100
www.scielo.br/rsbmt
Address to: Dra. Kátia Regina Netto dos Santos. Lab. Infecções Hospitalares/ Depto. Microbiologia Médica/IMPG/UFRJ. Av. Carlos Chagas Filho 373, Centro de Ciências da Saúde, Bloco I, Sala I2-10, Cidade Universitária, 21941-590 Rio de Janeiro, RJ, Brasil.
Phone: 55 21 2560-8344/r: 135; Fax: 55 21 2560-8028
e-mail: santoskrn@micro.ufrj.br
Received in 27/02/2011
Accepted in 20/04/2011
Tetracycline and trimethoprim/sulfamethoxazole at clinical
laboratory: Can they help to characterize Staphylococcus
aureus carrying different SCCmec types?
Fernanda Sampaio Cavalcante
[1], Ricardo Pinto Schuenck
[2],
Roberta Mello Ferreira Caboclo
[1],
Dennis de Carvalho Ferreira
[1],
Simone Aranha Nouér
[3],[4]and Kátia Regina Netto dos Santos
[1][1].Laboratório de Infecção Hospitalar, Departamento de Microbiologia Médica, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ. [2].Departamento de Patologia, Universidade Federal do Espírito Santo, Vitória, ES. [3].Faculdade de Medicina, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ. [4]. Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ.
ABSTRACT
Introduction: Methicillin-resistant Staphylococcus aureus (MRSA) can be dificult to detect at the clinical practice. Methods: We analyzed 140 MRSA isolates from inpatients to correlate the antimicrobial susceptibility with the SCCmec types. Results: Type III (n = 63) isolates were more resistant to ciproloxacin, clindamycin, cloramphenicol, erythromycin, gentamicin, and rifampin than type IV (n = 65) ones (p < 0.05). Moreover, type IV isolates were susceptible to tetracycline (100%) and trimethoprim/ sulfamethoxazole (98%), while type III isolates presented resistance to them. Conclusions: In regions where these SCCmec
types are prevalent, the detection of speciic resistant phenotypes could help to predict them, mainly when there are no technical conditions to SCCmec typing.
Keywords: MRSA. SCCmec. Trimethoprim/sulfamethoxazole. Tetracycline. Methicillin-resistant Staphylococcus aureus (MRSA) is
an important pathogen, which presents a penicillin-binding protein 2a (PBP2a) encoded by the mecA gene that is located in a mobile genetic element called staphylococcal cassette chromosome (SCCmec). The analysis of SCCmec has revealed eight different allotypes, which are designated as types I–VIII. Worldwide, most hospital-derived isolates belong to types I, II, and III; most community-derived isolates belong to type IV1. In Brazil, isolates carrying typesIII and IV have been more frequently found in hospitals2-4.
Recently, type IV MRSA isolates emerged in hospitals, and this change in hospital epidemiology had impact on the therapeutic choices for infections related to these strains and their control3. Thus, in this study, we analyzed MRSA isolates from inpatients to verify the possibility to use trimethoprim/ sulfamethoxazole (TMP/STX) and/or tetracycline at the clinical laboratory to predict the SCCmec type on MRSA isolates.
One hundred and forty isolates of MRSA, obtained from patients in four hospitals in Rio de Janeiro City between 2004 and 2007, were randomly selected. These clinical strains were isolated from different sites: nares (57%), blood (8.5%), prosthesis secretion (7.8%), wound (6.4%), bone (4.3%), and
others (16%). Pulsed ield gel electrophoresis (PFGE) analysis of genomic DNA that was performed previously revealed many restriction proiles (68) clustered in 18 different genotypes, demonstrating a high genomic diversity among the isolates.
The genomic DNA was extracted with guanidinium thyocianate5, and the SCCmec typing was performed by
multiplex PCR6. Control strains used in the multiplex PCR were
Mu50 (SCCmec type II), HU25 (SCCmec type III), and HU39 (SCCmec type IV)7.
The susceptibility to 13 antimicrobials (ciprofloxacin, clindamycin, chloramphenicol, erythromycin, gentamicin, linezolid, mupirocin, rifampin, teicoplanin, TMP/STX, tetracycline, tigecycline, and vancomycin) was determined using the disk diffusion method8. The minimum inhibitory
concentration (MIC) determination for oxacillin, TMP/ STX, and tetracycline (Sigma Chemical Company, St Louis, MO, USA) was performed using the broth microdilution method9, which utilized Mueller Hinton broth (Difco
Laboratories, Detroit, Michigan, USA) that does not contain sulfonamide inhibitors. Bacterial resistance was considered when the MIC values were ≥ 4, ≥ 4/76, and ≥ 16µg/mL for oxacillin, TMP/STX, and tetracycline, respectively10.
ATCC 29213 and ATCC 33591 strains were used as methicillin susceptibility and resistance controls, respectively.
Correlation analyses were carried out using Fischer’s exact test. The p-values < 0.05 were considered statistically signiicant.
We found three SCCmec types among 140 MRSA isolates
(II, n = 12; III, n = 63; and IV, n = 65). When type III isolates
were compared with type IV isolates, the susceptibility of the
Revista da Sociedade Brasileira de Medicina Tropical 46(1):100-102, Jan-Feb, 2013 http://dx.doi.org/10.1590/0037-868216062013
101 www.scielo.br/rsbmt
Cavalcante FS et al - S. aureus SCCmec type detection
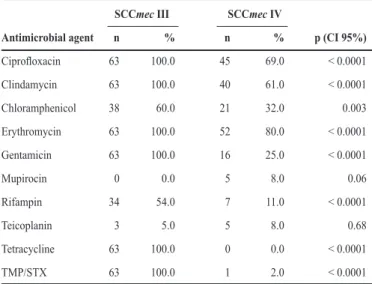
TABLE 1 - Antimicrobial resistance rates in 128 MRSA isolates carrying types III (63 isolates)and IV (65 isolates) SCCmec.
SCCmec III SCCmec IV
Antimicrobial agent n % n % p (CI 95%)
Ciproloxacin 63 100.0 45 69.0 < 0.0001
Clindamycin 63 100.0 40 61.0 < 0.0001
Chloramphenicol 38 60.0 21 32.0 0.003
Erythromycin 63 100.0 52 80.0 < 0.0001
Gentamicin 63 100.0 16 25.0 < 0.0001
Mupirocin 0 0.0 5 8.0 0.06
Rifampin 34 54.0 7 11.0 < 0.0001
Teicoplanin 3 5.0 5 8.0 0.68
Tetracycline 63 100.0 0 0.0 < 0.0001
TMP/STX 63 100.0 1 2.0 < 0.0001
SCCmec: Staphylococcal chromosome cassette mec; MRSA: methicillin-resistant Staphylococcus aureus;TMP/STX: Trimethoprim/sulfamethoxazole; p-values < 0.05 were considered statistically signiicant.
TABLE 2 - Range, MIC50, and MIC90 to oxacillin, TMP/STX, and tetracycline in 128 MRSA isolates carrying types III (63)and IV (65) SCCmec.
SCCmec III (n = 63) SCCmec IV (n = 65)
Antimicrobial agent Range* MIC50* MIC90* Range MIC50 MIC90
Oxacillin 64 - ≥ 256 256 ≥ 256 2 - 256 8 32
TMP/STX 16/304 - ≥ 32/608 ≥32/608 ≥ 32/608 ≤ 0.125/2.375 – ≥ 32/608 ≤ 0.125/2.375 0.25/4.75
Tetracycline 16 - ≥ 32 16 32 0.125 - 0.5 0.25 0.5
SCCmec: Staphylococcal chromosome cassette mec; MRSA: methicillin-resistant Staphylococcus aureus;TMP/STX: Trimethoprim/sulfamethoxazole; MIC50 and MIC90: minimum inhibitory concentration at which 50% and 90% of the strains are inhibited, respectively.*Concentrations are in μg/ml.
latter was statistically higher for all antibiotics (p< 0.05), except for mupirocin (p = 0.06) and teicoplanin (p = 0.68) (Table 1). All type III and 83% of type IV isolates were susceptible to mupirocin, but half (6/12) of type II isolates were resistant to this drug. Teicoplanin resistance was observed for a few isolates from type II (8%), III (5%), and IV (8%). All type III isolates were resistant to tetracycline and TMP/STX, while all type II and IV isolates were susceptible to both drugs, except for one type IV isolate that was resistant to TMP/STX.
As more than 98% of the isolates from type IV were susceptible to TMP/STX and tetracycline disks as compared to that of type III isolates, all strains were submitted to the broth microdilution test to determine the MIC for these drugs, conirm the results, and verify if they could be used as susceptibility markers. For TMP/STX, the values of MIC ranged from 16/304 to ≥ 32/608µg/mL among type III isolates, having MIC90
≥ 32/608µg/mL. However, for those isolates that harbored SCCmec IV, the MIC90 was 0.25/4.75µg/mL, similar to that
found among type II isolates. Only one type IV isolate showed MIC = 32/608µg/mL for TMP/STX. For tetracycline MRSA, isolates with SCCmec III showed MIC90 = 32µg/mL, and all
isolates with SCCmec IV presented MIC between ≤ 0.125 and 0.5µg/mL for this antimicrobial. Type II isolates showed MIC ranging from ≤ 0.125 to 8µg/mL.
The MIC test performed with oxacillin showed values ranging from 2 to > 256µg/mL. Among type III isolates, the MIC90 was ≥ 256µg/mL, whereas type IV isolates showed MIC90
= 32µg/mL for oxacillin. The only strain with MIC = 256µg/ mL to oxacillin was also resistant to TMP/STX. The MIC90 of
type II isolates was 128µg/mL (Table 2).
To ensure a reliable therapy and to control MRSA strains in the hospital environment, a better characterization of isolates presenting different types of SCCmec became necessary. We evaluated the susceptibility proiles of 140 MRSA clinical isolates to verify the possibility of using them as phenotypic markers. The disk diffusion method found that all type IV isolates were susceptible to tetracycline and 98% were susceptible to TMP/STX. Additionally, the MIC tests conirmed these results. Despite this, all type III isolates were resistant to both drugs. Moreover, type III isolates were more resistant to ciproloxacin, clindamycin, cloramphenicol, erythromycin, gentamicin, and rifampin than type IV ones (p < 0.05). Similar results have been found in Brazil2.
Among all type IV MRSA isolates, only one showed high MIC to TMP/STX (≥ 32µg/mL), but it was susceptible to
tetracycline (MIC = 0.5µg/mL). Trindade et al.11 did not ind
any type IV isolate resistant to TMP/STX and suggested the use of this drug as susceptibility marker for these strains. On
the other hand, Amorim et al.12 proposed the use of TMP/STX as resistance marker among isolates with SCCmec III. A recent study involving strains carrying SCCmec IV from hospitalized patients has shown emergence of multiresistant variants among them4. The authors found about 7% isolates resistant to TMP/ STX or tetracycline. However, only 2% of the isolates presented resistance to both antibiotics. Moreover, these isolates presented equal proiles of antimicrobial susceptibility, demonstrating that they belonged to the same genotypic proiles.
102
www.scielo.br/rsbmt
Rev Soc Bras Med Trop 46(1):100-102, Jan-Feb, 2013
REFERENCES
The authors declare that there is no conlict of interest.
CONFLICT OF INTEREST
FINANCIAL SUPPORT
This study is supported by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento Pessoal de Nível Superior, Fundação Universitária José Bonifácio, and Programa de Núcleos de Excelência.
1. Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the
antibiotic era. Nat Rev Microbiol 2009; 7:629-641.
2. Reinert C, McCulloch JA, Watanabe S, Ito T, Hiramatsu K, Mamizuka EM.
Type IV SCCmec found in decade old Brazilian MRSA isolates. Braz J Infect
Dis 2008; 12:213-216.
3. Schuenck RP, Nouér SA, Winter CO, Cavalcante FS, Scotti TD, Ferreira AL, et al. Polyclonal presence of non-multiresistant methicillin-resistant Staphylococcus aureus isolates carrying SCCmec IV in health care-associated infections in a hospital in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis 2009; 64:434-441.
4. Silva-Carvalho MC, Bonelli RR, Souza RR, Moreira S, dos Santos LC, Souza-Conceição M, et al. Emergence of multiresistant variants of the community-acquired methicillin-resistant Staphylococcus aureus lineage ST1-SCCmecIV in 2 hospitals in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis 2009; 65:300-305.
5. Pitcher DG, Sauders NA, Owen RJ. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 1989; 8:151-156.
6. Milheiriço CM, Oliveira DC, De Lencastre H. Update to the multiplex PCR strategy assignment the mec element types in Staphylococcus aureus. Antimicrob Agents Chemother 2007; 51:3374-3377.
7. Vivoni AM, Diep BA, Magalhães ACG, Santos KRN, Riley LW, Sensabaugh GF, et al. Clonal composition of Staphylococcus aureus isolates at a Brazilian university hospital: identiication of international circulating lineages. J Clin Microbiol 2006; 44:1686-1691.
8. Clinical Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. Approved standard. M02-A10. 10th edition. Wayne (PA):
CLSI; 2009a.
9. Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Informational supplement M100-S19. 19th. Wayne (PA):
CLSI; 2009b.
10. Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Informational supplement M100-S20. 20th. Wayne (PA):
CLSI; 2010.
11. Trindade PA, Pacheco RL, Costa SF, Rossi F, Barone AA, Mamizuka EM, et al. Prevalence of SCCmec type IV in nosocomial bloodstream isolates of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2005; 43:3435-3437. 12. Amorim ML, Faria NA, Oliveira DC, Vasconcelos C, Cabeda JC, Mendes AC,
et al. Changes in the clonal nature and antibiotic resistance profiles of methicillin-resistant Staphylococcus aureus isolates associated with spread of
the EMRSA-15 clone in a tertiary care Portuguese hospital. J ClinMicrobiol
2007; 45:2881-2888.
13. Bordon J, Master RN, Clark RB, Duwuri P, Karlowsky JA, Ayesu K, et al. Methicillin-resistant Staphylococcusaureus resistance to non–β-lactam antimicrobials in the United States from 1996 to 2008. Diagn Microbiol Infect Dis 2010; 67:395-398
14. Ruhe JJ, Menon A. Tetracyclines as an oral treatment option for patients with community onset skin and soft tissues infections caused by methicillin-resistant
Staphylococcus aureus. Antimicrob Agents Chemother 2007; 51:3298-3303. 15. Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC.
Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin Infect Dis 2008; 46:668-674.
Susceptibility to tetracycline and TMP/STX was also observed for type II isolates, which showed susceptibility similar to type IV isolates. However, in Brazil, isolates carrying SCCmec II are rare2-4.