Meta-analysis
Risk factors and peripheral biomarkers for
schizophrenia spectrum disorders: an
umbrella review of meta-analyses
Belbasis L, K€ohler CA, Stefanis N, Stubbs B, van Os J, Vieta E, Seeman MV, Arango C, Carvalho AF, Evangelou E. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses
Objective: This study aimed to systematically appraise the meta-analyses of observational studies on risk factors and peripheral biomarkers for schizophrenia spectrum disorders.
Methods: We conducted an umbrella review to capture all meta-analyses and Mendelian randomization studies that examined associations between non-genetic risk factors and schizophrenia spectrum disorders. For each eligible meta-analysis, we estimated the summary effect size estimate, its 95% confidence and prediction intervals and the I2metric. Additionally, evidence for small-study effects and excess significance bias was assessed.
Results: Overall, we found 41 eligible papers including 98 associations. Sixty-two associations had a nominally significant (P-value<0.05) effect. Seventy-two of the associations exhibited large or very large between-study heterogeneity, while 13 associations had evidence for small-study effects. Excess significance bias was found in 18
associations. Only five factors (childhood adversities, cannabis use, history of obstetric complications, stressful events during adulthood, and serum folate level) showed robust evidence.
Conclusion: Despite identifying 98 associations, there is only robust evidence to suggest that cannabis use, exposure to stressful events during childhood and adulthood, history of obstetric complications, and low serum folate level confer a higher risk for developing schizophrenia spectrum disorders. The evidence on peripheral biomarkers for schizophrenia spectrum disorders remains limited.
L. Belbasis
1, C. A. K
€
ohler
2,
N. Stefanis
3, B. Stubbs
4,5,
J. van Os
6,7, E. Vieta
8,
M. V. Seeman
9, C. Arango
10,
A. F. Carvalho
11,12,
E. Evangelou
1,131
Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioannina, Greece,
2
Translational Psychiatry Research Group, Department of Clinical Medicine, Federal University of Ceara Medical School, Fortaleza, Brazil,3Department of Psychiatry,
Eginition Hospital, National and Kapodistrian University of Athens Medical School, Athens, Greece,4Department
of Physiotherapy, South London and Maudsley NHS Foundation Trust, London, UK,5Department of Health
Service and Population Research, Institute of Psychiatry, King’s College London, London, UK,6Department of
Psychiatry, Brain Center Rudolf Magnus, University Medical Centre Utrecht, Utrecht, The Netherlands,
7Department of Psychosis Studies, Institute of
Psychiatry, King’s College London, London, UK,8Bipolar
Disorder Unit, Institute of Neuroscience, University of Barcelona, IDIBAPS and CIBERSAM, Barcelona, Spain,
9
Institute of Medical Science, University of Toronto, Toronto, ON, Canada,10Department of Child and Adolescent Psychiatry, University Hospital Gregorio Mara~non, Complutense University of Madrid Medical School, CIBERSAM, Madrid, Spain,11Department of
Psychiatry, University of Toronto, Toronto, ON, Canada,
12Centre for Addiction & Mental Health (CAMH),
Toronto, ON, Canada and13Department of Epidemiology
and Biostatistics, School of Public Health, Imperial College London, London, UK
Key words: epidemiology; meta-analysis; psychosis; risk factors; schizophrenia
Evangelos Evangelou, Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioannina, Greece.
E-mail: vangelis@cc.uoi.gr
Summations
•
This large-scale umbrella review assessed 98 associations of non-genetic risk factors withschizophrenia that were examined in meta-analyses and mendelian randomization studies.
•
Even though 62 associations were nominally statistical significant (P-value<0.05) most of themeta-analyses showed large heterogeneity, evidence of small-study effects and excess of significant findings.
•
Cannabis use, childhood adversities, history of obstetric complications, stressful events duringadult-hood and serum folate level showed robust evidence of association.
Considerations
•
We considered only associations that have been examined in a meta-analysis, therefore potentiallyinteresting risk factors that have been assessed in single studies, such as socioeconomic status, have not been considered.
•
The associations of adult stressful events, obstetric complications and serum folate level were notexamined in prospective studies.
Introduction
Schizophrenia is a mental disorder with a med-ian lifetime prevalence of 4.0 per 1000 individu-als (1). Its onset characteristically occurs in adolescence or early adulthood (2). A diagnosis of schizophrenia is associated with significant disability and with premature all-cause mortality (3, 4). The death rate is predominantly due to suicide early in the course of the disorder and cardio-metabolic disturbances later in life (1, 4, 5). Accumulating evidence indicates that a com-plex interplay of genetic and environmental risk factors probably underpins the emergence of schizophrenia (6).
Over the past twenty-five years, the field has witnessed an explosion in observational studies investigating putative environmental risk factors. Two previously published field-wide systematic reviews found that few non-genetic risk factors were supported by good quality evidence (7, 8). These reviews, however, did not provide a quanti-tative appraisal of epidemiological credibility nor did they explore potential biases (7, 8).
Aims of the study
In this study, we conducted an umbrella review of meta-analyses on risk factors and peripheral biomarkers for schizophrenia and other psychotic disorders. We assessed the potential for bias in this literature, and we identified associations sup-ported by the most robust epidemiological evi-dence. The potential causal association between risk factors and schizophrenia was examined by systematically searching for Mendelian random-ization (MR) studies, a novel epidemiological
study design allowing for better control of reverse causality and confounding (9).
Methods
This study is an umbrella review, which is a sys-tematic collection and critical evaluation of multi-ple systematic reviews and meta-analyses on a specific research topic (10). Similar efforts have been published for other chronic medical condi-tions. (11–16)
Search strategy and eligibility criteria
We systematically searched PubMed from inception to January 5, 2017 to identify meta-analyses of observational studies examining associations of schizophrenia spectrum disorders in adults and either environmental (i.e., non-genetic) risk factors or peripheral biomarkers. The following search algorithm was used: (schizophrenia OR psychosis) AND (‘systematic review*’ OR meta-analys*). Peripheral biomarkers were defined as biomarkers measurable in serum/plasma, saliva, or urine. Based on a definition by the World Health Organization (17), a risk factor was defined as any attribute, char-acteristic or exposure of an individual that increases the likelihood of developing a disease or injury.
guage restrictions were not applied. When more than one meta-analysis was available on the same association, we included the one with the largest number of non-overlapping prospective observa-tional studies.
Moreover, we performed an additional search on PubMed to systematically capture MR studies for schizophrenia spectrum disorders using the fol-lowing search algorithm: ‘mendelian randomiza-tion’ OR ‘mendelian randomisarandomiza-tion’. An MR study is an epidemiological study design using tri-angulation methods to address the causal relation-ship between an exposure and an outcome in observational studies (19). It is an application of the technique of instrumental variables with geno-type acting as an instrument for the exposure of interest. The term ‘Mendelian randomization’ is a method of using measured variation in genes of known function to examine the effect of an expo-sure on a disease (20). We did not include MR studies that used only summary-level data from candidate gene association studies.
Initial title screening was performed by one researcher (LB), and two independent researchers extracted the data (LB and EE). Discrepancies were discussed, and consensus was reached.
Data extraction
From each meta-analysis, we abstracted informa-tion on first author, year and journal of publica-tion, examined risk factors, number and study design of component studies, and study-specific risk estimates (i.e., risk ratio, odds ratio, hazard ratio, Cohen’s d, or Hedges’ g). We additionally recorded whether the eligible meta-analyses per-formed a qualitative assessment of component studies based on predefined quality scores or scales, such as the Newcastle–Ottawa scale. For
each eligible meta-analysis, we examined whether component studies included overlapping samples; in such circumstances, we considered only the component study with the largest sample size.
From each MR study, we extracted the follow-ing information: first author, year and journal of publication, sample size, effect size metric, causal effect size estimate along its 95% confidence interval (CI), P-value, study design, and genetic instrument.
Statistical analysis
Measures of standardized mean difference (i.e., Cohen’s d and Hedges’ g) were transformed to odds ratio. For each meta-analysis, we estimated
using both fixed-effect and random-effects models (21, 22). We also estimated the 95% prediction interval, which accounts for between-study hetero-geneity and evaluates the uncertainty for the effect that would be expected in a new study addressing that same association (23, 24). For the largest study of each meta-analysis, we estimated the SE of the effect size to examine whether it was less than 0.10.
Between-study heterogeneity was quantified using the I2 metric. I2 ranges between 0% and 100% and quantifies the variability in effect esti-mates that is due to heterogeneity rather than to sampling error (25). Values exceeding 50% or 75% are considered to represent large or very large heterogeneity respectively.
We assessed small-study effects using the Egger’s regression asymmetry test (26, 27). A P-value <0.10 combined with a more conservative effect in the largest study than in random-effects meta-ana-lysis was judged to provide adequate evidence for small-study effects. We also applied the excess sta-tistical significance test, which evaluates whether there is a relative excess of statistically significant findings in the published literature (28). It is a sta-tistical test that assesses whether the observed number of studies with statistically significant results is larger than expected. Excess statistical significance was claimed at two-sided P-value <0.10 (29).
Assessment of epidemiological credibility
To identify associations with robust evidence, we applied a set of methodological criteria, which have been previously applied in other research fields (11–16). For evidence to be convincing,
>1000 cases were required as well as a highly signif-icant association (P-value<10 6by random-effects model), no evidence of small-study effects or excess significance bias, a 95% prediction interval exclud-ing the null value and no large between-study heterogeneity (I2< 50%). Highly suggestive evi-dence required >1000 cases, a highly significant association (P-value<10 6 by random-effects model) and a statistically significant effect in the largest study. Suggestive evidence required >1000 cases and P-value <0.001 by the random-effects model, whereas the remaining nominally signifi-cant risk factors (P-value<0.05) were supported by weak evidence.
evidence from prospective cohort studies only. An additional sensitivity analysis was performed for component studies that applied a structured diag-nostic interview for case definition.
Results
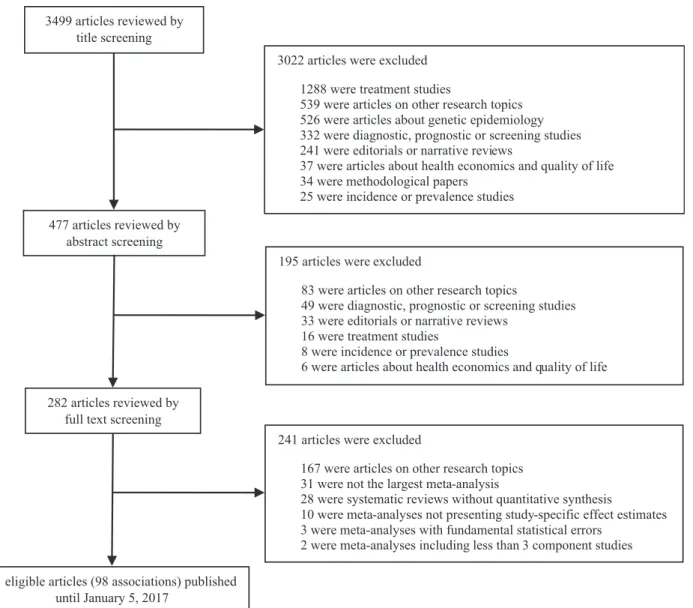
Overall, we searched 3499 articles and 41 articles fulfilled the eligibility criteria (Fig. 1). During full-text screening, 31 articles were excluded, because a larger meta-analysis examining the same associa-tion was available, whereas 28 systematic reviews were excluded because there was no quantitative synthesis of the evidence. The 41 eligible papers examined a total of 98 associations (41 environ-mental factors and 57 peripheral biomarkers).
Ten of the 41 papers (24%) reported a qualita-tive assessment of component studies through a standardized tool. Seven of these used the
Newcastle–Ottawa scale, and three papers used a
scoring system based on the STROBE statement (30). Six additional papers (15%) used a tailor-made assessment tool.
Environmental risk factors
Overall, 41 environmental risk factors were exam-ined for an association with schizophrenia. Eleven associations (stressful events during adulthood, Borna disease virus infection, general academic achievement, handedness, cannabis use, tobacco smoking, traumatic brain injury, obstetric compli-cations, advanced paternal age, childhood adversi-ties, and urbanicity) were studied in at least 1000 cases.
Thirty associations (73%) presented a nomi-nally significant summary effect, 21 associations remained significant at P-value <0.001, but only 3499 articles reviewed by
title screening
477 articles reviewed by abstract screening
41 eligible articles (98 associations) published until January 5, 2017
282 articles reviewed by full text screening
241 articles were excluded
167 were articles on other research topics 31 were not the largest meta-analysis
28 were systematic reviews without quantitative synthesis
10 were meta-analyses not presenting study-specific effect estimates 3 were meta-analyses with fundamental statistical errors
2 were meta-analyses including less than 3 component studies 3022 articles were excluded
1288 were treatment studies
539 were articles on other research topics 526 were articles about genetic epidemiology 332 were diagnostic, prognostic or screening studies 241 were editorials or narrative reviews
37 were articles about health economics and quality of life 34 were methodological papers
25 were incidence or prevalence studies
195 articles were excluded
83 were articles on other research topics
49 were diagnostic, prognostic or screening studies 33 were editorials or narrative reviews
16 were treatment studies
8 were incidence or prevalence studies
6 were articles about health economics and quality of life
the random-effects model (Table S1). Only four associations (stressful events during adulthood, cannabis use, childhood adversities, and obstetric complications) had a P-value <10 6 and were studied in at least 1000 cases. Seven associations (23%) were reported in meta-analyses that included a largest study with a SE <0.10 (stress-ful events during adulthood, general academic achievement, handedness, tobacco smoking, trau-matic brain injury, paternal age, and urbanicity). The result of the largest study was more conser-vative than the summary result in 19 associations (46%).
In twenty associations (49%), small or moderate between-study heterogeneity was found (I2< 50%). In 10 meta-analyses (24%), 95% pre-diction intervals excluded the null value. Five asso-ciations (handedness, childhood social withdrawal, Toxoplasma gondii infection, traumatic brain injury, and cooperativeness) were reported in meta-analyses with evidence for small-study effects. Six associations (handedness, childhood social withdrawal, Toxoplasma gondii infection, cooperativeness, openness, and agreeableness) were reported in meta-analyses with evidence for excess significance bias (Table S1). The excess sta-tistical significance test was not performed in four associations (parental communication deviance, tobacco smoking, paternal age, and urbanicity), because the study-specific sample sizes were not available and power calculations could not be per-formed.
Peripheral biomarkers
Fifty-seven associations of peripheral biomarkers and risk for schizophrenia were identified. Twelve associations (21%), pertaining to serum BDNF, serum vitamin B12, serum CRP, serum interleukin-6, serum antigliandin IgA and IgG, serum anti-TTG2 IgA, serum leptin, serum folate, serum TNF-a, serum morning cortisol, and plasma
adi-ponectin, were studied in a total sample of more than 1000 cases.
Thirty-two of 57 associations (56%) presented a nominally significant effect, 16 associations remained significant atP-value<0.001, and four of these (serum S100B, serum DPA, serum folate, and plasma TAS) had aP-value<10 6(Table S2). Only the association of schizophrenia with serum folate level had a P-value <10 6 and included a total number of cases greater than 1000. Also, only the association with serum TNF-a had largest
study with a SE <0.10. The effect of the largest
effect in 35 associations (61%).
Six associations (11%) presented small or mod-erate between-study heterogeneity (I2 < 50%), and 42 associations (77%) had anI2 > 75%. Only the association with impaired glucose tolerance had a 95% prediction interval excluding the null value, but this association was supported by a trivial number of cases. Thirteen associations (23%) rested on evidence suggestive of small-study effects, and 21 associations (37%) showed evidence for excess statistical significance (Table S2).
Assessment of epidemiological credibility
By applying a standardized procedure, we found one association supported by convincing evidence (i.e., history of obstetric complications). Also, we identified four associations (4%) supported by highly suggestive evidence. These associations were as follows: exposure to stressful events during adulthood, exposure to childhood adversities, can-nabis use, and serum folate level. Seven associa-tions (7%) had suggestive evidence (urbanicity, Borna disease virus infection, advanced paternal age, tobacco smoking, serum interleukin-6, serum BDNF, and serum CRP). The associations supported by convincing, highly suggestive, and suggestive evidence are presented in Table 1. Fifty-one associations (52%) presented weak evidence for an association with schizophrenia. The remain-ing associations had a non-significant effect (P-value>0.05).
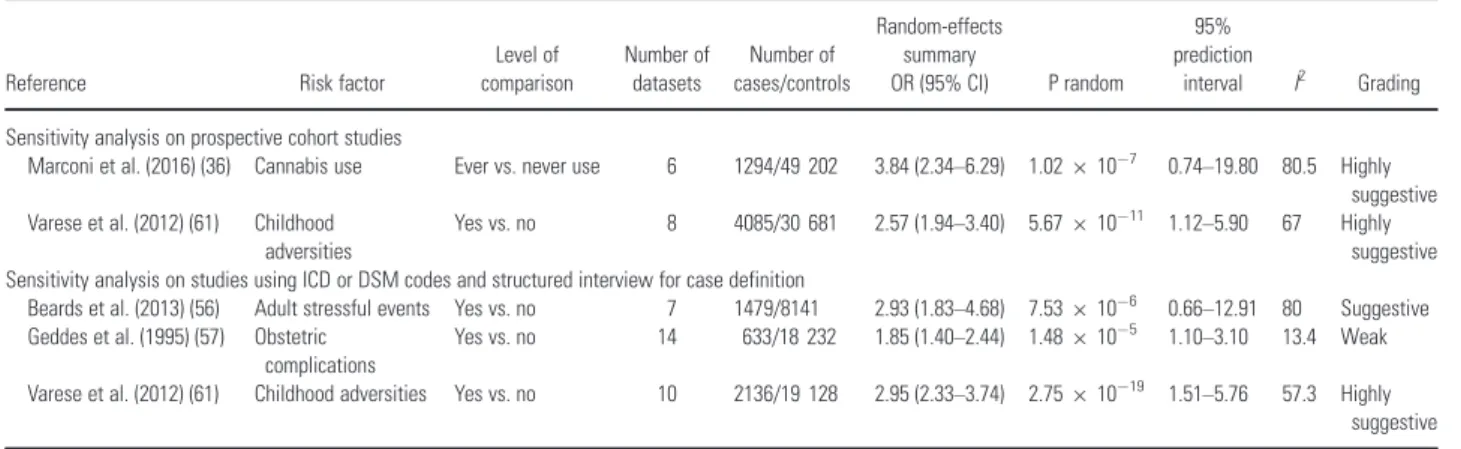
In the sensitivity analysis that included only prospective cohort studies, the evidence for the association of cannabis use and exposure to child-hood adversities with schizophrenia risk remained highly suggestive (Table 2). The association of exposure to stressful events during adulthood, his-tory of obstetric complications, and serum folate level were not studied in any prospective cohort study. In the sensitivity analysis that included only component studies using a structured diagnostic interview, the association of childhood adversities remained highly suggestive, the association of stressful events during adulthood became sugges-tive only, and the association of obstetric compli-cations became weak (Table 2). For cannabis use and serum folate level, fewer than three component studies using a structured diagnostic interview were available.
Mendelian randomization studies in schizophrenia
Table 2. Sensitivity analyses among associations with convincing and highly suggestive evidence
Reference Risk factor
Level of comparison
Number of datasets
Number of cases/controls
Random-effects summary
OR (95% CI) P random
95% prediction
interval I2 Grading
Sensitivity analysis on prospective cohort studies
Marconi et al. (2016) (36) Cannabis use Ever vs. never use 6 1294/49 202 3.84 (2.34–6.29) 1.02910
7 0.74
–19.80 80.5 Highly
suggestive Varese et al. (2012) (61) Childhood
adversities
Yes vs. no 8 4085/30 681 2.57 (1.94–3.40) 5.67910 11 1.12–5.90 67 Highly
suggestive Sensitivity analysis on studies using ICD or DSM codes and structured interview for case definition
Beards et al. (2013) (56) Adult stressful events Yes vs. no 7 1479/8141 2.93 (1.83–4.68) 7.53910
6 0.66
–12.91 80 Suggestive
Geddes et al. (1995) (57) Obstetric complications
Yes vs. no 14 633/18 232 1.85 (1.40–2.44) 1.48910 5 1.10–3.10 13.4 Weak
Varese et al. (2012) (61) Childhood adversities Yes vs. no 10 2136/19 128 2.95 (2.33–3.74) 2.75910 19 1.51–5.76 57.3 Highly
suggestive
CI, confidence interval; OR, odds ratio.
Table 1. Characteristics of associations with convincing, highly suggestive, and suggestive evidence
Reference Risk factor
Level of comparison
Number of cases/controls
Number of datasets
Random-effects summary
OR (95% CI) P random
95% prediction
interval I2
Small-study effects/Excess
significance bias Grading
Arias et al. (2012) (45)
BDV infection Yes vs. no 1930/2150 17 2.23 (1.47–3.39) 1.77910 4 0.76–6.59 36.4 No/No Suggestive
Beards et al. (2013) (56)
Adult stressful events
Yes vs. no 2218/17 628 13 3.11 (2.31–4.18) 5.21910 14 1.19–8.13 74.8 No/No Highly
suggestive Geddes and
Lawrie (1995) (57)
Obstetric complications
Yes vs. no 1000/19 101 18 1.97 (1.55–2.50) 2.87910
8 1.16
–3.36 18.9 No/No Convincing
Goldsmith et al. (2016) (58)
Serum IL-6 High levels vs. low levels
1398/1391 29 2.61 (1.55–4.40) 3.25910 4 0.15–44.49 91.6 No/Yes Suggestive
Green et al. (2011) (59)
Serum BDNF High levels vs. low levels
1189/969 17 0.34 (0.18–0.62) 5.29910
4 0.02
–4.66 91.5 No/No Suggestive
Gurillo et al. (2015) (60)
Tobacco smoking
Ever vs. never NA/NA 17 2.34 (1.65–3.33) 2.15910 6 0.63–8.75 88.3 No/NA Suggestive
Inoshita et al. (2016) (53)
Serum CRP High levels vs. low levels
1664/3070 15 2.96 (1.75–5.01) 5.28910 5 0.34–25.43 93.7 No/No Suggestive
Marconi et al. (2016) (36)
Cannabis use Ever vs. never 4036/62 780 10 3.90 (2.84–5.35) 3.41910 17 1.33–11.45 81.7 No/No Highly
suggestive Torrey et al.
(2009) (43)
Paternal age >35 years vs.
<35 years
NA/NA 10 1.28 (1.11–1.48) 9.11910 4 0.82–1.99 74.9 No/NA Suggestive
Varese et al. (2012) (61)
Childhood adversities
Yes vs. no 7738/67 009 34 2.80 (2.34–3.34) 5.33910
30 1.22
–6.42 72.5 No/No Highly
suggestive Vassos et al.
(2012) (42)
Urbanicity Highest vs. lowest category
NA/NA 8 2.39 (1.63–3.51) 9.37910 6 0.57–9.93 99.1 No/NA Suggestive
Wang et al. (2016) (62)
Serum folate High levels vs. low levels
1773/1930 26 0.37 (0.27–0.51) 1.23910 9 0.08–1.73 83.4 No/No Highly
suggestive
BDV, Borna disease virus; BDNF, brain-derived neurotrophic factor; CI, confidence interval; CRP, C-reactive protein; IL-6, interleukin-6; NA, not available; OR, odds ratio.
Table 3. Characteristics of Mendelian randomization studies for schizophrenia
Author, Year Risk factor Level of comparison
Genetic instrument
Number of SNPs in genetic instrument
Number of cases
Effect size metric
Causal effect
size (95% CI) P-value
Gage et al. (2016) (32) Cannabis use Users vs. non-users GRS 12 36 989 OR 1.01 (0.93–1.10) NS
Prins et al. (2016) (33) Serum CRP Per 1 mg/l increase in ln(CRP) GRS 3 34 241 OR 0.90 (0.82–0.99) NS
Prins et al. (2016) (33) Serum CRP Per 1 mg/l increase in ln(CRP) GRS 15 25 629 OR 0.86 (0.79–0.94) 0.001
Taylor et al. (2016) (35) Serum 25(OH)D Per 10% increase GRS 4 34 241 OR 0.99 (0.97–1.02) NS
Vaucher et al. (2017) (31) Cannabis use Users vs. non-users GRS 10 34 241 OR 1.41 (1.09–1.83) NR
Wium-Andersen et al. (2014) (34)
Serum CRP Highest vs. lowest quartile GRS 4 168 HR 1.40 (0.46–4.25) NS
CRP (33, 34), and serum vitamin D (35) with risk for schizophrenia (Table 3). All MR studies con-structed a genetic risk score as an instrumental variable. Three MR studies used summary-level data, one MR study used individual-level data, whereas one MR study used both summary-level and individual-level data. One MR study was based on a trivial number of schizophrenia cases (34), whereas the remaining MR studies included at least 25 000 cases. A significant protective effect was observed for higher serum level of CRP, and a non-significant effect was found for serum level of vitamin D. The MR studies on cannabis use showed conflicting results.
Discussion
We critically appraised almost 100 associations between risk factors and peripheral biomarkers for psychotic disorders in the schizophrenia spectrum. More than two-thirds of the examined associations presented a nominally significant effect, but most of these associations were based on weak evidence due to either a small number of cases or aP-value close to the significance threshold. This is a com-mon phenomenon which has also been observed in previously published umbrella reviews on other chronic conditions (11–15). Our analysis indicated that the association of schizophrenia with exposure to physical or psychological adversities during childhood and adulthood and with cannabis use was based on robust evidence and showed a large effect size (i.e., an odds ratio >2). Furthermore, a history of obstetric complications was associated with increased risk for schizophrenia in offspring. Also, serum folate level was lower in patients with schizophrenia than in healthy controls, and this association was also supported by robust evidence. Cannabis use was associated with a very large risk for schizophrenia spectrum disorders with no evidence of bias. Large between-study heterogene-ity was observed, but the 95% prediction interval excluded the null value. Several methodological aspects deserve consideration. First, the degree of cannabis exposure across studies varied, whereas available evidence indicates that heavy use may confer a higher risk for psychosis than light use (36). In addition, the increasing availability of high-potency cannabis and synthetic cannabinoids could modify the magnitude of this association (37). Cannabis use is associated with motivation and cognitive impairments (38), where there is evi-dence indicating more pronounced effects if canna-bis use starts in adolescence (38). Such factors may therefore influence the role of cannabis use as a
published MR studies on this association showed conflicting results (31, 32)
Exposure to physical and psychological adversi-ties during childhood showed highly suggestive evi-dence for an increased risk for schizophrenia. This meta-analysis presented a very large heterogeneity estimate, but the 95% prediction interval excluded the null value. Also, this association remained sig-nificant in our sensitivity analyses. Exposure to childhood adversities has been linked with later drug use disorders, indicating a possible correla-tion between the two highly suggestive risk factors (39, 40).
Furthermore, a history of obstetric complica-tions was associated with an almost twofold increase in risk for schizophrenia in offspring. This meta-analysis presented a highly significant effect, a small between-study heterogeneity, and a 95% prediction interval excluding the null value, whereas evidence for small-study effects and excess significance bias was absent. This finding is aligned with the neurodevelopmental hypothesis for schizophrenia, which supports that risk factors for schizophrenia affect early neurodevelopment dur-ing pregnancy (41).
Traditional risk factors, such as urban environ-ment (42), advanced paternal age (43), a history of traumatic brain injury (44), and perinatal infec-tions (45), did not present robust evidence as risk factors for schizophrenia spectrum disorders. This observation does not mean that these factors or other factors and exposures that are difficult to be measured should be ignored or ruled out from fur-ther research. For example, in the case of urban environment, nearly all the criteria for highly sug-gestive evidence were fulfilled, but theP-value was slightly larger than 10 6. Further well-designed prospective studies may provide convincing evi-dence for an association with schizophrenia spec-trum disorders.
Migration status is also considered a traditional risk factor for schizophrenia, and our literature search captured three meta-analyses that could be considered eligible and examined this association (46–48). These studies showed significant
course, observed heterogeneity may rather point at the necessity to explore underlying sources of vari-ation that may be genuine, especially for findings that have been consistently replicated in the past.
Fifty-seven biomarkers for schizophrenia spec-trum disorders have been studied in meta-analyses of observational studies. The identification of robust biomarkers associated with the schizophre-nia spectrum could lead to a better understanding of the pathophysiology and at the same time could offer clinicians a valuable tool for diagnosis in the emerging framework of precision psychiatry (49). However, in most cases, the sample size of compo-nent studies was small, or theP-value was close to the nominal significance threshold. Similar findings were observed in umbrella reviews on peripheral biomarkers for depression and bipolar disorder (50, 51).
Serum folate level was significantly lower in patients with schizophrenia than in healthy con-trols, and this association was supported by highly suggestive evidence. A field synopsis for genetic associations in schizophrenia has shown strong epidemiological credibility between rs1801131, a polymorphism in the methylene tetrahydrofolate reductase gene, and risk for schizophrenia (52). However, the evidence for the link between serum folate level and schizophrenia is based on case– control studies and there is a lack of prospective cohort studies supporting this association; there-fore, results should be interpreted with caution.
Increased serum CRP level presented suggestive evidence for an increased risk for schizophrenia spectrum disorders (53). In contrast, the available MR study indicated a causal protective effect for elevated levels of serum CRP level (33). Although there is accumulating evidence that peripheral immune activation could play a pathophysiological role in schizophrenia, the results of the MR study questions whether the observed CRP elevation in schizophrenia is a consequence of illness activity rather than a risk factor for schizophrenia (33). Previous studies could have been affected by potential biases regarding the causes of elevated CRP level in patients with schizophrenia, such as reverse causality (33).
Limitations
Our umbrella review has some limitations. We did not conduct a qualitative assessment of component studies as this should be performed in the original systematic reviews and meta-analyses through standardized tools, such as Newcastle–Ottawa
scale. However, only a small proportion of eligible meta-analyses included a standardized qualitative
assessment of component studies. Also, we consid-ered only associations that have been examined in a meta-analysis, so we did not include potentially important factors such as socioeconomic status. Furthermore, psychotic disorders are a very hetero-geneous group of psychiatric conditions, and the combination of studies on various schizophrenia spectrum disorders in the same meta-analysis could be a potential source of between-study heterogene-ity. However, it was not feasible to identify the component studies that are focused exclusively on schizophrenia, given that this process is beyond the scope of an umbrella review.
To conclude, our umbrella review found a wide range of risk factors and biomarkers for schizophrenia spectrum disorders. Although the majority of associations were statistically signifi-cant atP-value<0.05, only exposure to childhood adversities and cannabis use were supported by robust evidence. The associations of adult stressful events, obstetric complications, and serum folate level with risk for schizophrenia presented convinc-ing or highly suggestive evidence, but these associa-tions were not examined in prospective studies. We have shown that the contribution of environmental factors and biomarkers to the development of psy-chotic disorders remains incompletely elucidated. Both child maltreatment and cannabis use are potentially modifiable leading to reduced incidence of schizophrenia (54, 55). Randomized evidence, however, is still needed before establishing a causal association between child maltreatment or canna-bis use and schizophrenia spectrum disorders.
References
1. McGrathJ,SahaS,ChantD,WelhamJ. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 2008;30:67–76.
2. Messias EL, Chen C-Y, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am 2007;30:323–338.
3. MurrayCJL,Vos T,Lozano R et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380:2197–2223.
4. HjorthøjC,St€urupAE,McGrathJJ,NordentoftM. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 2017;4:295–301.
5. WalkerER,McGee RE,DrussBG. Mortality in mental disorders and global disease burden implications: a sys-tematic review and meta-analysis. JAMA Psychiatry 2015;72:334–341.
2014;40:729–736.
7. MathesonSL,ShepherdAM,LaurensKR,CarrVJ. A sys-tematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophr Res 2011;133:133–142.
8. LaurensKR,LuoL,MathesonSL et al. Common or dis-tinct pathways to psychosis? A systematic review of evi-dence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum dis-orders and affective psychoses. BMC Psychiatry 2015;15:205.
9. Smith GD, Ebrahim S. Mendelian randomization: pro-spects, potentials, and limitations. Int J Epidemiol 2004;33:30–42.
10. IoannidisJPA. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment net-works and multiple treatments meta-analyses. CMAJ 2009;181:488–493.
11. BelbasisL,BellouV,EvangelouE,IoannidisJPA,Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015;14:263–273.
12. BellouV, Belbasis L,TzoulakiI, EvangelouE, Ioannidis JPA. Environmental risk factors and Parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord 2016;23:1–9.
13. BellouV,BelbasisL,TzoulakiI,MiddletonLT,Ioannidis JPA, EvangelouE. Systematic evaluation of the associa-tions between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement 2017;13:406–418.
14. Belbasis L, Bellou V,Evangelou E. Environmental risk factors and amyotrophic lateral sclerosis: an umbrella review and critical assessment of current evidence from systematic reviews and meta-analyses of observational studies. Neuroepidemiology 2016;46:96– 105.
15. BelbasisL,StefanakiI,StratigosAJ,EvangelouE. Non-genetic risk factors for cutaneous melanoma and ker-atinocyte skin cancers: an umbrella review of meta-ana-lyses. J Dermatol Sci 2016;84:330–339.
16. BelbasisL,SavvidouMD,KanuC,EvangelouE,Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-ana-lyses. BMC Med 2016;14:147.
17. World Health Organization. Health topics: Risk factors. http://www.who.int/topics/risk_factors/en/
18. Fusar-Poli P, Tantardini M, De Simone S et al. Decon-structing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry 2016;40:65–75.
19. Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol 2017: dyw314.
20. LawlorDA,HarbordRM,SterneJAC,TimpsonN,Davey SMITHG. Mendelian randomization: using genes as
instru-ments for making causal inferences in epidemiology. Stat Med 2008;27:1133–1163.
21. DerSimonian R,Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188.
22. LauJ,IoannidisJP,SchmidCH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–826. 23. HigginsJPT,ThompsonSG,SpiegelhalterDJ. A
re-evalua-tion of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–159.
should be expected and appropriately quantified. Int J Epidemiol 2008;37:1158–1160.
25. HigginsJPT,ThompsonSG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558.
26. SterneJAC,SuttonAJ,IoannidisJPA et al. Recommenda-tions for examining and interpreting funnel plot asymme-try in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002.
27. EggerM,Davey SmithG,SchneiderM,MinderC. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634.
28. IoannidisJPA, TrikalinosTA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245–253. 29. IoannidisJPA. Clarifications on the application and
inter-pretation of the test for excess significance and its exten-sions. J Math Psychol 2013;57:184–187.
30. vonElmE,AltmanDG,EggerM et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observa-tional studies. J Clin Epidemiol 2008;61:344–349.
31. VaucherJ,KeatingBJ, LasserreAM et al. Cannabis use and risk of schizophrenia: a Mendelian randomization study. Mol Psychiatry 2017. https://doi.org/10.1038/mp. 2016.252
32. GageSH,JonesHJ,BurgessS et al. Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychol Med 2017;47:971–980.
33. PrinsBP,AbbasiA,WongA et al. Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium mendelian randomization study. PLoS Med 2016;13: e1001976.
34. Wium-AndersenMK,ØrstedDD, NordestgaardBG. Ele-vated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: a prospective study. Schizophr Bull 2014;40:1117–1127. 35. TaylorAE,BurgessS,WareJJ et al. Investigating
causal-ity in the association between 25(OH)D and schizophre-nia. Sci Rep 2016;6:26496.
36. MarconiA,Di FortiM,LewisCM,MurrayRM,VassosE. Meta-analysis of the association between the level of can-nabis use and risk of psychosis. Schizophr Bull 2016;42:1262–1269.
37. MurrayRM,QuigleyH,QuattroneD,EnglundA,Di Forti M. Traditional marijuana, high-potency cannabis and syn-thetic cannabinoids: increasing risk for psychosis. World Psychiatry 2016;15:195–204.
38. VolkowND,SwansonJM,EvinsAE et al. Effects of can-nabis use on human behavior, including cognition, moti-vation, and psychosis: a review. JAMA Psychiatry 2016;73:292–297.
39. McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitiza-tion hypothesis in a populasensitiza-tion-based sample of adults. Psychol Med 2010;40:1647–1658.
40. MyersB,McLaughlinKA,WangS, BlancoC,SteinDJ. Associations between childhood adversity, adult stressful life events, and past-year drug use disorders in the National Epidemiological Study of Alcohol and Related Conditions (NESARC). Psychol Addict Behav 2014;28:1117–1126.
42. Vassos E,PedersenCB,MurrayRM,Collier DA,Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull 2012;38:1118–1123. 43. TorreyEF,BukaS,CannonTD et al. Paternal age as a risk
factor for schizophrenia: how important is it? Schizophr Res 2009;114:1–5.
44. MolloyC,ConroyRM,Cotter DR,CannonM. Is trau-matic brain injury a risk factor for schizophrenia? A meta-analysis of case-controlled population-based studies. Schi-zophr Bull 2011;37:1104–1110.
45. AriasI,Sorlozano A,Villegas E et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res 2012;136:128–136.
46. Bourque F, van der Ven E, Malla A. A meta-analysis of the risk for psychotic disorders among first- and sec-ond-generation immigrants. Psychol Med 2011;41:897– 910.
47. Cantor-GraaeE,SeltenJ-P. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry 2005;162:12– 24.
48. van der Ven E, Veling W, Tortelli A et al. Evidence of an excessive gender gap in the risk of psychotic disorder among North African immigrants in Europe: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 2016;51:1603–1613.
49. GandalMJ, LeppaV,Won H, ParikshakNN, Geschwind DH. The road to precision psychiatry: translating genetics into disease mechanisms. Nat Neurosci 2016;19:1397– 1407.
50. Carvalho AF, K€ohler CA, Brunoni AR et al. Bias in peripheral depression biomarkers. Psychother Psychosom 2016;85:81–90.
51. Carvalho AF, K€ohler CA, Fernandes BS et al. Bias in emerging biomarkers for bipolar disorder. Psychol Med 2016;46:2287–2297.
52. AllenNC,BagadeS,McQueenMB et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 2008;40:827–834.
53. InoshitaM,NumataS,TajimaA et al. A significant causal association between C-reactive protein levels and schizophrenia. Sci Rep 2016;6:26105.
54. LundahlB,NimerJ,ParsonsB. Preventing child abuse: a meta-analysis of parent training programs. Res Soc Work Pract 2006;16:251–262.
55. NorbergMM,KezelmanS,Lim-HoweN. Primary preven-tion of cannabis use: a systematic review of randomized controlled trials. PLoS ONE 2013;8:e53187.
56. BeardsS,Gayer-AndersonC,BorgesS,DeweyME,Fisher HL,Morgan C. Life events and psychosis: a review and meta-analysis. Schizophr Bull 2013;39:740–747.
57. Geddes JR, Lawrie SM. Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry 1995;167:786–793.
58. GoldsmithDR,RapaportMH,MillerBJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016;21:1696– 1709.
59. GreenMJ,MathesonSL,ShepherdA,WeickertCS,Carr VJ. Brain-derived neurotrophic factor levels in schizophre-nia: a systematic review with meta-analysis. Mol Psychia-try 2011;16:960–972.
60. Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry 2015;2:718–725.
61. VareseF,SmeetsF,DrukkerM et al. Childhood adversi-ties increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 2012;38:661–671.
62. Wang D, Zhai J-X, Liu D-W. Serum folate levels in schizophrenia: a meta-analysis. Psychiatry Res 2016;235: 83–89.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1.Characteristics of 41 associations between environ-mental factors and risk for schizophrenia spectrum disorders.