Universidade
de
Évora
X'actores
ambientais
que
afectam
a
riqueza
especíÍica
de
macrofungos
em montados de
azinho
-
Implicações
para
a
Gestão
e
Conseruação
ffi
t a\ { ', J'Rogério Filipe Agostinho
Louro
Dissertação apresentada para a obtenção do grau de mestre em
Biologia da Conseruação
Orientador: Prof.u
Dr.'
Celeste Santos-Silvaffi
-{
Évora
r20l0
ffi
E
7 ,à 'ltl''lUniversidade
de lÚvora
Factores ambientais
que afectam
a
riqueza
especÍÍica
de
macrofungos
em
montados
de azinho
-
Implicações
para
a
Gestão
e
Conservação
Rogério
Filipe
Agostinho
Louro
Dlssertação apresentada para a obtenção do grau de mestre em Biologia da Conseruaçâo
Orientador: Profl" Dn" Celeste Santos-Silva
{ts
6;3
AGRADECIMENTOS
A
realização desta dissertação não teria sido possível sem a intervenção das seguintes pessoasàs quais estou eternarnente agradecido:
Agradeço
à
miúa
orientadoraProf."
Dr."
Celeste Santos-Silva pelasvaliosas
sugestões e correcções,bem como
por todo o
tempo,
esforço,apoio
e
dedicaçãoque ao
longo
deste trabalhofoi
demonstrando.Agradeço ao Prof.
Dr.
RussellAlpizar
Jara peloterrpo
e esforço que dedicou na revisão deste trabalho e pelas úteis sugestões acerca do tratame, to estatístico dos dados.Agradeço à Doutora Isabel Santos-Silva a pronta revisão deste
tabalho.
Agradeço à
EDIA
-
Empresa para o desenvolvimento de infra-estruturas do Alqueva-
porter
permitido e financiado este estudo.
Agradeço
à
Maria da Lrrz
Calado
pela valiosa ajuda
durante
o
processo
moroso
de amostragem.Agradeço
ao meu
innão
e à
minha
mãepor todo
o
apoio, paciênciae
incentivo que
meprestaram ao longo deste trabalho.
Agradeço aos meus amigos a paci&rcia e apoio qlre me deram.
Por
fim,
gostaria de agradecer a todas as pessoasaqui
não mencionadas mas que de algumÍrorcr
Acnepecnagl.ITos.Rssuuo
AgsTRAct
INTRODUÇÃoI
1I
2 3 4 OBJECTTVOS.BTOTTC AND ABIOTIC FACTORS AEFECTING MACROF1JNGAL RICHNESS IN HOLM OAK STA}IDS OF
SounrrnxPonrucer,
..
6AssmAcr
6INrnonucnoN
6MersRIALs AND
MBUrO»S.
9Rrsur,rs...
12DrscussroN
16AcrNowr.nocMENTs...
l7
ÁnnaoB EsruDo....
LmrnerunrCrreo.
RrrBnÊucns
emlIocRÁFrces...
Auexo
18 23 24 26tr
FactoÍ€s ambieotais que afecüm ariqueza ospecífca de macroflmgoa
X'actores ambientais que afectam
I
riqueza espocíÍics de macrofungos em montados de azinho -Implicações para a Gestão e ConservaçãoRESI'MO
Ariqrcza
específica de macrofungos e os mecanismos natuÍais que a influe,nciam são aindapouco coúecidos. Foram utilizados
modelos
de
regressãolinear para
inferir
a
relação existenteente
a riqueza de macrofungos e diversas variáveis ambie,ntais.De Novembro
de2005 a
Abril
de 2007foram
amosffidas me,lrsalmelrte as espécies de macrofimgos presentesem
montadosde
azinho
(Qtercus rotundifolia Lam.) no
Parquede Natureza de
Noudar(Alentejo,
Portugal).Verificou-se que
a
riqueza
de
espéciesmicorrízicas
aume,ntacom
a classee*ia
das árvorese com
a penceirtage,m de coberto arbustivo, enquantoa
riqueza de espécies sapróbias aumentacom
as percentagens de cobertos arbustivo e herbáceo, sendo ocoberto arbustivo relevante
para
ambosos
grupos
üóficos. Pelo
exposto
anteriormente,propomos que sejam mantidas faixas e pequenas manchas de vegetação arbustiva natural e
que o
contolo
de matos seja efechrado deforrra
a manter intocada a rizosfera, manimizandodesta forma a riqueza de macrofimgos.
Palawas-chave:
iqrrcza
específica de macrofimgos, regressÍlo linear, perce,ntagemde
cobertura arbustiva, montados de azinho, Portugal.Environmental factors affec'ting macrofungal richness in holm oakstands
-
Implicationsfor
Managemenú and Conservation
ABSTRACT
Macrofimgal rictrness
is
still
poorly known and
the
natural
meçhanisms
enhancingmacrofungal
diversity
still
remain
rmclear.We
usedlinear
regressionmodels
to
infEr
therelationship between mushroom richness
and
several environme,ntal variables. Thenofore, amacrofungal inventorybased on
fruit
bodies was conduckd monthly, from November 2005to
Apnl
200?,in holm
oak
stands (Quercusrotundifulia
l^am.) onthe
Parque de Natureza deNoudar (Ale,ntej o, Portugal).
According
to
our results,mycorrhizal
richness increaseswith
nee age class and shnrb cov€tr,while
saprohophicrichness
increaseswith
shrub cover
and hçrbaceouscovetr Although,
mycorúizal and saprorophic
riúness
models
üffered
ftom
eachother,
results
seernto
emphasize
trat
úrubs
areof
tlre
upmost importancefor
bothmycorúizal
and saprotrophic maorofiurgiin
holm
oak stands. Thuswe
proposethat
strips and patchesof
natural
shrubbyvegetation
úould
be maintained and shnrb control methodsúouldkeep
the rhizosphere intactin
orderto
enhance macrofungal richness.,
FactoÍ$ amblentais çe afectam ariçeza específica de macoofimgooINTRODUÇÃO
A
necessidade de protegere
conservar a biodiversidadee
os recursosnatrais
temvindo
aassumir
um
papel
de
destaque,uma
prioridade
e
uma
preocupação generalizada nacomunidade
científiça"
face às
constantes ameaçase
impactos
negativos
inerentes
àinsustentabilidade dos padrões de consumo
e
alteragões arnbientais que resultam directa ouindirectame,nte do aumento da população humana (RSPB 2003). Há
muito
que a maioria dosestudos
e
actividades relacionadas
com
a
protecção
da
Natureza
e
conservação daBiodiversidade incidern quase exclusivarne,nte sobre as comunidades faunísticas e florísticas,
menosprezando
a import&roia,
rJrqrezae
diversidade do micobiota,bem
como dos factoresque ameaçarn as comrmidades
micológicas.
Ditosamente, esta tendência estáa
inverter-se, tendo-se registado nas últimas décadasum
orescente interesse pelo recurso micológico,factor
que
tem
atraído
mais
investigadorespara esta
áreae
conseque,nteme,ntepromovido
arealização
de mais
estudos sobretaxonomiq
distribúção, biologia
e
ecologiados
firngos.Contudo, diversas entidades empenhadas
na
conservação domicobiota,
de queo
EuropeanCouncil
for
the Conservation ofFungi
é exemplo, continuama
alertar paru a necessidade de aumentaro
conhecimento da diversidade micológica, nomeadamerrte afuavés de"checklists"
locais, estudos prolongados e elaboração de *Red
Lists",
eÍl
particular nos países onde afalta
de estudos base é evidente (p. ex. Albânia, Grécia e Portugal) (Senn-hlet et a12007).
Importa ainda considerar a gestão do habitat oomo uma ferramenta esse,ncial no delineame,nto
de
estratégias paraa
conservação dos recursos micológicos(Molina et al
2001).
SegundoAmold
(2001), a conservação insitu
das comrmidades micológicas deverá passar emprimeiro
lugarpela
conseroaçãoe
gestâo adequada dos seus habitats.No
entanto,no
nosso País, a inclusão do micobiota em planos de conservação está ainda longe de se tornar uma realidade.É
reconhecido o papelfulqal
que osfiingos
desempenham aonível
do
eqúlíbrio
da cadeiatrófica
de
vários
ecossistemas,assegurando
a
reciclagern
da
matéria org&rica
edisponibilizando
nutrientespara as
espécies vegetais existentes (sapróbios),eliminando
asespécies vegetais me,nos saudáveis (parasitas), favorece,lrdo o crescimento e desenvolvime,nto
de
várias
espéciesvegetais,
na
medida
em
que lhes fomecem
nutrie,ntes essenciais(principalmente fósforo
e
azoto)e ágw
e as protegem de agentes patogénicos (micorrízicos)(Alexopoulos
andMims
1979,Smith
and
Read 2008). Paraalém
do
seu relevantç papelecológico,
muitos
dessesfungos produzem
estnrturas reprodutoras-
cogumelos-
múto
apreciadas
pelo
seuvalor
gastronómicoe
actualmente consideradoscomo
um
importanteÍecurso florestal
não-lenhoso. Seno
passado,a
colheita de
cogumelos representava umaFac'toee ambientaisque aftotam a riqueza específica de oacrofungoo
actiüdade
oultural$taizafu,
e umavaliosa
fonte de rendim€,nto apems para as populações locais(Arnold
2008, Garibay-Orijel etal
z}Og),hoje em dia caberá também aos proprietários gerir sustentavelmente este Íecurso, como preüsto no Código Florestal(MADRP
2009).Em Portugal,
o
conhecimento sobre a ecologia e diversidade dos macrofungos está longe deigualar
a
informação
existe,ntesobre
a
flora
e
fauna
com que
interage
e coúita.
Este desconhecimento deriva, em grande medida, das particularidades do método de estudo destes organismos: precariedade das fruüficações (órgão mais fiável para uma idenüficação segtra), sazonalidadecom que fiutificam,
complexidade
no
recoúecimento das
espéciese
nataxonomia de
alguns grupos,tudo isto
agravadopelo
insuficiente númerode
micologistas (Moore etaI2001).
A
necessidade decoúecer
as comunidades de macrofiingos,bem
como os factores que asafectam
positiva
ou negativame,nte évital,
pois este grupobiológico
esüí sujeito a umaforte
pressão humana, motivada pela colheita das suas esüuturas reprodutoras (cogumelos).
Sabe-se que são inrlrreros os frctores
bióticos
e abióticos que influenciam a presença efrutificação
dos maçrofungos. Cada
um
desses factores revela-se mais ou menos importante depende,ndo das características das especies e dos grupostróficos
a que pertencem. Os factores climáticosa
pm
de outros agentes abióticos, nomeadamente, edáficos(tipo
de solo,pH,
teor de matériaorgânica)
e
geomorfológicos
(altitude,
exposição, inclinaçãodo
terreno), condicionam
apresença e a produtividade dos macnofungos (Villeneuve et
al
1989, Brunner etal l992,Baar
1996,l-aganà et
al
1999, Kernaghan and Harper 2001, Kranabetter and Kroeger 2001, Bonetet
al
2004).
Vários
autores defendem
a
exist&tcia
de
uma
forte
correlação
enhe
ascomunidades de macrofungos e a composição e estrutura das comunidades vegetais (Laganà
etal
1999, Bonetetal2004,Richard
etaTzüM,Kernaghan
etaI2003).
Outros strgerem ainda que o controlo de matos, as mobilizações do solo e o pastoreio afectam a disnibuição espaciale
temporal dos
macrofungos (Courtecuisse2001,
Pilz
andMolina
2001,Wiensczyk
et
al
2002).Importa
pois,clarificar
quais os factores que influe,nciam directame,nte as comrmidadesde macrofirngos, de forma a promover medidas de gestâo zustentável para este recuÍso.
ObjeAivos.' De forma a fundamentm estratégias de conservação e gestão pam as comrmidades
de macrofungos
em
montadosde
azirúo, no
Sul
dePortugal,
secom
o
presemteestudo
identificar quais
os
factores
arrbie,ntais (relacionadoscom
a
vegetação,solo
egeomorfologia do
t1rr*o)
queinfluenciam
a riqueza específica de macrofirngosna
área doParque de Nattueza de Noudar
(PNN)
e
averiguar a existência de relações lineares e,nte osFactores ambientaisque afectam a riqueza específica de macrofungos
desenvolvida
cúminando
na
redacção
do
artigo
"Biotic
and
úiotic
factoÍs
affecting
macrofungal richnessin holm
oak
standsof
SouthernPortugal",
enquadrado nesta tesepor
uma introdução geral e considerações finais.Aücionalmente,
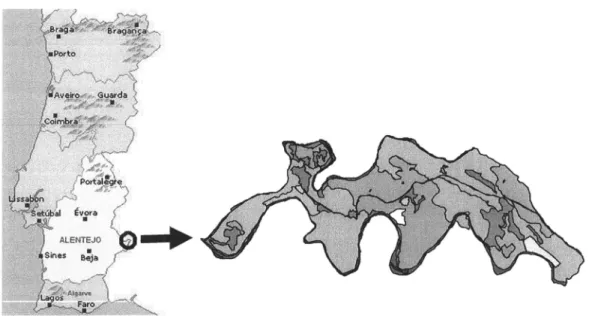
aprese,nta-se a lista das especies consideradas no âmbito do tratamento de dados efectuado (Anexo).Área
deEstudo: O
elevado pote,ncial ecológicoe
paisagísticodo
Parque de Natureza deNoudar, contando com mais de 400 espécies vegetais e uma fauna diversa e abundante @orto
2006)
motivou
a sua escolha paraa
elaboração do prese,nte estudo. Situadono
concelho de Barrancos,dishito
de Bejao PNN
(Fla.
1) e,ngloba uma áreatotal
de 994,5 ha e encontra-sedelimitado a norte/noroeste pelo rio
Ardila
e a sul pela ribeira de Múrtega.Fig.
1. Área do Parque de Natureza de Noudar e respectiva localizagão em PortugalInserido numa
área classificadada
Rede Natura2000, forma
um
conjuntoecológico
comoutras
áreas proüegdasem
Espanha,com as q;riis faz
fronteira,
nomeadarnentecom
osParques
Naturais
da
SErra
de
Arace,nae
Picos
de
Aroche,
Serra
Norte
e
Serra
de Hornachuelos. Relativarnenteao
e,nquadramentogeológico, integra-se
na
7-onada
OssaMorena,
uma
das
grandes unidades
paleogeográficasque dividem
o
Maciço
lbfuico,
constituído por formações das Eras Pré-Câmbrica e Paleozóica.Na
área do PNN predominamos
"Litossolos
dos
climas
de
regime xérico",
de
xistos
ou
grauvaques,
surgindoFactoúçs anbientris que afeotam ariqueza específica de macmfrmgos
frequenteme,
te
associadosa
afloramentos rochososde xistos ou
grÍuvaques (Piçarraet
al2001).
O
PNN
enquadra-sena
área
do
macrobioclima
Mediterrâneo,bioclima
Pluüestacional
Ocefuiiço.
A
temperatura média anual do ar é de 15,8t
e a precipitação anualde
525,6nm,
ocorre,Írdo quase exclusivame,lrtena
estaçãomais
fria.
O
período seco@<2T) é longo
eprolonga-se geralme,rte de Jrmho a Setenrbro.
A
especie arbórea dominante é aaziúeira
(Queransrotundiftlia
L*.),
ocupando cerca de 70%
dafueatotal
doPNN,
formando povoarnentos com densidades arbustivas variáveis.De
acordo
com
Gomes
(1999)
os
azinhais
e
montados
enquadram-senas
formações .$zro bourgaeana-Qaercetumrotundifolia
e Asparagoalbi-Rhannion
oleoides, surgindo nas zonasmais
degradadasformações
da
classe
CistoJanndulaea.
Nos
montados abertos,
sãocaraoterísticas
as
pastagensde
üevo-subte,rrâneo (Poetea bulbosae)e
as
comunidades de Tuberarietea, zujeitas a pastoreio por gado bovino.Num
estudo previamente efectuado(Louro et
al
2009)
foram identificadas 162 espécies de macrofungos, para todos os biótopos presentes noPNN.
Destas, foram referidas pelaprimeira
vez77
espécies para a regrão do Alentejo e 8 para Portugal. Registou-se ainda a presença de 6 espécies consideradas raras na Península Ibérica, nomeadamente Agaricusporplryrizon
Orton,Ileodictyon
gracile
Berk.,
Lactarius
camphoratus(Bull.)
Fr., Lepiota oreadiformls
Velen.,Lancoagaricas melanotrichzs
var.
melanotrichns (Malençon
and-Bertault) Trimbach
e Phaeomarasmius erinacans(Pers)
Scherff.
ex
Romagn.. Adicionalmente registaram-se asespécies Amanita verna
(Butl.)
Lam., Cortinarius orellanus Fr.,Qnoporus
castanazs@ull.)
QuéI. e Hygrocybeconica
var.. conica (Scop.) P.Kumm.),
consideradas como potencialme,lrte ameaçadas(SMM
2008).Factorcs amhientais Ere afecrom a riçreza específica de maotofimgos
Biotic
and
abiotic factors affecting macrofungal
richness
in
holm
oak stands
of
Southern
Portugal
Rogério F. Louro I
Celeste
M.
Santos-SilvaInstitute of Mediterranean Agricultural md Environmental Sciences
Biology Deparhen! University of Érorc Apartado 94
7 OO2-S 5 4 ÉVORA, PoÍtugal
lConesponding
author: rog.louro@gmail.com
Ábsffact:
We used linear regression models to infer the relationship between macrofungal richnessand several environmelrtal variables, in holm oak stands. Therefore a macrofirngal inventory based on
fruit
bodies,was
conducted monthly,from
November 2005to April
2007,tn
pwe
Quercusronndifolia Lam stands on the Parque de Natureza de Noudar, located in Alentejo Province, Porhrgal. According to ouÍ rcsults, vegetation characteristics were the best descriptors of macrofungal richness
in
the studied area. Thus, mycorrhizal richness increases with tree age class and shrub oovetr, whilesaprotophic richness increases
with
shrub cover and herbaceous cover. Although, mycorrhizal and saprohophic richness models ditr€red from each other, results seem to ç,mphasize that shrubs me of theupmost importance
for
both
mycorrhizaland
sap,rotophic macrofungiin
holm
oak
montadoecosystems.
Key Words: microfimgal richness, linear regression, shrub covsr, holn oak stands, Portugal.
INTRODUCTION
Montado
ecosystemsare
Mediterraneâil ssvannah-like
rangelands characterisedby
the
p̀senceof
an opentee
stratum (40-50 treesper ha) mainly
composedof
evergreen oaks(Quercus
rotunddolta
Lam
or
Qtercus
suberL.)
(Gallardo
2003,Azul
2010). The
rmder-canopy stratum is generally comprised of pastures and agricultuml fields
in
a rotation schemethat includes fallows,
with
Mediterranean shrubsartificially
kept atlow
de,nsities (Carreiraset
Ll2006,
Pereira and Fonseca2003, Pecoet
aL2006,Pinto-Correia1993).
These ecosysterns have resultedfrom
a fransformation púocess of theoriginal
cork oak andholm
oak fo,rests,by
human activities, and are maintainedby
constant human intervention(Aztil
2002). Montadoecosysüems
ocsupy exteÍrsive
areas
in
southem Porfugal
and
are
the
most
commonagroforesty
systernsin
theAlelrtejo
Province (Porttrgal)(DGF
2001,Azul
2010). Montadosare
known
to
simultaneouslyprovide
multiple
goods and services(Vogiatzakis et
al
2406)
and
to
sustain avery high biodiversity
(Plieningerard
Wilbrand 2001), thus representing anFactores ubieúis çe afecüm a riqueza especlfica de macrofrrngos
example of ecologically sustainable agroforestry systÉ,m
(Azul
2002).Montado ecosysteÍns are
§ryically
managedfor
three main purposes: forestry, agriculture, aúrdextensive graz.ng @ereira and Fonseca2003), Cork removal and acom production have been
traditionally
accompaniedby mixed
livestock raising atlow
stocking de,nsiües andby
arablesystems
with
long-period rotations and
closednutient
cycles,without
extemal inputs
of
fodder, fertilizers
and
agro-chemicals
(Plieninger
and
WilbraÍld 2001).
Nevertheless,socioeconomic and land use changes over
the
secondhalf of
the twentiettr ce,lrttrry, such asintensive graang,
mechanizationof
agiculture
and
rural
desertification, resulted
in
soil
degradation,
tee
scarce,ress,lack
of
regeneration and shrub encroachment that may increaseconsiderably
the
rislc
of
forest fires,
pests and
diseases(Ferreira 2001,
ICN
2006).
For
instance, shrub clearing was
customarily
carriedout
manuallyonly
within
selected patches,minimizing
the damaged area. However, due topublic
forestry subsidies and motor powered machinery, shrub clearingis
nowadaysfar more
intensive, producing a more homogeneous and intense disturbançe (Perez-Ramos etaI2008).
Macrofungi
are arnong the most important organismsin
both natural and semi-natural forestecosysterns.
Without saprotophic
fungi, theprimary
decomposers of wood andlitter
(tlobbie
et
al
1999, Robinson et al 2005, 7-elleret
ad2007),Dffiy nutient
cycleswould
be drastically alte,red and forest ecosystemproductiüties
greatly reduced (Ricklefs andMiller
2000). Others, such asmycorrhizal
fungi are capableof
establishingslmbiotic
associationswith
the rootsof
most plant species, aiding in plant
nutient
and wateracqüsition
@runner 2001, Hartnett andWilson
20V2,Gúdot
et
aL2003, Smith and Read 2008), altering the competitive relationshipsamong
plants
of
different
species(Kennedy
et al
2003,
Egerton-Warburtonet
al
2007),protecting
their
hosts
from soil
pathoge,lrs (Amaranthus 1998,Harhett
and
Wilson
2002,
Smiú
and Read 2008) andfrom
environmental extemes (Amaranthus 1998, Smith and Read2008), and
play
an important rolein
the sequestrationof
Cin
soil (Treseder andAllen
2000).Even pathogenic
ftngi
enhance forestdiversity by
killing
&ees thatlatter
can become snags and logs that can be used by avariety
of other organisms(O'Dell
etal
1996).However, the importance
of fimgi
goesfar
beyond their fimdamentalrole in
many ecosystemfuirctions and processes since
they
influence humans and various human-related activities aswell
(Mueller
andBills
2004).Wild
mushrooms, thefruit
bodiesof
many forest macrofungi,are nowadays regarded as one
of
the
most important non-wood forest products @onetet al
2004,Garibay-Oriiel
et
al
2009).In
fact,
the fruit
bodiesof
morethan 3000
maorofungal species are consumed around theworld (Garibay-Orijel
etal
2009), and the economic valueof
some of them surlrassesby
far the valueof
timber(Arnolds
1995, Honrubia 2007).Moreoveç
Factores ambimais que efectam ariqueza espeoífica dc macroftmgos
mushrooms
sudden appeararrceand
beauty always
fascinatedpeople, making
them
alsoimportant
in
the contextof
nature conservation and manageÍnent (Süaatsma et al 2001).Most
surveys dealing
with
masofungi
are
basedon fruiting
bodies, since
fruit
bodies can
beidentified to
species and large numberof
plots can be continuously moniüored over a numberof
years (Schimt etal 1999,Dúlberg
etal
1997).The
biotic
andabiotic
factorsinfluencing
macrofungal species occuÍt€lrces and distributionsaÍe numerous and interactive (Beare et
al
1995). Plant species composition is known to be animportant
fastü
shaping macrofurtgal communities@ills et
al
1986,Bnmner
et
al
1992, Villerreuve etal
1989, Kernaghan and Harper 2001, Cavender-Bares et a12009, Jrmrpponenet
al
2010) since plants co,nstitute both habitat and energy sourcefor
most macrofungi andmany
of
themexhibit
some degreeof
plant host or substratum qpecificity(Iodge
et al 2004). Plantcommunity's stucürre is
also an influe,ntial factor determining the macrofimgal communitiesprese,nt
in
a site (Yang et al 2006). Treeor
canopy cover's influence on macrofungi has beenshown
in
many
studies,mostly
in
Boreal
forestsor
conifer trees (Villeneuve
et
al
1989,Rúling
andTyler
1990, Laganà etal
1999, Senn-klet andBieri
1999, Jansen 1991, Bonetet
al
2004, Richardet al2004,
De
Bellis
et
al
2006). Shrub cover and herbaceous cover alsohave
been shownto
influence macrofungi
occuÍreÍrce anddistribution
@úling
andTyler
1990, Richard et
al
2004), as many macrofungi are knownto
associatewith
particular shnrb species and evç,n show a certain degreeof
host specificity (Comandini et al 2006, Eberhardt etal
2009). Plus,
mostHygrophoraceae
species areknown
to
occur
chiefly
in
open,grass-dominated
sites(Ruhling and
Tyler
1990). Stand'successional changesmay
alsohave
animpact on
macrofungal communities(Keizer
andAmolds
1994, Se,nn-hlet andBieri
1999,Nordén
and Paltto 2001, Gates etal
2005,Twieg
et aI 2009),through the
establishmelrtof
new hosts and changes on the amowrt and/or
quallty of
bothlitter
and organic matter content(Lodge
et al2004) or simply
through
variationson
stand photosynthetic rates andgpwth
efficiency
@úlberg
2002, Naraet
al2003,
Bonet etal
2008). Edaphic and geomorphologicfeatues
such as soil nutrients, base saturation, organic matter, slope, aspest and altitude are alsoknown to
influence macrofungal commrmities presentin
a site@onet
eta72004, Engolast
ú
2007, Hansen 1988, Kernaghan and Harper 2001,Rúling
andTyler
1990,Twieg
etal
2009,Yang
etaI2006).
The limited
and oftetr
scatteredknowledge
on how silvicultural
treafrnentsatrect
fungalpopulations, make
it
extremely
difficult for
most
forest managenlto
integrate mushroom species,in
manageme,ntprograms (Martínez-Aragón
et
al
2007).Forest
managersneed
abetter
understandingof
how their
choioeswill
influe,ncemacrofimgal communities
andFactorcs ambieotris que afectam ariqreza especlficade macrofungos
mushoom
yields
if
they want
to
explorethis
resource(Pilz
andMolina
2001).Yet,
to
our
knowledgeonly
afew
models regarding mushrooms have been developed sofar
(Yang et al2006).
For
instance, Hansen(1988)
usedsoil
and
litter
variablesto
predict
macrofimgaloccure,nces
in
Swedish beech forests, Yang et al (2006) e'nrployed a logistic regression and aGIS
expert
systemto
model
the
fine-scale spatial
distibution
of
matsutakein
Ytmnan,southwest China, Martínez-Aragón et
al
Q007) and Bonet etal
(2008) developed predicüve equationsfor
ectomycorrhizal and selectededible
saprotrophicfilngr productiüties in
pine
forests
of
the
pre-Pyreneesmountains
of
Spain and
Dúl
et
al
(2008) used
weatherinforrration
to predict
mushroom abundancein
Norway. According
to
somearthors,
thedevelopme,lrt
of
mechanistic models based onempirical
studiesover
a broad rarrgeof
forest§pes,
stand conditions, andsite factors might facilitate
forestersto
better
evaluate habitatconditions
for
mushrooms@ilz
et
al
2001, Bonnetet
a12004,Dúl
etal
2008). Moreover,land-use changes,
particularly
in
foresüry andagiculture,
are themajor
causesof
change anddecline
of
macrofungal
diversity
in
Ernope(Senn-hlet
et
al
2007). Therefore,
sustaining appropriate forest habitatis
esse,ntialfor
sustaining mushroom diversity andyields @ilz
et al 2001).In
orderto find
simple and applicable models that can explainhow
environme,ntal conditioninflue,nces macrofungal richness
we
developed linear regression modelsfor wild
mushroom richnessin holm
oak(Querax rotundiftlia
Lam)
montado ecosystemin
Alentejo
Province, Southem Portugal.MATERIAL AND METHODS
Site
deseription.-T\e
study was conductedin holm
oak(Qtercus rotundifolia l-am)
stands on the Parque de Natureza de Noudar(PI[}$.
Located nearBarancos
(38o 08'N
and 6o59'W)
in
Alentejo
Province,Portugal. The
PNN
elrcompassesa total
areaof
994.5ha
andit
is
bordered o,n the north-west by theArdila
River and on the southby
theMírtega
stueam. Theclimate
istlpically
Mediüerranean pluviseasonal oceanicwith
a mean annual air temperafureof
15.8'C
and a mean annualrainfall
of
525.6mm.
Thedry
season isusually
from
Juneto
September
(Mendes
et
al
1991).
The
study
area
is
dominated
by
4ro
bourgaeana
-Quercetum
rotundiftlia
wtdAsparago
albi-Rhamnionoluides
formations,úhough
various elementsof
the
Cisto-lavanduleteaarc
rather freque,ntin
the most
degraded areas (Gomesleee).
Factues ambiemtais quc afecm a riqueza especlfica de macnofinges
Noveruber 2005 to
April
2007,in
33 permane,nt circular plots (250 m2 each) randomly chosenin
pnre Q.rotundifotiaLam
stands.All
observed macrofimgi epigeous spoÍocarpswithin
thesarrpling
plots were
collected
in
orderto
ensurethe
detectionof
thosetma
difficult
to
distinguish
in
thefield (O' Dell et alzüM).Identification
was done on-site at the momentof
detection, and whenever necessary specimenswere
kept
in
a
freezerat
3
"C
for
further
verification.
Represe,lrtative vouchEr collectionsfor all
observedtma
were deposited atthe
Évora University Herbarium(UEVH-
FUNGD. Each macrofungaltmon
was includedin
oneof
the
three
main
üophic
groups: saprotophic,
parasitic
or
mycorhizal,
according
to
@reitenbach and
Krânzlin
1984, 1986, 1991,1995,2000, Frade andAfonso
z003,More,no etal
1986,kânzlin
2005).
Those
taxa with more than
one
fiophic group,
depending on ecological and environmental conditions, were included in the mostlikely
trophic group.Plot chmacterization.-Envronrrrcntalvariables
were recorded at plot level and encompassedvegetation
characteristics(composition
and
structure),
soil
descriptors,slope and
aspect(TegLE
I).
Vegetation cover was calculated separatelyby
layer as the percentageof
canopy (tree, shnrb,herb
or
moss andlichen)
pnojection areaper
plot
area.To
estimatetree
4ge, annual ring counts were made on the cross sectisns of the Gore sâmples removed from the tree stems,at
0.5
m
height
with
an
increme,ntborer. Tree
ageswere
groupedinto
5
classes,respectively: 10-19 years, 20-29 years, 30-39 yearsl,
4049
years and 50-59 years. Diametersat breast height were measured
with
a tree calliper at 1.30 m height. Soil sarrples (4 replicates perplot)
were collected,using a
5
cm diametersoil
probe.Soil
organic matter content was calculated by weight differe,nce afterignition
(550'C
for
5 h in amuffle
fumace§abertherm
L9lC6).
Soil pH measureme,lrts were donein
an aqueous solutionof
l:2
(earttr:distilled water)
using a pH meter (Metuohm 691). Soil§pe
was consultedin
Portugal soil map(SROA
1973), lett€rs no.44-A
and44-8,
scale 1:50000, and was groupedin
5 classes ranging betweem 0.2 and 1. He,nce 0.2 was athibuted úo the shallower leptosols whereas the most evolvedluvisols
correspondedtol.
Statistical Analysis.-Envfuonme,ntal
variablesnormality
was
assessedusing
Kolmogorov-Smirnov tests. Square
root
andlogarithmic
transformations wene usedto
achievenormality
when
necessary. Leve,ne'stests w€re
e,mployedto
assessthe
variance
homocedasticity assumption.fire
strength
of
the
linean association amongthe
depe,lrdentvariables (total
macrofimgal richness,
mycorrhizal
richness
and saprotophic
richness)
and
independent variables(Tarm I)
was quantified using the Pearson product-momelrt correlation coefficient.ll
Foctues ambientois que afectam a riçeza específicade macrofungos
EEEEEEg§EEEfiEEEE
ui
a B S
=
ü
o.i
r
oi
ct P d I H I
-.ioo(oo=oer\ooooo=oo
eoHox$oHoou.)Noxoo
O€O-ÊOOee\Ov-) eYíON-ioF-oR+dooo++oBcid
.t .ír t\
93
!l É \o
rô ôr ('r É §: § \o
o\88R+{F8RSã§Çõ3SN
..:oodãEóddôioôiaN§r-o
oo
oeu.)oot-oôloooooooo
o r.l t\ o (> N o \o (> o o Ê (ã o o o
qqqedqqqqqqqq<jqtq
r"l o g\ o o sf o o cn o F k.l Ô \o N o
É(ô
ooPPPrnF(noooooEgo
oç=xxo\ochoÉoNoo)<o
o cô 1. Y
=
r^ c'! q q q o! \o o ; Y t
c.;
o
=
R I,/i
o ci + o
od
vi a
Si
3
o
ü§n§[§fiEEÊf;üE$§§
e.i
€i
=
I § d o
o-
c"i
o ci vi 3 ãi =
€,§b
ÉEsssEEsggs§.§.#]
àEE-l
Bl
ɧÊs§ãEsg§§,ÊB,i
Êl
rãü(36EtrÉ
ãEIEEt
õrl
I E a Ex
6 É E ÉlE
É
rn o Eo ã 6 .t, í)E
É 6 !) 0 ÉI
Ê
() a lDn
0) -cr'cl I 6 d C) aD GI a or
o o, c)'o
E, E çr) É á d) t Ê oÀ
aÀ o E o.o
(D o) o o. ht€
C)E
o o (t) k ÍÀ o .Cl 6l 6l GI É (D É cl o É ril E É É-,'FactorGs mbientais que decÍm a riquea espeoífica de mauofirngos
The
thresholdvalue
for
deciding
on
Íedundancy arnong independent variables was setto
asignificant
correlationcoefficient
of
around0.6.
Stepwisemultiple
linea
regression analysis was then conductedto idenüff
the setof
independe,nt variables that bçtter explainvmiation
in
the response variables. Variable additions to the models continued
until
the significance levelof
F'values drop below the entry value (P < 0.05). Among all models computed we chose the ones
with
the
bestadjusünent
namelythe
oneswith:
highest adjusted coeffrcientof
determinationG'uj),
negligible residuals autocorrelation @urbin-Watson coefiEcientvalue
near2)and
with
anormal
residualsdisüibuüon.
AII
calculations were performedwith
SPSS 15.0for
Windows(SPSS Inc., Chicago,
IL).
RESI]LTS
Throughout
the
studyperiod a
total
of
lll
tma
belongtragto
57 genero were accountedfor,
encompassing79
saprotrophic
and
38
mycorrhizal macrofimgi. Members
in
the
order Agaricales comprised 81%
of thetotal identified
tma
(Ftc. 2).At
the genuslwel
Entoloma(6
sp.),Inocybe
(7
sp.),
Lepiota
(6
sp.),
À[ycena(7
sp)
and
Russula(s
sp)
were
the
mostrepresented genera 00 EO 70 ú €eo
$ro
E à40 .a Eao = 20 ío 0*d.P'/d"/
d/-//ry^C
Hc.
2. Species richness of several macrofirngal orders thnoughoú the study periodAround
9
Yoof
the
total
identified
Íara úowed a
widespread ocsuÍÍence, namely, Astraeus hygrometricas (Pçrs.) Morga,o, Gymnopusdrlnphilus (Bull.)
Murill,
Laccwia
laccato (Scop.)Cookg
LycoperdonatropurpneunYiltad.,
Lycoperdonp*latum
Pers., Iúycenapura
(Pers.) P.Kumm., Pmasola
mricoma
(Pat.)Redhea{ Vilgalys
and Hopple,Psathyrella
spadiceogrisea(Schaetr)
MaiÍe,
Psilocybe crobula
(Fr.)
Sing€r,Sclerofurma
verrucossurn(Bull.)
Pers. andFactores mbiGútaisque afectmr ariçrczaespeclfca de mcrofrntgos
Tubwia
romagnesiaruArnolds.
In
conüast,27
Yoof
the
total
identifred taxa
appearedonly
once
in
one plot, during the study period.None significant linear
corÍelations werefound
betweensoil
descriptors, slopeor
aspect andany
dependentvariable
(totat
macrofungal richness,mycorrhizal
richnessand
saprotrophicrichness).
In
what regards the vegetation characteristics, shrub cover, numberof
sbnrb species,mean shrub
height
herbaceous cover andlitter
height showed shonglinear
associationswith
some dependent
variables
(Teme
II).
Additionally,
a
large number
of
correlations amongvegetation characteristics
were
also
formd.
For
instance,
highly
significant
correlations(P<0.001,
n:33)
were found betweenúrub
cover and respective§, meanúÍub
height (0.114),úrub
species number (0.776) and meanlitter
height(0.7il).
Although
the large numberof
correlations among stand characteristics was expected,it
limited
the number
of
variablesto
be usedin
the models dueto
collinearity problems. Hence,only
thefollowing
variables were usedin
the multiple
linear regression analysis:tree
cover,tee
age class,úrub
coveÍ, aspect, slope, soil pH and organic matter content.Taslr
II. Pearson's product-moment correlation coefficient @-values). Dependentvariables: mycorrhizal richness
(Mr),
saprofophic richness (Sr),total
macrofirngalrichness
(Tr).
* rqrese,nt significant correldions at P < 0.05 and**
at P < 0.001.Variables
Tr
NIT SrTree qge class Trce cover
Shrub cover
Horbaoeous cover
Mean tneo height Mean shrub height
Mean herbaceous height
Shrub species number Mean litterheight
Soil organio matter content Soil pH
Soil humidity
Moss and liohen covetr
Aspect Slope Soil type 0.395* 0.204
0.638**
-0.360 0.065 0.227 -0.342 0.467*0.493**
0.085 -0.115 -0.436 0.505 0.024 -0.072 -0.238 0.381 0.090 0.149 0.182 0.1470.479**
0.346*-0.382*
0.203 -0.203 -0.315-0,24t
-0.133 -0.266 0.429 0.188 -0.045 0.2580.746**
-0.678**
-0.158 0.479* -0.4810.608**
0.708**
0.243 -0.031 -0.273 0.158 -0.097 -0.045 -0.410FactoÍes mbientais qrn aftcam a riqueza específ ca de mamofimgos
The best adjusted models are
úown
in
TInLB
III.
Shrub cover, hee age class and herbaceouscover
were
the most significant
explanatoryvariables
of
total
macrofimgal richness. For
mycorrhizal
richness the best setof
explanatory variables wasúnrb
cover andüee
age class,whereas
for
saprotrophic richness rryasúnrb
covetrand
herbapeouslcover.
All
regressionsforcefully
passedthrough
the origin
sincethe
constantalways
showeda
significance
value higher than 0.05 inall
regressions.TasLE Itr. Multiple linear regression models for the anatysed response variables. Ty - Tree age class, Cs - Shnrb cover, Ch - Herbaceous cover
Response Variables Model summary
Rz
P-ValuesTotal maorofirngal richness (Tr)
Mycorrhizal richness (Mr) Saprotophic richness (Sr) Tr = 0.177Cs + 1.435Ty + 0.068Ch Mr=0.123Cs+0.739Ty Sr=0.058Cs+0.096Ch 0.94E 0.830 0.929 < 0.001 < 0.001 < 0.001
In
orderto
examine exactlyhow
the
numberof
macrofimgal speciesvaried
in
regardto
the explanatory variables,we
plotted
the
response variablesas
a
function
of
each independentvariúle,
while
maintaining the other factors equal to their mean(Flc.
3,A-C).
Accordingto
ourresults
úrub
cover
exert more
influence
on
mycorrhizal
richnessthan on the
number
of
saprotrophic species. Thus,total
macrofimgal richness augmentwith úrub
cover seeÍnsmainly
athibutable
to
its
influence
on
mycorrhizal
richness.In
regardto
tee
age class,botfr total
macrofungal and mycorrhizal richness increaselinearly
with ree
age. Nevertheless, theformer
rises more steeply fhan the laÍer, possibly due to a positive, but not linear, saprohophic richness response
to
tee
age class. Herbaceous covetr,on the
other
handpositively
influences
bothsaprotrophio and
total macrofimgal
richness. However,the
less pronounced slopeon
thetotal
macrofimgal richness linear equation seerulto point out
a negative effectof
herbaceouscovet
on mycorrhizal richness.
Factü€s mbienAis que deorem ariErzaespeoíficade macroffmgos
A
25 0 20 20 G at aa*ts
t ê L atÉro
z
0 25 20 l5 0 0 a) I !) EI 0 li. c i. atê
Ez
5B
4íJ60
Shrubcovcr(7o) 3the
age chss4060
Ecúaccous cwer(Vo) 80 100 5 6 80 100 0 5 25 0 0 I 2 4C
20 e É) CIârs
b. c L oÊro
Itz
5 0 20FIG.3. Macrofimgal richness
(o-
total"
[
-mycorúizal,
A-
saproüophic) as a fimction of shnrb covor (À),tee
age class @) and herbaceous cover (C) accoding the equations shown onTeglp
III.
Fachúps ubienuis gue afectu a riquezr específica de mocrofungm
DISCUSSION
Many
authors consider edaphic and geomorphologicat features asimportant
factors a,ffectingthe macrofungal communities present
in
a site. However neither edaphionor
geomorphologic variables werelinearly
correlatedwith
mactofirogal ricbnessor
were included
in
any
of
the models computed.It
is
possible that those variables are only relevantin
awider
spatial scale, and therefore our results are merely a reflectionof
the naÍrolv spectreof this
sfudy, or perhapsthe
relationúip
betweenthem
and macrofungal richnessis
other thanlinear. On the
contrary vegetation descriptors seemedto
zuitably
explain the variationin
the macrofiugal
richnessof
the studied area.Rezutts
úowed
that
macrofimgat richnesswas
strongly relatedto
both tree
age and under-canopy vegetationcover
andthat the
numberof
macrofungal species increaseslinearly
with
them.
Yet, total
macrofungal,mycorrhizal
and saprotrophic richness modelsdiffer form
each other.Concerning
mycorrhizal
richness,resúts
úowed
that
it
only
increaseslinemly with
tree
ageand
with
the
percentageof úrub
coveÍ.
Concerninghee
age, recentworks
proposethat
thesuccessional changes effects
on
stand photosynthetic rates andgrowth efficiency may explain
the
variation
onthe
abundanceof
ectomycorrhizas and theirfruit
body production
(Dúlberg
2002,Nara
et alz003,Bonet
et al 2008). Asfor
shrub coveÍ's influence on mycorrhizal richness,Richard et al (2004)
working
in
an old-growth Mediterraneanforeit
dominatedby
Quercusilex
L.,
formdboú
ectomycorrhizal andsaprotophic
richness positively correlated ta Arbutusurudo
L.
density.
Also,
Comandini
et
al
(2006)
recognizedmore
than 200 fungal
speciesto
be associatedwith
Cisras spp. (the most abundant shrubtmon in
our study area),which
pointsout
the importance
of úrubs
as potential hostsfor
macrofungiin
Mediterranean forest eoosystems. Furthermore, accordingto
some authors, microenvironmental conditions such aslight,
moistureand
temperature encompassedby
densercanopy oover can
enhancemycorrhizal
divetsity
(KÍanabett€r andKroeger
2001,Bonnet etaL2004,
Santos-Silva etal
2010).In
asimilar
way,we propose that
is
also plausible that misroenvironmental conditions underúrubby
vegetationmay
allow
theestabliúment
of those mycorrhizal speoies that prefer shadier aÍeas, especiallyin
cases where tree density is
low.
Saprotrophic richness increases
linearly
with
shrub and herbaceouÍt coveÍ. Resourceavailability
is probably the more
likely
explanationfor
the linearrelationúip
between saproúophic richnessand
úrub
cover. Saprotrophicfimgr
areintimately
associated tothe
substatefrom
which
theyfee{,
and mâny show preferencefor
a specific shrublitter
(Robertset
al 2004). Moreover, theFac'toúes ebiêoteis qu€ a&cüm a riqueza sspecífica de maclofungoo
amount and
quality
of
available plantlitter
are knownto
alter the composition andstucture
of
decomposercommunities
(Wardle and Lavelle
1997,Wardle
et
al
2006). Thus,
it
seems possiblethat
shrubcontibution
to
úe
amount andquality
of
soil
litter in
ourplots
may have favoured saprohophic richness aswell.
As for
herbaceouscover,
Maggi
et
al
(2005) found
that
it
can affeot
microenvironmentalconditions at soil surface,
proüding
shade, increasing soil moisture content and thusproüding
agreater
protection
from
heat.
This may explain the
increasein
saprotrophicrichness,
as microenvironmental conditions are beüeved to directlyinpact
the fruitbodies transpiraüon rates(Kenmghan
and Harper 2001) and
consequently constrain mushroomproduction.
Substatespecificity also
oanexplain
someof
the
variation
in
saproúophic richness.In
fact,
severalhundred saprotrophic basidiomycete
fimgr
aÍe
moÍEoften found
in
grasslands(Griffith
andRoderick 2008),
or
areknown
to
occur mostly
io op"rt
grass-dominated sites(Ruhling
andTyler
1990). Additionatly, cattle's
presenceis
frequent
associatedto
open
areas whereherbaceous
cover
dominates.This probably
enablesthe
occurrenceof
many
coprophilousor
subcoprophilous macrofungi, usually linked to herbivorous dung.
In
summary,with this
studywe
aimedto
develop simple and accurate macrofungal richnessmodels
to
aid
nafire
conservation managercand
forestersto
better
undetstandhow their
decisions can
affect
macrofungal richness. Our results are therefore encouraging becausethey
demonstratethat
macrofirnsal richness
variations can
be
fairly
explained
by
vegetationcharacterisücs
that
can bereadily
influencedby
silvioultural
interventions.We
alsoúow
that
shrub cover is probably of the upmost importance
for
macrofimgal richness.If
we considerthat
in
this
ecosysüem shrubs are usually clearedout or
artificially
kept
atlow
densitiesto
preventthe
risk of
forestfiles,
new strategies may haveto
bç deüsedin
orderto
successfully man4gemacrofimgal ricbness.
This work is
a contributionto
the knowledgeof
fungal ecologyin
holm
oak montado
ecosystemsand furttrer
studies
in
other forest t),pes
with
different
stand characteristics arestill
necessaryin
orderto
fully
understand the natural mechanisms affecting macrofimgal richness.ACKNOWLEDGEMENTS
The research
was
supportedby EDIA (Alquwa
Infrastnrctures Development Enterprise).We
are grateful to PbD. Russell
/úprzrr
Jarafor
discussion on statistical analysis and to PhD.Isúel
Factores mbientais que afechm a riqueza específioa de nasroftuges
LITERATT'RE CITED
Amaranthus MP. 1998. The importancê and conservafion
of
ectomycorrizal fungat diversrtyin
forest ecosystems: lossons from Europe and the Pacific Northwest.In:
Gen. Tech. Rep. PNWGTR- 431. Portland. U.S. Deparünent of Agriculture, Forest Service. Pacific Northwest Resoarch Station. pl-15.
Arnolds
E.
1995. Conservation and managementof
naturalpopolrtiom
of
ediblefimgi.
Canadian Journal of Botany 7 3(l):987498.
Azul
AIv[, Sousa JP, Agsrer R, Martín MP, Freitas H. 2010. Land use practices and ectomycorrhizal fungal communities from oak woodlands dominded by Qaercus suberL. considering dÍought scenarios.Mycorrhiza 20:73-88.
Azul
AM.
2002. Díversidadede
fungos ectomicorrízicffrem
ecossistpmasde
Montado [Doctoraldissertationl. Coimbra, Porhrgal. Universidade de Coimbra.77í p.
Beare
MII,
Coleman DC,Cross§
Jr.DA,
Hendrix PF, Odum EP. 1995.A
hierarchical apprroach to evaluatingúe
sifficance
of soil biodiversity to biogeochemical cycling. Plant md Soil 170:5-22.Bills
G, Holtzmann G,Miller O.
1986. Comparison of ectomycorúizal basidiomycete communitiesin
red spruce versus northern hardwood forests of West
Virginia
Canadian Journal of Botanyil:76F768.
Bonet J, Fischer C, Colinas C.2004. The relationship betwee,n forest age and aspect on tho productionof
sporooarps of ectomycorrhizal fungt in Pímts sylvestris forests of the central §renees. Forest
Ecolory
and }úanagement 203 :l 57 -17 5.
Bonet J, Pukkala T, Fischer CR,
Palúí
M,
Martínez-'AragónI
Colinas C. 200&. Empirieal modelsfor
predicting the production
of wild
muúrooms in Scots pme(Pimn
sylvestrts L,) forests in the Celrtral$menees. Annals of Forest §cience 65(2):206.9 p.
Breitonbach
J,
trfuânzlinF.
1984. ChamFignonsde
Suisse,Tome
l:
Les
Ascomycêtes. Lucerne.MycologiaEd.313 p.
Breitenbach J,
Krâülin
F.
1986. Champignons de Suisse, Tome 2: Champignons sans lamos. Lucerne. MycologiaEd.4l2p.
Breite,lrbach J, KÍânzlitr F. 1991. Champignons de Sússe, Tomo 3: Bolets et champignons à lames lêne partie. Lucerne. Mycologia Ed. 360 p.
Breitenbach
I
KrenzünF.
1995. Champignons de Sússe, Tome 4: Champignons à lames2tu
partie.Luoerne. Mycologia Ed. 368 p.
Breitenbach J, Krânzlin F. 2000. Champignons de §uisse, Tome 5: Champignons à lames
3tu
partie.Luoerne. Mycologia Ed. 338 p.
Bruoner
I,
Brunner F, Laursen G.1992. Characterization and comparison of macrofimgal communiüesn
n, Alrustmuiftlia
and an Álnuscrisry
forest in Alaska. Canadian Journal of Botany 70:1247-1258.Brunner
I.
2001. Ectomycorrhizas:their role
in
forest ecosystems under the impactof
acidifring
pollutants. Perspectives in Plant Ecolory, Evolúion and Systematics
4(l):13-27.
Carreiras
JMB,
Pereira JMC, Pereira JS. 2006. Estimaüonof
fiee
canopÍy coverin
wergreen oak woodlands using remote sensing. Forest Ecolory and Mamagement 223:45-53.Cavender-Bares
J,Izzn
A,
Robinson R, Lovelock CÊ^ 2009. Changesin
ectomycorrhizal community structureon
two
containerizedoak
hosts across an experimental hydrologic gradient. Mycorrhiza L9:133-142.Faotdes mbientais 4re &cta,m ariquua específca do mamofiutgps
Comandini O, Contu lrd, Rinaldi AC. 2006. An overview
of
CfuÍas eotomycorrhizalfung.
Mycorrhiza l6:381-395.Dahl
FA" Galteland!
GjelsvikR
2008. Statistical modellingof
wildwood mushroom abundance.Scandinaüan Journal of Forest Research 23Q):2a+-V19.
Dúlberg
A, Jonsson L, Nylund 18.1997. Species diversity and distibtltion of biomass above and below ground among ectomycorrhizal fungr in a old-growth Norway spruce forest in south Sweden. Canadian Journal of Botany 15 :1323-1335.Dúlberg
A.
2002. Effectsof
fire on
ectomycorrhizal fungtin
Fennoscandian boneal forests. SilvaFennica
36(l):6H0.
De Bellis T, Kernaghan G, Bradley R, Widden P. 2006. Relationships between stand composition and ectomycorrhizal community structure in boreal mixed-wood foresB, Mcrobial Ecolory 52:11Ç126. DGF. 2001. Inventfuio Florestal Nacional: Porhgal Continental, 3o Rsvisão. Lisboa. Direcç,ão-Geral das
Florestas.233 p.
Eberhardt U, Beker HJ, Vila J, Vesterholt J, Llimona X, Gadjieva R. 2009. Hebelorn species associated
with Crsrn§. Mycological Rssearch
ll3
:153-162.Egerton-Warburton
IÀI,
Querej@JI, Allen MF.
2007. Common mycorrhizal networks provide apotential pathway for the transfer of hydraulically lifted water between plants. Joumal of Experimental Botany 58(6): 1473-1483.
Engola APO, Eilu G, Kabasa JD, Kisovi L, Munishi PKT, Olila D.2007. Ecolory of edible indigenous müshrooms ofthe lake VitoriaBasin (Uganda). Reserirch Journal of Biological Soiences
2(l):62-ó8.
Ferreira
DB.
2001. Evolução da paisagem de montadono
Alentejo Interiorao
longodo
séc)O*
Dinâmica e incidências ambientais. Finisterra 36(72):179-193.Frade B, Alfonso A.2003. Atlas fotogrrófico de los hongos de la Península Ibérica. León. Celarap Ed.
547 p.
Gallaldo
A.
2003. Effect of tree canopy on tho spanial distribution of soil nutientsin
a Mediterraneandehesa. Pedobiologia 47
:ll7
-125.Garibay-Orijel
&
Córdova J, Cifue,ntes J, Valenzuela R, EsEada-TorresA,
KongA.
2009.Integrating wildmuúroorls
use into a model of zustainable management for indigenous community forests. ForestEcolory aod I!{anagement 2582122-13
l.
Gates
GIú,
RatkowskyDÀ
Grove SJ. 2005.A
comparisonof
macrofungiin
yormg silvioulhrralregenemation and maturp forest at the Warra LTER Site in the southern forests of Tasmania. Tasforests
t6-.127-152.
Gomes
L.
1999. Herdade daCoitadiúa
-
Flora e Vegetação. Relatório complementar realizado pela Naturibérica para aEDIÀ
Empnesa de Desenvolvimento e Infra-estuturas do Alqueva.4l
p.GÍiffith
GW, RodericklC 2008. Saprofophic basidiomycetes in grasslands: distibution and fimction.In:Boddy
L,
FrarHandI
Van
Wost P, eds. 2008.Ecolory
of
Saprotnophio Basidiomyoete. Kew. The British Mycologioal Society. Elsevier. p 27 5-297.Guidot A, Debaud J-C, Effosse A, Marmeisse R. 2003. Below-ground distribution and persistence of an
FactoÍes mbiemtaisque &cüm a riqum específica de mrcÍofingos
Ilanse,n PA. 1988. Prediction of macrofungal occrrrence
in
Swediú beech forests from soil and littervariable models. Vegetatio 7
8:3144.
Harhett DC, Wilson GT. 2002. The role
of
mycotrhizasin
plant commtmity süucture and dlmamics: Lessons from grasslands. Plant and §oil 2442319-331.Hobbie EA" Macko
SÀ
ShugaÍtH.
1999. Insights into nitrogen and carbon dlmâmics of ectomycorrhizaland saprotuophic fimgr from isotopic evidence. Oecologia llE:353-360.
Honrubia M.2OO7. Aprovechamientos micológicos
y
desarrollo sostenible.In:
Junta de AndaluciqConsejeria de Medio Ambiente, Ed. World Fungt 2007. Proceedings of the first world confercnce on the conservation and sustainable use of wild fungi. Cordoba, Spain. p 46.
ICN. 2006. Plano Sectorial da RedeNatura 2000. Instituto de Conservação daNaturezq Lisboa. [online] URL: http://www.icn.pt/psrn2000/caÍacterizaoao_valores_naturais/habitatV9340.pdf.
Jansen
AE.
1991, The mycorrhizal status of douglasfir
in The Neúerlands: its relationwith
stand age,regional facto,rs, atmospherio pollutants and
tee
vitality.
Agriculture, Ecosystems and Envircnment35@-3):t9t-208.
Jumpponen
A,
LonesIJÇ
IvÍattox JD, Yaege C. 2010. Massively parallel 454-sequencingof
fungal communitiesn
Querats spp. ectomycorúizas indioates seasonal dynamicsin
urban and rural sites.Molecular Ecolory 19(1
):al-53.
Keizer PJ, Arnolds
E.
1994. Successionof
eotomycorrhizal fungtin
roadside verges planted withcommon oak(Qtwcus robtn L.) in Dr,enúe, theNetherlands. Mycorrhiza 4:147-159.
Keonedy PG,
lnb
AD,
BrunsTD.
2003. Thoreis
high
potentialfor
the
formationof
common mycorrhizal networks betrreen understorey and canopy treesin
a mixed evergreÊn forest. Jotrnalof
Ecolory 91:1071-1080.
Kernaghan
G,IlarperK.
2001. Community structure of ectomycorrhizal fungr across an alpine/subalpineecotone. Ecography 24(2): I 8 1-l 88.
Ikanabetter JUI, Kroeger P. 2001. Ectomyoorrhizal mushroom response to partial cuüing
in
a western hemlock-
western redcedar forest. Canadian Joumal of Forest Research 3l:978-987.KÍânzlin F. 2005. Champignons de Suisse, Tome 6: Russulaceae. Luçerno. Mycologia Ed. 319 p.
Laganà
A,
Loppi S,
h
DominicisV.
1999. Rolationship betnveen environmental factors and the proportionsof
fungpl trophic groupsin
forest ecosystemsof
the oenüal Mediterranean area. ForestEcolory and Management 124:145-151.
Iodge
DJ, Ammfuati JF,O'Dell
E, Mueller GM. 2004. Collecting and describing macrofimgi.In:
G.Mueller G, Bills G, Foster
M
eds. 2004. Biodivosity of Fungi - Inventory and Monitoring Merthods. SanDiego. ElsevierAcademic Press.
p
128-158.Maggi O, Persiani
AM,
CasadoMA,
Pineda FD. 2005. Effects of elwation, slope position and livestock exclusion on microfuirgi isolated from soils ofMediterranean grasslands. Mycologia 97(5):98ç995,Martínez-Aragón
J,
BonetJA
FischerC&
ColinasC.
2007. Productivityof
ectomycorrhizal andseleoted edible saprotrophic fungr
in
pine forestsof
the
pre-$nenees mountains, Spain: h,edictive equations for forest managsment of mycological resources. Forest Ecolory and Management252:239-256.