthe role ofchronic inflam-mation, such as the individual genetic predisposition and perhapsenvironmentalinfluencessuchasairpollution, nutri-tion andstress.Therole ofthe environment,despitebeing astillpoor-describedtopic inthe literature,hasbeen stud-ied asa potentialcontributing factor tothe triggering and reactivation ofjuvenile autoimmune diseases such as sys-temic lupus erythematosus, dermatomyositis, and juvenile idiopathicarthritis;andconsideringtheimportanceof envi-ronmental influence in the etiology of neoplasms, future studiesmayrevealtherelevanceofthisfactorintheetiology ofcancerandrheumaticdiseases.18,22
On the other hand, never it will be too repetitive to underlinethegreatimportanceofthewatchfuleyeof rheuma-tologists, clinicians and pediatricians for the diagnosis of malignantdiseasesinchildrenandadolescentswith osteoar-ticularcomplaints,especiallywhentheclinicalpicturedoes not include a specific rheumatic disease. Many children andadolescentswithleukemiahavecomplaintsthatmimic rheumaticdiseasesandthataremisdiagnosed,resultingina delayinpropertreatmentanddiagnosis.23
The perception of the risk of malignancy in children with rheumaticdisease duetothe use ofTNF-␣ inhibitors
is obscured by the lack of knowledge regarding the risk that derives from the own natural course and from the chronicinflammatoryprocessofthisdiseaseinitssubtypes and severities, an aspect that has already been investi-gated in studies with adults; but so far the reports are reassuring.
Theassociationbetweentheuseofbiologicalagentsand cancer suggested by the FDA in 20084 has not been
con-firmed;however,thereisascarcityofstudiesontheriskof neoplasticdiseasesinthepopulationsufferingfromjuvenile rheumaticdisease.Thus,thelimitednumberofendpointsin theliteratureisinsufficientfortheextractionofanydefinitive conclusion.Studieswithlongerfollow-upandmorepatients areneeded,soonecananswermoreconsistentlythequestion posedinthisstudy.
Conclusion
Patients with juvenile rheumatic disease appear to be at increasedriskofdevelopingcancer.Inchildrenand adoles-centswithJIA,themostobserveddiagnosisinthepopulations studied,theriskisuptothreetimeshigherwhencomparedto thegeneralpopulation,andmostcancersareofhematological andlymphoproliferativenature.Thegreatestriskofincidence ofmalignantdiseaseassociatedwithjuvenilerheumatic dis-easeisnotentirelyattributabletotreatmentwithbiological agents.
Conflicts
of
interest
rev bras reumatol.2017;57(2):174–181
181
r
e
f
e
r
e
n
c
e
s
1. MannionML,BeukelmanT.Riskofmalignancyassociated withbiologicagentsinpediatricrheumaticdisease.Curr OpinRheumatol.2014;26:538–42.
2. OnelKB,OnelK.Anti-tumornecrosisfactortherapyand cancerriskinpatientswithautoimmunedisorders.Arthritis CareRes(Hoboken).2010;62:1024–8.
3. CronRQ,BeukelmanT.Guiltbyassociation–whatisthetrue riskofmalignancyinchildrentreatedwithetanerceptforJIA? PediatrRheumatolOnlineJ.2010;8:23.
4. U.S.FoodandDrugAdministration.FDA:cancerwarnings requiredforTNFblockers;2009.RetrievedAugust4th2008. Availablein:http://www.fda.gov/NewsEvents/Newsroom/ PressAnnouncements/ucm175803.htm[accessed05.04.14]. 5. SmittenAL,SimonTA,HochbergMC,SuissS.Ameta-analysis
oftheincidenceofmalignancyinadultpatientswith rheumatoidarthritis.ArthritisResTher.2008;10:R45.
6. SymmonsDPM.Lymphomaandrheumatoidarthritis–again. Rheumatology.2007;46:1–2.
7. PaulaJFFV,SkareTL.Prevalênciadedoenc¸asneoplásicasem pacientescomartritereumatoide.ArqCatarinMed. 2013;42:21–6.
8. BernatskyS,JosephL,BoivinJF,GordonC,UrowitzM, GladmanD,etal.Therelationshipbetweencancerand medicationexposuresinsystemiclupuserythaematosus:a case-cohortstudy.AnnRheumDis.2008;67:
74–9.
9. LiberatiA,AltmanDG,TetzlaffJ,MulrowC,GøtzschePC, IoannidisJPA,etal.ThePRiSMAstatementforreporting systematicreviewsandmeta-analysesofstudiesthat evaluatehealthcareinterventions:explanationand elaboration.BMJ.2009;339:b2700.
10.LopesAA.Medicinabaseadaemevidências:aartedeaplicar oconhecimentocientíficonapráticaclínica.RevAssocMed Bras.2000;46:285–8.
11.MaltaM,CardosoLO,BastosFI,MagnaniniMMF,SilvaCMFP. IniciativaStrobe:subsídiosparaacomunicac¸ãodeestudos observacionais.RevSaúdePública.2010;44:559–65.
12.SimardJF,NeoviusM,HagelbergS,AsklingJ.Juvenile idiopathicarthritisandriskofcancer:anationwidecohort study.ArthritisRheum.2010;62:3776–82.
13.HorneffG.Malignancyandtumornecrosisfactorinhibitorsin juvenileidiopathicarthritis.ZRheumatol.2010;69:516–26.
14.HorneffG,FoeldvariI,MindenK,MoebiusD,HospachT. ReportonmalignanciesintheGermanjuvenileidiopathic arthritisregistry.Rheumatology.2011;50:2306.
15.BernatskyS,RosenbergAM,OenKG,DuffyCM, Ramsey-GoldmanR,LabrecqueJ,etal.Malignanciesin juvenileidiopathicarthritis:apreliminaryreport.J Rheumatol.2011;38:760–3.
16.BernatskyS,RosenbergAM,OenKG,DuffyCM, Ramsey-GoldmanR,LabrecqueJ,etal.Cancerriskin childhood-onset&systemiclupus.ArthritisResTher. 2013;15:R198.
17.NordstromBL,MinesD,GuY,MercaldiC,AquinoP,Harrison MJ.Riskofmalignancyinchildrenwithjuvenileidiopathic arthritisnottreatedwithbiologicagents.ArthritisCareRes (Hoboken).2012;64:1357–64.
18.BeukelmanT,HaynesK,CurtisJR,XieF,ChenL,
Bemrich-StolzCJ,etal.Ratesofmalignancyassociatedwith juvenileidiopathicarthritisanditstreatment.Arthritis Rheum.2012;64:1263–71.
19.HasijaRP,SilvermanED,ChoS,FungL,BenselerSM,Cameron B,etal.A170:Neoplasmsinpediatricpatientswithrheumatic diseasesexposedtobiologics–aquarternarycentre’s experience.ArthritisRheumatol.2014;66Suppl.11: S220–1.
20.KokVC,HorngJT,HuangJL,YehKW,GauJJ,ChangCW,etal. Population-basedcohortstudyontheriskofmalignancyin EastAsianchildrenwithjuvenileidiopathicarthritis.BMC Cancer.2014;14:634.
21.ClearyAG,McDowellH,SillsJA.Polyarticularjuvenile idiopathicarthritistreatedwithmethotrexatecomplicatedby thedevelopmentofnon-Hodgkin’slymphoma.ArchDis Child.2002;86:47–9.
22.Franc¸aCMP,SallumAM,SilvaCAA,AikawaEN,BraAL,Farhat SC.PediatrReumatolOnlineJ.2014;12Suppl.1:P27.