Peripheral iron levels in children with autism
spectrum disorders vs controls: a systematic review
and meta-analysis
Ping-Tao Tseng
a,b,1, Yu-Shian Cheng
a,1, Yen-Wen Chen
c,1, Brendon Stubbs
d,e,f,
Paul Whiteley
g, Andre F. Carvalho
h, Dian-Jeng Li
i,j, Tien-Yu Chen
k, Wei-Cheng Yang
l,
Chia-Hung Tang
m, Che-Sheng Chu
n,o, Wei-Chieh Yang
p, Hsin-Yi Liang
q,
Ching-Kuan Wu
a, Cheng-Fang Yen
r,s,2, Pao-Yen Lin
t,u,⁎
,2 aDepartment of Psychiatry, Tsyr-Huey Mental Hospital, Kaohsiung Jen-Ai's Home, Taiwan bWinShine Clinics in Specialty of Psychiatry, Kaohsiung, Taiwan
cProspect Clinic for Otorhinolaryngology & Neurology, Kaohsiung, Taiwan
dPhysiotherapy Department, South London and Maudsley NHS Foundation Trust, London, UK
eHealth Service and Population Research Department, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), King's College London, De
Crespigny Park, London, UK
f
Faculty of Health, Social Care and Education, Anglia Ruskin University, Chelmsford, UK
gESPA Research, 2A Hylton Park Rd, Sunderland SR5 3HD, UK
hTranslational Psychiatry Research Group and Department of Clinical Medicine, Faculty of Medicine, Federal University of Ceará, Fortaleza, CE, Brazil iGraduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
jDepartment of Addiction Science, Kaohsiung Municipal Kai-Syuan Psychiatric Hospital, Kaohsiung, Taiwan
kDepartment of Psychiatry, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan l
Department of Adult Psychiatry, Kaohsiung Municipal Kai-Syuan Psychiatric Hospital, Kaohsiung, Taiwan
mDepartment of Psychiatry, Tainan Hospital, Ministry of Health and Welfare, Tainan, Taiwan nDepartment of Psychiatry, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
oCenter for Geriatric and Gerontology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan pDepartment of Pediatrics, DA-AN Women and Children Hospital, Tainan, Taiwan
q
Department of Child Psychiatry, Chang Gung Memorial Hospital at Taoyuan and Chang Gung University, Taoyuan, Taiwan
rDepartment of Psychiatry, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
sDepartment of Psychiatry, School of Medicine, and Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan tDepartment of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan uInstitute for Translational Research in Biomedical Sciences, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
A R T I C L E I N F O A B S T R A C T
Article history:
Received 30 January 2017
Autism spectrum disorder (ASD) is a common neurodevelopmental disorder, and nutritional deficiency may play a role in the development of ASD. A relationship between
Abbreviations:ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CI, confidence interval; ES, effect size; HC, healthy control; ID, iron deficiency; OR, odds ratio.
⁎ Corresponding author at: Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital, 123 Dapi Rd, Niaosong District, Kaohsiung City 833, Taiwan. Tel.: +886 7 7317123x8751; fax: +886 7 7326817.
E-mail address:py1029@adm.cgmh.org.tw(P.-Y. Lin). 1Contributed equally as first authors.
2Contributed equally as corresponding authors.
https://doi.org/10.1016/j.nutres.2017.11.004 0271-5317/© 2017 Elsevier Inc. All rights reserved.
A v a i l a b l e o n l i n e a tw w w . s c i e n c e d i r e c t . c o m
ScienceDirect
Revised 14 October 2017 Accepted 17 November 2017
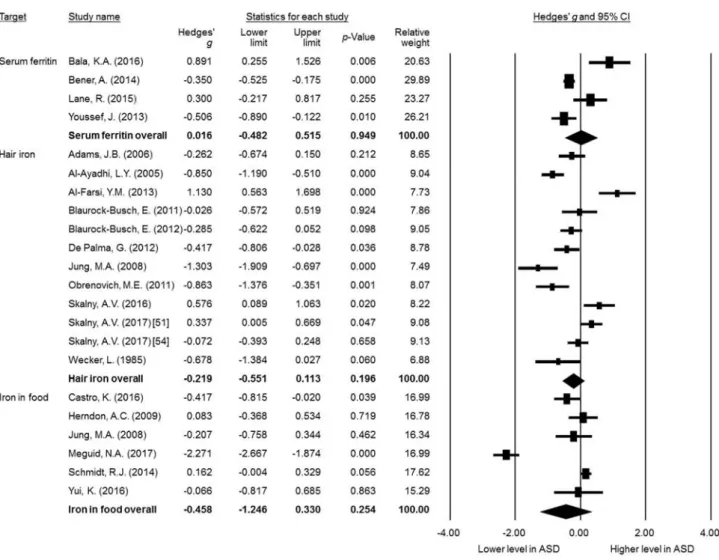
ASD and iron levels/iron deficiency (ID) has been reported; however, the results have been inconsistent. Therefore, we conducted this meta-analysis to examine the relationship between ASD and ID following the Meta-Analysis of Observational Studies in Epidemiology guidelines. We performed a systematic search of PubMed, ScienceDirect, Embase, ProQuest,ClinicalTrials.gov, and Cochrane CENTRAL databases up to September 22, 2017. Studies providing data on peripheral iron levels and/or the prevalence of ID in children with ASD vs those without ASD (non-ASD) were included. Primary outcomes included the difference in peripheral iron levels in children with ASD compared with those without ASD, and the odds ratio of ASD in children with ID compared with those without ID. Twenty-five articles met the inclusion criteria. We found that peripheral iron levels were not significantly different between the ASD and non-ASD groups, including serum ferritin (k = 4,Hedgesg= 0.016, 95% confidence interval [CI] =−0.482 to 0.515,P= .949) or hair iron (k= 12; Hedgesg
=−0.219, 95% CI =−0.551 to 0.113,P= .196). There was no significant difference in the amount of iron
in food content between the ASD and non-ASD groups (k= 6; Hedgesg=−0.458, 95% CI =−1.246 to
0.330,P= .254). However, the reciprocal comorbidity of ASD and ID was significantly higher than in the children without these disorders. Our analysis showed that the available evidence is inconsistent with regard to whether children with ASD have lower iron levels. Future longitudinal studies are required to confirm or refute these associations and elucidate potential mechanisms.
© 2017 Elsevier Inc. All rights reserved.
Keywords:
Autism spectrum disorder (ASD) Ferritin
Iron
Meta-analysis Nutrition Transferrin
1.
Introduction
Autism spectrum disorder (ASD) is a common neurodevelopmental condition. The estimated global prevalence of ASD is approx-imately 1.0%[1]in the pediatric population, with an increasing amount of research focusing on the presence of a dual diagnosis [2]. ASD has been associated with substantial disabilities and impaired quality of life[1,3,4]. In addition, ASD has been consistently associated with a higher prevalence of co-occurring medical and psychiatric conditions[3,4].
ASD is thought to be a predominantly heritable condition, with genetic polymorphisms of dopamine receptors and dopamine transporters playing an important role[5]. The pathoetiology of ASD has yet to be completely elucidated[6], possibly because this diagnostic category encompasses a more heterogeneous group of conditions. Various studies have linked genetic polymorphisms [5], brain connectivity abnormalities[7], dysfunction in neuro-transmitters [8], abnormal transcription/translation of certain genes[9], and prenatal infections or toxin exposure[10]to cases of ASD. In addition, increasing evidence has suggested the possible role of environmental (nongenetic) factors in the etiology of ASD [11]. Such environmental factors (or their effects) are potentially modifiable and may represent preventative targets for ASD and lead to the development of novel therapies for certain aspects of this condition [12]. Among these environmental risk factors, nutritional factors including deficiencies in certain micronutrients have been increasingly implicated[13].
One potential environmental factor linked to ASD is iron, an essential element in child development. Iron plays important roles in homeostasis, including the formation of hemoglobin, antioxi-dants, genetic repair, and in particular central nervous system development[14,15]. Iron is present in various biological“states.”
Bound to transferrin, iron circulates around the body in the bloodstream seeking suitable receptors on the surface of cells. Bound to ferritin, iron is stored safely in the body, protecting it from
the deleterious effects of free iron. Measurements of ferritin levels can provide an important snapshot of iron levels and possible storage disorders[16]. Insufficient iron intake and subsequent iron deficiency (ID) during pre- and postnatal periods may significantly impair neurodevelopment, with long-lasting and possibly irreversible consequences[17]. Moreover, ID may lead to aberrant myelination and impaired synaptogenesis[18,19], and possibly impair the development and functioning of mono-amine neurotransmission systems [17]. For example, ID has been reported to reduce dopamine synthesis and the expres-sions of cognate D1 and D2 receptors[20]. The timing of ID also seems to be relevant, with more detrimental and long-lasting behavioral consequences being observed when ID occurs in the prenatal and early postnatal periods (first 3 years of life) when myelination is occurring at a rapid pace [17]. Adequate iron intake and peripheral iron levels may therefore be important factors modifying the onset of ASD. However, although several studies have reported that ID is prevalent in children diagnosed with ASD [14,21], other studies have reported no difference in iron levels between children with ASD[22,23]and controls.
To date, no meta-analysis has evaluated peripheral iron levels in children with ASD. Therefore, the aim of the current work was to conduct a systematic review and meta-analysis considering differences in peripheral iron levels between children with ASD and healthy (asymptomatic) controls (HCs). In addition, we aimed to explore potential sources of heterogeneity across the included studies.
2.
Approach
defined but unpublished protocol. The current meta-analysis was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-105-12).
2.1. Eligibility criteria
The inclusion criteria were as follows: (a) observational studies, including a cohort or cross-sectional study design comparing peripheral levels of iron, ferritin, or transferrin in children with ASD (confirmed by either a structured or nonstructured diagnostic interview) and controls; (b) studies as formal published articles; and (c) trials in humans. We excluded preclinical studies, review articles, meeting ab-stracts, nonhuman studies, and peer-reviewed original arti-cles published in languages other than English.
2.2. Search strategy and study selection
Two independent authors performed a systematic literature search of PubMed from inception until September 22, 2017, using the keywords(iron OR ferritin OR ferrous) AND (autism OR Asperger). Similar searches were also conducted using ScienceDirect, Embase, ProQuest, ClinicalTrials.gov, and Cochrane CENTRAL databases. The reference lists of the included articles and recent reviews were also searched to identify additional articles[25,26]. Two authors independently screened the titles and abstracts of all retrieved results for potential eligibility. Both authors reviewed the full-text articles of the potentially eligible papers, and a final list of included studies was drafted. Any inconsis-tencies were resolved through discussion with a third reviewer.
2.3. Data extraction
The co-primary outcomes were differences in peripheral iron levels (including iron, ferritin, or transferrin) in children with ASD compared with controls (calculated as Hedgesgstatistic) and the odds ratio (OR) of ASD in children with or without ID. We did not choose differences in means as effect sizes (ESs) of our primary outcome because of presumed differences in units used among each study. The control groups were defined as those without the condition in question (eg, ASD or ID). If data were available, we used HCs (asymptomatic) as the control group. The diagnostic criteria for ID were based on previous studies[27-29]. When data were not available from the included studies, we contacted the primary authors to request the original data. We contacted the authors via e-mail on 2 occasions if required (a second email was sent a week later if no response was received following an initial email). If there were no relevant data in the paper regarding peripheral iron levels or the prevalence of ID, we attempted to use another compatible statistical parameter (eg, OR,t, orPvalue and sample size) to estimate the ESs.
Two independent authors extracted data from the afore-mentioned databases. The variables of interest included the following: prevalence/incidence rates of ASD; prevalence rates of ID; peripheral iron levels in the form of blood iron, blood ferritin, blood transferrin, or hair iron; amount of food iron intake; mean age; sex distribution (% female); mean body mass index; cognitive performance (in the form of mean IQ); parental tobacco smoking and alcohol consumption; ethnic background
(including African, Caucasian, Asian, and Hispanic); parental history of ASD; geographical latitude of where the study was conducted; and the type of assay used to measure iron levels.
2.4. Methodology appraisal
We used the Jadad scale, a so-called Oxford quality scoring system, to evaluate the methodological quality of the recruited studies, including interventional and observational studies. This scale consists of a 3-point questionnaire to assess whether the study described was randomized and double blind, and whether there was a description of study noncompleters (participants who withdrew or dropped out). The summarized score ranged from 0 (poor quality) to 5 (high quality)[30].
2.5. Statistical analyses
The current study was conducted in 2 parts. We first calculated the data considering iron in relation to children with ASD compared with controls. Given the anticipated heterogeneity in the basic population of the studies, we then conducted the meta-analysis with a random-effects model [31]. In brief, random-effects modeling assumes that a genuine diversity among study results is present, and incorporates a between-study variance into the calculations[32]. The primary ES was estimated as Hedgesg
with 95% confidence intervals (CIs) to compare peripheral iron levels in the children with ASD vs controls and the OR and 95% CIs to compare the prevalence of ASD among children with/without ID. The current meta-analyses were conducted using Compre-hensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ). The threshold for statistical significance was set at a 2-tailedP
value < .05.
2.5.1. Heterogeneity, publication bias, and sensitivity test
Heterogeneity was assessed using the Cochran Qtest and its correspondingPvalue[33]. TheI2statistic should be interpreted as the proportion of heterogeneity a study estimates that is due to heterogeneity. We assessed publication bias via the inspection of funnel plots when fewer than 10 data sets were available[34]and with the Egger regression test when more than 10 data sets were available[35]. If there was evidence of publication bias, we used the Duval and Tweedie trim-and-fill procedure, which is a validated model to estimate an ES[36]. Sensitivity analysis was performed by removing one study at a time to detect changes in statistical results, a method which has been widely used in meta-analyses, to verify whether a single study could bias the summary ES estimates[37].
2.5.2. Meta-regression and subgroup meta-analysis
data on each potential moderator were available in at least 5 different studies. We also conducted sensitivity analyses in which 1 study was excluded at a time from the analysis to observe whether an outlier could potentially bias our ES estimates. Subgroup analyses were conducted to determine whether the results differed as a function of the measurement of peripheral iron, ferritin, and transferrin levels in the children with ASD compared with the controls. We performed subgroup analyses whenever data from at least 3 independent data sets were available[38].
3.
Findings
3.1. Study selection
Fig. 1summarizes the details of the search results. In brief, a total of 69 studies were entered into the full-text review stage. Forty-four articles were excluded for various reasons (Fig. 1and Supplemental Fig. S1). A list of the excluded articles is presented in Supplemental Table S2. The remaining 25 articles met the inclusion criteria, and their details are summarized inTable 1.
Among the included studies, 24 compared peripheral iron levels in children diagnosed with ASD vs those without ASD [12,22,23,39-59](ASD children = 1767, HCs = 1887). Two studies compared the prevalence rate of ASD in children with ID vs those without ID[15,40](ID = 3211, controls = 12 082).
3.2. Characteristics and methodological quality of the included studies
Among the recruited studies, the diagnosis of ASD was made according to widely accepted criteria, including the Diagnostic and Statistical Manual of Mental Disorders and the International Classification of Disease codes. The criterion-standard Autism Diagnostic Observation Schedule was also considered [60]. The assays used to detect iron levels included radioimmu-noassay, turbidimetric assay, enzyme-linked immunosorbent
assay, Western blot, the ferrozine method, and chemilumi-nescence (Table 1). Peripheral levels of iron were measured either in serum, plasma, or whole blood and are discussed separately in the following meta-analysis sections. Regarding the methodological quality of the included studies, the average Jadad score was 1.0 (Supplemental Table S3).
3.3. Meta-analysis of peripheral iron levels and prevalence of ASD vs controls
3.3.1. Peripheral ferritin
Our meta-analysis suggested that levels of serum ferritin in the children diagnosed with ASD (n = 391, mean age = 5.4, mean female proportion = 32.6%) did not differ significantly compared with the children without ASD (n = 352, mean age = 6.0, mean female proportion = 43.8%) (k = 4,Hedgesg= 0.016, 95% CI =−0.482 to 0.515,P= .949) (Fig. 2). There was evidence
of significant heterogeneity (Qvalue = 19.70,df= 3,I2= 84.77%,
P < .001,τ = 0.457) but no evidence of publication bias via inspection of funnel plots (Supplemental Fig. S2A). The results were not altered after removing any single study, which indicated that the main results were not affected by any outliers among the recruited studies. Meta-analysis was not performed on plasma ferritin or peripheral blood ferritin[23] because fewer than 3 data sets were available.
3.3.2. Blood iron
The meta-analysis of differences in serum iron between the ASD and control groups was not performed because only 2 data sets were available[51,55]. There were no significant differences in serum iron noted between the ASD and control groups in these 2 studies (P= .409 in Skalny et al (2017)[55]andP= .99 in Skalny et al (2017)[51]). The meta-analysis of differences in plasma iron between the ASD and control groups was not performed because only 1 data set was available[42], in which there was a significantly higher concentration of plasma iron in the ASD group compared with the controls (P< .0001).
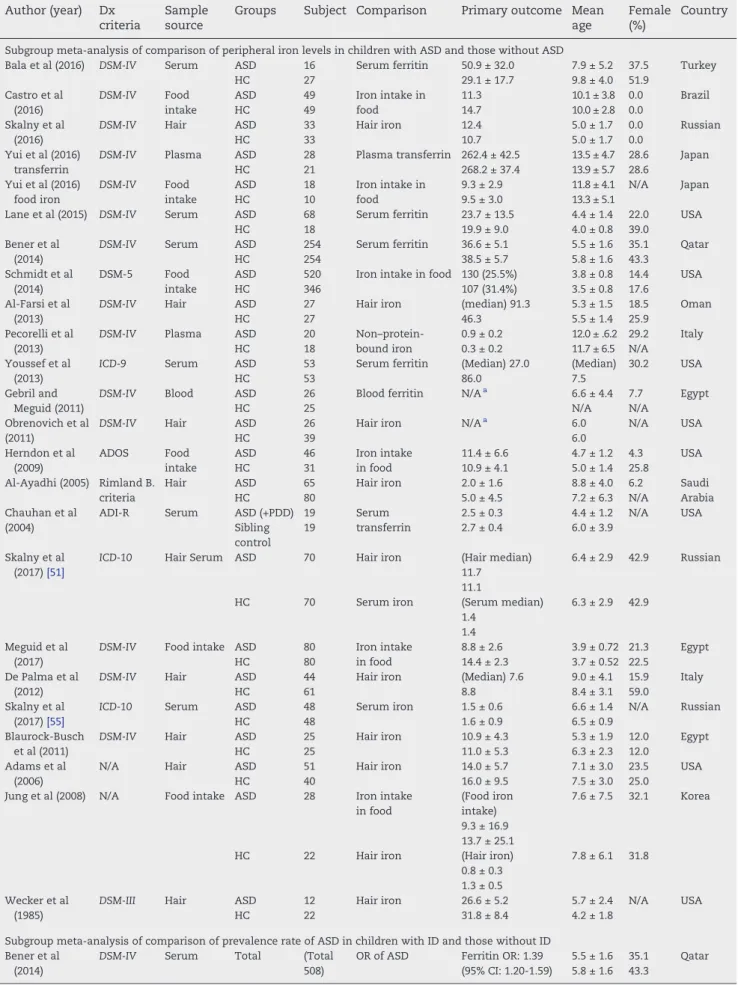
Table 1–Summary of included studies comparing comorbidity of ID and ASD Author (year) Dx
criteria
Sample source
Groups Subject Comparison Primary outcome Mean age
Female (%)
Country
Subgroup meta-analysis of comparison of peripheral iron levels in children with ASD and those without ASD
Bala et al (2016) DSM-IV Serum ASD 16 Serum ferritin 50.9 ± 32.0 7.9 ± 5.2 37.5 Turkey
HC 27 29.1 ± 17.7 9.8 ± 4.0 51.9
Castro et al (2016)
DSM-IV Food intake
ASD 49 Iron intake in
food
11.3 10.1 ± 3.8 0.0 Brazil
HC 49 14.7 10.0 ± 2.8 0.0
Skalny et al (2016)
DSM-IV Hair ASD 33 Hair iron 12.4 5.0 ± 1.7 0.0 Russian
HC 33 10.7 5.0 ± 1.7 0.0
Yui et al (2016) transferrin
DSM-IV Plasma ASD 28 Plasma transferrin 262.4 ± 42.5 13.5 ± 4.7 28.6 Japan
HC 21 268.2 ± 37.4 13.9 ± 5.7 28.6
Yui et al (2016) food iron
DSM-IV Food intake
ASD 18 Iron intake in
food
9.3 ± 2.9 11.8 ± 4.1 N/A Japan
HC 10 9.5 ± 3.0 13.3 ± 5.1
Lane et al (2015) DSM-IV Serum ASD 68 Serum ferritin 23.7 ± 13.5 4.4 ± 1.4 22.0 USA
HC 18 19.9 ± 9.0 4.0 ± 0.8 39.0
Bener et al (2014)
DSM-IV Serum ASD 254 Serum ferritin 36.6 ± 5.1 5.5 ± 1.6 35.1 Qatar
HC 254 38.5 ± 5.7 5.8 ± 1.6 43.3
Schmidt et al (2014)
DSM-5 Food
intake
ASD 520 Iron intake in food 130 (25.5%) 3.8 ± 0.8 14.4 USA
HC 346 107 (31.4%) 3.5 ± 0.8 17.6
Al-Farsi et al (2013)
DSM-IV Hair ASD 27 Hair iron (median) 91.3 5.3 ± 1.5 18.5 Oman
HC 27 46.3 5.5 ± 1.4 25.9
Pecorelli et al (2013)
DSM-IV Plasma ASD 20 Non–protein-bound iron
0.9 ± 0.2 12.0 ± .6.2 29.2 Italy
HC 18 0.3 ± 0.2 11.7 ± 6.5 N/A
Youssef et al (2013)
ICD-9 Serum ASD 53 Serum ferritin (Median) 27.0 (Median) 30.2 USA
HC 53 86.0 7.5
Gebril and Meguid (2011)
DSM-IV Blood ASD 26 Blood ferritin N/Aa 6.6 ± 4.4 7.7 Egypt
HC 25 N/A N/A
Obrenovich et al (2011)
DSM-IV Hair ASD 26 Hair iron N/Aa 6.0 N/A USA
HC 39 6.0
Herndon et al (2009)
ADOS Food
intake
ASD 46 Iron intake 11.4 ± 6.6 4.7 ± 1.2 4.3 USA
HC 31 in food 10.9 ± 4.1 5.0 ± 1.4 25.8
Al-Ayadhi (2005) Rimland B. criteria
Hair ASD 65 Hair iron 2.0 ± 1.6 8.8 ± 4.0 6.2 Saudi
Arabia
HC 80 5.0 ± 4.5 7.2 ± 6.3 N/A
Chauhan et al (2004)
ADI-R Serum ASD (+PDD) 19 Serum
transferrin
2.5 ± 0.3 4.4 ± 1.2 N/A USA
Sibling control
19 2.7 ± 0.4 6.0 ± 3.9
Skalny et al (2017)[51]
ICD-10 Hair Serum ASD 70 Hair iron (Hair median) 11.7 11.1
6.4 ± 2.9 42.9 Russian
HC 70 Serum iron (Serum median)
1.4 1.4
6.3 ± 2.9 42.9
Meguid et al (2017)
DSM-IV Food intake ASD 80 Iron intake in food
8.8 ± 2.6 3.9 ± 0.72 21.3 Egypt
HC 80 14.4 ± 2.3 3.7 ± 0.52 22.5
De Palma et al (2012)
DSM-IV Hair ASD 44 Hair iron (Median) 7.6 9.0 ± 4.1 15.9 Italy
HC 61 8.8 8.4 ± 3.1 59.0
Skalny et al (2017)[55]
ICD-10 Serum ASD 48 Serum iron 1.5 ± 0.6 6.6 ± 1.4 N/A Russian
HC 48 1.6 ± 0.9 6.5 ± 0.9
Blaurock-Busch et al (2011)
DSM-IV Hair ASD 25 Hair iron 10.9 ± 4.3 5.3 ± 1.9 12.0 Egypt
HC 25 11.0 ± 5.3 6.3 ± 2.3 12.0
Adams et al (2006)
N/A Hair ASD 51 Hair iron 14.0 ± 5.7 7.1 ± 3.0 23.5 USA
HC 40 16.0 ± 9.5 7.5 ± 3.0 25.0
Jung et al (2008) N/A Food intake ASD 28 Iron intake in food
(Food iron intake) 9.3 ± 16.9 13.7 ± 25.1
7.6 ± 7.5 32.1 Korea
HC 22 Hair iron (Hair iron)
0.8 ± 0.3 1.3 ± 0.5
7.8 ± 6.1 31.8
Wecker et al (1985)
DSM-III Hair ASD 12 Hair iron 26.6 ± 5.2 5.7 ± 2.4 N/A USA
HC 22 31.8 ± 8.4 4.2 ± 1.8
Subgroup meta-analysis of comparison of prevalence rate of ASD in children with ID and those without ID Bener et al
(2014)
DSM-IV Serum Total (Total 508)
3.3.3. Hair iron
Meta-analysis suggested that the level of hair iron in the children diagnosed with ASD (n = 471, mean age = 6.5, mean female proportion = 19.0%) did not differ significantly compared with those without ASD (n = 617, mean age = 6.4, mean female proportion = 29.4%) (k= 12; Hedgesg=−0.219, 95% CI =−0.551 to
0.113, P = .196) (Fig. 2). There was evidence of significant heterogeneity (Qvalue = 78.418,df= 11,I2= 85.973%,P< .001,
τ= 0.536) but no evidence of publication bias according to the Egger regression test (t= 0.14,df= 10,P= .893). However, after removing the data set by Al-Farsi et al (2013), the results of the meta-analysis showed a significantly lower hair iron level in the ASD group compared with the controls (Hedgesg=−0.328, 95% CI =−
0.630 to−0.026,P= .033)[41]. To explore potential sources of
heterogeneity, we performed meta-regression analysis, which revealed no significant association between the differences in hair iron and clinical variables, including mean age (P= .080) or female proportion (P= .369). Meta-regression analysis of other clinical variables was not performed because fewer than 5 studies provided further information about other clinical variables.
3.3.4. Food iron
Meta-analysis suggested that food iron intake in the children diagnosed with ASD (n = 741, mean age = 4.6, mean female proportion = 14.2%) did not differ significantly compared with those without ASD (n = 538, mean age = 4.6, mean female proportion = 17.8%) (k= 6; Hedgesg=−0.458, 95% CI =−1.246 to
0.330,P= .254) (Fig. 2). There were significant heterogeneity (Q
value = 125.88, df = 5, I2 = 96.03%, P < .001,
τ = 0.954) and significant publication bias according to the inspection of funnel plots (Supplemental Fig. S2B). The adjusted ESs via the Duval and Tweedie trim-and-fill test still revealed insignificant results (Hedgesg=−0.758, 95% CI =−1.518 to 0.002). Moreover,
the results were not altered by the removal of any single study, which indicated that the main results were not affected by any outliers among the recruited studies. To explore potential sources of heterogeneity, we performed meta-regression anal-ysis, which revealed no significant association between differ-ences in hair iron and clinical variables, including mean age (P= .546) and female proportion (P= .732).
3.3.5. Blood transferrin
The meta-analysis of differences in serum transferrin be-tween the ASD and control groups was not performed
because only 1 data set was available[47], in which the level of serum transferrin was significantly lower in the ASD group than in the control group (P < .05). The meta-analysis of differences in plasma transferrin between the ASD and control groups was not performed because only 1 data set was available[22]. The meta-analysis of differences in blood transferrin in the ASD and control groups was not performed because only 1 data set was available [47], in which no significant difference in plasma transferrin was found be-tween the ASD and control groups (P= .56).
3.4. Prevalence and odds of ASD and ID
Meta-analysis of the prevalence rate of ASD in the children with ID was not performed because only 2 studies were included in our analysis [15,40]. The ORs of ASD in the children with ID were, however, significantly elevated (total n with ID = 2957, total n HCs = 11 828), OR = 2.66 in the male children and OR = 4.07 in the female children in the study by Chen et al[15]and OR = 1.39 (P< .001) in the study by Bener et al (total n with ASD = 254, total n of HCs = 254)[40].
4.
Discussion
The results of this review and meta-analysis suggest that there were no differences in the levels of mean serum ferritin or hair iron in the children diagnosed with ASD compared with asymptomatic controls even though children with ID have been reported to be more likely to have ASD than those without ID in previous reports[15,40]. Although we could only perform subgroup meta-analysis of serum ferritin and hair iron levels but not peripheral serum iron or serum transferrin levels because of an insufficient number of studies, previous studies have reported a higher prevalence rate of ASD in children with ID than in those without ID[15,40]. Neverthe-less, there were no significant differences in the levels of serum ferritin or hair iron between the children diagnosed with and without ASD. This could be due to several factors. First is the low number of recruited studies and participants in this part of our meta-analysis. Second, in clinical practice, the dual presentation of ASD and attention-deficit/hyperac-tivity disorder (ADHD) may not be uncommon[61], suggesting that a significantly lower level of serum ferritin in ADHD
Table 1(continued)
Author (year) Dx criteria
Sample source
Groups Subject Comparison Primary outcome Mean age
Female (%)
Country
Chen et al (2013)
ICD-9 Diagnosis IDA 2957 ASD diagnosis 24 (0.8%) 10.6 ± 6.0 64.2 Taiwan
HC 11828 32 (0.3%) 10.6 ± 6.0 64.2
Values are presented as means ± SD or median if specified.
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; AS, Asperger syndrome; CPRS, Conners' Parents Rating Scales; CTRS, Conners' Teacher Rating Scales;DSM-III,Diagnostic and Statistical Manual of Mental Disorders, Third Edition; DSM-IV,Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; Dx, diagnosis;ICD-9,International Statistical Classification of Diseases and Related Health Problems, Ninth Revision;ICD-10,International Statistical Classification of Diseases and Related Health Problems, 10th Revision; IDA, iron deficiency anemia; MA, meta-analysis; Mx, medication; N/A, not available; PDD, pervasive developmental disorder.
Based on the hypothesis of normal distribution of the peripheral iron levels and prevalence of ADHD, we merged the different outcomes from recruited studies into 1 single outcome, the Hedges G (Reference: Hedges LV:Statistical considerations. In:The handbook of research synthesis and meta-analysis. 2nd ed. Edited by Cooper H, Hedges LV, Valentine JC. New York: Russell SAGE foundation; 2009: 38-46).
(standardized mean difference =−0.40, 95% CI =−0.66 to−0.14)
[62]may also occur in those with ASD. Among the 2 studies that examined the prevalence rate of ASD in children with ID [15,40], one was conducted using a database search method rather than direct interviews[15], which may have incurred a higher risk of misdiagnosis due to indirect diagnostic ascer-tainment methods. Third, differences in peripheral iron levels were mainly determined by iron intake [63], although no significant difference was found in the amount of dietary iron intake between the children with and without ASD in our meta-analysis. Finally, the neurotransmitters linked to ASD include dopamine, serotonin, glutamate, andγ-aminobutyric acid[64]. Furthermore, maternal intake of iron supplements was associated with a lower risk of ASD in their offspring, especially in relation to advanced maternal age[12]. Although the symptoms of ASD may not be observed during the early years of life, the activation of brain dysregulation may occur during fetal brain development [65]. A deficiency in iron storage could occur during the critical periods of fetal brain development in ASD, and this may not be measurable after birth. Further studies are needed to investigate iron storage
during fetal development in ASD to elucidate more about the role of iron in the pathogenesis of ASD.
Some limitations of the current study deserve consider-ation. First, the total number of included studies was relatively small, potentially leading to type I and type II errors. Second, the brain represents a central organ with regard to research and hypotheses about ASD. Our primary outcomes focused on peripheral blood or hair iron levels rather than central iron levels because of the lack of studies providing such information. Third, we could not further explore other potential moderators (eg, assay type) because of the inconsistent reporting of these data across the included studies. Fourth, we could not conduct subgroup analyses of cross-sectional studies and cohort studies because prospec-tive data were not provided by any of the studies. Fifth, we could not perform subgroup meta-analysis of plasma ferritin, iron, or transferrin because of the limited number of data sets provided by the studies. Finally, the basic limitation of a meta-analysis is that we could only evaluate the findings from a meta-analysis rather than discovering the actual pathophysiology behind the findings.
5.
Conclusion
The results of our meta-analysis suggest that the relationship between ASD and iron is unclear with respect to serum ferritin, hair iron, and food iron intake. Future longitudinal research is required to evaluate the relationship we observed and to elucidate potential pathophysiological mechanisms of dysregulation of iron metabolism in aberrant brain develop-ment related to ASD. In addition, further studies are required to examine whether early iron supplementation is beneficial for reducing the prevalence rate of ASD.
Supplemental materials to this article can be found online athttps://doi.org/10.1016/j.nutres.2017.11.004.
Acknowledgment
The authors declare that there are no conflicts of interest or funding in relation to the subject of this study. We thank the following authors for providing additional data for our meta-analysis: Al-Ayadhi LY, Bener A, Blaurock-Busch E, Chauhan A, De Felice C, Gebril OH, Obrenovich ME, and Schmidt RJ. The authors state that there are no competing interests in the current study. In addition, the authors declare that no extramural or intramural funding was received for this study.
R E F E R E N C E S
[1] Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383:896–910.
[2] Antshel KM, Zhang-James Y, Faraone SV. The comorbidity of ADHD and autism spectrum disorder. Expert Rev Neurother 2013;13:1117–28.
[3] Matson ML, Matson JL, Beighley JS. Comorbidity of physical and motor problems in children with autism. Res Dev Disabil 2011;32:2304–8.
[4] King BH. Psychiatric comorbidities in neurodevelopmental disorders. Curr Opin Neurol 2016;29:113–7.
[5] Yang MS, Gill M. A review of gene linkage, association and expression studies in autism and an assessment of conver-gent evidence. Int J Dev Neurosci 2007;25:69–85.
[6] Martin A, Volkmar FR, Lewis M. Lewis's child and adolescent psychiatry: a comprehensive textbook. 4th ed. London: Lippincott, Williams & Wilkins; 2007.
[7] Ecker C. The neuroanatomy of autism spectrum disor-der: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2017;21:18–28.
[8] Muller CL, Anacker AM, Veenstra-VanderWeele J. The sero-tonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 2016;321:24–41.
[9] Anitha A, Thanseem I. microRNA and autism. Adv Exp Med Biol 2015;888:71–83.
[10] Murphy CM, Wilson CE, Robertson DM, Ecker C, Daly EM, Hammond N, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016;12:1669–86.
[11] Mandy W, Lai MC. Annual research review: the role of the environment in the developmental psychopathology of autism spectrum condition. J Child Psychol Psychiatry 2016; 57:271–92.
[12] Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol 2014;180:890–900. [13] Fujiwara T, Morisaki N, Honda Y, Sampei M, Tani Y.
Chemicals, nutrition, and autism Spectrum disorder: a mini-review. Front Neurosci 2016;10:174.
[14] Krebs NF. Dietary zinc and iron sources, physical growth and cognitive development of breastfed infants. J Nutr 2000;130: 358S–60S.
[15] Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry 2013;13: 161.
[16] Duck KA, Connor JR. Iron uptake and transport across physiological barriers. Biometals 2016;29:573–91. [17] Doom JR, Georgieff MK. Striking while the iron is hot:
understanding the biological and neurodevelopmental ef-fects of iron deficiency to optimize intervention in early childhood. Curr Pediatr Rep 2014;2:291–8.
[18] Franco PG, Pasquini LA, Perez MJ, Rosato-Siri MV, Silvestroff L, Pasquini JM. Paving the way for adequate myelination: the contribution of galectin-3, transferrin and iron. FEBS Lett 2015;589:3388–95.
[19] Bakoyiannis I, Gkioka E, Daskalopoulou A, Korou LM, Perrea D, Pergialiotis V. An explanation of the pathophysiology of adverse neurodevelopmental outcomes in iron deficiency. Rev Neurosci 2015;26:479–88.
[20] Unger EL, Hurst AR, Georgieff MK, Schallert T, Rao R, Connor JR, et al. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. J Nutr 2012;142: 2040–9.
[21] Verlaet AA, Noriega DB, Hermans N, Savelkoul HF. Nutrition, immunological mechanisms and dietary
immunomodulation in ADHD. Eur Child Adolesc Psychiatry 2014;23:519–29.
[22] Yui K, Imataka G, Kawasak Y, Yamada H. Increased omega-3 polyunsaturated fatty acid/arachidonic acid ratios and up-regulation of signaling mediator in individuals with autism spectrum disorders. Life Sci 2016;145:205–12.
[23] Gebril OH, Meguid NA. HFE gene polymorphisms and the risk for autism in Egyptian children and impact on the effect of oxidative stress. Dis Markers 2011;31:289–94.
[24] Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000;283:2008–12.
[25] Yui K, Imataka G, Nakamura H, Ohara N, Naito Y. Eicosanoids derived from arachidonic acid and their family prostaglan-dins and cyclooxygenase in psychiatric disorders. Curr Neuropharmacol 2015;13:776–85.
[26] Chauhan A, Chauhan V. Oxidative stress in autism. Patho-physiology 2006;13:171–81.
[27] Sidrak S, Yoong T, Woolfenden S. Iron deficiency in children with global developmental delay and autism spectrum disorder. J Paediatr Child Health 2014;50:356–61.
[28] Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997; 277:973–6.
[29] Fuglestad AJ, Georgieff MK, Iverson SL, Miller BS, Petryk A, Johnson DE, et al. Iron deficiency after arrival is associated with general cognitive and behavioral impairment in post-institutionalized children adopted from Eastern Europe. Matern Child Health J 2013;17:1080–7.
randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12.
[31] Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–59.
[32] Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. In: Higgins J, Green S, editors. The Cochrane collaboration; 2009.
[33] Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58.
[34] Higgins JP, Green S. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. 5.1.0 ed. Cochrane Library; 2011.
[35] Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315: 629–34.
[36] Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63.
[37] Tobias A. Assessing the influence of a single study in meta-analysis. STATA Tech Bull 1999;47:15–7.
[38] Davey J, Turner RM, Clarke MJ, Higgins JP. Characteristics of meta-analyses and their component studies in the Cochrane database of systematic reviews: a cross-sectional, descriptive analysis. BMC Med Res Methodol 2011;11:160.
[39] Lane R, Kessler R, Buckley AW, Rodriguez A, Farmer C, Thurm A, et al. Evaluation of periodic limb movements in sleep and iron status in children with autism. Pediatr Neurol 2015;53:343–9. [40] Bener A, Khattab AO, Al-Dabbagh MM. Is high prevalence of
vitamin D deficiency evidence for autism disorder?: in a highly endogamous population. J Pediatr Neurosci 2014;9: 227–33.
[41] Al-Farsi YM, Waly MI, Al-Sharbati MM, Al-Shafaee MA, Al-Farsi OA, Al-Khaduri MM, et al. Levels of heavy metals and essential minerals in hair samples of children with autism in Oman: a case-control study. Biol Trace Elem Res 2013;151:181–6. [42] Pecorelli A, Leoncini S, De Felice C, Signorini C, Cerrone C,
Valacchi G, et al. Non-protein-bound iron and
4-hydroxynonenal protein adducts in classic autism. Brain Dev 2013;35:146–54.
[43] Youssef J, Singh K, Huntington N, Becker R, Kothare SV. Relationship of serum ferritin levels to sleep fragmentation and periodic limb movements of sleep on polysomnography in autism spectrum disorders. Pediatr Neurol 2013;49:274–8. [44] Obrenovich ME, Shamberger RJ, Lonsdale D. Altered heavy
metals and transketolase found in autistic spectrum disor-der. Biol Trace Elem Res 2011;144:475–86.
[45] Herndon AC, DiGuiseppi C, Johnson SL, Leiferman J, Reynolds A. Does nutritional intake differ between children with autism spectrum disorders and children with typical devel-opment? J Autism Dev Disord 2009;39:212–22.
[46] Al-Ayadhi LY. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neuro-sciences (Riyadh) 2005;10:213–8.
[47] Chauhan A, Chauhan V, Brown WT, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin—the antioxidant proteins. Life Sci 2004;75:2539–49.
[48] Bala KA, Dogan M, Kaba S, Mutluer T, Aslan O, Dogan SZ. Hormone disorder and vitamin deficiency in attention deficit hyperactivity disorder (ADHD) and autism spectrum disor-ders (ASDs). J Pediatr Endocrinol Metab 2016;29:1077–82.
[49] Castro K, Faccioli LS, Baronio D, Gottfried C, Perry IS, Riesgo R. Feeding behavior and dietary intake of male children and adolescents with autism spectrum disorder: a case-control study. Int J Dev Neurosci 2016;53:68–74.
[50] Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Radysh IV, Skalnaya MG, et al. Analysis of hair trace elements in children with autism spectrum disorders and communi-cation disorders. Biol Trace Elem Res 2017;177:215–23. [51] Skalny AV, Simashkova NV, Skalnaya AA, Klyushnik TP,
Bjorklund G, Skalnaya MG, et al. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab Brain Dis 2017;32: 1675–84.
[52] Meguid NA, Anwar M, Bjorklund G, Hashish A, Chirumbolo S, Hemimi M, et al. Dietary adequacy of Egyptian children with autism spectrum disorder compared to healthy developing children. Metab Brain Dis 2017;32:607–15.
[53] De Palma G, Catalani S, Franco A, Brighenti M, Apostoli P. Lack of correlation between metallic elements analyzed in hair by ICP-MS and autism. J Autism Dev Disord 2012;42: 342–53.
[54] Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Bjorklund G, Skalnaya MG, et al. Hair toxic and essential trace elements in children with autism spectrum disorder. Metab Brain Dis 2017;32:195–202.
[55] Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Radysh IV, Skalnaya MG, et al. Assessment of serum trace elements and electrolytes in children with childhood and atypical autism. J Trace Elem Med Biol 2017;43:9–14. [56] Blaurock-Busch E, Amin OR, Rabah T. Heavy metals and trace
elements in hair and urine of a sample of arab children with autistic spectrum disorder. Maedica (Buchar) 2011;6:247–57. [57] Adams JB, Holloway CE, George F, Quig D. Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol Trace Elem Res 2006;110:193–209.
[58] Jung MA, Jang HS, Park EJ, Lee HW, Choi JH. Study on the mineral and heavy metal contents in the hair of preschool aged autistic children. J Korean Soc Food Sci Nutr 2008;37:1422–6. [59] Wecker L, Miller SB, Cochran SR, Dugger DL, Johnson WD.
Trace element concentrations in hair from autistic children. J Ment Defic Res 1985;29(Pt 1):15–22.
[60] Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule—WPS edition. Western Psychological Services: Los Angeles, CA; 1999.
[61] Grzadzinski R, Dick C, Lord C, Bishop S. Parent-reported and clinician-observed autism spectrum disorder (ASD) symp-toms in children with attention deficit/hyperactivity disorder (ADHD): implications for practice under DSM-5. Mol Autism 2016;7:7.
[62] Wang Y, Huang L, Zhang L, Qu Y, Mu D. Iron status in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. PLoS One 2017;12:e0169145.
[63] Bhattacharya PT, Misra SR, Hussain M. Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica (Cairo) 2016;2016:5464373. [64] Zurcher NR, Bhanot A, McDougle CJ, Hooker JM. A systematic
review of molecular imaging (PET and SPECT) in autism spectrum disorder: current state and future research oppor-tunities. Neurosci Biobehav Rev 2015;52:56–73.